HAL Id: hal-01713243
https://hal-univ-tlse3.archives-ouvertes.fr/hal-01713243
Submitted on 16 Jul 2018
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Generalized method for determining fluoroacidity by
electrochemical diffusion coefficient measurement
(application to HfF4)
Mickaël Kergoat, Mathieu Gibilaro, Laurent Massot, Pierre Chamelot
To cite this version:
Mickaël Kergoat, Mathieu Gibilaro, Laurent Massot, Pierre Chamelot. Generalized method for
deter-mining fluoroacidity by electrochemical diffusion coefficient measurement (application to HfF4).
Elec-trochimica Acta, Elsevier, 2015, 176, pp.265 - 269. �10.1016/j.electacta.2015.06.124�. �hal-01713243�
To cite this version :
Kergoat, Mickaël
and Gibilaro, Mathieu
and
Massot, Laurent
and Chamelot, Pierre
Generalized method for
determining fluoroacidity by electrochemical diffusion coefficient
measurement (application to HfF4). (2015) Electrochimica Acta, 176.
265-269. ISSN 0013-4686
O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 20336
To link to this article: Doi :
10.1016/j.electacta.2015.06.124
URL :
http://doi.org/10.1016/j.electacta.2015.06.124
Any correspondence concerning this service should be sent to the repository
Generalized
method
for
determining
fluoroacidity
by
electrochemical
diffusion
coefficient
measurement
(application
to
HfF
4
)
M.
Kergoat,
M.
Gibilaro
*
,
L.
Massot,
P.
Chamelot
UniversitédeToulouse,UPS,CNRS,LaboratoiredeGénieChimique,118RoutedeNarbonne,F-31062Toulouse,France
Keywords: Fluoroacidity hafniumfluoride diffusioncoefficient masstransport ABSTRACT
Auniversalmethodforfluoroacidityevaluationwasdevelopedandbasedonamasstransportapproach: itsimplyconsistsinmeasuringthediffusioncoefficientofanelectroactivespeciesinvariousmolten media. Thereductionbehaviour ofHf(IV) ions was investigated inmolten fluoridesand diffusion coefficientsofHf(IV)ions weremeasured. Resultsshowed thatdiffusioncoefficientsdecreasewith fluoroacidity,due totheeffectof solventviscosity(whichis linked tobridgedfluorines). Aglobal approachofmasstransportinsolutionwasthenproposed,takingintoaccountbothsoluteandsolvent. TheSchmidtnumber(Sc)definedastheratiobetweensolventviscosityandsolutediffusivitywas calculatedinordertotakeintoaccountthesetwoparameters.ResultsshowedthatScincreaseswith fluoroacidity, in amuch more sensitive way than D. This universal method can extended to all electroactivespeciesandtoallbathfluoroacidity.
1.Introduction
Byanalogywithaqueoussolutions,notionofacidityinmolten fluoridesolventsisdefinedbyfluoroacidity(pF)andbasedonthe freefluoridesexchangeinmoltenmixturesasdescribedinEq.(1): fluorobaseÐfluoroacid +nF" (1)
pF="log(aF") (2)
Theactivityoffreefluorides(aF"),which quantifies
fluoroa-cidity, depends on the nature of the cations constituting the solvent,thecompositionandtemperature,andinfluences solva-tion,ionstabilityandprocessesreaction.Itthereforeneedstobe studied and quantified for a better understanding of physico-chemicalpropertiesofsolventsandsolutes.
Usual molten salts solvents are mixtures of alkaline and alkaline-earth fluorides (LiF, NaF, KF, MgF2, CaF2...) and the
amountoffreeF"ionsdependsontheirnatureandcomposition:
themoreamoltensaltcontainsfreefluorides(i.e.fluorodonor),the higheritsbasicityis.Asinaqueousmedia,theacidityisessentialas it influences so many parameters; however pF values are not availableyetsincetheF"activitycan'tbemeasured:onlyarelative
fluoroacidityscalewasestablishedbyindirectevaluations[1–3].
Oneelectrochemicalmethodtosortmoltenfluoridesmixtures inregardoftheirfluoroaciditywasproposedinourlaboratoryby Bieberetal.[4] andKergoat etal.[5].Basedonsilicon[4] and completedwithboron[5],itconsistsinstudyingtheequilibrium betweenadissolvedspeciesandagaseouscompoundsgivenby Eq.(3)forSiandEq.(4)forB:
SiF4+xx"(bulk)ÐSiF4(g)+xF" (3)
BF3+xx"(bulk)ÐBF3(g)+xF" (4)
Thus,bydefinition,afluorobasicbath(high[F"]
free)stabilizesa
speciesinsolution,whileafluoroacidbath(low[F"]
free)promotes
thereactionofgaseousspeciesrelease.
