HAL Id: hal-03086966
https://hal.archives-ouvertes.fr/hal-03086966
Preprint submitted on 23 Dec 2020HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Mechanical Strength Enhancement by Grain Size
Reduction in a Soft Colloidal Polycrystal
Ahmed Mourchid, Imane Boucenna, Florent Carn
To cite this version:
Ahmed Mourchid, Imane Boucenna, Florent Carn. Mechanical Strength Enhancement by Grain Size Reduction in a Soft Colloidal Polycrystal. 2020. �hal-03086966�
1
Mechanical Strength Enhancement by Grain Size Reduction in a Soft
Colloidal Polycrystal
Ahmed Mourchid*, Imane Boucenna, Florent Carn
Matière et Systèmes Complexes (MSC), UMR 7057 CNRS and Université de Paris 10 rue Alice Domon et Léonie Duquet, 75205 Paris Cedex 13, France
* ahmed.mourchid@univ-paris-diderot.fr
Keywords: colloids; polycrystals; grain refinement; mechanical properties; Hall-Petch.
Abstract
The mechanical strength of finely-grained solid state polycrystals is substantially enhanced when the grain size is reduced. This key well-known Hall-Petch relationship is used to engineer structural metals and alloys. To date, no similar behavior has been reported in other materials. Here, we design a soft colloidal alloy made of micellar polycrystalline grains and tune their size by inserting nanoparticles in the grain boundaries. We show that the size of the polycrystallites decreases as nanoparticle concentration increases, up to a nanoparticle threshold, then increases. In concert, both yield stress and elastic modulus are found to respectively increase and decrease, with yield stress
rigorously obeying Hall-Petch law. The investigated soft colloidal system shows striking features
similar to those reported in solid alloys. This experimental approach offers the possibility to study poorly understood mechanical aspects of polycrystalline and nanocrystalline structures, such as their plasticity, with non-destructive techniques.
2
Topological defects are well-known to impact many important properties of crystalline materials such as their mechanical behavior [1-6]. One such effect that has been beneficially implemented in applications for decades is strength hardening in solid state metals. Indeed, the formation of grain boundaries and their multiplication by grain refinement lead to peculiar yield stress enhancement which usually shows a 2 to 10 folds increase as compared to the unrefined material [1, 3, 7]. This well-known relation between yield, flow and cleavage in solid polycrystalline metals, theorized by Hall and Petch in the early 1950’s, is commonly used to engineer polycrystalline solids by refining the size of grains to less than 1 µm. It predicts that the yield stress follows a linear relation as a function of the inverse square root of grain diameter [8]. The yield stress enhancement is due to the presence of many interfaces, i.e., grain boundaries, which constitute an obstacle to dislocation motion and act as pinning points [8-10]. The strength enhancement usually comes at the expense of poor ductility hampering the effectiveness of micro- and nano-engineered materials and thus limiting their applications. However, deviations from this pathway are being reported as the grain size falls into the nanometric scale. Indeed, in this range, there is agreement that the mechanical properties are furthermore dictated by grain boundary sliding, rotation, or diffusion rather than by dislocation pile-up [11, 12]. This points again to widened size impact which, in some cases, is at the origin of novel properties of nanomaterials [13-17]. Remarkably, in the nanometric size range, super-plasticity, which gives rise to highly ductile materials, was observed and is believed to be the consequence of adequate tailoring of the microstructure [14, 15]. Size-dependent softening in small systems was also discovered in nanocrystalline materials [16] and nano-objects [17]. The last findings were observed in nanometric metallic particles subjected to small mechanical solicitations and at relatively low temperature. The observed shape modification was ascribed to surface-diffusion mediated by pseudoelasticity.
All these mechanisms have attracted huge interest owing to the various opportunities they could offer for applications in the light of the large number of small systems that could be both easily designed and studied with newly advanced nanoscopic techniques [18, 19]. Historically, the reports have focused only on solid state metals and their alloys because they constitute an important class of structural materials and are now widely used in nanodevices. However, studying the above properties at the nanoscale is a difficult task [13]. It is particularly challenging to probe nanomaterials by using current in-situ techniques which could easily induce sample damage and
3
further complicate data analysis. It is thus barely assured that the observed effects are exempt from technique-induced artefacts at the nanometric size.
