HAL Id: hal-01890092
https://hal.archives-ouvertes.fr/hal-01890092
Submitted on 8 Oct 2018
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Neodymium electrowinning into copper-neodymium
alloys by mixed oxide reduction in molten fluoride media
Mihaela Raluca Ciumag, Mathieu Gibilaro, Laurent Massot, Richard
Laucournet, Pierre Chamelot
To cite this version:
Mihaela Raluca Ciumag, Mathieu Gibilaro, Laurent Massot, Richard Laucournet, Pierre Chamelot.
Neodymium electrowinning into copper-neodymium alloys by mixed oxide reduction in molten fluoride
media. Journal of Fluorine Chemistry, Elsevier, 2016, 184, pp.1-7. �10.1016/j.jfluchem.2016.02.001�.
�hal-01890092�
OATAO is an open access repository that collects the work of Toulouse
researchers and makes it freely available over the web where possible
Any correspondence concerning this service should be sent
to the repository administrator:
tech-oatao@listes-diff.inp-toulouse.fr
This is an author’s version published in: http://oatao.univ-toulouse.fr/20547
To cite this version:
Ciumag, Mihaela Raluca
and Gibilaro, Mathieu
and Massot, Laurent
and
Laucournet, Richard and Chamelot, Pierre
Neodymium electrowinning into
copper-neodymium alloys by mixed oxide reduction in molten fluoride media. (2016) Journal
of Fluorine Chemistry, 184. 1-7. ISSN 0022-1139
Official URL:
https://doi.org/10.1016/j.jfluchem.2016.02.001
Neodymium
electrowinning
into
copper-neodymium
alloys
by
mixed
oxide
reduction
in
molten
fluoride
media
M.
Ciumag
a,b,
M.
Gibilaro
a,*
,
L.
Massot
a,
R.
Laucournet
c,
P.
Chamelot
aaLaboratoiredeGénieChimiqueUMRCNRS5503,UniversitéPaulSabatier,118routedeNarbonne,31069ToulouseCedex9,France bCEA-TechMidi-Pyrénées,135avenuedeRangueil,31400Toulouse,France
cCEAGrenobleLITEN,17ruedesMartyrs,38054GrenobleCedex9,France
ABSTRACT
Thepossibility ofneodymiumelectrowinningfromneodymiumoxideusing areducingagent(RA) producedin-situbysolventreductionwasinvestigatedinmoltenLiF–CaF2–Li2Obetween900!Cand 1040!C.Nd2O3galvanostaticelectrolyseswereperformedandreactionproductswereanalyzedbySEM, XRDandelectron-probemicroscopy.Metallicneodymiumwasnotdirectlyobtained,duetoseveral limitationsforNd2O3reduction:lowelectricalconductivity,unfavourablePilling–Bedworthcoefficient andformationofadenseinsulatingCaOlayeronthesamplesurface.
Consequently,thereductionofapelletmadeofaneodymiumoxide-metallicoxidemixturewascarried outasanalternativepathway.Nd2O3-CuOreductionledtometallicneodymiumproductionintheform ofliquidCu-Ndalloys.Areactionmechanismwasproposedbasedontheseexperimentalresults:
1.Introduction
Needs of metallic neodymium and its alloys are lately increasing,particularly in the fields of magnetism, energy and hightechnology,asinpermanentmagnets,lampphosphorsand rechargeable NiMH batteries [1]. Neodymium is industrially produced by both calciothermic reduction of NdF3, requiring severalpurificationsteps,and bymoltensaltelectrolysis,which enablescontinuousoperationandismoresuitableformassmetal production.
Metallicneodymiumelectrodepositioncantakeplaceinfused chloridesalts[2–13],butthisprocesshasseveraldrawbacks,such astheevolutionofchlorinegasattheanodeandlowfaradicyield. Thus,neodymiummetaliscurrentlyproducedbyelectrowinning fromneodymiumoxideinamoltenfluoridemediacontaininghigh amountsofNdF3(upto87mass%)[14].
Severalauthorssuggesteddifferentreductionmechanismsof Nd2O3inNdF3-basedmoltensalt[16–19],butnoagreementhas been found yet. Stefanidaki et al. [15,16] detailed neodymium
production in LiF–Nd2O3 and LiF–NdF3–Nd2O3 systems. In LiF– Nd2O3nocharacteristicsignalforneodymiumoxidereductioninto metalwasobservedandmetallicneodymiumdepositiondidnot occurin this molten mixture.In LiF–NdF3–Nd2O3, fluoride and oxyfluoride complexes such as [NdF6]3" and [NdOF5]4" were proposedtobeformed,andmetallicneodymiumwasobserved. Thefollowingelectrontransfermechanismwassuggested: Cathode:[NdF6]3"+3e"!Nd(s)+6F"
Anode:3[NdOF5]4"!3/2O2(g)+3Nd3++15F"+6e"
The authors concludedthat electrowinning of metallic neo-dymiumcanbeperformedbycontrolledcell–voltageelectrolysis inLiF–NdF3–Nd2O3.
