Differential effects of lycopene consumed in tomato paste and
lycopene in the form of a purified extract on target genes of cancer
prostatic cells
1–3Je´re´mie Talvas, Catherine Caris-Veyrat, Laurent Guy, Mathieu Rambeau, Bernard Lyan, Re´gine Minet-Quinard, Jean-Marc Adolphe Lobaccaro, Marie-Paule Vasson, Ste´phane George´, Andrzej Mazur, and Edmond Rock ABSTRACT
Background: Prospective studies indicate that tomato consumers
are protected against prostate cancer. Lycopene has been hypothe-sized to be responsible for tomato health benefits.
Objective:Our aim was to differentiate the effects of tomato matrix
from those of lycopene by using lycopene-rich red tomatoes, lyco-pene-free yellow tomatoes, and purified lycopene.
Design: Thirty healthy men (aged 50–70 y old) were randomly
assigned to 2 groups after a 2-wk washout period. In a crossover design, each group consumed yellow and red tomato paste (200 g/d, which provided 0 and 16 mg lycopene, respectively) as part of their regular diet for 1 wk separated by 2 wk of washout. Then, in a parallel design, the first group underwent supplementation with purified lycopene (16 mg/d) for 1 wk, whereas the second group received a placebo. Sera collected before and after the interventions were incubated with lymph node cancer prostate cells to measure the expression of 45 target genes.
Results: Circulating lycopene concentration increased only after
consumption of red tomato paste and purified lycopene. Lipid pro-file, antioxidant status, prostate-specific antigen, and insulin-like growth factor I were not modified by consumption of tomato pastes and lycopene. We observed significant up-regulation of IGFBP-3 and Bax:Bcl-2 ratio and down-regulation of cyclin-D1, p53, and Nrf-2 after cell incubation with sera from men who consumed red tomato paste when compared with sera collected after the first washout period, with intermediate values for yellow tomato paste consumption. Cell incubation with sera from men who consumed purified lycopene led to significant up-regulation of IGFBP-3, c-fos, and uPAR compared with sera collected after placebo consumption.
Conclusion:Dietary lycopene can affect gene expression whether
or not it is included in its food matrix. This trial was registered by the French Health Ministry at http://www.sante-sports.gouv.fr as
2006-A00396-45. Am J Clin Nutr 2010;91:1716–24.
INTRODUCTION
Investigations into a causal relation linking food and cancer have been a challenge for scientists in the nutrition/cancer re-search area. However, extending the effects of a complex food to one of its components is controversial. Tomato/lycopene intake and its relation with prostate cancer illustrates this fact and is still a matter of debate (1). However, systematic reviews by the World Cancer Research Foundation (2) and an evidence-based review of studies (1) both concluded that the strongest evidence for an
association between tomato consumption and prostate cancer risk came from 2 large prospective studies (3, 4).
Establishing a causal relation between tomato consumption and prostate cancer risk has led researchers to focus on lycopene. Tomatoes are the main, if not exclusive, provider of dietary lycopene, the carotenoid that gives the fruit its characteristic red color. Lycopene is a potent antioxidant (5), and it accumulates in the prostate (6) where it could protect DNA from oxidative damage, which is a starting point for cancer development (7).
In vitro studies have reported potential mechanisms to explain the effects of lycopene on cancer cell development, including inhibition of cell cycle progression/cell proliferation, inhibition of cell growth via modulation of insulin-like growth factor (IGF) axis, apoptosis induction, improvement in gap-junction com-munication, and antimetastatic activity (for reviews, see refer-ences 8 and 9). Unfortunately, most in vitro results have often been obtained with supranutritional amounts of lycopene.
In vivo, lycopene supplementation in the MatLyLu Dunning rat model induced beneficial effects on genes involved in prostate cancer development concomitantly with a reduction of tumor growth (10). Interestingly, in N-methyl-N-nitrosourea testoster-one–treated rats, greater survival was observed in tomato pow-der–fed animals than in those receiving lycopene (11).
Two independent clinical studies with similar designs in-vestigated the effect of either lycopene supplementation (30 mg/
1From the Human Nutrition Unit (JT, MR, BL, AM, and ER) and Quality
and Security of Plant Products (CC-V), National Institute of Agronomical Research, Clermont-Ferrand, France; the Departments of Urology (LG) and Biochemistry and Molecular Biology (RM-Q), Hospital of Clermont-Ferrand, Clermont-Clermont-Ferrand, France; the Department of Genetics, Reproduc-tion, and Development, University of Blaise Pascal, Clermont-Ferrand, France (J-MAL); the Laboratory of Biochemistry, Molecular Biology and Nutrition, University of Auvergne, Clermont-Ferrand, France (M-PV); and the Nutrition Department, Technical Center for the Conservation of Agricul-tural Products, Avignon, France (SG).
2
Supported by the Institut National du Cancer (postdoctoral grant for JT), La Ligue Contre le Cancer d’Auvergne, and La Re´gion Auvergne. Lycored (Beer-Sheva, Israel) kindly provided the cold-water-dispersible (CWD) ly-copene product (Tomat-O-Red 10% CWD) and the corresponding placebo.
3
Address correspondence to E Rock, Edmond Human Nutrition Unit, Human Nutrition Research Center, Clermont Ferrand-Theix Center, 63122 Saint Gene`s Champanelle, France. E-mail: rock@clermont.inra.fr.
Received September 14, 2009. Accepted for publication March 25, 2010. First published online April 14, 2010; doi: 10.3945/ajcn.2009.28666.
1716 Am J Clin Nutr 2010;91:1716–24. Printed in USA.Ó 2010 American Society for Nutrition
d) (12) or the consumption of tomato-based foods, also providing 30 mg lycopene/d (13), in patients with prostate cancer. In contrast with lycopene alone, the whole-food intervention de-creased the concentration of prostate-specific antigen (PSA) and oxidized DNA in prostate tissue, without affecting the mean Gleason scores. Others have also reported the lack of an effect of purified lycopene (14, 15). In a study in patients with high-grade prostate intraepithelial neoplasia, a transient decrease in PSA concentrations was found with purified lycopene (15) as well as with tomato oleoresin (14).
More investigations are needed to determine whether lycopene alone can provide the bioactivity of that present in whole to-matoes, even if whole-food intervention may present the com-plication of lacking a placebo control (16). On the basis of previous work in rats (17), we used red and yellow tomato species; the latter lacks lycopene but provides a comparable dietary matrix. To further investigate a potential anticarcinogenic role attributable specifically to lycopene, the participants re-ceived supplements in the form of capsules containing either lycopene or a placebo. The biological effects of tomato/lycopene consumption were tested with respect to the expression of target genes after incubation of lymph node cancer prostate (LNCaP) cells with sera provided by the volunteers, collected before and after consumption of tomato pastes and lycopene/placebo capsules.
