HAL Id: hal-01940965
https://hal.archives-ouvertes.fr/hal-01940965
Submitted on 30 Nov 2018
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Is there a difference between various presentations of
valproate for cognitive outcome after in utero exposure?
Philippe Gelisse, Pierre Genton, Arielle Crespel
To cite this version:
Philippe Gelisse, Pierre Genton, Arielle Crespel. Is there a difference between various presentations
of valproate for cognitive outcome after in utero exposure?. Epilepsia, Wiley, 2016, 57 (3), pp.523–4.
�10.1111/epi.13301�. �hal-01940965�
G
RAY
M
ATTERS
L
ETTERS
Is there a difference between various
presentations of valproate for cognitive
outcome after in utero exposure?
To the Editors:
The works of the Neurodevelopmental Effects of Antiepileptic Drugs study group has had a profound impact.1–3Prenatal valproate exposure is associated with a dose-dependent reduction of postnatal cognitive abilities.3 Children exposed to valproic acid above 800 mg daily have the lowest mean scores for IQ and for verbal, nonver-bal, and spatial subscale, whereas lower doses were not associated with reduced IQ but only with impaired verbal abilities.3Several presentations of valproate exist, includ-ing chemical variants (valproic acid alone, valproate salts alone, sodium valproate in combination with valproic acid, and divalproex sodium) and this may raise some specific questions about their bioequivalence and their side effects. Most commonly used formulations worldwide are sodium valproate alone or a combination of sodium valproate with valproic acid in controlled release tablets; in North America, however, divalproex semisodium or valproic acid alone are used. Divalproex sodium consists of sodium valproate and valproic acid in a 1:1 molar rela-tionship. If all forms of valproate act through the final common pathway of the valproate molecule, comparison of divalproex sodium with valproate salts alone or in com-bination with valproic acid are not equivalent for valproic acid (Table 1). Each 500 mg of divalproex sodium tablet contains 538.2 mg of the active substance, valproate semi-sodium, equivalent to 500 mg of valproic acid.4 Each tablet of sodium valproate in combination with valproic acid contains 333 mg of sodium valproate and 145 mg of valproic acid, equivalent to 500 mg of sodium valproate, equivalent to 433 mg of valproic acid.4It is important to note that divalproex sodium 500 mg or a formulation with
valproic acid alone contains 15.5% more than sodium val-proate 500 mg alone or in combination with valproic acid. If we want to demonstrate a dose-dependent side effect of valproate, it is important to precise the galenic form of val-proate. It can only be assessed as valproic acid equivalent, especially for statistical tests.
Whenever valproate treatment appears unavoidable dur-ing pregnancy, prevention of major malformation is based on the lowest possible dosage,5but also now on the lowest possible dosage to prevent cognitive impairment. It is nec-essary to achieve the most precise recommendation possi-ble. As the target seems to be 800 mg for divalproate/ valproic acid,3 it is probably higher (800+ 15.5% = 924 mg) for sodium valproate alone or in combination with valproic acid in controlled release tablets, which are the presentations most commonly marketed and used around the world.
DISCLOSURE OFCONFLICT OFINTEREST
Dr. Gelisse has received support from Pharmaceutical Companies for teaching programs (Sanofi-Aventis, UCB, Psicofarma). He received a research grant from the French League Against Epilepsy and the Janssen-Cilag company. He was a paid consultant for Eisai-France in 2011. Dr. Genton has received speaker honoraria from Sanofi, UCB, Eisai, GSK, Novartis, and Pfizer, and support for teaching programs from UCB, Sanofi. He has been a consultant for Sanofi, Actelion, Eisai, and UCB. Dr. Crespel has received support from pharmaceutical companies for teaching programs (Sanofi-Aventis, UCB). Dr Crespel served as a board member for Eisai-France. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consis-tent with those guidelines.
Philippe Gelisse1,2 p_gelisse@hotmail.com Pierre Genton3 Arielle Crespel1,2
1
Epilepsy Unit, Gui de Chauliac Hospital, Montpellier, France;
2Department of Neurobiology, Research Unit“Movement
Disorders” (URMA), Institute of Functional Genomics, CNRS UMR5203– INSERM U661 – UM1, Montpellier, France; and
3Sant-Paul Center-Henri Gastaut, Marseille, France
Table 1. Comparaison of different marketed forms of valproate
International nonproprietary name Content Equivalent of valproic acid Divalproex sodium 500 mg Valproate semisodium: 538.2 mg 500 mg Sodium valproate enteric coated tablets (500 mg) Sodium valproate: 500 mg 433 mg Sodium valproate and valproic acid in controlled
release formulations
Sodium valproate: 333 mg Valproic acid: 145 mg 433 mg Valproic acid enteric coated tablets (500 mg) Valproic acid: 500 mg 500 mg
REFERENCES
1. Meador KJ, Baker GA, Browning N, et al. NEAD Study Group. Cog-nitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009;360:1597–1605.
2. Meador KJ, Baker GA, Browning N. et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 2013;12:244–252. 3. Baker GA, Bromley RL, Briggs M, et al. IQ at 6 years after in utero
exposure to antiepileptic drugs: a controlled cohort study. Neurology 2015;84:382–390.