Thudumetal.[17]proposedadifferentreductionpathwayfor Nd2O3inLiF-CaF2-NdF3,suggestingboth[NdFO5]4"and[NdF6]3" complexeswerereduced tometallic neodymium,dependingon theOF/Fmolarratio:forlowratios,[NdF6]3"wasreduced,whereas aboveacriticalvalue,[NdFO5]4"wasreduced.
DysingerandMurphy[18]testedNd2O3reductioninLiF–CaF2– NdF3 on tungsten cathode, for temperatures from 1030!C to
1060!C, andhighlightedseveral side reactionsoccurringatthe
anode:
Theauthorsconcludedmetallicneodymiumelectrowinningfrom LiF–CaF2–NdF3–Nd2O3wasonlypossibleathighcurrentdensity (1Acm"2)andhightemperature(T>1021!C=Ndfusion
tempera-ture).
Fe–NdalloyswereobtainedbyMorriceet al.[19],byNd2O3 electrolysisonreactiveironcathodeat990!CinLiF–NdF
3(89mass %).Later,Nd2O3electrolysisinfusedfluoridesforFe–Ndfabrication was patented by Pechiney [20]. Alloys were obtained by long duration (>25h) Nd2O3 electrolyzes on reactive iron cathode, performedinLiF–NdF3–BaF2–B2O3,between750!and1000!C,and atconstantcellvoltage.
Nowadays, neodymium is industrially produced by Nd2O3 reduction inNdF3-basedmixtures(NdF3>70mass%[21])using Mo, W or Fe as cathodic materials [22]. Nevertheless, several drawbackscanbepointedoutforthisprocess:
– ThesolubilityofNd2O3isonly5mass%(at1100!C)[23],which makestheoxidefeedingintotheelectrolytedifficulttocontrol andcanleadtoformationofsludgeatthecellbottom[24]. – Theoverallcostoftheindustrialprocessishighduetotheprice
ofNdF3($1000euros/kg).
Theaimofthisworkistoinvestigatealowercostproduction process for neodymium electrowinning from Nd2O3 in molten fluoridemedia,inabsenceofNdF3(LiF–CaF2eutecticscontaining Li2O2mass%),byanewapproach:theneodymiumoxideisusedas solidpelletsatthecathode,similarlytotheFFC—Cambridge[25] andOSprocesses[26],andnomoreasthefeedmaterial(current industrialprocess).Thetwomainadvantagesofthisapproachare: firstly, Nd2O3solubilitylimitation,encountered for the conven-tionalelectrolyticsystem,iseliminated.Secondly,theoverallprice oftheprocessisreduced,duetothelowercostofLiF–CaF2–Li2O: 1kgofLiF–CaF2–Li2Omixturecosts200euros($60mass%LiFat 100euros/kg,$40mass%CaF2at60euros/kgand2mass%Li2Oat 4800euros/kg);while1kgofLiF–NdF3mixturecosts$750euros ($30 mass % LiF at 100euros/kg, and 70 mass % NdF3, at 1000euros/kg).
Withthisnewapproach,Nd2O3electroreductionwasstudiedby linear voltammetry and galvanostatic electrolyses at different operating parameters:temperaturesfrom900!Cto1040!C and
imposed currents from 0.15 to 0.8A. Elemental analyses of compositionsandmicrostructures(SEM,XRDandelectron-probe microanalyser)wereperformed,andthelimitingfactorsforNd2O3 reduction intoNdinmoltenfluorides, inabsenceofNdF3,were elucidatedforthefirsttime.
Nd2O3electroreductioninpresenceofanelectricalconducting metallic oxide was also investigated.Indeed, this strategy was already shown to be suitable for preparing Nd–Co alloys by electrochemical reduction of Nd2O3–Co3O4 mixtures in molten chloridemedia[27].Otherrareearthsalloyswithheavymetals, suchasLa–Ni[28],Ce–Co[29],Ce–Ni[30],Tb–Fe[31]andTb–Ni [32],werealsoobtainedbyelectrochemicalreductionofrareearth oxidesandmetallicoxidesmixtures.