SUBJECTS AND METHODS Subject and ethics
Thirty healthy, nonsmoking men (aged 50–70 y old) partici-pated in the study. A history of clinical events was recorded for all the subjects, and a physical examination was performed before recruitment. All recruited volunteers had normal blood bio-chemical profiles and appeared to be healthy at physical ex-amination, without any chronic disease and/or medication. Exclusion criteria included use of dietary supplements and specific dietary habits (vegetarians). The experimental protocol was approved by the local ethics committee (Clermont-Ferrand, France) and conducted according to the Declaration of Helsinki and French legislation (the Huriet law). The nature and potential risks of the study were fully explained, and written informed consent was obtained from each participant.
Products
Yellow Lorenzo (De Ruiter, Bergschenhoek, Netherlands) and red Cheers (Seminis, St Louis, MO) tomato varieties were cul-tivated following producers’ guidelines for good practice. The tomatoes were harvested over a period of 3 wk and were delivered to the Technical Center for the Conservation of Agricultural Products (CTCPA Avignon, Avignon, France), which is equipped and certified for food processing. The tomatoes were matured to yield the maximal lycopene concentration from red tomatoes and the lowest concentration of acidity for both red and yellow to-matoes. Low acidity was expected to increase the sugar content of tomatoes and thereby to increase their palatability and the compliance of the volunteers. Moreover, to minimize differences in other constituents, the tomatoes were grown in the same field, harvested on the same days, and transformed with similar pro-cesses. After sorting and washing, the freshly chopped tomatoes
were rapidly heated to .90°C. This hot-break treatment was followed by a refining process. The resulting product was con-centrated, pasteurized, and canned. Chemical contents of the tomato pastes were determined by HPLC as previously de-scribed (18) (Table 1). The purified lycopene and placebo for-mulations are shown in Table 2.
Experimental design
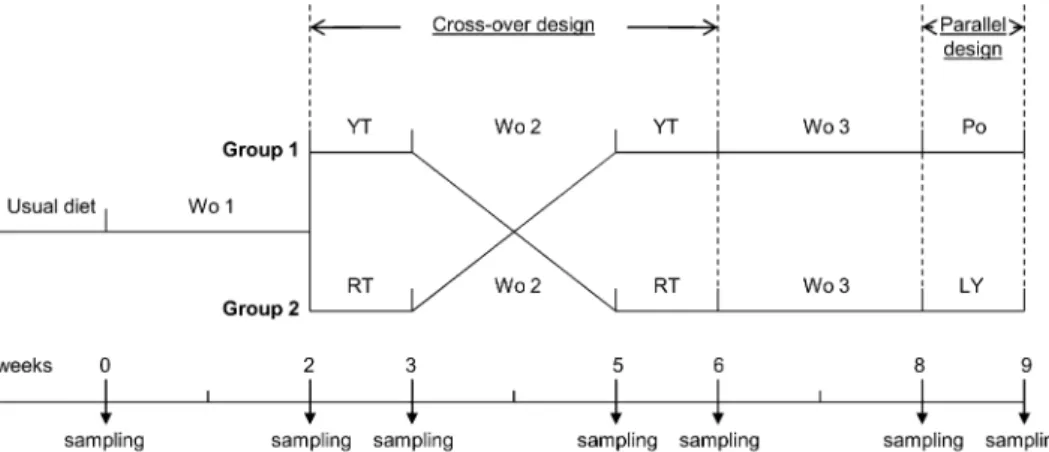
The study protocol was registered with the General Health Department of the French Ministry of Health and approved by the regional Medical School Ethics Committee. The study design consisted of a randomized, single-blinded crossover study for tomato paste studies and a parallel study for lycopene studies (Figure 1). After inclusion, the volunteers continued consuming their usual diet for 1 wk followed by a 2-wk washout period on a lycopene-free diet. The individuals were then randomly as-signed into 2 groups: group 1 consumed a daily supplement (200 g/d for 1 wk) of yellow tomato paste (YT), whereas group 2 received the same amount of red tomato paste (RT). After an additional washout period (2 wk), group 1 consumed the RT and group 2 consumed the YT. Then, after a final washout period (2 wk), group 1 was given a daily capsule providing lycopene at the same concentration as that contained in the RT (16 mg/d), whereas group 2 daily received a placebo capsule for 1 wk. Tomato pastes and capsules were consumed offsite. During all of the study periods, volunteers were asked not to use sources of tomato/lycopene other than those provided. Pastes were used by the volunteers for processing their meals (eg, lasagna, pizza, tomato sauce) or diluted with water (tomato juice). Participants otherwise maintained their habitual diets and lifestyle. Before each experimental period, volunteers were given cans of either YT or RT for their weekly consumption. At the end of each experimental period, volunteers returned the unused cans, and compliance was also evaluated by an interview with a dietitian.
Blood and urine collection
At the end of each period, 24-h urine samples were collected and separated into aliquots after volume determination and stored at280˚C. Blood samples were obtained from each volunteer in a dry-gel separating tube (Vacutainer; BD Biosciences, Le Pont
TABLE 1
Composition of tomato pastes1
Tomato paste content (n = 3)
YT RT lg/g dry matter Lycopene 0 5426 82 b-Carotene 3.636 0.8 75.86 2.82 Lutein 4.86 0.4 9.36 1.43 Zeaxanthin 0.686 0.2 2.86 1.23 Vitamin A ND ND Vitamin E 1146 3 2016 43 Vitamin C 2626 4 3606 43 1
All values are means 6 SEMs. YT, yellow tomato paste; RT, red tomato paste; ND, not detectable.
2,3
Significantly different from YT (unpaired t test):2P, 0.001,3P, 0.05.
de Claix, France) and in EDTA-coated tubes (Vacutainer; BD Biosciences). Plasma was obtained after centrifugation (12,000· g, 4 min, 4°C) and stored at 280°C until further analyses. For plasma vitamin C determination, samples were immediately de-proteinized with the addition of 5% metaphosphoric acid (2:1, vol:vol) before storage at280°C. Serum was obtained after 1 h clotting, centrifuged for 10 min at 1000 · g, separated into ali-quots, and stored at280°C until further analysis.
Carotenoids and vitamins A, E, and C
One volume of serum was first deproteinized with 1 volume of ethanol, and then carotenoids and vitamins A and E were extracted twice with 2 volumes of hexane. Tomato paste extracts were made as described (18). The extracts were analyzed by HPLC as scribed (19). Total vitamin C in deproteinized serum was de-termined according to the method previously described (20).
Total cholesterol and triglycerides
Plasma triglycerides and total cholesterol were enzymatically determined by using appropriate commercial kits (PAP 150 and RTU, respectively; BioMe´rieux, Marcy l’Etoile, France). Oxidative stress parameters
The ferric reducing ability of plasma (FRAP) was determined in the plasma samples (25lL), which were diluted twice with
distilled water by using the Benzie and Strain method (21) as described previously (18).