An alternative to the solid state materials is provided by soft colloidal materials which are frequently and successfully investigated as model materials to study fundamental physical phenomena ranging from phase transition, structuring in a variety of ordered phases, to glassy dynamics on a notably widened length and time scales [20-24]. Their extensive use is also motivated by the facile means that leads to their synthesis and manipulation, coupled to their direct characterization by several non-destructive techniques [25]. Among the recent studies in this field, a new strategy emerged and consisted of adding small amounts of nanoparticles (NPs) to a system of different nature [26-34]. It was successfully shown that when a phase transition is induced in such a system, microscopic segregation occurs and results in polycrystalline grains separated by nanoparticle (NP) rich interstices. In this case the formers act as impurities and promote the formation of a polycrystal, rather than a monocrystal in the absence of added impurities, with well-defined grain boundaries. This well-known property in solid state systems, specially solid state metallic alloys, is now well documented both in molecular [35] and in distinct colloidal systems [27]. Moreover, the soft methods used to induce micro- and nano-structuring have identified the key solution parameters that influence the microstructure and yield accurate tailoring of it. Such easily functional tuning factors could pave the way for potentially numerous explorations of fundamental mechanisms, such as those mentioned above and reported for solid state atomic systems, particularly the mechanical properties. Indeed, besides the fundamental interest, many soft colloidal systems are exploited in a huge number of manufactured products, such as paints, coatings, adhesives, cosmetics care, and oil-drilling fluids for which the mechanical properties are of significance. Extensive theoretical and experimental literature exists on their interesting rheology. However, to date, no data were reported on the effect of grain boundaries and grain size on the rheology of soft colloidal polycrystals even though their occurrence should be much more common than thought given the fact that a small amount of impurities is expected to lead to boundaries formation and grain refinement in colloidal crystals.
The purpose of this study is to explore the evolution of the mechanical properties in a soft colloidal polycrystal designed so that the size of the grains is advantageously tuned on a wide length range. To this end, we produce aqueous solutions of (EO)100-(PO)65-(EO)100 copolymer micelles (i.e.
4
nm in diameter. The chosen system is structurally homogeneous fluidlike free-flowing solution at room temperature. Upon heating above the fluid-crystal transition temperature, the micellar solution forms polycrystalline grains, instead of a single crystal, in the presence of NPs which segregate in the grain boundaries. We study the mechanical behavior of this soft system by shear rheology and correlate the changes in mechanical parameters to the evolution of the microstructure, such as the grain size, monitored by phase contrast optical microscopy and small angle radiation scattering. The data clearly show that the formation of micellar polycrystalline grains and their refinement concur with yield stress and elastic modulus enhancements and remarkably agrees with the Hall-Petch relation historically established in solid state atomic systems. We also show that the evolution of the grain size is non-monotonous, a finding that is also observed in solid state alloys. Remarkably, the yield stress is found to rigorously follow grain size variations, obeying Hall-Petch law, in both decreasing and increasing grain size regimes.
Typical optical microscopy images of samples at a fixed copolymer concentration involved in micellization of 15.2 vol. %, and with increasingly added silica NPs from 0 to 2.92 vol. % are shown in Figure 1 (a-h). These images were recorded at a temperature of 42 °C, well above the transition temperature of 29 °C for this fixed micellar volume fraction. On this figure we observe that pure micellar solutions (figure 1a) do not show any noticeable difference in the microstructure below and above 29 °C. In contrast, samples with added NPs show, upon heating, the appearance of micrograins. They start to form below the onset of fluid to crystal phase transition temperature and occupy the whole sample slightly above it. Similar data were also observed by fluorescence microscopy on micelles with labelled NPs which showed non-fluorescent grains separated by fluorescent interstices [26]. On the images of figure 1 we notice that the number of contrasted micrograins per unit surface area increases as NP concentration increases from 0 to 1.67 vol. % while it decreases when the NP concentration increases from 1.67 to 2.92 vol. %. The variation of the average grain size, d, with NP concentration is also shown in this figure (1i) and ranges from almost 100 to less than 10 µm. These averages are known to depend on a variety of solution parameters such as crystallization heating rate, micellar volume fraction, as well as NP concentration [26, 28].