Inthisstudy,theelectrochemicalbehaviourofNd2O3–CuOwas investigatedatdifferenttemperaturesbylinearvoltammetryand
constantcurrentelectrolyses.Analysisofmicrostructuresshowed formation of Cu–Nd alloys and allowed proposing a reduction pathway.ForhighpuritymetallicNdrecovery,afurtherseparation of Cu and Nd can be easily realised with electrorefining, by exploitingtheelectrochemicalpotentialsofthetwoconstituents. 2. Resultsanddiscussion
2.1.Preliminarydiscussionandsolventselection
Rareearth oxides can beelectrochemically reduced by two differentpathways,dependingontheirelectricalconductivity: 1.Highlyconductiveoxidesaresubjecttodirectelectrochemical
reductionbyFFC—Cambridgeprocess[25],wheretheoxideis reducedintometalonthecathodesurface.
2. Oxides presenting low electrical conductivity are subject to indirectreductionbyOSprocess[26].Inthiscase,areducing agent(producedbysolventreduction)reactswiththerareearth oxidetoformametallicphase.
Nd2O3 has a very low electrical conductivity (
s
650!,0,2barO2= 1,45% 10"6V
"1cm"1[33])thereforeitisexpectedtobereduced followingtheindirectOSprocess,asillustratedintheequation: Nd2O3+3xRA!2Nd+3RAxO (1) whereRAisthereducingagentandRAxOistheoxidizedformof thereducingagent.The reducing agent (RA) couldbe an alkaline (K,Na, Li)or alkaline-earth metal (Ca) obtained by cathodic reduction of fluoride solvents (KF, NaF, LiF, CaF2). The Gibbs energy of the reaction(
D
rG!)betweenneodymiumoxideandthereducingagent indicateswhethertheneodymiumoxideisreducedintometallic neodymium or not: ifD
rG! is negative, the reduction of neodymiumoxidebythealkalineoralkaline-earthis thermody-namicallyachievable.AspresentedinTable1,neodymiumoxide canbereducedintometallicneodymiumbyCaonly.Nevertheless,becauseofCaF2toohighfusiontemperature(1 418!C), a lower working temperature was chosen: LiF–CaF
2 eutectics(Tfusion=767!C).BecausethereductionpotentialsofLi+ andCa2+areclose(ELi+/Li=
"5.31VvsF2/F"andECa2+/Ca="5.33 VvsF2/F"),theirsimultaneousreductionatthecathodesurface wasalreadyobserved[34].
Table1
StandardfreeenergydataforNd2O3reductionwithdifferentalkaliand
alkaline-earthmetals,followingequation(1)at900!C.
RA K Na Li Ca
2.2.ElectrochemicalcharacterizationinLiF–CaF2bylinearsweep voltammetry
ToprovideO2"ionsinthemolten
fluoridemediaandensurethe anodicreaction,Li2Owasused.Gibilaroetal.[35]showedinLiF– CaF2–Li2Omixture,Li2Ohadtobemaintainedataconcentration higherthan1mass%topreventtheAuanodeoxidationand to ensuretheoxidationofO2"intogaseousO
2[36].
Inthiswork,Nd2O3reductionmechanismwasstudiedinLiF– CaF2–Li2O(2mass%).Thelinearvoltammogramobtainedat10mV. s"1and900!C,ispresentedinFig.1,comparedwiththesolvent.
Thetwocurvesdisplaynosignificantdifference,meaningthat nospecificNd2O3 electrochemicalreactionoccurs.Therefore,its reduction isindirectand theproposedreaction pathwayisthe following:
Cathode:Ca2++2e"!Ca (2)
Spontaneousredoxreaction:Nd2O3+3Ca!2Nd+3CaO (3)
Anode:2O2"
!O2+4e" (4)
The Ca reducing agent [34] produced on the Mo cathode reducestheneodymiumoxideintometallicneodymium,andthe resultedO2"ionsareoxidizedintogaseousO
2onthegoldanode. 2.3.Galvanostaticelectrolysesofneodymiumoxide
2.3.1.Neodymiumoxidereduction
ToinvestigateNd2O3reduction,experimentswerecarriedout inconstantcurrentmode.Becausestablereferenceelectrodesin
fluoridesaltsarenotavailableyet(noaccuratecontrolofcathodic potential), the constant potential mode was not used during electrolyzes.
Several reduction tests were performed in LiF–CaF2–Li2O (2mass%) at900!C. ThetheoreticalchargeQ
thneededfor the reductionofoneneodymiumoxidepelletiscalculatedasfollows: Qth¼mNd2O3
MNd2O3
nF ð5Þ
whereQthisthetheoreticalchargecalculated(C),mNd2O3isthe oxidepelletweight(g),MNd2O3istheoxidemolecular weight (336.48g.mol"1),nisthenumberofelectronsexchangedpermole ofNd2O3,FistheFaradayconstant(96,480Cmol"1).
