C.
ANTITHROMBOGENIC DIALYSIS MEMBRANES FOR THE ARTIFICIAL KIDNEY
by
Ben J. Lipps, Jr.
B.S., Purdue University (1962)
M.S., Massachusetts Institute of Technology (1963)
Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Science at the
Massachusetts Institute of Technology February 1966
Signature of Author:
Certified by:
Signature redacted
Department of Chemicagl EngineeringSignature redacted
E.W. Merrill, Thesis Supervisor
Signature redacted
E.R. Gillilhnd, 'esis Supervisor
E.W. Salzman, Thesis Supervisor
Accepted by:
G.C. Williams, Chairman
DEDICATED TO
MY PARENTS
for their wise counsel and continual generosity MY NEW BRIDE
ANTITHROMBOGENIC DIALYSIS MEMBRANES FOR THE ARTIFICIAL KIDNEY
by
Ben J. Lipps, Jr.
Submitted to the Department of Chemical Engineering on February 19, 1966, in partial fulfillment of the requirements
for the degree of Doctor of Science at the Massachusetts Institute of Technology.
ABSTRACT
The purpose of this study was to establish certain funda-mentals necessary for the eventual design of a compatible and efficient artificial kidney. Specifically, regenerated
cellu-lose films and cellucellu-lose esters were treated to impart an anti-thrombogenic surface to them and studies of urea diffusion in human blood were performed to aid in such a design.
This new type of surface appears superior to existing materials in its versatility and its compatibility with blood. Heparin has been chemically bonded to these flexible and rigid materials after amination through graft polymerioation with
ethylenimine. Experiments employing radioactive heparin indi-cate that such chemically-adsorbed heparin surfaces are per-manently coated even under conditions of high fluid shear, in contrast to previously reported processes by which heparin has been bonded to graphite substrates through physical adsorption.
In vitro coagulation tests have shown that such chemically-adsorbed heparin surfaces become "passivated" in contact with blood, and that they achieve a powerful antithrombogenic effect without loss of heparin from the surface to anticoagulate the contained blood by heparin in solution. This "passivation" appears to be the result of clotting factor adsorption onto the heparin surface. This adsorption has also been demonstrated by studies with radioactive plasma. In vivo implantation of intra-vascular prostheses prepared from such materials has resulted
in extended patency in regions of low blood flow rate. Regener-ated cellulose dialysis membranes, of the type currently used in the artificial kidney, were heparinized by this procedure
and were observed to be at least the equal of the original membranes in their mechanical strength and permeability and
far superior to the original materials in their compatibility with blood.
Thesis Supervisors: Edward W. Merrill
Professor of Chemical Engineering
Edwin R. Gilliland
Professor of Chemical Engineering Edwin W. Salzman, M.D.
Massachusetts General Hospital
ii
Department of Chemical Engineering Massachusetts Institute of Technology
February 19, 1966
Professor William C. Greene Secretary of the Faculty
Massachusetts Institute of Technology Cambridge, Massachusetts
Dear Professor Greene:
In accordance with the regulations of the Faculty, I
hereby submit a thesis entitled "Antithrombogenic Dialysis
Membranes for the Artificial Kidney," in partial fulfill-ment of the requirefulfill-ments for the degree of Doctor of Science
in Chemical Engineering. Respectfully submitted,
Signature redacted
Ben J. Lipps, Jr. iii---..
..
...
, . ...
-- 1
11I
II I I 1
ACKNOWLEDGEMENTS
Special acknowledgements are due to Professor E.W. Merrill for his continual advice and enthusiastic contributions. His keen insight into the physical problem proved to be an
invalu-able asset during this study. Special appreciation is also ex-pressed to Dr. E.W. Salzman for his patient and enlightening
instructions in the area of hemotology. Without his assistance this study would have been next to impossible.
Thanks are due Dr. Julio Joison for his undaunted perser-verance during the in vivo evaluation studies which he conducted at the Massachusetts General Hospital. Thanks are also due
Dr. W.G. Austen for providing the laboratory facilities and counsel during these in vivo studies.
Mr. G.A. Pelletier deserves particular thanks for his
assistance and advice throughout this study. Likewise Mr. M.M. Bolton of M.I.T.'s Occupational Medicine Department deserves special thanks for his assistance with the radioactive isotope studies.
This author also greatly appreciates the contributions of Mr. D. Lumgair, Mr. W. Jouris, Miss Y.Y. Hsieh, Miss E. Bergman and Mrs. B.L. Lynch for her services as typist and proofreader.
TABLE OF CONTENTS
I II
SUMMARY . . . . . . . . . . . . . . INTRODUCTION . . . . . . . . .
A. Purpose of the Study. . . . . .
B. Physiology of Human Blood . . .
11. Composition of Human Blood. 2. The Red Blood Cell. . . . .
3. The White Blood Cell . . .
4. The Platelet. . . . . . . .
C. Human Renal Function. . . . . .
1. Renal Anatomy . .. . o... 2. Renal Functioning . . . . . a) Glomular Filtration . . b) Tubular Reabsorption. . c) Tubular Secretion . . . D. Extracorporeal Hemodialysis . . 1. Extracorporeal Hemodialysis 2. Dialyzer Comparisons . . . Operation
3. Dialyzer Mass Transfer Coefficients . 4. Overall Urea Transfer Coefficients. . 5. Dialysate-side Mass Transfer
Coeffi-cient . . . . . . . . . . . . . . . . 6. Membrane Mass Transfer Coefficient. .
47 48 52 54 57 59 Page 31 31 31 .. .. . ... 31 33 . . . .33 35 36 40 . .40 .. .41 41 41 45 45
TABLE OF CONTENTS (Cont)
7. Blood-side Mass Transfer Coefficient E. Ion Related Artificial Kidneys . .
F. Human Blood Coagulation . . . . .
1. Thrombin-Fibrinogen Reaction a) Fibrinogen . . .. .... b) Thrombin . . . . . . . . . . c) Fibrin Formation... 2. Prothrombin-Thrombin Reaction. 3. Extrinsic Activation System .
a) Factor V . . . . . . . . . . b) Factor VII . . . . . .. . . c) Factor X . . . . . . . . . . 4. Intrinsic Prothrombin Activator.
a) Factor XII and Factor XI . .
G. An 1. 2. 3. 4. 5. b) Factor IX . . . . . c) Factor VIII... ticoagulant Heparin. . . . Heparin Stability. . . . Structure of Heparin . .
Reaction with Proteins Biological Activity .
Role of Surface Heparin.
... 69 ... 72 ... 77 77 78 79 80 82 83 83 84 84 85 86 87 89 93 94 96 99 100 vi Page 66 . . . . . . . . . .. . . . . . . .