Moreover, as the phase transition temperature is reached, the NPs are being segregated outside the micrograins whose equilibrium size is, in turn, controlled by the NPs. This effect was explained by studying the evolution of grain size as a function of the two tuning parameters: NP volume fraction
5
and crystallization rate in a nearly similar colloidal system [28, 29]. Accordingly, it was shown that the presence of NPs decreases the energy barrier to nucleation and favors the formation of smaller crystalline nuclei through reduction of the surface term in Gibbs energy. Increasing the number of impurities in the polycrystalline colloidal material, or adding segregating NPs, ultimately increases the number of nuclei and hence yields smaller polycrystalline grains. Such relation between free energy barrier to nucleation and grain size is in agreement with data previously reported in metallic samples [36]. Overall, the nucleation and growth model was easily adjustable to the multicomponent colloidal system by means of polycrystalline grain size calculation as a function of NP concentration [37]. By following the strategy mentioned above and adjusting the fit parameters to the experimental variation of d as a function of NP concentration, resulting best fits were found to reasonably agree with the experimental decrease of grain size down to the minimum observed at the NP concentration of 1.67 vol. %. However, the fitted curve, which is solely a monotonous decreasing size as a function of increasing NP concentration, does not capture the subsequent experimental increase which we measure for d and no effort has been made to account for it at the present time. Nevertheless, it is worth adding that similar second stage size increases were experimentally reported in solid state metallic alloys, where the effect is termed as a “poisoning” because it leads to a growing grain size and hence to weakened strength, which is exactly what our system shows [38]. Its cause is not resolved and there is still controversy.
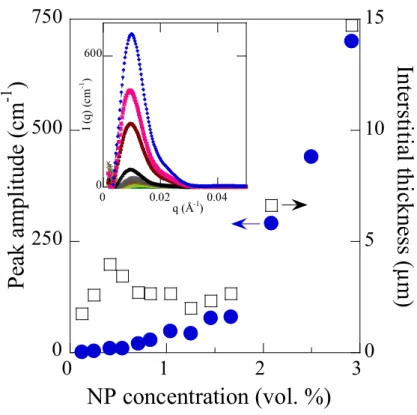
The microscopically observed segregation of NPs in the constrained space between the grains inexorably hints to inhomogeneous NP dispersion in the samples in the polycrystalline phase. As a result, we anticipate to see structural differences between fluid samples where NPs are homogeneously dispersed and polycrystalline samples, i.e., below and above the phase transition temperature respectively. Previous studies have already shown that the micrograins in the polycrystalline phase are formed of micellar polycrystals ordered into a fcc structure and that the NPs are located inside the interstices between them, which were visible on the images as dark gaps [26]. Moreover, structural analysis showed that the lattice parameters are not impacted by the presence of NPs. Their effect is displayed in small angle X-ray scattering (SAXS) data, carried out at 42 °C, and shown in the inset of Figure 2. The raw results and the procedure of extracting these partial signals are given in the supporting materials. Data in the inset of figure 2 display the appearance of a single NP correlation peak located at scattering wavevector q ~ 0.01 Å-1 for all the
6
with NP concentration, its intensity progressively increases as a function of average NP concentration in the samples. The invariability of the peak position with NP concentration strongly suggests that the average distance between them in the interstitial space is constant, ~ 63 nm, when confinement is achieved upon polycrystallization and corresponds to a locally invariable NP concentration ~ 6.2 vol. %. This value is considerably higher than the average NP concentration in the samples. It is remarkably in agreement with previous observation performed on Greenland and Antarctic glacial ice, where local impurity concentration in the veins of the ice was found to be considerably higher than in the melted samples [35]. A close look at the scattering data also suggests that there is a key difference between this microstructured colloidal system and solid state materials. Indeed, the thickness of the interstices, which corresponds to the space between adjacent micrograins in which the NPs are segregated, is probably of the order of 1 micrometer, i.e., several NP layers. The variation of the thickness in the samples can be estimated from geometrical arguments by assuming that the micrograins are spherical and taking into account the micellar volume fraction in the grains and NP volume fraction in the interstices. We also assume that all the NPs are expelled from the crystalline micellar grains so that the amplitude of NP correlation peak is solely proportional to the NP number. These assumptions yield an estimated interstitial thickness represented in figure 2 along with the amplitude of the NP correlation single peak in the scattering curves of figure 2 (inset). The data also show that the interstitial thickness has almost a constant value of few micrometers, and the amplitude of the peak increases linearly with NP concentration in the concentration range of 0 to 1.67 vol. %. Above this concentration threshold, we notice a regime change, where both the interstitial thickness and peak amplitude steeply increase, that coincides with the transition observed from decreasing to increasing grain size, around the NP concentration of 1.67 vol. %.