The pellet cross-section after electrolysis at I="0.4A and t=1320s(500%Qth),observedbySEM,showsthreedifferentzones (Fig.2).Quantitativeelectron-probemicroanalysisallowed iden-tifyingthecompositionofthesezones,asfollows:
– Theinternalzone(1)ismainlyNdOF,formedbyspontaneous decomplexationofNd2O3:
Nd2O3+2F"!2NdOF+O2" (6) ThisphenomenonwasalsoobservedbyNourry[37]whenadding O2"ionsin
LiF–CaF2–NdF3.
– Zone2isformedbyrecrystallizedfluoridesalts(LiFandCaF2). – AdenselayermainlycomposedoflowelectricalconductingCaO, formedbyO2"andCa2+co-precipitation,isobservedoverallthe surfaceoftheneodymiumoxidepellet(zone3).TheCaOlayer actsasabarrier, limitingthediffusionofO2"fromthepellet towardsthemoltensalt,preventingtheNd2O3reductiontotake place.ThisphenomenonexplainstheabsenceofmetallicNdat theendofelectrolysis.
2.3.2.Temperatureinfluence
ToincreaseCaOsolubilityinLiF-CaF2(from0.5mol%at730!C to2.9mol%at1000!C,[38])andtoattempttoremovethelayeron
the sample surface, experiments were performed at higher temperature,whereliquidmetallicneodymiumcouldbeformed. Fig. 3 illustrates the cross-section of Nd2O3 sample after electrolysisat1040!Cand"0.4A(500%Q
th).
Asexpected,noCaOlayerwasformedonthesamplesurface, but X-ray diffractommetry (Fig. 4) didnot highlight either the presenceofmetallicNd.
WhencomparingFig.2(900!C)andFig.3(1040!C),itcanbe
pointedout thatthepelletstructureand compositionchanged: Nd2O3chemicaldecomplexationat900!CledtoNdOF,whileat1 040!C Nd
2O3 clusters appeared, enhancing significantly the
Fig. 1.LinearsweepvoltammogramsonMogridinLiF–CaF2–Li2O(2mass%Li2O)at
10mVs"1and900!C.Continuousline:solvent,dottedline:Nd2O3pellet.
porosity. This phenomena was also confirmed by the X-ray diffractogram (Fig. 4) where Nd2O3, LiF and CaF2 are present, butnoNdOFwasdetected.
To investigate the temperature effect, a Nd2O3 pellet was exposedat1040!Cinthemoltensaltbathwithoutpolarisation,
andSEManalysisshowedsimilarstructurestothosepresentedin Fig.3.Batsanovetal.[39]hadalreadyobservedasimilarbehaviour of neodymiumoxide powder (grain size 1–10
m
m) under high pressure and temperature, where 100–1000m
m crystalline aggregateswerecreated.Theinfluenceoftheappliedcathodiccurrent(from 0.15Ato 0.8A)andneodymiumoxidepelletsfabrication(pressureapplied for sinterizing the oxide powder from 2 to 4tcm"2) was also studied,butmetallicneodymiumwasstillnotobtained.
To resume, Nd2O3 electrochemical reductiondidnot lead to metallicneodymium,whatevertheoperatingparameters. More-over,severallimitingphenomenawerehighlighted:
1RelatedtoNd2O3physicochemicalcharacteristics:
- low electrical conductivity (
s
650!C,0,2barO2=1,45% 10"6V
"1 cm"1)[33];- unfavourable Pilling-Bedworth coefficient (VNd/VNd2O3=0.89). Pilling–Bedworthruleconsiderations[40],asillustratedbyLi etal. [41]and Gibilaroetal. [42],indicatethat duringoxide reduction,ifthemolarvolumeoftheformedmetalVmissmaller thanthemolarvolumeoftheoxideVo,themetalobtainedis porousenoughtoallowthemoltensaltelectrolyteaccessingthe underlying oxide. For neodymium, themetal tooxide molar volume ratio is VNd/V Nd2O3=0.89, meaning that volume constrictionisnotenoughduringNd2O3conversionintometal: themetallicneodymiumformedwouldthusactasadiffusion barrier.
2Relatedtosolvent:
- neodymiumoxidecanonlybereducedbyCametal,limitingthe selectiontoCaF2-basedsolvents;
- inCaF2-based solvent, at900!C, theprecipitationof a dense insoluble CaO layer on the sample surface is observed, preventingNd2O3reduction.