III
TABLE OF CONTENTS (Cont)
Page
APPARATUS AND PROCEDURE. . . . . . . . . . . . . . 105
A. GBH Wall Shear Measurements. . . . . . . . . . 105
1. Equipment . . . . . . . . ... . ..... 105
2. Experimental Procedure .. . . . . . . . .106
B. Heparinization of Regenerated Cellulose Dialysis Tubing. . . . 108
1. Surface Hepariln Detection. . . . . . . . . 108
a) Radioactive Decay. . . . . . . . . . . 110
b) Beta Particle Detection. . . . . . . .111
c) Solid Scintillation Detection Sys-tem . . . . . . . . . . . . . . ... 113
d) H Heparin Adsorption and Measure-ment Procedure . . . . . . . . . . . . 116
2. Regenerated Cellulose Surface Amina-tion . . . 121
a) Reaction Procedure and Equipment . . . 122
C. In Vitro Testing of CIH Surfaces with Human Blood . . . 125
D. Membrane Surface Microphotographs. . . . . . . 127
E. CIH Membrane Stress-Strain Measurements. . . . 127
F. CIH Membrane Permeability Measurements . . . . 128
G. In Vivo Studies with Heparinized Surfaces. . . 133
1. Description of Testing Procedure ... 135
H. Urea Diffusion Through Human Blood . . . . . . 138
1. Diffusion Capillary and Instrumenta-tion . . . . . . . . . . . . . . . . .. 145
TABLE OF CONTENTS (Cont)
2. Procedure. . . . . . . . . . . . . . .
IV RESULTS AND DISCUSSION . . . . . . . . . . . . A. Tritiated Heparin . . . . . . . . . . . .
B. GBH - Wall Shear Measurements. . . . . . . C. Heparinization of Regenerated Cellulose
Film (CIH Membranes) . . . . . . . . . . .
D. In Vitro Compatibility Studies with Human Blood. . . . . . . . . . . . . . . . . . . E. CIH Membrane Surface Microphotograph . . . F. Proposed Interaction Between Surface
Heparin and Human Blood. . . . . . . . . .
G. Preliminary In Vivo Compatibility Studies
in Canine Vein . . . . . Page . .146 . . 151 . . 151 . . 154 . . 160 . . 193 . . 206 . . 219 . . . . .. . . .. . 235 H. CIH Membrane Stress-Strain Measurements.
I. CIH Membrane Permeability Measurements . J. Urea Diffusion in Whole Human Blood. . . K. Design Considerations for Compatible
Artificial Kidney. . . . . . . . . . . . V CONCLUSIONS. . . . . . . . . . . . . . . . .
VI RECOMMENDATIONS. . . . . . . . . . . . . . .
APPENDICES . . . . . . . . . . . . . . . . . . .
A. Derivation of Relationship Between Overal Membrane Mass Transfer Coefficient and Dialysance . . . . . . . . . . . . . . . . B. Conversion of Urea to Ionic Products . . . C. In Vitro Blood Coagulation Test Procedures
.. 256 269 . . . 274 287 . . 290 . . . 294 . . . 295 1 295 297 298 viii
TABLE OF CONTENTS (Cont)
Page
D. Couette Wall Shear Cell. . . . . . . . . . . . 301
E. Calculations Pertaining to H -Heparin Detection. . . . . . . . . . . . . . . . . . . 304
F. Ethylenimine Health Hazards . . . . . . . . . 305
G. Heparin Monolayer Calculations . . . . . . . . 307
H. Membrane Diffusion Calculations . . . . . . . 308
I. Urea Diffusion in Human Blood . . . . . . . . 309
J. Literature Citations . . . . . . . . . . . . . 311
K. Nomenclature and Abbreviations . . . . . . . . 321
LIST OF TABLES Table No. A-l 2-1 2-2 4-1 4-2 Page Typical Solute Concentrations of Plasma,
Urine, and Artificial Kidney Dialysate. Summary of Artificial Kidney
Character-istics and Performance. . . . . . . . .
Roman Numeral Nomenclature for Human
Blood Coagulation Factors . . . . . . .
Calculated Surface Heparin(Monolayer)-GBH Surface . . . . . . . . . . . . . . Urea Diffusion Through Membranes and
Solutions . . . . . . . . . . . . . . . LIST OF FIGURES . . . 296 . . . 49 . . . 74 . . . 184 ., . 273 Figure No. 2-1 2-2 2-3 2-4 2-5 2-6 2-7 2-8 2-9 3-1 3-2 3-3 Page Illustration of Kidney Nephron. . . . . . . . 43 Schematic Diagram of Kidney Nephron . . . . . 44 Schematic of Artificial
Kidney-Patient System. . . . . . . . . . . . . . . . 50 Modified Wilson Plot. . . . . . . . . . . . . 58 Fringed Micelle Model . . . . . . . . . . . . 64 Effect of Increasing Membrane Permeability
with No Reduction in Blood-side Resistance. 75 Human Blood Coagulation Mechanism . . . . . . 76
Proposed Heparin Molecule . . . . . . . . . . 97
Proposed Crosslink in Heparin Molecule. . . . 97 Couette Surface Shear Cell. . . . . . . . . . 107 Schematic Beta Scintillation System ... 114 H 3-Heparin Solid Scintillation Detector . . . 117
x
LIST OF FIGURES (Cont) Figure No. 3-4 3-5 3-6 3-7 3-8 3-9 3-10 3-11 3-12 3-13 4-1 4-2 4-3 4-4 4-5 4-6 4-7
Scintillation Detector with Amplification Instrumentation. . . . . . . . . . . . . . .
Typical H3 - Heparin Membrane Measurement.
Resin-Kettle Amination Reactor . . . . . . .
Membrane Diffusion Cell. . . . . . . . . . .
Membrane Permeability Measuring System . . .
Microphotograph of CAIH Surface, I. . . . . Schematic of Canine Inferior Vena Cava
Importance of Second + Terms in the
Capillary Cell Series Expansion. . . . . . .
Human Erythrocyte Sedimentation Rate as a Function of Hematocrit . . . . . . . . . . . Urea Diffusivity Measuring Equipment . . . .
Anticoagulant Activity of Tritiated Heparin. Transfer of Tritium From Heparin to Water. Typical Surface Adsorption Curve with H3 _
Heparin . . . . . . . . . . . . . . . . . .
Effect of Wall Shear on GBH Surface
Heparin . . . . . . . . . . . . . . . . . .
Effect of Various Ethylenimine and RCF React Schemes on the Quantity of Adsorbed Heparin. Effect of Catalyst Loading and Ethylenimine Reaction Time on the Quantity of Adsorbed Heparin . . . . . . . . . . . . . . . . . .