We then went forward and measured the evolution of the rheological properties as a function of temperature and NP concentration which are shown in Figure 3 and 4. First, we show, the variation of the elastic modulus, G’, of the mixtures as a function of temperature and NP concentration in figure 3. We notice on this figure that when the temperature increases above 27 °C, G’ sharply increases, and shows the expected crossover from viscous liquidlike behavior, defined by a weaker G’ modulus than the viscous modulus, to elastic solidlike behavior [39]. The elastic modulus increases within a very narrow temperature window centered on the transition temperature point of 29 °C. Remarkably, upon addition of NPs, G’ curves show two important features. First, the
7
transition temperature does not shift, which points to a fixed copolymer volume fraction involved in micellization in all solutions. Second, the increase of NP concentration indeed leads to an increase of the plateau elastic modulus up to a concentration of 1.67 vol. % followed by a slight decrease above this NP concentration as it is highlighted in the inset of figure 3. Thorough investigation of the yield stress at this fixed micellar volume fraction as a function of NP volume fraction was achieved at 42 °C and two heating rates of 0.1 and 1 °C min-1 as it is shown in figure
4. First, we observe that both data superimpose in the entire investigated NP concentration range. Second, the data display an almost linear increase of the yield stress as a function of addition of NPs into the micellar crystal when the NP concentration increases from 0 to 1.67 vol. % followed by a decrease above this concentration threshold. Remarkably, the yield stress reaches its maximum value for the sample concentration which displays the shortest grain size. By combining both data of figure 1i and 4a, the yield stress results are plotted in figure 4b and show its variation as a function of the inverse square root of grain size, d-1/2. The variation displays a linear increase of
yield stress as a function of d-1/2 for all the studied samples including concentrated samples above
1.67 vol. % for which the yield stress values are also distributed around the straight line. It is thus straightforwardly inferred that in finely grained materials, the prediction of Hall and Petch for the variation of yield stress with grain size is fully verified including in colloidal polycrystalline solutions. The yield stress follows in that the variation of the grain size which is found to be non-monotonous in this system. The decrease in grain size prompts an increase in yield stress while its increase triggers a decrease of it. A straightforward comparison of data in figure 4b with those previously in solid state metallic systems clearly shows that Hall-Petch relation validity extends to soft colloidal systems which involve considerably large length scales including NP and grain sizes.
As a conclusion, we have used a soft colloidal material that undergoes fluid-polycrystal phase transition induced by temperature and in which the size of the polycrystals is easily tuned by nanoparticle impurity number. The induced size decrease or grain refinement thereby exhibits strength enhancement obeying in that the Hall and Petch relation. This equation constitutes a major constitutive law governing mechanical behavior in response to grain size variations. The soft colloidal system studied here also shows a non-monotonous grain size variation as a function of added nanoparticle impurities in the space between the grains. This feature bears resemblance to similar observations reported in solid state metallic alloys which occur above an impurity concentration threshold. Remarkably, a strict linear variation of the yield stress as a function of the
8
inverse square root of grain diameter is obeyed both in the first stage decreasing grain size and the second stage increasing grain size regimes. As we mentioned earlier, this followed experimental approach, as well as newly reported soft strategies, offers the possibility to study new aspects of polycrystalline and nanocrystalline structures and their mechanics with non-destructive experimental methods. They include important issues frequently reported in structural solid state materials such as super-plasticity, the ‘poisoning effect’ or the inverse Hall-Petch phenomenon whose origins remain poorly understood.