The study of these equilibria, moved by the free fluorides concentration, is then an indicator of fluoroacidity. Indeed the releaseofSiF4(g)orBF3(g)leadstoadecreaseofSi(IV)andB(III)ions
concentrationsrespectively,controlledbyin-situelectrochemical titrations.
Bycalculatingkineticconstantsofsilicon(kSi)andboron(kB)
releases, ranking of various eutectic mixtures was established (Fig.1a) and a fluoroacidity scale of fluoride compounds was proposed(Fig.1b)[5].
Howeverseverallimitationswerepointedout:
- thechoiceofthesoluteiscriticalasithastoformagaseous speciesandbeinequilibriumwithit,
- thesolutemustnotreactwiththesolvent,
* correspondingauthor:GibilaroMathieuTel.:+33561557219fax:+3356155
6139
- hastobemoreacidthanthesolventtocapturefreefluorides, - forthemostacidicbaths,thegaseousspeciesreleaseistoofast
todeterminethekineticconstants.
To avoid these drastic conditions, a universal method for fluoroacidity evaluation was developed and based on a mass transportapproach:itsimplyconsistsinmeasuringthediffusion coefficientofaspeciesinvariousmoltenmedia[5].However,small differenceswereobservedbetweenthediffusioncoefficientsand thediscriminationwascomplicatedbyalackofselectivity.Aglobal approachofmasstransportinsolutionwasthenproposed,taking intoaccountbothsoluteandsolvent.
Severalauthorsalreadyshowedthatanincreaseoffluoroacidity directlyimpactsthechemical structureofthemoltensalt[6–8]
and leads to a decrease of the free F" which are involved in
coordinationofonecomplex.Forthemostacidicbaths,complexes shares fluorinesbyforming bridges leading toa structure asa network-likeliquid[9–11].Thischemicalbehaviour(coordination and bridging) affects physico-chemical parameters as solute solubility,vaporpressureandviscosity[12].
BymeasuringthediffusioncoefficientsofSi(IV)andB(III)ions and calculating theSchmidt number(Sc),defined asthe ration between solvent kinematic viscosity
y
(in m2s"1) and solutediffusivityD(inm2s"1)invariousmolten
fluoridesmixtures,itwas shown thatDdecreasesandSc (Sc=
y
/D)increaseswithacidityvalidatingthepreviousscaleobtained.
This novel approach is easier to set up than kinetic rates determination,asanaccuratemeasurementcouldbeperformed by electrochemical techniques; moreover, it could be certainly extendedtoallelectroactivespeciesandtoallbathfluoroacidity. Inordertoconfirmthisassumption,hafniumtetrafluorideHfF4
solutewas selected:itis stablein solution,inerttothesolvent constitutants,anddonotformgaseousspeciesinourexperimental conditions.
OnlyfewstudiesoftheelectrochemicalbehaviourofHf(IV)ions are presented in the bibliography. The available results were mainlyacquiredinmoltenchloridesorchloro-fluoridesmedia.In moltenchlorides,Poinsoetal.[13,14]andAdhoumetal.[15]did notagreeontheonHf(IV)ionreductionmechanisminNaCl-KCl mixture.Spinketal.[16]inCsCl,showedthatHf(IV)ionsreduction is a one step process exchanging 4 electrons leading to the formationofHfmetal.Quitethecontrary,Guang-Senetal.[17]and Serrano [18] demonstrated inNaCl-KClthat reduction ofHf(IV) ionstakesplaceinatwostagesprocess,Hf(IV)toHf(II)toHf(0). However, all theseauthors agree that fluorideions addition in moltenchloridesstabilizes Hf(IV)ionstoformhafniumfluoride complexHfF62"(Eq.(5))inmoltenchloro-fluoridesmedia,asthe
reductionofHf(IV)ionstoHf(0)isperformedinasingle4electrons reversiblestepunderdiffusioncontrol(Eq.(6)):
HfCl62"+6F"ÐHfF62"+6Cl" (5)
HfF62"+4e"ÐHf+ 6F" (6)
Inthefirstpartofthispaper,thereductionbehaviourofHf(IV) ionswasinvestigatedinmolteneutecticLiF-NaF(61–39mol.%)at 750#C by cyclic voltammetry, square wave voltammetry and
chronopotentiometricmethods.Then,diffusioncoefficientsofHf (IV) ionsweredeterminedindifferentconditionsvalidatingthe previousobtainedfluoroacidityscale.