2.4.Reductionofneodymiumoxideinpresenceofcopperoxide ToobtainmetallicNd,theadditionofanelectricalconducting oxideintheneodymiumoxidepelletwastested.CuOwaschosen foritsgoodelectricalconductivity(
s
127!C=10"1V
"1cm"1[43]) and its favourable Pilling–Bedworth coefficient VCu/VCuO=0.56Fig.4. X-raydiffractogramofNd2O3pelletafterelectrolysis(I="0.4A,500%Qth)at1040!CinLiF–CaF2–Li2O(2mass%).
Fig.3.MicrographofNd2O3pelletcross-sectionafterelectrolysis(I="0.4A,500%
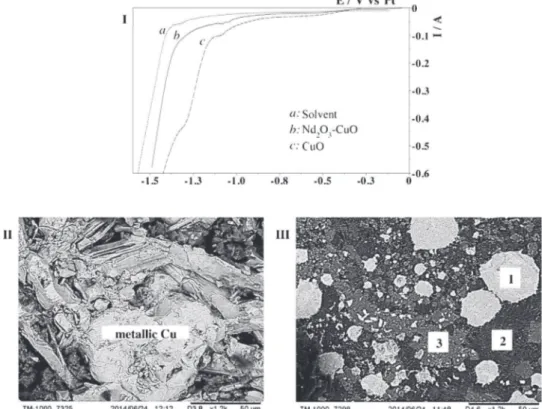
[36], enhancing pellet porosity. CuO is therefore expected to undergodirectelectrochemicalreductionintometallicCu,andto confirmit,CuOreductionin LiF–CaF2–Li2O(2mass%) wasfirst studied. The linear voltammogram obtained at 10mVs"1 (vol-tammogramcinFig.5,I)displaysareductionsignalat"1.3VvsPt, assigned to the direct electrochemical reduction of CuO into metallicCu,followingtheequation:
CuO+2e"!Cu+O2" (7) Moreover,theelectrolysisof aCuOpelletat"0.15Aand 900!C
confirmedthis result: 100
m
mto1000m
mmetallic Cu clusters (micrographinFig.5,II)wereobtained.The current efficiency was also calculated, following the equation:
h
¼mexperimental mtheoreticalwheremexperimentalisthemassofthemetallicdepositobtainedby electrolysis(g)andmtheoreticalisthetheoreticalmassofmetallic depositexpectedtobeobtainedbyelectrolysis(g),calculatedas following:
mtheoretical¼I)t!
n)F)Mmetal
whereIistheintensityoftheimposedcurrent(A),tiselectrolysis duration(s),nisthenumberofexchangedelectronspermoleof metallic oxide (n=2 for CuO!Cu reduction), F is the Farady constant(96480Cmol"1), and M
metalis theatomicmassofthe metal(forCu,M=63,54gmol"1).
The calculated current efficiency for CuO reduction into metallicCuwasverycloseto100%.
Nd2O3reductioninpresenceofCuOwasfurtherinvestigated. Cu–Ndphasediagram[44]presentsseveralliquidandsolidalloys, andcompositionofNd2O3–CuOpelletswaschosentobeinthe zonewhereliquidalloysareformed:65atomic%Ndand35atomic %Cu.ThelinearvoltammogramofNd2O3–CuOat10mVs"1inLiF–
CaF2–Li2O(2mass%)(voltammogrambinFig.5,I)showsalsoa reduction signal starting from "1.4V vs Pt, due to the direct electrochemical reduction of CuO. Additional cathodic signals, between"1.0and"1.3VvsPt,wereassignedtoimpuritiespresent inthesolvent(comparedtovoltammogramainFig.5,I)andinthe 99%purityCuO(comparisonwithvoltammogramcin Fig.5,I). Afterelectrolysis at"0.15A (500% Qth)and 900!C, thesample presentedseveralzones(Fig.5,III):
– Zone 1, composed of NdCu4, NdCu5 and NdCu6 droplets (size<50
m
m);– Zone2,composedofrecrystallizedLiFandCaF2;
– Zone3,composed ofNdOFandCaO,but nocompactlayeris observedonsamplesurface.
TheseresultsconfirmNd2O3reductionispossiblewhenCuOis simultaneouslypresent,leadingtoformationofCu–Ndalloys,and amulti-stepreductionmechanismwasproposed,asfollows:
1ststep:ChemicaldecomplexationofNd2O3intoNdOF: Nd2O3+2F"!2NdOF+O2" (6)
2nd step: CuO direct reduction at the cathode, leading to metallicCu,behavingasreactivecathode:
Mocathode:CuO+2e"
!Cu+O2"+porosity (7) 3rdstep:NdOFreductiononthesurfaceofmetallicCu,leading toCu-Ndalloys:
Metallic Cu reactive cathode: Cu+NdOF+3e"!Cu–Nd+O2"+
F" (8)
Thethirdstepofthismechanismwasconfirmedbyelectrolysesof Nd2O3-Cu metallic powder mixture, which also led to the formation ofCu–Nd alloys.Nourryetal. presentedalso similar results, showing that Nd3+reduction oncopper cathodeled to formationofCu–Ndalloys[37,45,46].