Comparison of Surface Heparin - GBH vs. CIH Procedures . . . . . . . . . . . . . . . . . xi Page 118 118 123 131 132 139 140 147 148 149 155 156 157 158 181 ion
.
182 183LIST OF FIGURES (Cont) Figure No. 4-8 4-9 4-10 4-11 4-12 4-13 4-14 4-15 4-16 4-17 4-18 4-19 4-20 4-21 4-22 Page 187
Amination of RCF Using Ethylenimine with
Various Catalysts. . . . . . . . . . . . . . .
Distribution of Amino Nitrogen with
Various Reaction Schemes . . . . . . . . . . . 188 Distribution of Amino Nitrogen with
Ethylene Oxide Pretreatment. . . . . . . . . . 191 Theoretical Substitution of Cellulose
as a Function of Nitrogen Content. . . . . . . 192 Effect of Wall Shear on Surface Heparin
of CIH Membranes . . . . . . . . . . . . . . . 199 Effect of Ethylenimine and Methanol on
Human Plasma . . . . . . . . . . . . . . . . . 200 Effect of Polyethylenimine and
Cati-onic Surfactants on Human Plasma . . . . . . . 201 Effect of Polyethylenimine on Human Plasma
Compatibility of Heparinized Cellulose Dialysis Tubing (CIH) With Human Blood Microphotographs of Regenerated
Cellu-lose Membrane Surface. . . . . . . . . . .
Microphotographs of CIH Membrane Surfaces, I. . . . . . . . . . . . . . . . . . . . .
Microphotographs of CIH Membrane Surfaces, II. . . . . . . . . . . . . . . . . . . .
Microphotographs of CIH Membrane Surfaces, III. . . . . . . . . . . . . . . . . . . .
Microphotographs of CIH Membrane Surfaces, IV. . . . . . . . . . . . . . . . . . . .
Microphotographs of CIH Membrane Surfaces, V. . . . . . . . . . . . . . . . . . . . . xii 202 209 210 211 212 213 214 215
LIST OF FIGURES (Cont)
Figure No. Page
4-23 Effect of CIH Tubes on Fresh Whole Human
Blood . . . . 223 4-24 Interaction of CIH Tubes With Fresh Human
Plasma - 20h Washing Prior to
Hepariniza-tion . . . . 224 4-25 Interaction of CIH Tubes With Fresh Human
Plasma - 120h Washing Prior to
Heparini-zation. . . . . . . . . . . . . . . . . . . . 225 4-26 Interaction of CIH Tubes with Fresh Human
Plasma - 360h Washing Prior to
Heparini-zation. . . . . 226 4-27 Adsorption of Plasma Components Onto
Hep-arinized Surfaces . . . . . . . . . . . . . . 231 4-28 Surface Heparinization of Cellulose
Acetate . . . . . . . . . . . . . . . . . . . 241 4-29 Effect of Reaction Impurities on
Prothrom-bin Time of Plasma Incubated in CAIH
Tubes . . . . . . . . . . . . . . . . . . . . 242 4-30 Effect of Surface Deacetylation On Whole
Blood Clotting Time . . . . . . . . . . . . . 247 4-31 Effect of Extended Prothrombin Time On
Whole Blood Clotting Time . . . . . . . . . . 248 4-32 Microphotographs of CAIH Surfaces, II. . . . 249 4-33 In Vivo Clotting Times of CIH Prostheses
Placed in the Abdominal IVC Below the
Renals. . o.. ... . . . . . . . . . . . 253 4-34 In Vivo Clotting Times of CAIH Prostheses
Placed in the Canine Abdominal IVC Below
the Renals . . . . . . . . . o ...o. 254 4-35 Force-Strain Curves for CIH
LIST OF FIGURES (Cont) Figure No. 4-36 4-37 4-38 4-39 4-40 4-41 4-42 4-43 4-44 4-45 4-46 4-47 4-48 4-49 4-50 Page Force-Strain Curves for CIH
Membranes-.04N HCl Catalyzed. . . . . . . . . . .
Force-Strain Curves for CIH Membranes-.2N HC1 Catalyzed . . . . . . . . . . . Force-Strain Curves for CIH
Membranes-.4N HCl Catalyzed . . . . . . . .. .. Effect of Reaction Conditions on Membrane Breaking Force . . . . . . . . . . . . .
Effect of Reaction Conditions on Membrane Thickness . . . . . . . . . . . . . . . . Stress-Strain Curves for Various Reaction Conditions. . . . . . . . . . . . . . . .
Effect of Reaction Conditions on Membrane Breaking Stress . . . . . . . . . . . . . Total Liquid Side Resistance for Membrane Diffusion Cell . . . . . . . . . . . . .
Urea Membrane Diffusion Results... Diffusivity of Urea Through Saline and Distilled Water . . . . . . . . . . . . . Diffusivity of Urea Through Human Plasma. Diffusivity of Urea Through Citrated
Human Blood - Hematocrit of 50 . . . . .
Diffusivity of Urea Through Citrated Human Blood - Hematocrit of 65 . . . . .
. . . . 260 . . . . 261 . . . . 262 265 266 267 S . 268 . . . 273 . . . 274 279 280 280 . . . 281
Diffusivity of Urea Through Heparinized
Human Blood - Hematocrit of 70 . . . . . . . 281 Diffusivity of Urea Through Human Red
Cell-Saline Suspension - Hematocrit of 70. . . . . . 282
xiv
LIST OF FIGURES (Cont)
Figure No. Page
4-51 Diffusivity of Urea Through Citrated
Human Blood - Hematocrit of 78. . . . . . . . . 282 4-52 Urea Diffusion Coefficients for Human
Blood . . . 283 4-53 Comparison of Theoretically Predicted
and Experimentally Measured Urea
Diffu-sion Coefficients for Human Blood . . . . . . . 284
I~ SUMMARY synopsis
The purpose of this study was to establish certain funda-mentals necessary for the eventual design of an efficient and compatible artificial kidney. By compatible is meant a
materi-al whose surface is sufficiently passive toward human blood to avoid completely, or minimize the activation of clotting fac-tor during blood-surface contact. Specifically regenerated cellulose films, henceforth abbreviated RCF, were treated to
impart an antithrombogenic property to them. The strength and permeability characteristics of the treated films were com-pared to the original RCF. The interaction of this film with human blood was studied and an hypothesis developed for the cause of the surface passivation. This hypothesis, confirmed partially with two independent tests, is believed to be one of primary importance in design of compatible artificial organs. Studies of urea diffusivities in human blood were also per-formed to aid in such a design.