Inasecondpart,measurementsofdiffusioncoefficientsofHf (IV) ionswereperformedintwoothersmoltenmedia:LiF-NaF-KF andLiF-CaF2selectedinregardsoftheirfluoroacidity(thefirstone
is more basic and thesecond one more acid than LiF-NaF:cf.
Fig.1a),inthe800–900#Ctemperaturerange. Resultsobtained
werecorrelatedtosolventviscositythankstotheSchmidtnumber previouslydefined.RelationshipsbetweenD,Scandfluoroacidity previouslydemonstratedwereconfirmedwiththis electrochemi-calspecies.
Thisglobalmasstransportapproachisvalidatedwithasimple determinationofadiffusioncoefficient,andisapowerfultoolin ordertodeterminefluoroacidity.
2. Experimental
The cell consisted in a vitreous carbon crucible placed in a cylindricalvesselmadeofrefractorysteelandclosedbyastainless steellidcooleddownbycirculatingwater.Theinnerpartofthewall wasprotectedagainstfluoridevapoursbyagraphiteliner.Thiscell hasalreadybeendescribedinpreviouswork[19].Theexperiments wereperformed underaninert argonatmosphere. Thecellwas heatedusingaprogrammablefurnaceandthetemperatureswere measuredusingachromel-alumelthermocouple.
Threeeutecticmixtures(CarloErbaReagents99.99%)wereused as electrolyteand selectedaccording totheir fluoroacidityand relativeeasyhandling:
- a basic bath: LiF-NaF-KF (46.5-11.5-42 mol%, melting point 452#C),
- anacidbath:LiF-CaF2(79.2-20.8mol%,meltingpoint767#C),
- an intermediate bath: LiF-NaF (61-39 mol%, melting point 652#C).
Allthe solvents were initially dehydrated by heating under vacuum from ambient temperature up to their melting point during4days.Hafniumionswereintroducedintothebathinthe formofhafniumtetrafluorideHfF4powder(SigmaAldrich99.9%).
Silverwires(1mmdiameter,Goodfellow)wereusedasworking electrode.Thesurfaceareaoftheworkingelectrodewasdetermined aftereachexperimentbymeasuringtheimmersiondepthinthe bath.Theauxiliaryelectrodewasavitreouscarbonrod(V25,3mm diameter)withalargesurfacearea(2.5cm2).Thepotentialswere
referredtoaplatinumwire(0.5mmdiameter,Goodfellow)actingas aquasi-referenceelectrodePt/PtOx/O2"[20].
AlltheelectrochemicalstudieswereperformedwithanAutolab PGSTAT30potentiostat/galvanostatcontrolledbyacomputerusing theresearchsoftwareGPES4.9.
3. Resultsanddiscussion 3.1.Hf(IV)reductionmechanism 3.1.1.Cyclicvoltammetry
Hf(IV)ionsreductionbehaviourwasinvestigatedinmolten LiF-NaFinthe750-900#Ctemperaturerange.Ashafniumandsilver
Fig.1.Fluoroacidity scaleof various eutectic mixtures. Fluoroacidity scaleof
arenotmiscibleatoperatingtemperature,silverwasselectedasan inertworkingelectrode[21].
Fig. 2 shows typical cyclic voltammogram of LiF-NaF-HfF4
(0.69molkg"1) on silver at 750#C and 100mVs"1. Only one
reduction peak and its corresponding reoxidation peak are observed at -1.43 and -1.25V vs. Pt respectively. The signal crossingbetweenforwardand backwardscansis typicalofthe formationofasolidphaseattheelectrode(crossover).Inaddition, theasymmetricalshapeofthereoxidationpeakischaracteristicsof ametaldepositeddissolutioninthecathodicrun(strippingpeak). AspresentedintheinsetofFig.2,novariationsofpeakpotentialat differentscan rateprovedthatHf(IV)electrochemicalreduction processisreversible[22].Thus,accordingtoequationsofcyclic voltammetryforareversiblesoluble/insolublesystem,thenumber of exchanged electrons can be calculated by measuring the differencebetweenthepotentialpeakandthehalfpeakpotential
[22]: jEp" Ep 2j¼ 0:77 RT nF (7)
whereEpisthepeakpotential(V)andEp/2isthehalfpeakpotential
(V),Rtheidealgasconstant(8.314Jmol"1K"1),Tastheabsolute
temperature(K),nthenumberofexchangedelectronsandFthe Faradayconstant(Cmol"1).