Oxygen radical absorbance capacity (ORAC) was based on the detection of oxidative damage of b-phycoerythrin through the decrease in its natural fluorescent emission after oxidative at-tack. AAPH [2,2#-azobis (2-amidino-propane) dihydrochloride] was used as a peroxylradical generator, and Trolox (Sigma-Aldrich, Saint-Quentin Fallavier, France) was used as an anti-oxidant standard. The assay was carried out at 37°C by using spectrofluorimetry (excitation wavelength, 540 nm; emission wavelength, 565 nm); data were recorded every 5 min for 60 min, until the final value was,5% of the initial one.
Urinary F2-isoprostane (15-F2t-isoprostane) concentration was determined by using a commercial kit (Oxford Biomedical Research, Oxford, United Kingdom) based on competitive ELISA and performed according to the manufacturer’s instructions.
Serum PSA and IGF-I
PSA concentrations were measured by sandwich electro-chemiluminescence immunoassay (Roche Modular Analytics E170; Roche Diagnostics, Meylan, France). The PSA detection limit of this system was 0.003–100 ng/mL. The analyte (PSA) formed a sandwich complex with 2 antibodies. One of the antibodies was marked with ruthenium-tris-(2,2-bispyridyl) and the other with biotin. The addition of streptavidin-coated, iron-containing microparticles provided the means for magnetic separation of the bound ternary complex in the measurement cell. Electroluminescence of the bound ruthenium-tris-(2,2-bispyridyl) was directly proportional to the analyte concentration in the sample.
IGF-I was measured by automated 2-site sandwich immu-noassay with chemiluminescent detection (Immulite 2000; Sie-mens Health Care Diagnostics, Saint-Denis, France). The IGF-I detection limit of this system was 25–1600 ng/mL. Sample pretreatment was first performed in an onboard dilution step. Then, the sample and alkaline phosphatase–conjugated anti-IGF-I antibodies were simultaneously incubated with antibody-coated beads. During this time, the IGF-I in the sample formed an antibody sandwich complex that bound to the streptavidin on the
TABLE 2
Purified lycopene and placebo formulations1
Ingredients Tomat-O-Red 10% CWD Placebo
% %
CWD natural tomato lycopene 10.1 0
Sucrose esters, soy lecithin, Arabic gum, modified starch, maltodextrin matrix
89.9 100
1
Lycopene product and placebo formulations were provided by Lycored, Beer-Sheva, Israel. CWD, cold-water-dispersible.
FIGURE 1.Clinical study design. Washout (Wo) refers to a 2-wk period during which the volunteers consumed their usual diet depleted of all sources of lycopene. In the crossover study, the volunteers consumed 200 g yellow tomato paste (YT) or 200 g red tomato paste (RT) daily for 1 wk, corresponding to 0 and 16 mg lycopene, respectively. During the 1-wk parallel study, the volunteers from the first group (Group 1) consumed 16 mg purified lycopene (LY) daily, and the volunteers from the second group (Group 2) consumed the placebo capsules (Po) daily. Wo 1, 2, and 3 indicate the first, second, and third washout periods, respectively.
beads. Unbound enzyme conjugate was then removed by washing, after which substrate was added. The chemiluminescent substrate, a phosphate ester of adamantyl dioxetane, underwent hydrolysis in the presence of alkaline phosphatase to yield an unstable intermediate. Continuous production of these inter-mediates resulted in the sustained emission of light. The photon output was directly proportional to the concentration of the IGF-I in the specimen.
Cell culture
The LNCaP prostatic cell line was obtained from the American Type Culture Collection (ATCC; Manassas, VA). The propaga-tion medium consisted of ATCC complete growth medium: RPMI 1640 medium with 2 mmol glutamine/L adjusted to contain 20 mmol sodium carbonate/L, 25 mmol glucose/L, 10 mmol HEPES/L, 1.0 mmol sodium pyruvate/L, and a mix of antibiotics (10 lg gentamycin/mL, 100 IU penicillin, 100 lg streptomycin/mL), supplemented with 10% (vol:vol) fetal bo-vine serum. For propagation, cells were routinely seeded in an 8-cm-diameter plate (5· 103cells/plate). For the cell proliferation assay, cells were seeded for 48 h in a 96-well plate in complete medium with 10% (vol:vol) human serum. For reverse tran-scriptase–quantitative polymerase chain reaction (RT-qPCR) analyses, cells were seeded in 5-cm-diameter plates under the same conditions in complete medium plus 10% human serum for 48 h. The 48-h incubation time was chosen on the basis of previous assays showing optimum cellular uptake of lycopene (17) and was also verified during the study.
Cell proliferation
The effect of sera on LNCaP cell proliferation was determined by using the nonradioactive bromodeoxyuridine (BrdU)–based cell proliferation assay (Roche, Basel, Switzerland) according to the manufacturer’s protocol with some modifications.
The effects of lycopene and its placebo were also assessed in a dose-response experiment (0–5 lmol/L). A solution of 0.1 mmol/L was prepared in the culture medium. The same amount of placebo powder was also dissolved in the culture medium. The equivalent volume of placebo was adjusted to the volume of tested lycopene solution. Lycopene and placebo effects were determined relative to a control value obtained by cell in-cubation in a medium with neither lycopene nor placebo.
LNCaP cells (5· 103 cells/well) were incubated for 24 h in a 96-well plastic plate in complete medium. For the assay, media from the plates were removed, and media containing human sera or lycopene (or its placebo) were added for 24 h. Twenty mi-croliters of BrdU was then added, and cells were cultured for another 24 h. Incorporation of BrdU into DNA was determined by measuring the absorbance at 450 nm on an ELISA plate reader. mRNA expression analyses by TaqMan low-density array RT-qPCR
Total RNA was prepared by using the RNeasy mini kit according to the manufacturer’s instructions (Qiagen, Courtabeuf, France). RNA concentration was determined with a NanoDrop 1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE).