Introduction
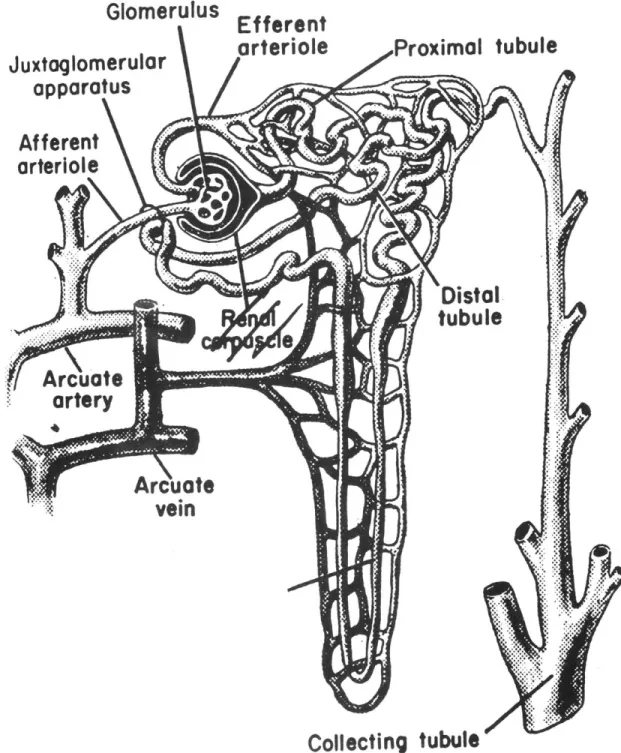
In the healthy human waste metabolites are removed in the form of urine, which is the product resulting from the action of the kidney nephrons on the blood. A schematic of a nephron is presented in Figure 2-2. Normally blood is "fil-tered" by the glomerulus producing a cell and protein
trate on one side of the glomerular membrane and a solution of small molecules and ions on the other side including the waste product. The two streams from the glomerulus pass cocurrently,
separated by the tubular membrane. This membrane has the unique property of actively transporting Na + and other necessary pro-ducts back into the concentrated blood stream. The water and electrolyte content of the blood are adjusted by this membrane to the desired level and the remaining ultrafiltrate becomes urine. About 99% of the ultrafiltrate is readsorbed into the blood stream.
Extracorporeal hemodialysis is substituted for human kid-ney functioning in the case of uremia. Currently this artifi-cial kidney takes over only for the function of the glomerulus and therefore the removal of impurities from the blood requires careful balancing of the dialysis fluid against blood. Common clinical designs consist of either a long dialysis tubing coil through which blood is pumped, with the dialysis bath outside, or a parallel design where alternating blood and dialysate channels are stacked tqgether to form a laminate. In both designs RCF is used as the dialysis membrane. A schematic of the "coil" artificial kidney in relation to the patient is
shown in Figure 2-3.
During hemodialysis using "regional heparinization" blood is drawn from the arterial cannula and is anticoagulated with
heparin, for otherwise it would rapidly clot in its progress through the artificial kidney as a result of surface activa-tion of some of the innate clotting factors. Subsequently the anticoagulated blood is pumped through the dialyzer and returned to the patient. Prior to entering the patient, the blood has its clotting mechanism reinstated by neutralizing the heparin with protamine. Regional heparinization,
al-though it produces large quantities of the heparin-protamine complex in the patient, is preferred over total hepariniza-tion of the patient's blood during hemodialysis because of the complications of internal bleeding and bone brittleness commonly associated with patient heparinization. (94).
Although the exact cause of the uremic syndrome is not as yet clear, unmistakably the artificial kidney must remove more waste metabolite urea than all the other solutes com-bined. This results from the body's 20 gm per day production rate for urea. Water may be removed by ultrafiltration
through the RCF membranes to correct any excess of fluid in the patient. Therefore it is appropriate to consider urea removal as the basis for an assessment of the efficiency of a given unit. An efficient artificial kidney should have a high urea removal rate with a minimum of membrane area or blood volume. A compilation of performance characteristics of the current "coil" and "parallel" kidneys is given in Table 2-1.
Human blood is a complex suspension of three general types of solid particles in a continuous medium, known as plasma. The solid particles are red cells (erythrocytes), white cells (leukocytes) and platelets. Plasma in turn, is
an exceedingly complex aqueous solution of organic and inor-ganic salts, proteins and other orinor-ganic macromolecules. The red cells, which account for the majority of blood solids, are biconcave disks 8.5/ in diameter with a volume of about
3
90/A . They are largely comprised of water and hemoglobin, the latter accounting for 90% of the solid matter. Water and many solutes pass rapidly across the cell membrane but
some ions such as sodium and potassium are transported against concentration gradients by a mechanism which requires energy from metabolism. These red cells themselves are not mobile, but rhythmical movements of the surface, which cause rapid fluctuations in the thickness of the cell, have been observed with phase-contrast microscopy (119).
The interaction between human blood and foreign sur-faces, usually manifested by rapid coagulation of the blood, is believed to be related to the globulin fraction of the
plasma proteins (124). Although the exact nature of the coagu-lation mechanism is still unknown, a cascade mechanism, which is initiated by foreign surface contact or tissue injury, has received much support in recent years and is important in the
interpretation of the results of this thesis as will be seen below. (71,9) The proposed mechanism is shown in Figure 2-7. Following tissue injury, coagulation can proceed through two parallel paths, the intrinsic clotting system or the extrinsic clotting system, to the prothrombin activation step. With the formation of either the intrinsic or extrinsic prothrombin activator, known as intrinsic or extrinsic thromboplastin, prothrombin is converted to thrombin, which in turn, converts fibrinogen to fibrin. Fibrin, a rapidly produced polymer of fibrinogen, forms three dimensional water insoluble networks which can be observed as the blood or plasma clot. All clot-ting factors required for coagulation are present in normal blood and are needed during the intrinsic clotting route. For the extrinsic route tissue extract is substituted for the phospholipids of the platelets. Few of the clotting factors of the extrinsic or intrinsic system have been isolated or rigorously identified and are presumed to exist only by the absence or presence of their clotting activity. For the sake of clarity the clotting factors have been given the Roman numeral designations widely adopted in current hematology.
Foreign surfaces normally produce blood coagulation via the intrinsic clotting system. Factors XII and XI are thought to initiate this coagulation by becoming activated at the
foreign surface. This activated Factor XII-XI complex in 5
turn activates other clotting factors, and so on through a series of consecutive activations until fibrin is formed.