In this study, the difference was found to be 18&2mV, correspondingtoavalueof3.8&0.3exchangedelectrons.
ThelinearrelationshipbetweenHf(IV)reductionpeakcurrent density at -1.43V vs. Pt and the square root of the scan rate presented in the inset of Fig. 2, proved that electrochemical reductionprocessiscontrolledbyHf(IV)diffusion[22].Diffusion coefficients were determined using Berzins-Delahay equation (Eq.(8))forareversiblesoluble/insolublesystem[23]:
Jp¼0:61nFCðnFDv RT Þ
1=2 (8)
where Jp is the peak current density (Am"2), C the solute
concentration (mol m"3), D the diffusion
coefficient (m2 s"1)
and
y
thepotentialscanrate(Vs"1).At750#C,avalueof(3.5&0.3)x10"9m2s"1forDwasfound.
Serrano [18] and Poinso [13] found a Hf(IV) ions diffusion coefficientinNaCl-KCl-NaFat750#Cequalto2.2and4.9)10"9
m2s"1respectively.
DiffusioncoefficientsweredeterminedinLiF-NaFinthe800– 900#C temperaturerangeand are presentedinTable 1. Results
showedthatDandTfollowanArrheniuslawtype,therelationship isexpressedasfollows:
InD¼"EA
RTþInDo¼" 7863:4
T "11:8 (9)
FromEq.(9),theactivationenergyisfoundtobe65.4&0.7kJ mol"1.Thisvalueisbythesameorderofmagnitudewithprevious
studies,asforinstance213kJmol"1forSi(IV)ions[4]or48.9kJ
mol"1forNd(III)ions[24].
3.1.2.Squarewavevoltammetry
The square wave voltammetry was used to confirm more accuratelythenumberofexchangedelectronsofHf(IV)reduction thancyclicvoltammetry.
Fig.3showsasquarewavevoltammogramoftheLiF-NaF-HfF4
(0.69molkg"1)systemonsilverelectrode at750#C and9Hz.A
singlepeakaround-1.40Vvs.Ptisobserved,inagreementwith cyclicvoltammetryandpresentinganasymmetricGaussianshape characteristicofasoluble/insolublesystem[25].
Theasymmetryofthepeakisduetothecurrentlessnucleation overvoltage needed for the formation of the first crystals of metallichafniumattheelectrodesurface.Thisphenomenondelays theappearanceofthefaradiccurrent,leadingtoasignaldistortion
[26]. The nucleation overvoltage
h
(V) can be determined bymeasuringthewidthatmid-height ofthetwohalfpeaksusing Eq.(10):
h
=2(10)(W2-W1) (10)The obtained value,
h
=32&2mV, is in agreement with previous overvoltage values for metals deposition available in theliterature[27].After checking in the frequency domain that the reduction reactionofHf(IV)hasareversiblebehavior(linearvariationofthe peakdifferentialcurrentdensitywiththesquarerootoffrequency (cf. inset Fig. 3)), the number of exchanged electrons was determinedfrom themeasurement of thewidthat mid-height W1/2(V)carriedoutonanisolatedpeak.
However,togetridofthenucleationprocess,thewidthat mid-heightW1/2measurementhasbeendeterminedbydoublingthe
half-widthatmid-heightW2(V)oftheGaussiancurve(Eq.(11)).
Thismethodologyhasalreadybeenvalidatedinmoltenfluorides fordifferentmetalsstudies[24].
W1=2¼2W2¼3:52 RT nF (11) -0.2 -0.1 0.0 0.1 0.2 0.3 -1.5 -1 -0.5 0 0.5 1 1.5
J/
A
cm
-2E vs. Pt/V
-0.3 -0.25 -0.2 -0.15 -0.1 -0.05 0 0 0.2 0.4 0.6 0.8 Jp /A cm -2 υ1/2/V1/2s-1/2 -1.45 -1.44 -1.43 -1.42 -1.41 -1.4 -1.5 -1 -0.5 0 Ep v s. P t/ V log υ/V s-1Fig.2.CyclicvoltammogramofLiF-NaF-HfF4(0.69molkg"1)at100mVs"1and
T=750#CWorkingEl.:Ag;AuxiliaryEl.:vitreouscarbon;ReferenceEl.:PtInsets:
LinearrelationshipofHf(IV)reductionpeakcurrentdensityversusthesquareroot
ofthescanningpotentialrate.Hf(IV)reductionpeakpotentialversusthelogarithm
ofthescanningpotentialrate.