Materials and Methods
Materials, techniques used, description of data analysis, additional results and references are provided in supplementary information.
Acknowledgements
We thank Dr Thomas Bizien (Soleil synchrotron, Swing beamline) for his assistance with carrying out SAXS experiments. Soleil synchrotron is gratefully acknowledged for beam time allocation. We also thank Dr Nicolas Sanson (ESPCI) for his help with TOC measurements and Dr Cyprien Gay for helpful discussions.
References
[1] Pande, C. S.; Cooper, K. P. Nanomechanics of Hall–Petch relationship in nanocrystalline materials. Prog. Mater. Sci. 2009, 54, 689-706.
[2] Zhong, L.; Sansoz, F.; He, Y.; Wang, C.; Zhang, Z.; Mao, S. X. Slip-activated surface creep with room-temperature super-elongation in metallic nanocrystals. Nat. Mater. 2017, 16, 439-445. [3] Chen, Z.; Kang, H.; Fan, G.; Li, J.; Lu, Y.; Jie, J.; Zhang, Y.; Li, T.; Jian, X.; Wang, T.Grain refinement of hypoeutectic Al-Si alloys with B. Acta Mater. 2016, 120, 168-178.
[4] Ovid'Ko, I.A.; Valiev, R.Z.; Zhu, Y.T. Review on superior strength and enhanced ductility of metallic Nanomaterials. Prog. Mater. Sci. 2018, 94, 462-540.
9
[5] Huang, P. Y.; Ruiz-Vargas, C. S.; Van Der Zande, A. M.; Whitney, W. S.; Levendorf, M. P.; Kevek, J. W.; Garg, S.; Alden, J.S.; Hustedt, C.J.; Zhu, Y.; Park, J. Grains and grain boundaries in single-layer graphene atomic patchwork quilts. Nature 2011, 469, 389-392.
[6] Lavergne, F. A.; Curran, A.; Aarts, D. G.; Dullens, R. P. Dislocation-controlled formation and kinetics of grain boundary loops in two-dimensional crystals. Proc. Nat. Acad. Sci. 2018, 115, 6922-6927.
[7] Masumura, R.A.; Hazzledine, P.M.; Pande, C.S.Yield stress of fine grained materials. Acta Mater. 1998, 46, 4527-4534.
[8] Li, Y.; Bushby, A.J.; Dunstan, D.J. The Hall–Petch effect as a manifestation of the general size effect. Proc. Roy. Soc. A 2016, 472, 20150890.
[9] Chen, L.Y.; He, M.R.; Shin, J.; Richter, G.; Gianola, D.S. Measuring surface dislocation nucleation in defect-scarce nanostructures. Nat. Mater. 2015, 14, 707-713.
[10] He, L.B.; Zhang, L.; Tang, L.P.; Sun, J.; Zhang, Q.B.; Sun, L.T. Novel behaviors/properties of nanometals induced by surface effects. Mater. Today Nano 2018, 1, 8-21.
[11] Ovid'Ko, I.A. Deformation of Nanostructures. Science 2002, 295, 2386-2386.
[12] Naik, S.N.; Walley, S.M. The Hall–Petch and inverse Hall–Petch relations and the hardness of nanocrystalline metals. J. Mater. Sci. 2020, 55, 2661-2681.
[13] Wang, S.; Shan, Z.; Huang, H. The Mechanical Properties of Nanowires. Adv. Sci. 2017, 4, 1600332.
[14] Wang, Y.; Chen, M.; Zhou, F.; Ma, E. High tensile ductility in a nanostructured metal. Nature 2002, 419, 912-915.
[15] Murayama, M.; Howe, J.M.; Hidaka, H.; Takaki, S. Atomic-level observation of disclination dipoles in mechanically milled, nanocrystalline Fe. Science 2002, 295, 2433-2435.