Three types of coagulation tests were used during this study to determine the potency of the intrinsic clotting sys-tem, extrinsic clotting syssys-tem, and the fibrinogen to fibrin step. These tests are respectively the kaolin-cephalin test,
the prothrombin test and the thrombin test. )
Because of the importance of heparin in blood coagulation and hemodialy-sis, as well as its most recent utilization in thrombus resistant surfaces, a brief discussion of its proper-ties will be given. Heparin is a mucopolysaccharide found in the liver and many tissues of the body. It is a highly sul-fonated polyanion which has been found to react with many pro-teins and complex bases. (20, Chapt. 3, 23) This reaction with proteins is considered to be a simple salt formation in which reversible dissociation of the salt occurs according to
1. With the kaolin-cephalin test, the time required for co-agulation of platelet-poor plasma in the presence of all clotting factors and kaolin at 370C is known as the
"kaolin-cephalin time." In this test the brain extract, cephalin, is substituted for platelets to give standardized results and kaolin supplies the surface for activation. The "pro-thrombin time" resulting from the pro"pro-thrombin test is the time required for coagulation of platelet-poor plasma at
370C when tissue extract has been added. The "thrombin time," which refers to the thrombin test, is the time required to
form fibrin from fibrinogen in the presence of thrombin.
the mass action law.
Heparin is known to interfere with coagulation in the intrinsic clotting system by inhibiting the formation of in-trinsic thromboplastin. In the presence of a plasma cofac-tor, "thrombin inhibitor," heparin also interferes with the
fibrinogen-thrombin reaction. The former anticoagulant
ef-fect is though to be the more important of the two. O'Brien (80,81) postulates that heparin reversibly complexes with Factor IX of the intrinsic syst.em, resulting in sufficient
activity reduction to produce anticoagulation. Because of the highly cationic nature of protamine sulfate, this heparin neutralant is thought to have a higher affinity for heparin
than the clotting factors. Hence the clotting factor in the heparin complex is probably replaced by protamine reinstating the coagulation activity.
The interference of heparin with the fibrinogen-thrombin reaction, in the presence of a plasma factor, was used in this
study as an indication of heparin's presence. Extended "throm-bin times" were a definite evidence of heparin activity.2
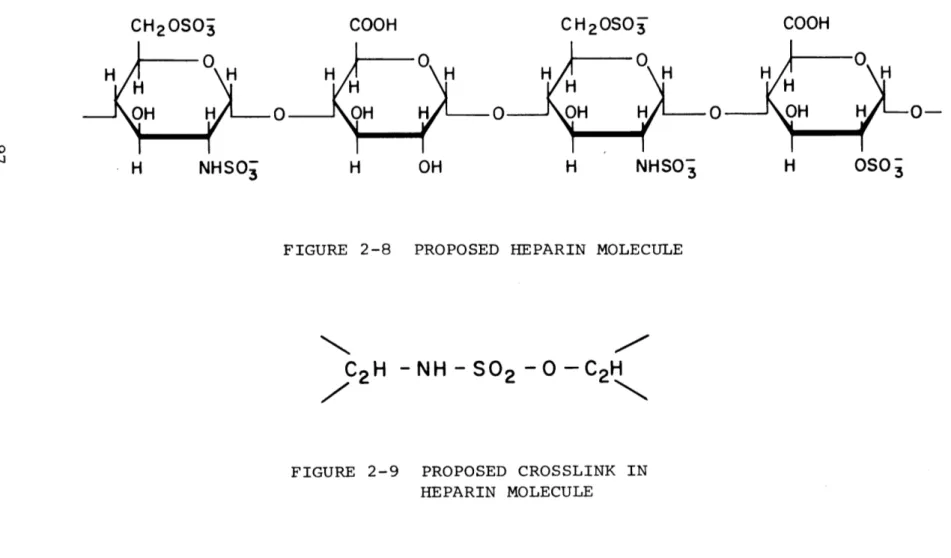
The molecular weight of heparin is thought to be around 15,000 to 16,000 (4). The most probable structure for the basic tetrasaccharide repeating unit of heparin can be seen
7 2. See footnote 1.
in Figure 2-8. Heparin is known to contain o((l-+ 4) linkages and hence a helical structure similar to that for amylQse has been proposed. (23,107,122,123). It has been further suggested that a certain amount of rigidity might be conferred on such a structure by means of a small number of intramolecular sul-fate bridges of the type shown in Figure 2-9. Considering this, the heparin molecule might be expected to be a collapsed helical structure with sulfate sites present around its peri-meter. In this configuration, heparin might be adeorbed to a solid substrate and still maintain anticoagulant activity since a portion of heparin's sulfate and amino sulfate groups will continue to be exposed to the solution contacting the substrate. It seems likely that the degree and distribution of sulfate
groups and the molecular shape of heparin contribute much towards its anticoagulant activity (11).
Studies by Whiffen, Gott, and Young (33,34,35,113,114, 115,116,117,118) indicate that the adsorption of heparin onto
their graphite-benzalkonium surfaces gives extended in vitro whole blood clotting times, hereafter abbreviated WBC, as well
as extended in vivo patency, when heparinized prostheses were placed in the canine circulatory system near the heart. How-ever, fresh whole blood when contacted with the graphite-ben-zalkonium-heparin surface, hereafter abbreviated GBH, was found in this study to be anticoagulated by heparin desorbed
from the surface. Thus the cause of long term in vivo patency is not immediately obvious. Possibly an irreversibly adsorbed heparin surface might be passive enough toward human blood to permit the design of compatible extracorporeal circuits which require no heparinization of the patient's blood.
Apparatus and Procedure
To gain a better understanding of the GBH surface, it was subjected to well defined fluid shears using fresh plasma and saline. The wall shear rates corresponded to those normally existing in the cannulas used in present heart-lung and hemo-dialysis circuits. The fluid shear was produced in the equi-valent of a Couette viscometer in which the inner cylinder
surface was coated with graphite-benzalkonium chloride-heparin. The heparin migrating from the surface into the solution as a result of shear was analyzed by means of a modified thrombin test. The total heparin appearing in solution was compared to the original heparin on the inner cylinder before shear, measured with tritiated heparin (radioactive counting).
This tritiated heparin was produced by exposure of hep-arin to tritium gas, commonly known as the Wilsbach technique
(120) and was used to determine the quantity of adsorbed sur-face heparin. The anticoagulant activity of the tritiated hepar-in closely resembled that of the non radioactive heparhepar-in. Triti-ated heparin was selected rather than S 35-heparin, which has been
produced by canine ingestion of NaS35 04 followed by heparin extraction of the dog's liver, because of the short half life of S3 5 and the ideal range of tritium emission. With tritium one could measure only the surface heparin present, even on thin (1 mil) membranes.
The adsorbed tritiated heparin was detected and measured with a solid scintillator counter assembly similar to the
schematic shown in Figure 3-2. A photograph of the actual photomultiplier tube assembly and preamplifier is presented in Figure 3-3.