Reverse transcription of RNA was performed by using the High-Capacity cDNA Reverse Transcription kit (Applied
Bio-systems, Foster City, CA). Predesigned TaqMan low-density array (TLDA; 48 TaqMan Gene Expression assays preconfigured in a 384-well format spotted on microfluidic cards; Applied Biosystems), were used in a 2-step RT-PCR process using the ABI Prism 7900HT Sequence Detection System (Applied Bio-systems) with a TLDA upgrade (Applied BioBio-systems). Each cDNA sample (100lL) was added to an equal volume of 2 · TaqMan Universal PCR Master Mix (Applied Biosystems). After gentle mixing, the solution was transferred into a loading port on a TLDA card (Applied Biosystems). Each sample was analyzed in a single TLDA run for each of the 10 TLDAs by using an Applied Biosystems 7900 HT Fast-Real-Time PCR System. Thermal cycle conditions were as follows: 2 min at 50° C, 10 min at 94.5°C, 30 s at 97°C, and 1 min at 59.7°C for 40 cycles. Data acquisition was performed according to the man-ufacturer’s suggestions. TaqMan gene expression assays (Ap-plied Biosystems) were used for quantification of mRNA of the respective genes. The expression of 16 housekeeping genes was measured by TLDA as a human endogenous control for each condition in triplicate. Glyceraldehyde-3-phosphate de-hydrogenase (GAPDH), transferrin receptor (TFRC), and pepti-dylprolyl isomerase A (cyclophilin A; PPIA) showed good average expression stability [,0.2 crossing threshold (Ct)] (data not shown) and were selected as housekeeping genes with the software program geNorm (Center for Medical Genetics, Ghent, Belgium; available from http://medgen.ugent.be/) (22). For anal-ysis, expression levels of target genes were normalized to GAPDH, TFRC, and PPIA for each plate. Gene expression values were then calculated on the basis of theDDCt method with nor-malized data. The washout sera (WoS) condition, collected after the first washout period, were used as reference for the yellow-tomato sera (YTS) and red yellow-tomato sera (RTS) collected after tomato paste consumption. Similarly, the placebo sera (PoS) col-lected after placebo capsule consumption were used as a reference for the lycopene supplement sera (LYS) collected after lycopene capsule consumption. Relative quantities (RQs) were determined by using the equation RQ = 22DDCt. Means of RQs obtained with the 3 housekeeping genes were used. Only genes with re-producible amplification curves were analyzed and presented.
Western blotting for IGF binding protein 3 (IGFBP-3) was performed as previously described (23)
Statistics
Repeated-measures analysis of variance followed by Tukey-Kramer posttests were performed by using data from tomato paste consumption (YTS and RTS) compared with those obtained after the first washout period (WoS). An unpaired t test was applied to data from lycopene (LYS) and placebo (PoS) con-sumption. All statistical analyses were performed with Graph-Pad InStat 3 software (GraphGraph-Pad, San Diego, CA).
RESULTS Clinical study
The tomato paste composition analyses showed that YT did not contain any trace of lycopene, whereas RT did contain ly-copene. RT also contained a significantly higher concentration of b-carotene (Table 1).
None of the 30 subjects recruited into the study dropped out. All volunteers complied with consumption rules for both RT and YT. No side effects (eg, diarrhea, carotenodermia) were described by the volunteers or noticed by the investigators. Measurements of physiologic and biochemical variables of the volunteers who were randomly assigned to each intervention group are presented in Table 3. None of the anthropometric, physiologic, and bio-chemical variables measured at the time of inclusion signifi-cantly differed between the 2 groups. Similar values for biomarkers of oxidant status, as determined by the plasma total antioxidant capacity (FRAP and ORAC) and the urinary con-centration of F2-isoprostanes, were measured in the 2 groups. There were no significant differences in serum concentrations of PSA and IGF-I.
As shown in Table 4, consumption of RT resulted in a sig-nificantly higher serum lycopene concentration compared with the washout and YT periods, whereas consumption of YT had no effect on plasma lycopene concentration compared with the washout periods. Serum concentrations of lutein and vitamins A and E were not affected by consumption of tomato pastes and depletions. A slight but significantly higher serum vitamin C concentration was observed in both RT and YT groups com-pared with the washout period. A significantly higher serum b-carotene concentration was measured in the RT group than in the YT group. All data from serum fat-soluble micronutrients were standardized with triglyceride concentrations.
As shown in Table 4, lycopene supplementation induced a higher lycopene concentration in the LYS compared with the PoS. The concentration attained after lycopene supplementation did not significantly differ from that attained after RT
con-sumption. The very low concentration of lycopene obtained after placebo intake is due to the long depletion period (ie, 6 wk) without any sources of lycopene. Note that plasma antioxidant capacity and concentrations of urinary F2-isoprostanes and of IGF-I and PSA were close to baseline measurements in all groups (Table 3) after both washout and intervention periods.
Ex vivo study: mRNA accumulation
The accumulation of mRNA was measured by using cus-tomized TLDAs after incubation of LNCaP cells with WoS, YTS, RTS, PoS, and LYS. Forty-five genes were chosen for the ex-periment because of their implication in prostate carcinogenesis and their susceptibility to regulation by nutrients. Among these genes, 8 were not amplified (namely, a-reductase-1, estrogen receptor-1, E-selectin, metalloproteinase-9, V-CAM, cyclo-oxygenase-2, IL-6 and IL-1-a), whereas 4 showed Ct values that were too high (.31 Ct) to be considered (FABP-3, IGF-1, connexin 43, and PTEN). The fold inductions of each gene were between 0.8 and 2.5 and are given in Table 5. In comparison with the WoS condition, only a few genes were significantly regulated when LNCaP cells were incubated with YTS and RTS. Cyclin D1, p53, and Nrf-2 were down-regulated after incubation with RTS, whereas the Bax:Bcl-2 ratio and IGFBP-3 were in-duced under the same conditions. Cell incubations with YTS showed intermediate values, without attaining statistical signif-icance.
Incubation of LNCaP cells with LYS induced an up-regulation of IGFBP-3 but also of c-Fos and uPAR . The 2 latter genes were
TABLE 3
Baseline characteristics of volunteers1
Characteristics Group 1 (n = 15) Group 2 (n = 15) Inclusion criteria (range)
Age (y) 60.76 1.42 60.96 1.9 50–70 y
BMI (kg/m2) 25.96 0.4 25.86 0.5 24–28 kg/m2
Systolic pressure (mm Hg) 1336 2 1366 2.9 No hypertension
Diastolic pressure (mm Hg) 786 2 836 2 No hypertension
Plasma
Glucose (mmol/L) 5.36 0.1 5.3 06 0.1 4.0–6.0 mmol/L
Total cholesterol (mmol/L) 5.36 0.2 5.46 0.2 3.0–8.0 mmol/L
Triglycerides (mmol/L) 1.16 0.1 1.26 0.2 0.5–3.0 mmol/L
Sodium (mEq/L) 142.46 0.4 143.76 0.5 135–145 mEq/L
Potassium (mEq/L) 4.516 0.1 4.456 0.1 3.8–4.9 mEq/L
Urea (lmol/L) 6.196 0.3 6.636 0.4 3–7.5 mmol/L
Creatinine (mmol/L) 84.606 1.8 84.076 2.9 65–120lmol/L
Aspartate amino transferase (IU/L) 286 2 256 1 10–40 IU/L
Alanine amino transferase (IU/L) 276 4 226 2 10–50 IU/L
Alkaline phosphatase (IU/L) 666 3 636 3 35–100 IU/L
c-Glutamyl-transpeptidase (IU/L) 346 3 276 3 10–50 IU/L
C-reactive protein (mg/L) ,2.3 6 0.5 ,1.9 6 0.26 ,6 mg/L
Ferric reducing ability of plasma (lmol Fe2+/L) 778.96 39.4 747.06 17.1 —
Oxygen radical absorbance capacity (lmol TE/L) 13,6386 436 13,2476 454 —
Serum
Prostatic-specific antigen (ng/mg) 2.426 0.83 1.556 0.41 —
Insulin-like growth factor I (ng/mg) 135.56 7.11 115.56 8.36 —
Urine samples (24 h)
F2-isoprostanes (ng/mg creatine) 2.326 0.66 1.996 0.56 —
1
TE, Trolox equivalent. No statistical differences were observed between groups as measured by unpaired t test.