[16] Schiøtz, J.; Di Tolla, F.D.; Jacobsen K.W. Softening of nanocrystalline metals at very small grain sizes. Nature 1998, 391, 561-563.
[17] Sun, J.; He, L.; Lo, Y.C.; Xu, T.; Bi, H.; Sun, L.; Zhang, Z.; Mao, S.X.; Li, J. Liquid-like pseudoelasticity of sub-10-nm crystalline silver particles. Nat. Mater. 2014, 13, 1007-1012. [18] Richter, G.; Hillerich, K.; Gianola, D. S.; Monig, R.; Kraft, O.; Volkert, C. A. Ultrahigh strength single crystalline nanowhiskers grown by physical vapor deposition. Nano Lett. 2009, 9, 3048-3052.
[19] Yue, Y.; Liu, P.; Zhang, Z.; Han, X.; Ma, E. Approaching the theoretical elastic strain limit in copper nanowires. Nano letters 2011, 11, 3151-3155.
10
[20] Pieranski, P. Colloidal crystals. Contemp. Phys. 1983, 24, 25-73.
[21] Gast, A.P.; Russel, W.B. Simple ordering in complex fluids. Phys. Today 1998, 51, 24-31. [22] Senyuk, B.; Liu, Q.; He, S.; Kamien, R.D.; Kusner, R.B.; Lubensky, T.C.; Smalyukh, I.I. Topological colloids. Nature 2013, 493, 200-205.
[23] Irvine, W.T.; Hollingsworth, A.D.; Grier, D.G.; Chaikin, P.M. Dislocation reactions, grain boundaries, and irreversibility in two-dimensional lattices using topological tweezers. Proc. Nat. Acad. Sci. 2013, 110, 15544-15548.
[24] Poon, W.Colloids as big atoms. Science 2004, 304, 830-831.
[25] Cash, C.E.; Wang, J.; Martirossyan, M.M.; Ludlow, B.K.; Baptista, A.E.; Brown, N.M.; Weissler, E.J.; Abacousnac, J.; Gerbode, S. J. Local melting attracts grain boundaries in colloidal polycrystals. Phys. Rev. Lett. 2018, 120, 018002.
[26] Boucenna, I.; Guedeau-Boudeville, M.A.; Lapp, A.; Colinart, P.; Proag, A.; Royon, L.; Mourchid, A. Temperature directed-assembly of coated-laponite nanoparticles in pluronic micellar solutions. Soft Matter 2013, 9, 170-176.
[27] Gokhale, S.; Nagamanasa, K.H.; Ganapathy, R.; Sood, A.K. Grain growth and grain boundary dynamics in colloidal polycrystals. Soft Matter 2013, 9, 6634-6644.
[28] Ghofraniha, N.; Tamborini, E.; Oberdisse, J.; Cipelletti, L.; Ramos, L. Grain refinement and partitioning of impurities in the grain boundaries of a colloidal polycrystal. Soft Matter 2012, 8, 6214-6219.
[29] Louhichi, A.; Tamborini, E.; Ghofraniha, N.; Caton, F.; Roux, D.; Oberdisse, J.; Cipelletti, L.; Ramos, L. Nucleation and growth of micellar polycrystals under time-dependent volume fraction conditions. Phys. Rev. E 2013, 87, 032306
[30] Wu, S.; Li, L.; Xue, H.; Liu, K.; Fan, Q.; Bai, G.; Wang, J. Size controllable, transparent, and flexible 2D silver meshes using recrystallized ice crystals as templates. ACS Nano 2017, 11, 9898-9905.
[31] Shen, X.; Chen, L.; Li, D.; Zhu, L.; Wang, H.; Liu, C.; Wang, Y.; Xiong, Q.; Chen, H. Assembly of colloidal nanoparticles directed by the microstructures of polycrystalline ice. ACS Nano 2011, 5, 8426-8433.
[32] Hu, S.; Nozawa, J.; Koizumi, H.; Fujiwara, K.; Uda, S. Grain boundary segregation of impurities during polycrystalline colloidal crystallization. Cryst. Growth Des. 2015, 15, 5685-5692.