RCF of the type currently used in hemodialysis was selected as the substrate for irreversible heparinization because of its
immediate and well founded biological potential. The general reaction scheme for producing this heparinized RCF consists of, 1) surface amination to attach cationic and basic groups to the RCF and 2) heparinization of the aminated film by
immer-sion in an aqueous sodium heparin solution, 5 to 100 Mg/ml, for 10 to 15 minutes. Thus, the surface heparin was chemically bonded to the substrate through the amino linkage. Various amination techniques were studied and evaluated by measuring the quantity of adsorbed surface heparin. Normally a balance between membrane strength and surface heparin dictated the usefulness of the amination technique.
Only RCF amination techniques employing ethylenimine as 10
the aminating agent gave adsorbed heparin quantities com-parable to those of the GBH surface with a retention of mem-brane strength. Generally, amination with ethylenimine took
place in the presence of a catalyst, such as water or acid, with ethylenimine vapor at temperatures between 70 and 900C.
The ethylenimiine was believed to graft polymerize onto the RCF in the following manner.
H Cel - OH + N -- Cel - 0 - CH2CH2NH2 H2C - CH2 H Cel - 0 - CH2CH2NH2 + N -- Cel-O-[CH2CH2NH]n-CH2CH2NH2 H2C -CH2 n
With or without protonation of the RCF - grafted
polyethyl-enimine surface a high affinity for heparin exists, givingfar
more adsorbed heparin than with the GBH procedure, but still maintaining adequate membrane strength.
To aid in the design of more efficient artificial kidneys, be they compatible or not, other studies were undertaken to determine experimentally the diffusion coefficient at 200C of
C 4 labelled urea through human blood, in a capillary diffusion cell. The same scintillation detection instrumentation as that used for the tritiated heparin detection was employed to follow the diffusion of the C 14-urea from a capillary, fabricated from
a solid scintillator, into a reservoir. A photograph of the
capillary, the reservoir, and the detection instrumentation is
presented in Figure 3-13.
Results and Discission
1) Fluid Shear Experiments with Couette Geometry-Graphite-Benzalkonium-Heparin Surface
As much as 20% of the original 2 gm/cm surface heparin on the Lucite-GBH surface can migrate into fresh plasma
in five hours when the surface and plasma are in contact but with no fluid shear. In the presence of a wall shear of 9.5
2
dynes/cm2, comparable to the maximum shear in the heart-lung
machine, approximately 80% of the original heparin on the inner cylindrical surface migrates from the surface into the fresh plasma within five hours. With a wall shear comparable to the maximum shear in the artificial kidney, about 40% of
the original GBH heparin is lost to the plasma in five hours. In contrast to the fluid shear results with plasma, vir-tually no GBH surface heparin loss from the inner cylindrical
surface observed when the shearing solution was saline, even with a wall shear of 9.5 dynes/cm2 for five hours.
This wall shear data indicates that a definite interaction exists between the GBH surface heparin and fresh plasma but appears to be absent between saline and the surface heparin. The data also points to a limitation on the utility of GBH
surfaces in regions of high shear but indicate that sufficient
surface heparin might remain in regions of low flow to give
extended patency.
2) Heparinization of Regenerated Cellulose Film
Amination procedures involving the action of chloro-alkylamines or aminoalkyl hydrogen sulfate with alkali cellu-lose were studied in an attempt to surface aminate RCF. These procedures were greatly limited by the sodium hydroxide
treat-ment of the RCF to produce alkali cellulose. A vigorous
sod-ium hydroxide treatment is necessary to produce amination but
results in destruction of the RCF structure. Maximum heparin
adsorption with an acceptable membrane structure was of the order of 0.3 /1 gm/cm2, well below that of the GBH surface.
Four different reaction schemes using ethylenimine were studied: 1) ethylene oxide pretreated RCF (air dry) +
ethylenimine vapor at 85 0C, 2) initially water swollen RCF + ethylenimine vapor at 70 C and 85 C, 3) RCF + NH4Cl and
ethyl-enimine in toluene, reflux 800C, and 4) initially acid swollen RCF + ethylenimine vapor at 85 C.
Ethylenimine is known to react with groups containing active hydrogen atoms, such as the hydroxyl groups on the
cellulose molecule, if the proper catalyst is present. Assum-ing that this reaction resembles homopolymerization of ethyl-enimine, the first step in the grafting reaction is probably the production of the ethylenimmonium ion according to
RX + NH - RNH + X where H2C-CH2 H2C - CH2
R may be H also. This ion is then believed to react with the RCF according to Cel-OH + RNH -p Cel-O-CH2CH2NH2 + H H2C - CH2 +H + Cel-O-CH2CH2NH2+ RNH -- Cel-O-CH2CH2NHCH2CH2NH2+H H2C- H 2
The grafted polyethylenimine successfully competes for the H+ ion and eventually limits the polymerization by depleting the acid present.(2,53,54) Branching of the polyethylenimine is possible through the interaction of the immonium ion and
secondary nitrogens of the polymer but no proof of its exist-ence has yet appeared.
The ethylene imine grafting procedure of this study used water, HCl, and NH4Cl as catalysts. The purpose of the
ethyl-ene oxide pretreatment was to produce a more uniform surface amination by converting the secondary cellulose hydroxyls to primary hydroxyls prior to amination through etherification.
The influence of these various reaction procedures on the quantity of adsorbed surface heparin can be seen in Fig-ure 4-5. The ethylene oxide pretreatment gave the highest values of adsorbed surface heparin. Additional water beyond
that contained in the initially swollen membrane was required to produce further amination after 50 hours of contact with ethylenimine vapor at one atmosphere and 700C. With a reac-tion temperature of 850C and an initially water swollen mem-brane, the quantity of absorbed heparin appeared to level at
50 hours also, but at significantly higher levels. Both
pro-cedures are probably terminated by evaporation of the original
water from the membrane, for dry cellulose will not react with
ethylenimine.
With the toluene phase amination procedure, the grafting of ethylene imine did not appear to level off with time indi-cating the possibility of unlimited and perhaps excessive sur-face polymerization. The procedure using acid catalysis showed the highest tendency toward leveling off of the subsequently adsorbed heparin as a function of ethylenimine exposure time. This equilibrium level was found to be inversely related to the initial acid loading of the swollen membrane. This indi-cates that the acid must play a part in the termination of the graft polymerization. The acid probably protonates the grafted polyethylenimine at an early stage causing termination, since protonated nitrogens and immonium ions have very little tendency to react. A consequence of this termination might be a more uniform amination, since after protonation of the grafted polyethylenimine the immonium ions have only cellulose
hydroxyls available for reaction.
Nitrogen analyses and the data of Figure 4-5 indicate that those membranes which were aminated in the presence of HCl have the deepest and possibly most uniform amination. The toluene amination proceedure tends to produce surface amina-tion. The ethylene oxide pretreatment probably results in a uniform but not necessarily as penetrating an amination as the acid catalyzed procedure. With water as the catalyst a penetration intermediate between the acid catalyst and ethyl-ene oxide process is expected.