Fig.5. ILinearsweepvoltammogramsinLiF–CaF2–Li2O(2mass%)at10mVs"1and900!C.Dottedline:solvent,continuousline:Nd2O3–CuO,interruptedline:CuO.II,III
Several authorsprovedthereductionof rareearth oxidesin presenceofmetallicoxidestobesuitableforobtainingrareearth alloysinmoltenchlorides.Moreover,theyalsoproposedreduction mechanismswherethemetallicoxide(Co3O4,NiO,Co3O4,Fe2O3) wasfirstreducedonthecathode,followedbythereductionofrare earthoxideandformationofalloys[27–32].
ThecurrentefficienciesforNd2O3reductioninpresenceofCuO ormetallicCupowdercouldnotbeestimated,duetothelackof homogeneityof thesample andthe smallsizeof Cu–Ndalloys droplets (<50
m
mdiameter,Fig.5,III),whichcouldnotbefully recoveredtobeweighted.Nevertheless,whenNd2O3–CuOelectrolyseswereperformedat higher temperature (1040!C), coalescing of Cu–Nd droplets
occurred (size$500
m
m to 2mm), facilitating theirrecovery at theendofelectrolysis.Inthiscase,theglobalcurrentefficiency couldbeestimatedusingtheequation(8)anditsvaluewas$70%. The remaining30% current fractioncould berelatedtosolvent reduction(Ca+andLi+)anduncompleterecoveryandseparationof Cu–Ndalloysfromthesalt.Thereductionofneodymiumoxideinpresenceofcopperoxide presentsseveraladvantages:
- CuO directreductionledtocreationofporosityin thepellet, enablingthepenetrationofmoltensaltinthebulkofthesample and thusenhancingtheinteractionbetweenthesolvent, the metallicCuobtainedbyreductionandtheneodymiumoxide; - Underpolarization,themetalliccopperobtainedbyCuOdirect
reduction behaves as reactive cathodic material, leading to formationofCu–Ndalloys.
TorecoverhighpuritymetallicNd,CuandNdseparationcanbe easilyaccomplishedbytheelectrorefiningprocess,duetothelarge differencesoftheirelectrochemicalpotentials:inLiF–CaF2at840! C,ECu2+/Cu=2.83V/Li+/LiandENd3+/Nd=0.35V/Li+/Li[37].Thus,Nd fromCu-NdalloyscanbeselectivelyoxidizedintoNd(III),which willbefurtherdepositedashighpuritymetallicNdonthecathode. Meanwhile, pure metallic Cu remaining at the anode can be recycledandreusedforNd2O3reduction.
3. Conclusions
Inmoltenfluoridemedia,thereductionofNd2O3wasshownto beindirect,similartotheOSprocess.Nevertheless,reductionof neodymiumoxideintometallicneodymiumdidnotoccurwhen usinganinertcathodicmaterial(Mo),regardlessoftheoperating parameters(temperature,current,fabricationoftheoxidepellet). Thelimitingfactorswererelatedtothe:
1.PhysicalcharacteristicsofNd2O3:lowconductivityand unfav-ourablePilling-Bedworthcoefficient.
2. Solvent: Indirect reduction of Nd2O3 in molten salts is thermodynamicallyachievable(
D
rG!<0)forCa-basedsolvents only,whenmetallicCaisobtained.However,adenseinsoluble CaO layer was formed overall the sample surface, acting as diffusionbarrierforO2",preventingthereductionoftheoxide pellet.Moreover,Nd2O3behaviorwasinfluencedbytemperature:at 900!C, the oxide was decomplexed into insoluble chemically
stable NdOF, while at 1040!C it ledto formation ofcrystalline
aggregates. Thus, electrowinning of metallic neodymium from neodymiumoxidecannotbeperformedinmoltenfluoridemedia inabsenceofNdF3.
Addition of a conductive metallic oxide to the neodymium oxidewasfurtherinvestigated.WhenNd2O3–CuOandNd2O3–Cu pelletswereelectrolyzed,metallicneodymiumwasrecoveredas
alloyedCu–Nddroplets,andathighertemperature(T>1040!C)a
bettercoalescencewasobserved.Thefollowingreduction mecha-nismwasproposed:
TheCu,initiallypresentinmixturewithNd2O3orproducedby CuOdirectelectroreduction,actsasadepolarizingreactivecathode forNd3+(NdOF)reduction,leadingto
Cu–Ndalloys.Thisstrategyis theonlyoneviable forobtainingmetallicNdfromneodymium oxideinmoltenfluorideswithoutNdF3.Furthermore,highpurity metallicNdcanberecoveredbyasimpleelectrorefiningprocess, while the metallic Cu can be recycled and reused for Nd2O3 reduction.