4. Sanofi UK. Depakote, Epilim 2015. Available at: http://www.sanofi.-co.uk. Accessed January 21, 2013.
5. Genton P, Gelisse P. Valproate: adverse effects. In Levy R, Mattson R, Meldrum B, Perucca E (Eds) Antiepileptic drugs. 5th Ed. New York: Lippincott Williams and Wilkins; 2002:519–536.
Clobazam-induced pedal edema:
“An
unrecognized side effect of a common
antiepileptic drug
”
To the Editors:
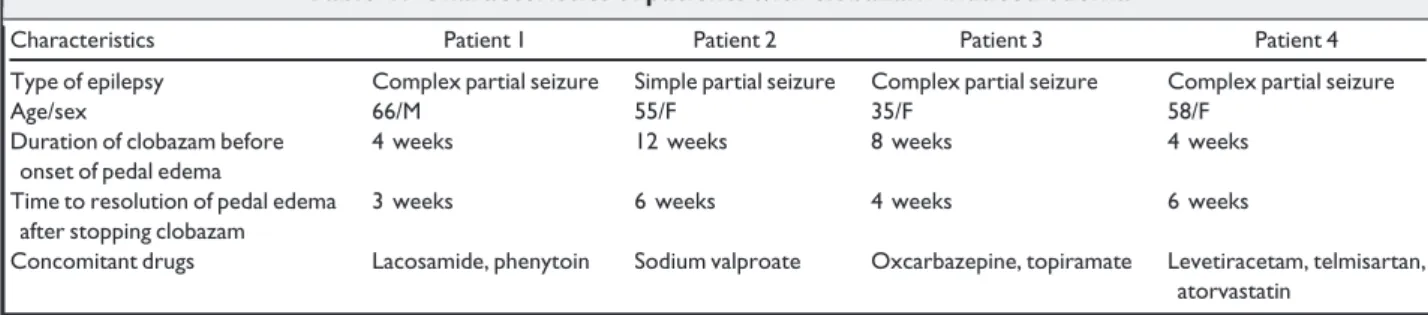
Clobazam is a 1,5-benzodiazepine derivative that is widely used as an add-on antiepileptic drug. It is a rela-tively safe drug with reported side effects including drowsiness, ataxia, diplopia, and, rarely, hypersensitivity.1 We have seen an unusual side effect of this drug in our patients with epilepsy. From July 2014 to May 2015, we had four patients with epilepsy who complained of pedal edema after starting clobazam (Table 1). Three were female and one was male. Their ages ranged from 35 to 66 years. Three had complex partial seizure and one had simple partial seizure. Clobazam was introduced as add-on therapy for uncontrolled seizures. Patients had no other comorbid diseases, such as thyroid, cardiac, renal, or respi-ratory disease. All four patients developed pedal edema within 1–3 months of starting clobazam. All had pitting nonpainful edema, with one of the patients developing ana-sarca. They were evaluated for other causes of pedal edema. Tests included hemoglobin, albumin, urine routine, thyroid function tests, echocardiography, ultrasound of the abdomen, and venous Doppler of the limbs. Results of the preceding investigations were unremarkable. In all four
patients, pedal edema completely subsided within 6 weeks of stopping clobazam. Most of these patients were widely investigated for other causes of edema and were treated with diuretics for the same without any benefit.
Benzodiazepines (BZDs) are a class of antiepileptics that bind toc-aminobutyric acid (GABA)Areceptors and
act by potentiating GABA-mediated action. Pedal edema as a side effect of BZDs has not been reported in the litera-ture. Antiepileptic drugs (AEDs) such as gabapentin, pre-gabalin, and valproate are known to cause pedal edema.2,3 Although the mechanism of pedal edema in patients taking gabapentin and pregabalin is thought to be the antagonistic action of these drugs on the calcium channels in the periph-eral vasculature, the exact mechanism in the case of val-proate is not yet known. The mechanism by which clobazam causes pedal edema has not yet been evaluated. However, there is evidence that BZDs have an influence on the autonomic neurocardiac regulation. BZDs are known to cause a rapid increase in resting heart rate and a concomitant reduction in vagal tone.4Probably by a simi-lar effect on peripheral vasculature, clobazam may cause pedal edema.
Physicians and neurologists treating epileptic patients with clobazam should be aware of this rare side effect to avoid unnecessary and elaborate investigations. Discontin-uation of clobazam invariably results in the resolution of the edema in few weeks.
DISCLOSURE
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical pub-lication and affirm that this report is consistent with those guidelines.
Thomas Mathew chakkuthom@hotmail.com Delon D’Souza Uday S. Nadimpally Raghunandan Nadig St. John’s Medical College
REFERENCES
1. Hanks GW. Clobazam: pharmacological and therapeutic profile. Br J Clin Pharmacol 1979;7(Suppl. 1):151S–155S.
Table 1. Characteristics of patients with clobazam-induced edema
Characteristics Patient 1 Patient 2 Patient 3 Patient 4 Type of epilepsy Complex partial seizure Simple partial seizure Complex partial seizure Complex partial seizure Age/sex 66/M 55/F 35/F 58/F
Duration of clobazam before onset of pedal edema
4 weeks 12 weeks 8 weeks 4 weeks Time to resolution of pedal edema
after stopping clobazam
3 weeks 6 weeks 4 weeks 6 weeks
Concomitant drugs Lacosamide, phenytoin Sodium valproate Oxcarbazepine, topiramate Levetiracetam, telmisartan, atorvastatin
Epilepsia, 57(3):523–527, 2016 524