2
Mean6 SEM (all such values).
not affected by sera from the same individuals who consumed RT.
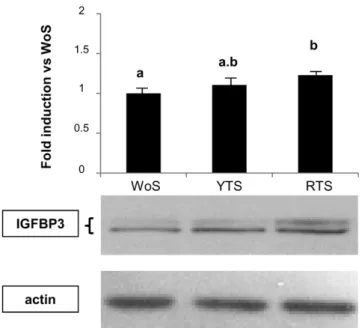
To validate the TLDA results, the expression of 4 genes regulated by RTS, LYS, or both conditions was examined. The up-regulation of IGFBP-3 and uPAR as well as the increase of Bax:Bcl-2 ratio were confirmed by RT-qPCR (data not shown). The IGFBP-3 result was further confirmed by Western blot analysis (Figure 2).
Cell proliferation
The BrdU incorporation assay failed to show any decrease of proliferation after the incubation of LNCaP cells with medium containing 10% human serum (YTS, RTS, and LYS) compared with WoS or PoS, as appropriate. To better assess potential effects of lycopene on cell proliferation, a dose-response experiment was performed with lycopene and its placebo. A dose of 5lM ly-copene was necessary to significantly reduce cell proliferation (Figure 3). This concentration was far above the concentrations of lycopene reached with the sera of the volunteers (60, 30, and 20 nM for RTS, YTS, and PoS, respectively). As expected, the placebo had no effect (Figure 3).
DISCUSSION Clinical study
The design of this study allowed us to increase serum lycopene concentration after consumption of RT and lycopene capsules (16 mg lycopene/d) and to decrease it after consumption of YT and placebo capsules. Similar intakes are achieved by diet in several countries (24). Minor effects on the status of other carotenoids and fat-soluble vitamins that were otherwise due to the usual diet of the volunteers were observed. No modification of the oxidative stress–related biomarkers PSA and IGF-I occurred after nutri-tional interventions, probably reflecting the good health status of the volunteers. After intervention with tomatoes, improvement of redox status, PSA, and IGF-I is often reported in patients with prostate cancer (13, 25) and in patients with benign prostatic hyperplasia (26).
Ex vivo study
The ex vivo approach aimed to determine the effect of sera on the expression of target genes of prostate cancer. Incubation of LNCaP cells with sera from healthy volunteers was performed to provide cells with near-physiologic concentrations of micro-nutrients and their metabolites.
mRNA accumulation
Only a few of the 45 genes plotted in the custom-made array were significantly modulated by sera obtained after either ly-copene or yellow/red-tomato consumption when compared with the PoS and WoS. The level of regulation was expectedly low when using nutrients close to physiologic concentrations (27).
Global comparison of the fold induction of regulated genes showed a systematic intermediate effect of YTS despite similar concen-trations of lycopene found in YTS and WoS. Therefore, yellow tomatoes cannot be considered a purely negative control for red tomatoes, and this also indicates that other compounds/metabolites in addition to lycopene are likely involved in the gene expression changes induced by sera from individuals who consume red tomatoes. Regulated genes were implicated in many functions, including cell cycle regulation (cyclin-D1), apoptosis (Bax:Bcl-2), stress response (p53, Nrf-2), and carcinogenesis (uPAR, c-Fos), and a more systemic role was implied for IGFBP-3, which is involved in cell proliferation and apoptosis (28). The weak down-regulation of p53 and Nrf-2 after RTS incubation might not be biologically relevant because these proteins are known to be regulated post-translationally by specific inhibitors (mouse double minute-2 and kelch-like ECH-associated protein-1, respectively) (29, 30). Moreover, MDM-2 mRNA and antioxidant response element (ARE)–responsive genes (SOD-1, GPX-1) regulated by nuclear factor (erythroid-derived 2)-related factor-2 were unaffected.
IGFBP-3 is the main binding protein for IGF-I in plasma (for review, see reference 28). Furthermore, IGFBP-3 not only modulates IGF-I action but also regulates cell growth, pro-liferation, and apoptosis in an IGF-I-independent manner. Liu et al (31) showed in ferrets exposed to cigarette smoke that synthetic lycopene supplementation was able to inhibit lung squamous metaplasia and to induce apoptosis via up-regulation
TABLE 4
Effects of tomato paste consumption and purified lycopene supplementation on serum concentrations of carotenoids and vitamins1
Carotenoids and vitamins
Serum concentration
WoS (n = 30) YTS (n = 30) RTS (n = 30) PoS (n = 15) LYS (n = 15)
lmol/lg TG Lycopene 0.436 0.07a 0.316 0.04a 0.786 0.11b 0.306 0.03 0.876 0.102 b-Carotene 1.046 0.18a,b 0.956 0.15a 1.256 0.18b 1.326 0.20 1.436 0.29 Lutein 0.586 0.11 0.626 0.09 0.606 0.10 0.786 0.12 0.796 0.19 Vitamin A 2.306 0.25 2.466 0.19 2.336 0.22 3.006 0.31 3.306 0.32 Vitamin E 32.376 3.37 34.806 2.55 34.806 3.18 44.136 4.53 48.166 4.15 Vitamin C 43.756 4.06a 49.506 3.78b 49.676 3.57b 49.556 4.14 51.986 4.06 1
All values are means 6 SEMs. Concentrations of lycopene, b-carotene, lutein, and vitamins A and E are normalized with plasma triglyceride concentrations. WoS, first washout serum; YTS, yellow tomato serum; RTS, red tomato serum; PoS, placebo serum; LYS, lycopene serum. Values with different superscript letters are significantly different (P, 0.05; ANOVA with repeated measures followed by Tukey-Kramer posttests for WoS, YTS, and RTS).
2
Significantly different from PoS, P, 0.05 (unpaired t test).
of IGFBP-3. Moreover, in human prostate cancer (PC-3) cells, another prostatic cell line, both secreted and cytosolic IGFBP-3 is induced after 48 h incubation with lycopene, concomitant with growth arrest and induction of apoptosis (32). We observed up-regulation of IGFBP-3 after incubation of LNCaP cells with RTS as well as with LYS, suggesting that lycopene contributes to the regulation of IGFBP-3 expression.