4. Experimental
Thecelldesignconsistedinavitreouscarboncrucibleplacedin acylindricalvesselmadeofrefractorysteel.Theinnerpartofthe cellwallwasprotectedagainstfluoridevapoursbyagraphiteliner. Experimentswereperformedunderinertargonatmosphere.The moltensalt(200g)wascomposedofLiF-CaF2eutectic,dehydrated byheatingundervacuumfromroomtemperatureuptomelting point(767!C).Li
2Opowder(Cerac99.5%)wasusedtoprovideO2" ionsinthebath,toensuretheanodicreaction(2O2"
!O2+4e"). Nd2O3(Aldrich99.9%),CuO(Merck99%)andCumetallicpowder (Goodfellow99.8%)wereusedintheformofpelletssinteredby applyingapressureof3.2tcm"2toseveralmilligrams(from100to 300mg) of powder, at 25!C. The pellets, attached with a
molybdenumgridconnectedtothecurrentleadbyamolybdenum wire,wereusedasworkingelectrodes.Theauxiliaryelectrodewas agoldwireorplate,withalargesurfacearea(S=3cm2),andall potentialswerereferredtoa platinumwire (0.5mmdiameter), actingasaquasi-referenceelectrodePt/PtOx/O2".
The electrochemical experiments were performed with an AutolabPGSTAT 30potentiostat-galvanostat.Afterresin embed-ding and polishing, the cathode bulk was examined with a scanningelectronmicroscopeSEM(LEO435VP)equippedwith and EDS probe (Oxford INCA200). XRDcharacterisations were performed with an Equinox 1000 diffractometer. Quantitative analysis wereperformed witha Cameca SXFive electronprobe microanalyser.Nevertheless,neitherSEM-EDSnorelectron-probe microanalysisdonotprovidedataonLi.Meanwhile,SEM-EDSdo notprovidedataanalysisonlightelementssuchasfluorideand oxygen.
References
[1]K.Binnemans,P.T.Jones,B.Blanpain,T.VanGerven,Y.Yang,A.Walton,M. Buchert,J.CleanerProd.51(2013)1–22.
[2]E.Morrice,E.S.Shed,T.A.Henrie,U.S.Bur.MinesRep.Invest.7146(1968). [3]E.Morrice,M.M.Wong,Miner.Sci.Eng.11(1979)125–136.
[4]Y.Qiqin,L.Guanqun,F.Zhongan,S.Lichang,RareMet.8(1989)9. [5]M.F.Chambers,J.E.Murphy,U.S.Bur.MinesRep.Invest.9391(1991). [6]A.Kaneko,Y.Yamamoto,C.Okada,J.AlloysCompd.193(1993)44–46. [7]C.Shiguan,Y.Xiaoyong,Yu.Zhongwing,Lu.Qingtao,RareMet.13(1994)46. [8]J.E.Murphy,D.K.Dysinger,M.F.Chambers,LightMet.(1995)1313–1320. [9] D.W.Dees,J.P.Ackerman,UniversityofChicago,USPatent0045835A1,(2004). [10]Y.H.Kang,S.C.Hwang,H.S.Lee,E.H.Kim,J.Met.Process.Technol.209(2009)
5008–5013.
[11] R.Fujita,H.Nakamura,K.Mizuguchi,S.Kanamura,T.Omori,K.Utsunomyia,S. Nomura,KabushikiKaishaToshiba,USPatent0314260A1,(2010). [12] B.H.Park,I.C.Choi,J.-M.Hur,J.Chem.Eng.Japan45(2012)888–892. [13]E.-Y.Choi,J.W.Lee,J.J.Park,J.-M.Hur,J.-K.Kim,Chem.Eng.J.514(2012)207–
208.
[14]S.Seetharaman(Ed.),TreatiseonProcessMetallurgy,vol.3,Elsevier,2014,pp. 995–1069.
[15]E.Stefanidaki,C.Hasiotis,C.Kontoyannis,Electrochim.Acta46(2001)2665– 2670.
[16]E.Stefanidaki,G.M.Photiadis,C.Kontoyannis,A.Vik,T.Østvold,J.Chem.Soc. (2002)2302–2307.