WBC tests with fresh human blood were used to determine the compatibility of the various heparinized surfaces. The results of these studies are presented in Figure 4-16. The data of Fig. 4-16 showing the extended whole blood clotting times obtained with these surfaces are definitely the result of the transformed nature of the surface and not artifacts such as elutable heparin or elutabl'e reaction impurities. The absence of heparin in the blood, that could have Come from elution or leaching of the membranes, is indicated by the nor-mal value of the "thrombin time" on aliquots of plasma incu-bated in the heparinized regenerated dialysis tubes. Addi-tional evidence of an irreversibly adsorbed heparin surface is afforded by the results of fluid shear measurements.
Heparinized regenerated cellulose membranes, hereafter called 16
CIH, sheared with fresh plasma at 9.5 dynes/cm2 for 15 hours showed no loss of heparin from the membrane.
Another potential artifact other than elutable heparin is
ethylenimine or its homopolymer formed during the amination.
Concentrations of greater than 30 ppm of monomer or polyethyl-ene imine produce an extended "prothrombin time." In fact, the prothrombin time serves as a semi--quantitative indicator of these impurities. Since these impurities are highly solu-ble in water, all aminated membranes were washed for a suffi-cient period of time with water to give a normal "prothrombin time" with aliquots of citrated plasma incubated in the CIH tubes.
The data of Figure 4-16 indicate that the cellulose mem-brane surfaces prepared via the water-ethylenimine vapor or
liquid phase amination schemes are incompletely covered in the subsequent heparinization. A more concentrated heparin-izing3 solution produced considerably more surface heparin on the water catalyzed membranes but only a slight improvement in compatibility was observed. The sensitivity of surface bound heparin as measured by tritium emission/to the
heparin-3. The heparinizing solution is the aqueous sodium heparin
solution described on page 10. The higher concentration
re-ferred to here is 100 Mg of heparin per ml of water.
ized solution concentration indicates a possible multiple attachment between the heparin and the grafted ethylenimine. Such bonding could render useless muchof the surface heparin by, in effect, completely sequestering it. Since a
propor-tionate increase in compatibility was not observed with this increase in surface heparin, one tentatively concludes that non-heparinized regions of membranes exist.
Microphotographs of the heparinized membrane surfaces and surfaces of the original RCF show surface crystallites of from 20 to 50,/4in size. Due to the hydrogen bonding be-tween hydroxyls in the cellulose crystallites, these crys-tallihe regions are notoriously less reactive than the amor-phous regions. (82,83) Consequently, these surface crystal-line regions might well be the non-heparinized portions of the surface.
The CIH surfaces with the ethylene oxide pretreatment and the lowest acid catalyst concentration, .04N HCl, have improved compatibility but the most compatible surfaces do produce a clot in 90 to 110 minutes. Surface crystals were observed with these surfaces also but to a lesser extent than with the water catalyzed schemes.
Surfaces shacing whole blood clotting times well in ex-cess of 110 minutes are obtained consistently only with an acid catalyzed amination achieved by prior swelling of the
18 11 dudmil"
membrane in a solution of hydrochloric acid having a con-centration of 0.2 N or greater. The time of exposure to
ethylenimine vapor at 85 0C in the presence of such acid cata-lysts need only be about two hours for successful amination. The maximum amination time and catalyst loading is usually governed by considerations of membrane strength , since the acid is known to hydrolyze the glucosidic linkags of cellu-lose in the presence of heat.
Microphotographs of these acid catalyzed CIH surfaces show a complete absence of surface crystallites. Since both intra- and intercrystalline swelling of cellulose has been noted with acids (83), the hydrochloric acid, when present
in sufficient concentrations, apparently penetrates the sur-face crystalline regions causing amination in the presence of ethylenimine and disruption of the crystallites.
Additional in vitro experimentation with the most pro-mising acid catalyzed CIH membranes indicates that an irre-versibly heparinized surface, when totally heparinized, pre-vents blood coagulation by: 1) actively adsorbing certain clotting factors thus rendering the blood indefinitely anti-coagulated, or 2) becoming passivated through the adsorption of the blood clotting factors, hence in the presence of suffi-cient clotting factor activity the blood-like layer adsorbed to the heparin surface has a much reduced tendency toward
contact activation of the clotting mechanism.
When fresh blood is incubated at 350C in CIH tubes for lengthening periods of time, the clotting time4 of the incubated 'blood' when it is removed and incubated in a glass test tube becomes progressively longer in proportion to its CIH incubation time. However, after a CIH incubation time of 70 to 90 minutes the fresh blood is permanently coagulated.
Incubations of fresh citrated plasma in CIH tubes show
prolonged "kaolin-cephalin times" and normal "prothrombin" and "thrombin" times. The extended "kaolin-cephalin times" were corrected to normal with a 1 to 1 part dilution of the
incubated plasma with normal plasma. This indicates that probably the prolonged "kaolin-cephalin time" was a result of clotting factor deficiencies in the intrinsic clotting system. These data do not completely rule out clotting factor deficien-cies in the extrinsic system but do indicate that their level is sufficient, after incubation with the CIH membrane, to give a normal "prothrombin time."
Since heparin is known to inhibit the formation of in-trinsic thromboplastin when used as an anticoagulant in solu-tion (13,9,23) the in vitro data of this study apparently
indi-4. This clotting time is exclusive of the CIH incubation time.
cate a similar anticoagulant ability for heparin irreversibly adsorbed to surfaces.
Considering O'Brien's hypothesis (81, 82) that heparin
in solution reversibly complexes with Factor IX of the intrin-sic clotting system, one might, on the basis of the above data, extend O'Brien's theory to include heparin irreversibly bonded to solid substrates, in such a way as to have a portion of
the active sites of heparin exposed to the contacting solution. From this point of view, the adsorbed heparin layer is thought
to be active and adsorb at least one or more intiinsic clotting factors resulting in anticoagulation of the initial aliquot of blood placed in the heparinized tube. With complete saturation
of the heparin surface a passive outer surface presumably con-sisting of plasma constituents such as proteins, clotting fac-tors, etc. is expected. This heparin-blood clotting factor complex is thought to result in little or no denaturing of the clotting factors as in the case of heparin in solution. This protein-like outer surface may behave as a permanent
anti-thrombogenic surface when not denatured by the atmosphere or other solutions, thus producing a far superior surface for in vitro or in vivo blood contacting devices.
Additional evidence of heparinized surface activity is presented in Figure 4-27. Tritiated human plasma was observed to strongly adsorb onto heparinized surfaces in much larger
quantities than those observed for silicone rubber and cellu-lose. This represents independent evidence of the activity of a heparinized surface. The quantity of plasma components adsorbed onto the surface bound heparin is seen to reach a saturated level of about an equal weight of components per weight of surface heparin.