Previous reports have indicated that IGFBP-3 induced apo-ptosis through regulation of the Bcl-2 protein family (33, 34). In PC-3 cells, lycopene induced apoptosis, altered the cell cycle distribution, down-regulated the expression of cyclin D1 and Bcl-2, and up-regulated Bax, resulting in an inhibition of cell pro-liferation (35). However, these effects were described for high amounts of lycopene. In the present study in LNCaP cells,
up-TABLE 5
Effects of sera derived from men who consumed tomato pastes and purified lycopene on mRNA accumulation in lymph node cancer prostate cells1
Fold changes
Genes Assay ID WoS (n = 20) YTS (n = 20) RTS (n = 20) PoS (n = 10) LYS (n = 10)
Cell to cell b-Catenin CTNNB1-Hs00170025_m1 1.006 0.02 1.036 0.03 1.016 0.04 1.006 0.04 1.006 0.05 Growth signaling AKT AKT1-Hs00178289_m1 1.006 0.02 1.006 0.03 1.026 0.03 1.006 0.03 1.066 0.05 GSK3b GSK3B-Hs00275656_m1 1.006 0.03 0.996 0.05 0.936 0.03 1.006 0.07 0.996 0.10 Apoptosis Bad BAD-Hs00188930_m1 1.006 0.02 0.966 0.05 1.066 0.06 1.006 0.18 0.846 0.09 Bax BAX-Hs00180269_m1 1.006 0.03 0.996 0.04 1.026 0.04 1.006 0.06 1.006 0.04 Bcl-2 BCL2-Hs00608023_m1 1.006 0.05 1.036 0.06 0.946 0.05 1.006 0.05 1.076 0.07 Bax/Bcl-2 — 1.006 0.06a 1.006 0.12a,b 1.126 0.06b 1.006 0.09 0.936 0.11 Caspase 3 CASP3-Hs00234387_m1 1.006 0.02 0.956 0.03 0.966 0.04 1.006 0.05 1.036 0.10 I-jB-a NFjBIA-Hs00153283_m1 1.006 0.03 1.016 0.04 0.986 0.03 1.006 0.04 1.046 0.05 Metastasis uPAR PLAUR-Hs00182181_m1 1.006 0.06 0.956 0.06 1.096 0.15 1.006 0.08 2.566 0.292 Cell cycle Cyclin D1 CCND1-Hs00277039_m1 1.006 0.02a 0.966 0.03a,b 0.926 0.02b 1.006 0.03 1.006 0.05 p21 CDKN1A-Hs00355782_m1 1.006 0.04 0.936 0.04 0.936 0.03 1.006 0.06 0.906 0.04 p27 CDKN1B-Hs00153277_m1 1.006 0.02 1.006 0.04 0.966 0.03 1.006 0.03 1.076 0.09 Biomarkers AMACR AMACR-Hs01091294_m1 1.006 0.04 0.986 0.03 0.986 0.06 1.006 0.06 1.186 0.12 PSA KLK3-Hs03063374_m1 1.006 0.05 1.036 0.05 1.016 0.03 1.006 0.06 1.086 0.05 Inflammatory response TNF-a TNF-Hs00174128_m1 1.006 0.10 0.986 0.09 1.056 0.11 1.006 0.14 1.026 0.14 STAT3 STAT3-Hs00374280_m1 1.006 0.03 1.006 0.04 0.986 0.03 1.006 0.03 1.026 0.04 Oncogenes c-Myc MYC-Hs00153408_m1 1.006 0.02 1.056 0.04 1.046 0.03 1.006 0.05 0.986 0.04 c-Fos FOS-Hs00170630_m1 1.006 0.04 1.206 0.12 1.056 0.05 1.006 0.09 1.206 0.102 c-Jun JUN-Hs99999141_s1 1.006 0.02 1.056 0.04 0.986 0.03 1.006 0.04 0.956 0.05 p53 TP53-Hs00153349_m1 1.006 0.03a 0.936 0.04a,b 0.906 0.04b 1.006 0.09 0.956 0.06 MDC-2 MDM2-Hs01066938_m1 1.006 0.05 1.006 0.09 0.926 0.06 1.006 0.16 0.826 0.10
Sexual hormone receptor
AR AR-Hs00171172_m1 1.006 0.03 1.016 0.03 0.986 0.02 1.006 0.05 1.016 0.04 Oxidative stress GPX-1 GPX1-Hs00829989_gH 1.006 0.03 1.096 0.07 1.026 0.06 1.006 0.09 1.126 0.12 Nrf-2 NFE2L2-Hs00232352_m1 1.006 0.02a 0.976 0.03a.b 0.916 0.02b 1.006 0.02 1.016 0.08 SOD-1 SOD1-Hs00533490_m1 1.006 0.02 1.036 0.04 1.006 0.04 1.006 0.05 1.076 0.05 Growth factor IGF-1R IGF1R-Hs00181385_m1 1.006 0.02 1.036 0.04 1.006 0.04 1.006 0.05 1.136 0.07
IGFBP-3 IGFBP3-Hs00181211_m1 1.006 0.05a 1.116 0.07a,b 1.296 0.14b 1.006 0.14 1.396 0.212
Angiogenesis
HIF-1a HIF1A-Hs00153153_m1 1.006 0.02 1.006 0.04 0.956 0.02 1.006 0.03 1.046 0.08
VEGF-1 VEGFA-Hs00900054_m1 1.006 0.06 0.966 0.06 0.926 0.05 1.006 0.08 1.076 0.08
IL-8 IL8-Hs00174103_m1 1.006 0.07 1.076 0.08 0.936 0.11 1.006 0.10 1.206 0.21
Lipid and steroid metabolism
PPAR-c PPARG-Hs01115513_m1 1.006 0.03 0.946 0.03 0.946 0.03 1.006 0.04 1.046 0.05
a-Reductase 1 SRD5A1-Hs00602694_mH 1.006 0.02 0.966 0.03 0.956 0.03 1.006 0.03 1.066 0.07
1
All values are means6 SEMs. WoS, washout serum; YTS, yellow tomato serum; RTS, red tomato serum; PoS, placebo serum; LYS, lycopene serum; ID, identification. Fold changes are defined as the ratio of mRNA expression of lymph node cancer prostate cells incubated with WoS, which was set to 1 for YTS and RTS conditions, and with PoS, which was set to 1 for LYS conditions. Fold changes with different superscript letters are significantly different (P, 0.05; repeated-measures ANOVA followed by Tukey-Kramer posttests for WoS, YTS, and RTS conditions).