Table1
Diffusioncoefficient(D),kinematicviscosity(y)andSchmidtnumber(Sc)ofHf(IV)
ionsfordifferentfluoridemediaatdifferenttemperature.
T(#C) LiF-NaF-KF LiF-NaF LiF-CaF
2 Molarcomp. 46.5–11.5–42 61–39 79.2–20.8 109D(m2s"1) 800 5.34 4.96 1.49 850 9.25 6.80 1.94 900 16.66 9.46 2.48 107y(m2s"1) 800 7.8 12.1 30.2 850 6.5 10.8 29.6 900 5.6 9.7 29.2 Sc=y/D 800 145 245 2028 850 71 158 1528 900 34 102 1178
82&4mVwasfoundforW1/2,correspondingtoanumberof
exchangedelectronsof3.8&0.2.
Theresultsobtainedbycyclicandsquarewavevoltammetries allowconcludingthatHf(IV)ionsreductioninmoltenfluoridesisa one-stepprocessexchanging4electronsunderdiffusioncontrol. 3.1.3.Chronopotentiometry
To confirm the diffusion-controlled process of the Hf(IV) electrochemicalreduction,chronopotentiogramswereperformed.
Fig.4showsthevariationofthechronopotentiogramsofHfF4with
theappliedcurrentonsilverelectrodeat750#C inLiF-NaF-HfF
4
(0.05mol kg"1). These curves exhibit a single wave, obviously
associatedtothereductionofhafniumionsinthepotentialrange (+-1.4Vvs.Pt)alreadyobservedonvoltammograms.
According to the data plotted in the inset of Fig. 4, Sand's equation(Eq.(12))wasverified[22]:
I
t
1=2C ¼ nFSD1=2
p
1=22 ¼constant
¼0:160&0:004Acm3s"1=2mol"1 (12)
whereIistheappliedcurrent(A),
t
thetransitiontime(s),andStheimmergedelectrodesurfacearea(m2).
Thushafniumreductionprocesswasconfirmedtobelimitedby the diffusionof Hf(IV) ionsin solution. Its diffusioncoefficient calculated using Eq. (12) and assumingthat n=4 is:(3.3&0.2)
x10"9m2s"1at750#C,inaccordancewithourpreviousresultby
cyclicvoltammetry((3.5&0.3)x10"9m2
s"1).
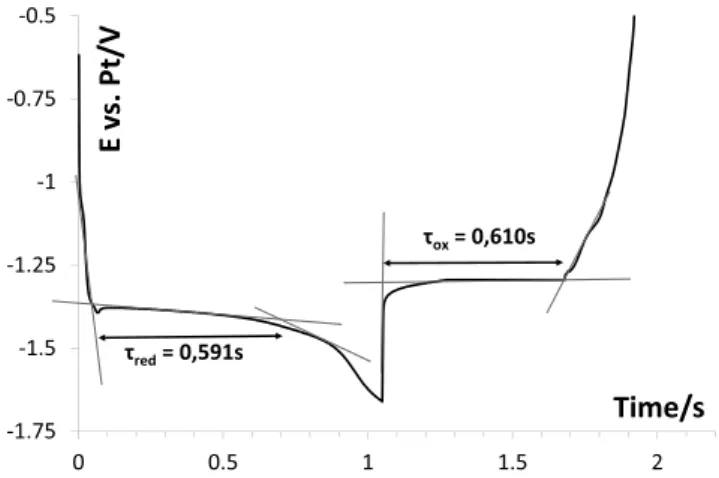
The reversal chronopotentiogram presentedin Fig. 5 proves thataninsolublecompoundisformedonsilverelectrodeduring thecathodicrun,asthetransitiontimeofthereductionwaveis veryclosetotheoxidationtime(
t
red+t
ox+0.6s)[28].ThisresultconfirmsonemoretimethatthereactionleadstoHfmetalonthe workingelectrode.