The effectiveness of the heparinized surface can be reduced considerably by a concentrated protamine sulfate
rinse, 10 Mg/ml. This has been demonstrated both in vitro
and to some extent with the radioactive plasma. With the tritiated plasma one cannot distinguish between surface pro-tamine replacement by the plasma components and plasma com-ponent adsorption on the protamine surface itself.
3) In vivo Compatibility Studies5
The limit of compatibility of the CIH surfaces can best be determined by controlled in vivo experimentation where the heparinized surface can become saturated with no exposure to alien environments. Since thin CIH membranes do not lend themselves to prostheses fabrication without some type of adhering support holder, prostheses for blood vessel replacement were fabricated from cellulose acetate. The
sur-5. These studies were performed at the Massachusetts General
Hospital in Dr. G. Austin's Laboratory.
faces of these cellulose acetate prostheses were deacetylated with a methanolic solution of 1y% sodium methylate. Amination and heparinization procedures resembled those for the
regener-ated cellulose films.
An erratically wide range of in vivo clotting times were observed for 3 cm. cellulose acetate prosthesis implanted into the canine thoracic inferior vena cava, the vein which returns blood to the heart. Therefore, the heparinized pros-theses of this study were substituted for sections of the canine inferior vena cava below where the renal veins enter the vena cava. In this region of low blood flow, controls of silicone rubber and cellulose acetate were observed to occlude totally in 1 to 1.5 hours after the implantation. The venous pressure was monitored to determine the occurence of clotting. Irreversibly heparinized cellulose acetate prostheses have remained patent for at least 8 1/2 to 9 hours, at which time the dogs were sacrificed to determine the degree of patency. This extended patency in such a severe thrombus producing
situ-ation is clear indicsitu-ation that an irreversibly heparinized sur-face is certainly antithrombogenic, probably because of its tendency to adsorb plasma components.
The results of these preliminary in vivo studies should in no way be construed as the limit of compatibility for the heparinized surface, since the actual prosthesis design and
fabrication procedure needs to be improved markedly. With these experimental heparinized cellulose acetate prostheses the surface was quite rough and the walls thick, .07 to .1 cm to prevent stress cracking. Even though the entrance and exit regions were bevelled, stagnation points existed pro-ducing slow vortex circulation and trauma. Each tend to produce coagulation in in vivo systems.
Mbmbrane Strength Measurements
Stress-strain measurements were performed on swollen CIH membranes at 370C to determine the effect of the acid cata-lyzed amination on membrane strength. None of the membranes measured, including the regenerated cellulose films, showed a yield stress under these stressing conditions. The
force-strain curves for the amination procedures using the minimum acid catalyst are given in Figure 4-37. With amination times of up to 12 hours no more than a 15% decrease in breaking force
is observed. With longer reaction times or higher initial acid concentrations more degradation in CIH membrane strength is observed. The influence of amination conditions on the breaking strength of CIH membranes can be observed from Fig-ure 4-39. Reaction conditions which produce simultaneously both acceptable surface compatibility and sufficient membrane
strength can be selected from this figure.
However, due to the membrane swelling which occurs dur-ing the amination as a result of the hydrochloric acid and ethylenimine treatment, the actual breaking stress and Young's modulus for the CIH membranes are somewhat lower than those for
regenerated cellulose. These values are given in Figure 4-41.
Diffusion Studies
Measurements of membrane permeability to urea, using C14 labelled urea, indicate that RCF and CIH membranes, the latter having been saturated with plasma, have comparable urea trans-port coefficients. However, since the CIH membrane swells during amination to about twice the thickness of the original RCF, the permeability of the CIH membrane (per unit area and compared at equal thicknesses) is about twice that of RCF. Nevertheless, the urea clearance of an artificial kidney, using a CIH membrane should be comparable to that of current hemodialyzers, since the increased permeability and membrane
swelling will have cancelling effects.
Urea diffusion coefficients measured for human blood are presented in Figure 4-53 as well as those predicted by the Prager (85,86) model for suspensions and the inverse viscosity rule. The experimentally measured values lie well above the predicted values. This is probably a result of assuming total
impermeability of the red cell by the Prager model or a result 25
of the red cell membrane flickering which has been described previously. The effect of the red cells becomes noticeable above a hematocrit of 50 which might very well indicate a damping out of the membrane flickering effect because of more pronounced red cell-red cell interaction at the high hemato-crits. For the purposes of mass transfer correlations and engineering designs a value of 0.75 x 10-5 cm 2/sec seems ap-propriate based on present data, for the urea diffusivity through normal human blood at 200C.
26
$
Conclusions
A) Artificial Kidney Performance
1. The current artificial kidneys appear to be operating at 20-30% of their membrane urea removal capacity.
2. These efficiencies apply equally well to extra-corporeal hemodialysis units dialyzing actual patient blood or saline as a blood simulant. Thus an excessive increase in membrane resistant due to the adsorption of blood com-ponents onto the membrane must be ruled out.
3. The major resistance to urea transport appears
to exist in the blood.
B) Graphite-Benzalkonium-Heparin Surface
1. About 2 /' gm/cm2 of adsorbed heparin is present
on the Lucite-GBH surface. This value is of the same order of magnitude as that calculated for a monolayer of heparin assuming a collapsed helical coil configuration for heparin.
2.
Surface heparin can be desorbed from the GBH
surface by fresh plasma with little or no fluid shear at the surface, but not with saline even at wall shears of 9.5 dyne/ cm2, suggesting strong complexing power of the plasma proteins.
3. The heparin removal rate from the GBH surface is a function of fluid shear at the surface when the shearing solution is plasma.
4. The weak bond in the GBH surface is probably the
physical bond between the graphite particle and the benzal-konium organic tail.
C) Heparinization of Regenerated Cellulose Films
1. Heparin can be irreversibly bonded to regener-ated cellulose dialysis tubing following amination with ethyl-enimine.
2. An acid catalyst must be present during the
amination to give effective surface amination if surface crys-talline regions are present on the substrate. The total ad-sorbed surface heparin ranges from 9 to 12/gm/cm2 depending on the acid concentration of the solution initially used to swell the cellulose.
3. Such heparinized surfaces give WBC times in ex-cess of 100 minutes whereas the controls are normally 20 min-utes.
4. The surface heparin, when adsorbed in the pre-ferred manner, interacts with plasma and whole blood. This interaction appears to be a result of complexing between the surface heparin and one or more intrinsic clotting factors.
5. The adsorption of clotting factors onto the hep-arinized surface is, in many cases, sufficient to anticoagu-lant the contacting aliquot of blood. Of course, the degree of saturation of the heparin surface dictates the surface ad-sorption power.