2Fold expressions were significantly different from PoS, P, 0.05 (unpaired t test).
regulation of the Bax:Bcl-2 ratio was observed after incubation with YTS, and was even more pronounced with RTS, but this ratio was unaffected with LYS. Because both RTS and LYS contained similar amounts of lycopene, it is likely that at low concentrations lycopene modulates the expression of these genes synergistically and/or additively with other compounds/metab-olites of tomatoes contained in the sera of the RT consumers. In support of this hypothesis, lycopene combined with other nu-trients was also found to be an efficient modulater of genes associated with apoptosis. In vivo, a combination of lycopene and S-allylcysteine limited the development of gastric cancer cells and induced apoptosis with modulation of Bcl-2, Bax, bim,
and caspases (36). In the Lady transgenic model of prostate cancer, a combination of lycopene with selenium and vitamin E increased the apoptotic index (37).
Cell cycle arrest is often associated with an increase in IGFBP-3 whose effects can be mediated via p21, p27, and cyclin-D1 (IGFBP-38, 39). Cyclin-D1 was down-regulated after incubation of LNCaP cells with RTS but not with incubation with LYS. Lycopene and/ or tomato extracts are known to decrease cyclin-D1 accumula-tion in various cancer cell lines such as human breast cancer (40), LNCaP, PC-3 (41), human colon carcinoma (42), human nasopharyngeal tumor (43), and the human prostate epithelial cell line (44). Thus, a reduction in cyclin-D1 expression by RTS may contribute to lower prostate cancer risk by limiting cell proliferation. Again, this gene was not regulated by LYS, in-dicating that other compounds and/or metabolites from red to-matoes may be involved in this regulation.
Except for IGFBP3, none of the aforementioned genes was modulated when LNCaP cells were incubated with LYS. However, c-fos and uPAR were shown to be specifically affected by these sera. The protein product of uPAR facilitates matrix degradation by inducing the activity of metalloproteinases involved in cancer metastasis. The observed up-regulation of uPAR is in agreement with the results from Forbes et al (45) on PC-3 cells. These results indicate that purified lycopene should be used with caution during the aggressive stages of prostate cancers. Up-regulation of the c-fos oncogene by LYS also reflected a potential adverse effect of pure lycopene consumption. Induced expression of c-fos in several cell lines (osteoblasts, chondrocytes, MCF-7) increases their motility, invasion capacity, and metastatic potential (46). Therefore, the up-regulation of u-PAR and c-fos, observed only with LYS and not with RTS, suggests that other components in the matrix of the red tomato are able to counteract the potential harmful induction of these genes by lycopene.
Cell proliferation
Our TLDA results indicate a cellular orientation toward ap-optotic induction (IGFBP-3 and Bax:Bcl-2 ratio induction) and cell cycle arrest (IGFBP3 induction, cyclin-D1 repression). However, the sera from volunteers receiving YT, RT, and LY had no effect on cell proliferation, as measured with BrdU in-corporation. This observation suggests that gene expression changes occurred well before phenotypic ones. Regarding the in vitro effects of lycopene, 5 lM is necessary to reduce pro-liferation, a concentration far above the 60 nM found in media containing RTS and LYS.
Together, our data clearly indicate that short-term intake of tomatoes induces changes in the concentrations of serum com-ponents that modulate potential cancer-related gene expression in LNCaP cells. Because these effects did not significantly differ from sera collected after the consumption of lycopene-free yellow tomatoes, we conclude that lycopene cannot be the sole component responsible for the putative protective role of whole tomatoes. The higher expression of IGFBP-3 found after in-cubation of LNCaP cells with sera of individuals who consumed red tomatoes and lycopene also suggests that this carotenoid may act on specific signaling pathways. However, lycopene intake without its matrix also led to up-regulation of procarcinogenic genes. Therefore, it can be stated that tomato consumption may be preferable to pure lycopene on the basis of the induction of these procarcinogenic genes.
FIGURE 2.Effects of yellow tomato sera (YTS) and red tomato sera (RTS) on insulin-like growth factor binding protein 3 (IGFBP3) expression of lymph node cancer prostate cells. Lymph node cancer prostate cells were incubated for 48 h with the corresponding sera. Results are expressed as means (6SEM) of fold change relative to washout sera (WoS; n = 20). Values with different superscript letters are significantly different (P, 0.05, repeated-measures ANOVA followed by Tukey-Kramer posttests).
FIGURE 3. Mean (6SEM) effect of purified lycopene and its
corresponding placebo on lymph node cancer prostate (LNCaP) cell proliferation. LNCaP cells were incubated for 48 h in a medium containing lycopene or placebo. A range of concentrations of lycopene was tested by serial dilutions of a 0.1-mmol/L solution prepared in the medium. For the placebo, an equivalent volume (eq Vol) was adjusted to the volume of lycopene solution added. Control values set to 100% (hatched bar) referred to LNCaP cells incubated with a medium without lycopene or placebo. The optical density was 1.176 0.02. The effects of placebo (white bars) and lycopene (black bars) were compared relative to the control.*P, 0.05 (unpaired t test).
The authors’ responsibilities were as follows—ER, LG, JT, M-PV, RM-Q, SG, and AM: design of the experiment; JT, M-PV, MR, and SG: collection of data; JT, M-PV, MR, BL, RM-Q, CC-V, and SG: analysis of data; and ER, JT, J-MAL, M-PV, CC-V, LG, and AM: writing of the manuscript and pro-vision of significant advice. None of the authors declared a conflict of interest.
REFERENCES
1. Kavanaugh CJ, Trumbo PR, Ellwood KC. The U.S. Food and Drug Administration’s evidence-based review for qualified health claims: to-matoes, lycopene, and cancer. J Natl Cancer Inst 2007;99:1074–85. 2. WCRF/AICR. Food, nutrition, physical activity, and the prevention of
cancer: a global perspective. Washington, DC: World Cancer Research Fund/American Institute for Cancer Research, 2007.
3. Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer 1989;64:598–604. 4. Giovannucci E. A review of epidemiologic studies of tomatoes, lyco-pene, and prostate cancer. Exp Biol Med (Maywood) 2002;227:852–9. 5. Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys 1989;274: 532–8.
6. Clinton SK, Emenhiser C, Schwartz SJ, et al. cis-trans Lycopene iso-mers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev 1996;5:823–33.
7. Ames BN. Dietary carcinogens and anticarcinogens: oxygen radicals and degenerative diseases. Science 1983;221:1256–64.
8. van Breemen RB, Pajkovic N. Multitargeted therapy of cancer by ly-copene. Cancer Lett 2008;269:339–51.
9. Erdman JW Jr, Ford NA, Lindshield BL. Are the health attributes of ly-copene related to its antioxidant function? Arch Biochem Biophys 2008; 483:229–35.
10. Siler U, Herzog A, Spitzer V, et al. Lycopene effects on rat normal prostate and prostate tumor tissue. J Nutr 2005;135:2050S–2S. 11. Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW Jr, Clinton SK.
Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testoster-one-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst 2003;95:1578–86.
12. Kucuk O, Sarkar FH, Sakr W, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epi-demiol Biomarkers Prev 2001;10:861–8.
13. Chen L, Stacewicz-Sapuntzakis M, Duncan C, et al. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based en-trees as a whole-food intervention. J Natl Cancer Inst 2001;93:1872–9. 14. Clark PE, Hall MC, Borden LS Jr, et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology 2006;67:1257–61. 15. Jatoi A, Burch P, Hillman D, et al. A tomato-based, lycopene-containing intervention for androgen-independent prostate cancer: results of a phase II study from the North Central Cancer Treatment Group. Urology 2007; 69:289–94.
16. Gann PH, Khachik F. Tomatoes or lycopene versus prostate cancer: is evolution anti-reductionist? J Natl Cancer Inst 2003;95:1563–5. 17. Gitenay D, Lyan B, Talvas J, et al. Serum from rats fed red or yellow
tomatoes induces connexin43 expression independently from lycopene in a prostate cancer cell line. Biochem Biophys Res Commun 2007;364: 578–82.
18. Gitenay D, Lyan B, Rambeau M, Mazur A, Rock E. Comparison of lycopene and tomato effects on biomarkers of oxidative stress in vitamin E deficient rats. Eur J Nutr 2007;46:468–75.
19. Lyan B, Azais-Braesco V, Cardinault N, et al. Simple method for clinical determination of 13 carotenoids in human plasma using an isocratic high-performance liquid chromatographic method. J Chromatogr B Bi-omed Sci Appl 2001;751:297–303.
20. Tessier F, Birlouez-Aragon I, Tjani C, Guilland JC. Validation of a mi-cromethod for determining oxidized and reduced vitamin C in plasma by HPLC-fluorescence. Int J Vitam Nutr Res 1996;66:166–70.
21. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996; 239:70–6.
22. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002;3:r34.1–11.
23. Talvas J, Obled A, Fafournoux P, Mordier S. Regulation of protein synthesis by leucine starvation involves distinct mechanisms in mouse C2C12 myoblasts and myotubes. J Nutr 2006;136:1466–71.
24. Porrini M, Riso P, Brusamolino A, Berti C, Guarnieri S, Visioli F. Daily intake of a formulated tomato drink affects carotenoid plasma and lymphocyte concentrations and improves cellular antioxidant protection. Br J Nutr 2005;93:93–9.
25. Mucci LA, Tamimi R, Lagiou P, et al. Are dietary influences on the risk of prostate cancer mediated through the insulin-like growth factor sys-tem? BJU Int 2001;87:814–20.
26. Edinger MS, Koff WJ. Effect of the consumption of tomato paste on plasma prostate-specific antigen levels in patients with benign prostate hyperplasia. Braz J Med Biol Res 2006;39:1115–9.
27. Afman L, Muller M. Nutrigenomics: from molecular nutrition to pre-vention of disease. J Am Diet Assoc 2006;106:569–76.
28. Yamada PM, Lee KW. Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. Am J Physiol Cell Physiol 2009;296:C954–76. 29. Kruse JP, Gu W. Modes of p53 regulation. Cell 2009;137:609–22. 30. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to
en-vironmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007;47:89–116.
31. Liu C, Lian F, Smith DE, Russell RM, Wang XD. Lycopene supple-mentation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res 2003;63:3138–44.
32. Kanagaraj P, Vijayababu MR, Ravisankar B, Anbalagan J, Aruldhas MM, Arunakaran J. Effect of lycopene on insulin-like growth factor-I, IGF binding protein-3 and IGF type-I receptor in prostate cancer cells. J Cancer Res Clin Oncol 2007;133:351–9.
33. Rajah R, Valentinis B, Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-beta1 on programmed cell death through a p53- and IG-F-independent mechanism. J Biol Chem 1997;272:12181–8.
34. Rajah R, Lee KW, Cohen P. Insulin-like growth factor binding protein-3 mediates tumor necrosis factor-alpha-induced apoptosis: role of Bcl-2 phosphorylation. Cell Growth Differ 2002;13:163–71.
35. Wang A, Zhang L. [Effect of lycopene on proliferation and cell cycle of hormone refractory prostate cancer PC-3 cell line]. Wei Sheng Yan Jiu 2007;36:575–8 (in Chinese).
36. Velmurugan B, Mani A, Nagini S. Combination of S-allylcysteine and lycopene induces apoptosis by modulating Bcl-2, Bax, Bim and caspases during experimental gastric carcinogenesis. Eur J Cancer Prev 2005;14: 387–93.
37. Venkateswaran V, Klotz LH, Ramani M, et al. A combination of mi-cronutrients is beneficial in reducing the incidence of prostate cancer and increasing survival in the Lady transgenic model. Cancer Prev Res (Phila Pa) 2009;2:473–83.
38. Peng L, Wang J, Malloy PJ, Feldman D. The role of insulin-like growth factor binding protein-3 in the growth inhibitory actions of androgens in LNCaP human prostate cancer cells. Int J Cancer 2008; 122:558–66.
39. Shukla S, Mishra A, Fu P, MacLennan GT, Resnick MI, Gupta S. Up-regulation of insulin-like growth factor binding protein-3 by apige-nin leads to growth inhibition and apoptosis of 22Rv1 xenograft in athymic nude mice. FASEB J 2005;19:2042–4.
40. Nahum A, Zeller L, Danilenko M, et al. Lycopene inhibition of IG-F-induced cancer cell growth depends on the level of cyclin D1. Eur J Nutr 2006;45:275–82.
41. Ivanov NI, Cowell SP, Brown P, Rennie PS, Guns ES, Cox ME. Lyco-pene differentially induces quiescence and apoptosis in androgen-responsive and -independent prostate cancer cell lines. Clin Nutr 2007; 26:252–63.
42. Tang FY, Shih CJ, Cheng LH, Ho HJ, Chen HJ. Lycopene inhibits growth of human colon cancer cells via suppression of the Akt signaling pathway. Mol Nutr Food Res 2008;52:646–54.
43. Cheng HC, Chien H, Liao CH, Yang YY, Huang SY. Carotenoids sup-press proliferating cell nuclear antigen and cyclin D1 exsup-pression in oral carcinogenic models. J Nutr Biochem 2007;18:667–75.
44. Obermuller-Jevic UC, Olano-Martin E, Corbacho AM, et al. Lycopene inhibits the growth of normal human prostate epithelial cells in vitro. J Nutr 2003;133:3356–60.
45. Forbes K, Gillette K, Sehgal I. Lycopene increases urokinase receptor and fails to inhibit growth or connexin expression in a metastatically passaged prostate cancer cell line: a brief communication. Exp Biol Med (Maywood) 2003;228:967–71.
46. Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer 2005;41:2449–61.