Allthese electrochemical techniques allow to concludethat reductionofHf(IV)ionsaddedasHfF4inmoltenfluorides:
- isaone-stepprocessexchanging4electrons, - iscontrolledbydiffusionofHf(IV)ions, - andleadstotheformationofmetallichafnium.
As it is stated in thelitterature that cations coordinancyis stronglyinfluenced byfluoroacidity,reductionmechanismofHf (IV) ionsinmoltenfluoridescanbewrittenasinEq.(13): HfF4+xx"+4e"ÐHf+(4+x)F" (13)
3.2.Diffusioncoefficientsandmasstransportapproach
The effect of fluoroacidity on Si(IV) and B(III) ions mass transportswaspreviouslydemonstrated[5].Resultsshowedthat anincrease ofthefluoroacidityleadstoa decreaseof diffusion coefficientsduetocoordinancyandbridgingsolventphenomena. Thesephysico-chemicalsparametersleadtoanincreaseofsolvent viscosity with fluoroacidity. As a consequence solutes mass transportbecomemoredifficult.
In order to confirm these resultson a simple electroactive species,investigationonhafniumsolutewasperformedinthree moltensystemswithdifferentfluoroacidities:
- abasicbath:LiF-NaF-KF, - anacidbath:LiF-CaF2,
- andanintermediatebath:LiF-NaF.
Diffusion coefficients of Hf(IV) ions were determined by calculating the average of cyclic voltammetry (Eq. (8)) and chronopotentiommetry (Eq. (11))values. Results are presented inTable1wheresolventsaresortedfromthemorebasictotheleft tothemoreacidtotheright.
ResultsonHf(IV)ionsshowthatdiffusioncoefficientsdecrease with fluoroacidity whatever the temperature.However for the lowesttemperature,theminordifferencesbetweenvaluesandthe -0.18 -0.16 -0.14 -0.12 -0.10 -0.08 -0.06 -0.04 -0.02 0.00 -1.6 -1.5 -1.4 -1.3 -1.2 -1.1 -1.0
δ
j/
A
cm
-2E vs. Pt/V
W
2W
1 -0.6 -0.5 -0.4 -0.3 -0.2 -0.1 0 0 2 4 6 8 10 δ j/ A cm -² f1/2/Hz1/2Fig.3.SquarewavevoltammogramofLiF-NaF-HfF4(0.69molkg"1)atfrequency=
9HzandT=750#CWorkingEl.:Ag;AuxiliaryEl.:vitreouscarbon;ReferenceEl.:Pt
Inset:LinearrelationshipofHf(IV)reductionpeakcurrentdensityversusthesquare
rootofthefrequency.
Fig.4.TypicalchronopotentiogramswiththeintensityofthesystemLiF-NaF-HfF4
(0.05molkg"1) at T=750#C Working El.: Ag; Auxiliary El.: vitreous carbon;
ReferenceEl.:Pt. -1.75 -1.5 -1.25 -1 -0.75 -0.5 0 0.5 1 1.5 2
E
v
s.
P
t/
V
Time/s
τred= 0,591s τox= 0,610sFig.5.ReversalchronopotentiogramofHfF4(0.083molkg"1)inLiF-NaF,J=&207
mAcm"2atT=750#CWorkingEl.:Ag;AuxiliaryEl.:vitreouscarbon;ReferenceEl.:
uncertaintyofmeasurement(&3)10"10m2s"1)donotallowthe
discriminationofsolventsfluoroacidity.
Itisobviousthattheionicradiusoftheelementinsolutionand itscoordinancyaffecttheabilityofthespeciestomovethroughthe solution(representedbyD).Inhighlyacidbaths,wherethecontent offreeF"islow,hafniumcomplexeshavetoshareoneormore
fluorinebybridging,andthenform,bypolymerization,largersizes species,whichslowsdownthemasstransportinsolution.
AsthehaniumHfF4+xx"coordinancyisafunctionof
fluoroa-cidityand cannot bedeterminebyelectrochemistry, thesolute transportresultshavetotakeinaccountthesolventcontribution throughitsviscosity:aviscosityincreaseleadstoamoredifficult solutemasstransport.
Kinematicviscosities
y
(inm2s"1)definedastheratiobetweendynamicviscosity
m
(inkgm"1s"1)anddensityr
(inkgm"3)werecalculatedfromtheMoltenSaltsHandbookvalues[29].Notethat data for LiF-CaF2 viscosity at operating temperatures are not
availableinliterature.Then,viscositywasextrapolatedfromthe work of Robelin et al. as thesum of the fluoridescompounds viscosities balanced by their molar fractions in the eutectic mixture[30].ThesevaluesaregatheredinTable1.