[17]R.Thudum,A.Srivastava,S.Nandi,A.Nagaraj,R.Shekhar,Min.Process.Extr. Metall.(Trans.Inst.Min.Metall.C)119(2010)88–92.
[18]D.K.Dysinger,J.E.Murphy,USBur.MinesRep.Invest.9504(1994). [19]E.Morrice,E.Shedd,T.Henrie,USBur.MinesRep.Invest.7146(1968). [20] Y.Bertaud,AluminiumPechiney,EuropeanPatentEP0289434(A1),1988. [21]D.Chen,SolutionofNeodymiumandformationofslimeduringthe
neodymiumelectrolysis,J.RareMet.32(2008)482–483Translatedfrom ChinesebyJ.Wang,inChinese.
[22]W.Li,Utilizationrateofneodymiumoxideinproducingmetallicneodymium, Non-ferrousSmelting4(50)(2001)35–36TranslatedfromChinesebyJ.Wang. [23]E.Morrice,R.G.Reddy,Solubilityofrareearthoxidesinfluoridemelts,
SymposiumonHighTemperatureandMaterialsChemistry,Berkeley, California,1989.
[24]X.Guo,J.Sietsma,Y.Yang,1stEuropeanRareEarthResourcesConference2014, MilosIsland,Greece,4–7September,2014,pp.149.
[25]G.Z.Chen,D.J.Fray,T.W.Farthing,Nature407(2000)361–364. [26]K.Ono,R.O.Suzuki,J.Min.MetalsMat.Soc.54(2002)59–61. [27]A.M.Abdelkader,D.J.Hyslop,A.Cox,D.J.Fray,J.Mater.Chem.20(2010)
6039–6049.
[28]B.J.Zhao,L.Wang,L.Dai,G.G.Cui,H.Z.Zhou,J.AlloysCompd.468(2009) 379–385.
[29]L.Dai,S.Wang,Y.-H.Li,L.Wang,Trans.NonferrousMet.Soc.China22(2012) 2007–2013.
[30]Y.Zhang,H.Yin,S.Zhang,D.Tang,Z.Yuan,T.Yan,W.Zheng,J.RareEarths30 (2012)923–927.
[31]G.Qiu,D.Wang,M.Ma,X.Jin,G.Z.Chen,J.Electroanal.Chem.589(2006) 139–147.
[33]K.A.GschneiderJr.,L.Eyring,HandbookonthePhysicsandChemistryofRare EarthsCh.27,North-HollandPublishingComp.,1979,pp.386.
[34]M.Gibilaro,S.Bolmont,L.Massot,P.Chamelot,J.Electroanal.Chem.726(2014) 84–90.
[35]M.Gibilaro,L.Cassayre,O.Lemoine,L.Massot,P.Chamelot,J.Nucl.Mater.414 (2011)169–173.
[36]L.Massot,L.Cassayre,P.Chamelot,P.Taxil,J.Electroanal.Chem.606(2007)17– 23.
[37]C.Nourry,Thèsededoctoratdel’UniversitéPaulSabatier,Toulouse(2007)51. [38]D.-G.Kim,M.-A.VanEnde,C.Liebske,C.vanHoek,S.vanderLaan,P.Hudon, I.-H. Jung,9thInternationalConferenceonMoltenSlags,FluxesandSalts,Beijing, China,27–30May,2012,pp.110.
[39]S.S.Batsanov,A.A.Deribas,FizikaGoreniyaIVzryva1(1965)103–108English version:Combustion,ExplosionandShockWaves1(1965)77–80(10.1007/ 2FB00757157)..
[40]N.B.Pilling,R.E.Bedworth,J.Inst.Met.29(1923)529–591. [41]W.Li,X.Jin,F.Huang,Angew.Chem.Int.Ed.49(2010)3203–3206. [42]M.Gibilaro,J.Pivato,L.Cassayre,L.Massot,P.Chamelot,Electrochim.Acta56
(2011)5410–5415.
[43] A.A.Samokhvalov,N.A.Viglin,B.A.Gizhevskîi,N.N.Loshkareva,V.V.Osipov,N. I. Solin,Y.P.Sukhorukov,Zh.Eksp.Teor.Fiz.103(1993)951–961English version:J.Exp.Theor.Phys.76(1993)462–468(http://www.jetp.ac.ru/cgi-bin/ dn/e_076_03_0463.pdf)..
[44] BinaryAlloyPhaseDiagrams,S.E.,ASMInternational,1996.
[45]C.Nourry,L.Massot,P.Chamelot,P.Taxil,J.Appl.Electrochem.39(2009) 927–933.
[46]C.Nourry,L.Massot,P.Chamelot,P.Taxil,J.Appl.Electrochem.39(2009) 2359–2367.