11
[33] Yoshizawa, K.; Okuzono, T.; Koga, T.; Taniji, T.; Yamanaka, J. Exclusion of impurity particles during grain growth in charged colloidal crystals. Langmuir 2011, 27, 13420-13427. [34] Lavergne, F. A.; Diana, S.; Aarts, D. G.; Dullens, R. P. Equilibrium grain boundary segregation and clustering of impurities in colloidal polycrystalline monolayers. Langmuir 2016, 32, 12716-12724.
[35] R.E. Barletta, R.E.; Priscu, J.C.; Mader, H.M.; Jones, W.L.; Roe, C.H. Chemical analysis of ice vein microenvironments: II. Analysis of glacial samples from Greenland and Antarctica. J. Glaciol. 2012, 58, 1109-1118.
[36] Shi, F.G.; Tong, H.Y.; Ayers, J.D. Free energy barrier to nucleation of amorphous‐to‐ crystalline transformation selects the scale of microstructure of crystallized materials. Appl. Phys. Lett. 1995, 67, 350-352.
[37] Auer, S.; Frenkel, D. Numerical prediction of absolute crystallization rates in hard-sphere colloids. J. Chem. Phy. 2004, 120, 3015-3029.
[38] Easton, M.A.; Qian, M.; Prasad, A.; StJohn, D.H. Recent advances in grain refinement of light metals and alloys. Curr. Opin. Solid State Mater. Sci. 2016, 20, 13-24.
[39] Boucenna, I.; Royon, L.; Guedeau-Boudeville, M.A.; Mourchid, A. Rheology and calorimetry of microtextured colloidal polycrystals with embedded laponite nanoparticles. J. Rheol. 2017, 61, 883-892.
12
Figures
Figure 1. Observation by optical microscopy of samples at a micellar concentration of 15.2 vol. % and nanoparticle concentration of 0 (a), 0.125 (b), 0.417 (c), 0.833 (d), 1.25 (e), 1.67 (f), 2.08 (g) and 2.92 (h) vol. %. The images were recorded at T = 42 °C after a standing time of 15 min. The scale bare is 100 µm and holds for all the images. Variation of the average grain size with nanoparticle concentration (i).
13
Figure 2. Evolution of the amplitude of the first oscillation of the nanoparticle structure factor measured by SAXS (full circles) and estimate of the thickness of the interstices between adjacent grains (squares) as a function of nanoparticle concentration. Inset: occurrence of a correlation peak in SAXS intensities for nanoparticle concentration ranging from 0 to 2.92 vol. % from bottom to top. 0 600 0 0.02 0.04 I (q ) (c m -1 ) q (Å-1)
0
250
500
750
0
5
10
15
0
1
2
3
P
ea
k
am
pl
it
ud
e
(c
m
-1)
In
te
rs
tit
ia
l th
ic
kn
es
s (
µ
m
)
NP concentration (vol. %)
14
Figure 3. Evolution of elastic modulus as a function of temperature at the micellar concentration of 15.2 vol. % and nanoparticle concentration of 0, 0.417, 0.833, 1.25, 1.67 and 2.08 vol. % (colored ●, ■, ◆, ▲, ▼ and ◢ respectively). Inset: zoom on the plateau elastic modulus as a function of temperature.
10
-310
-210
-110
010
110
210
310
410
15
20
25
30
35
40
E
la
st
ic
m
ou
lu
s
(P
a)
T (°C)
5103 104 30 35 4015
Figure 4. (a) Variation of yield stress as a function of nanoparticle concentration at 42 °C and heating rate of 1 (full circles) and 0.1 (empty squares) °C min-1. Each square and plus symbol point
is an average of 3 to 5 experiments; the dashed curve is a guide to the eye. (b) Representation of yield stress recorded at 42 °C as a function of inverse square root of micellar grain diameter at a heating rate of 1 (full circles) and 0.1 °C min-1 (squares and pluses). The squares correspond to the
increasing branch of figure 4a (nanoparticle concentration from 0 to 1.67 vol. %) while the pluses correspond to its decreasing branch (nanoparticle concentration from 1.67 to 2.92 vol. %). The solid line is a least squares linear fit at heating rate of 0.1 °C min-1.