It shows that molten fluorides viscosity increase with fluoroacidity, and could be correlated to a bridging phenomen by sharing available free fluorides, modifying the molten salt structure and forming a network-like liquid. This behaviour is particularlyimportantwiththepresenceofcalcium,which was demonstratedasoneofthemoreacidcompounds(Fig.1a).
InordertotakeintoaccounttheeffectoftheviscosityonHf(IV) diffusioncoefficients,theadimensionalSchmidtnumber(Sc=
y
/D)wascalculated tocharacterizethe soluteglobalmass transport throughitsenvironment.ThecalculatedScnumberaregatheredin
Table1.Atagiventemperature,theresultsshowedthatScforHf (IV) ionsincreaseswithfluoroacidity, allowing tosort melts in regardoftheirfluoroacidity.
Thus relationships between fluoroacidity, bridging fluorines andviscositydirectlyimpactmasstransportofasimplespeciesin solution.Thedeterminationofdiffusioncoefficientsofaspecies, whichisdirectlyaffectedbyacumulativeeffectofviscosityand ionicradiusofthesolute,allowstodiscriminatemoltenfluorides mediaasa function of fluoroacidity.However, ifthe difference betweenD values is very low, the Schmidt number by taking accountviscosityincreasethisdifference,asforinstancebetween LiF-NaF-KFandLiF-NaFat800#Cwherethedifferenceisaround7%
onDand69%onScinthesameconditions. 4. Conclusion
TheHfF4electrochemicalbehaviourwasinvestigatedinmolten
fluorides. By cyclic voltammetry, square wave voltammetry, chronopotentiometry and reversal choronpotentiometry, Hf(IV) ionsreduction mechanism was demonstrated tobe a one step processexchanging4electronsunderHf(IV)ionsdiffusioncontrol leadingtotheformationofhafniummetal:HfF4+xx"+4e"ÐHf+
xF".
Diffusion coefficients of Hf(IV) were determinated in three moltenfluoridessolvents,withdifferentfluoroacidities,between 800 and 900#C. Hf(IV) diffusion coefficients decrease with
fluoroacidity:thisphenomenumisduetoacumulativeeffectof solventviscosity(whichislinkedtobridgedfluorines)andionic radiusofthesolutewithfluoroacidity. Asviscosityreferstothe solventanddiffusioncoefficienttothesolute,theSchmidtnumber wascalculatedinordertotakeintoaccountthesetwoparameters. Resultsshowed that Sc increases withfluoroacidity, in a much moresensitivewaythanD.
Thismasstransportapproachconsistinginthedetermination of thediffusion coefficient and the calculation of the Schmidt number is easier to set up than kinetic rates determination. Moreover,thisuniversalmethodcanextendedtoallelectroactive speciesandtoallbathfluoroacidity.
References
[1]D.Elwell,Electrowinningofsiliconfromsolutionsofsilicainalkalimetal fluoride/alkalineearthfluorideeutectics,Sol.Energ.Mater.5(1981)205. [2]E.W.Dewing,Theeffectsofadditivesonactivitiesincryolitemelts,Metall.
Trans.B20B(1989)657.
[3]B. Gilbert, E. Robert, E. Tixhon, J.E. Olsen, T. Østvold, Structure and ThermodynamicsofNaF-AlF3MeltswithAdditionofCaF2andMgF2,Inorg. Chem.35(1996)4198.
[4]A.L. Bieber, L. Massot, M. Gibilaro, L. Cassayre, P. Chamelot, P. Taxil, Fluoroacidityevaluationinmoltensalts,Electrochim.Acta56(2011)5022. [5]M.Kergoat,L.Massot,M.Gibilaro,P.Chamelot,Investigationonfluoroacidity
ofmoltenfluoridessolutionsinrelationwithmasstransport,Electrochim. Acta120(2014).
[6]C.Bessada,A.-L.Rollet,A.Rakhmatullin,I.Nuta,P.Florian,D.Massiot,Insitu NMRapproachofthelocalstructureofmoltenmaterialsathightemperature, C. R.Chim9(2006)374.
[7]A.-L.Rollet,S.Godier,C.Bessada,HightemperatureNMRstudyofthelocal structureofmoltenLaF3-AF(A=LiNa,KandRb)mixtures,PCCP10(2008) 3222.
[8]C.Bessada,A.Rakhmatullin,A.-L.Rollet,D.Zanghi,HightemperatureNMR approachofmixturesofrareearthandalkalifluorides:Aninsightintothelocal structure,J.FluorineChem.130(2009)45.
[9]C.F.BaesJr.,ApolymermodelforBeF2andSiO2melts,J.SolidStateChem.1 (1970)159.
[10]V.Dracopoulos,J.Vagelatos,G.N.Papatheodorou,Ramanspectroscopicstudies ofmoltenZrF4-KFmixturesandofA2ZrF6A3ZrF7(A=LiKorCs)compounds,J. Chem.Soc.DaltonTrans.(2001)1117.
[11]M.Salanne,C.Simon,P.Turq,P.A.Madden,Conductivity-Viscosity-Structure: UnpickingtheRelationshipinanIonicLiquidy,J.Phys.Chem.B111(2007) 4678.
[12]D.Williams,L.Toth,K.Clarno,AssessmentofCandidateMoltenSaltCoolants for the Advanced High-Temperature Reactor (AHTR), ORNL/TM-2006/12 (2006).
[13] J.Y.Poinso,Ph.DThesis,(1993).
[14]J.Y. Poinso, S.Bouvet, P. Ozil, J.C. Poignet, J. Bouteillon, Electrochemical ReductionofHafniumTetrachlorideinMoltenNaCl-KCl,J.Electrochem.Soc. 140(1993).
[15]N.Adhoum,L.Arurault,J.Bouteillon,A.Cotarta,J.C.Gabriel,J.C.Poignet, ElectrochemistryofRefractoryMetals:Hf,Mo,Cr,in:D.Kerridge,E.Polyakov (Eds.),RefractoryMetalsinMoltenSalts,vol.53,Springer,Netherlands,1998, pp.61–72.
[16]D.R.Spink,C.P.Vijayan,HafniumElectrowinningStudies,J.Electrochem.Soc. 121(1974).
[17]C.Guang-Sen,M.Okido,T.Oki,Electrochemicalstudiesofzirconiumand hafniuminalkalichlorideandalkalifluoride-chloridemoltensalts,J.Appl. Electrochem.20(1990).
[18] K.Serrano,Ph.DThesis,UniversitéToulouseIII-PaulSabatier,(1998).
[19]L.Massot,P.Chamelot,F.Bouyer,P.Taxil,Electrodepositionofcarbonfilms frommoltenalkalinefluoridemedia,Electrochim.Acta47(2002)1949. [20]A.D.Graves,D.Inman,Adsorptionandthedifferencialcapacitanceofthe
electricaldouble-layeratplatinum/halidemetalinterface,Nature208(1965) 481.
[21]H.Okamoto,Ag-Hf(silver-hafnium),J.PhaseEquilib17(1996).
[22]A.J. Bard, L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications,2ed.,JohnWiley&Sons,NewYork,2001.
[23]T.Berzins,P.Delahay,OscillographicPolarographicWavesfortheReversible DepositionofMetalsonSolidElectrodes,J.Am.Chem.Soc.75(1953). [24]C.Hamel,P.Chamelot,P.Taxil,Neodymium(III)cathodicprocessesinmolten
fluorides,Electrochim.Acta49(2004).
[25]L.Ramaley,M.S.Krause,Theoryofsquarewavevoltammetry,Anal.Chem.41 (1969).
[26]G.J.Hills,D.J.Schiffrin,J.Thompson,Electrochemicalnucleationfrommolten salts—I.Diffusioncontrolledelectrodepositionofsilverfromalkalimolten nitrates,Electrochim.Acta19(1974).
[27]C.Nourry,L.Massot,P.Chamelot,P.Taxil,Dataacquisitioninthermodynamic andelectrochemicalreductioninaGd(III)/Gdsystem inLiF–CaF2media, Electrochim.Acta53(2008).
[28]H.B. Herman, A.J. Bard, CyclicChronopotentiometry.Diffusion Controlled ElectrodeReactionofaSingleComponentSystem,Anal.Chem35(1963). [29]G.J.Janz,MoltenSaltsHandbook,ElsevierScience, 2013.
[30]C.Robelin,P.Chartrand,Aviscositymodelforthe(NaF+AlF3+CaF2+Al2O3) electrolyte,TheJournalofChemicalThermodynamics43(2011).