HAL Id: hal-01774703
https://hal.archives-ouvertes.fr/hal-01774703
Submitted on 7 May 2018
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
First Detection of VIM-2
Metallo-beta-Lactamase-Producing Pseudomonas putida
in Blattella germanica Cockroaches in an Algerian
Hospital
Lotfi Loucif, Zineb Cherak, Naima Chamlal, Esma Bendjama, Djamila
Gacemi-Kirane, Nadia Grainat, Jean-Marc Rolain
To cite this version:
Lotfi Loucif, Zineb Cherak, Naima Chamlal, Esma Bendjama, Djamila Gacemi-Kirane, et al.. First
Detection of VIM-2 Metallo-beta-Lactamase-Producing Pseudomonas putida in Blattella germanica
Cockroaches in an Algerian Hospital. Antimicrobial Agents and Chemotherapy, American Society for
Microbiology, 2017, 61 (8), �10.1128/AAC.00357-17�. �hal-01774703�
First Detection of VIM-2 Metallo-
-Lactamase-Producing Pseudomonas
putida in Blattella germanica
Cockroaches in an Algerian Hospital
Lotfi Loucif,
a,bZineb Cherak,
cNaima Chamlal,
dEsma Bendjama,
a,bDjamila Gacemi-Kirane,
eNadia Grainat,
fJean-Marc Rolain
bLaboratoire de Biotechnologie des Molécules Bioactives et de la Physiopathologie Cellulaire (LBMBPC), Faculté des Sciences de la Nature et de la Vie, Université de Batna 2, Batna, Algeriaa; Unité de Recherche sur les
Maladies Infectieuses et Tropicales Émergentes (URMITE), UM 63, CNRS 7278, IRD 198, INSERM 1095, IHU Méditérranée Infection, Faculté de Médecine et de Pharmacie, Aix-Marseille-Université, Marseille, Franceb;
Laboratoire de Génétique, Biotechnologie, et Valorisation des Bio-ressources (GBVB), Faculté des Sciences Exactes et des Sciences de la Nature et de la Vie, Université Mohamed Khider, Biskra, Algeriac; Département de
Biologie, Faculté des Sciences de la Nature et de la Vie et Sciences de la Terre et de l'Univers, Université 08 Mai 1945, Guelma, Algeriad; Département de Biochimie, Faculté des Sciences, Université Badji Mokhtar, Annaba,
Algeriae; Faculté de Médecine, Université de Batna 2, Batna, Algeriaf
KEYWORDS
VIM-2 MBL, Pseudomonas putida, Blattella germanica, Algeria
I
n the last few years, carbapenem resistance has increasingly been reported in
Gram-negative bacilli (GNB), particularly via carbapenemase production (1).
Metallo--lactamases (MBLs) are the most commonly acquired carbapenemases identified
among Pseudomonas spp. (2). Moreover, the VIM-2 MBL, initially reported in a
Pseu-domonas aeruginosa clinical isolate from France (3), is now endemic in many parts of
the world (4). In our recent investigation of hospital cockroaches of the Blattella
germanica species, we reported the carriage of carbapenemase-producing
Enterobac-teriaceae by these insects, which may present a serious health threat (5). Here we report
the first detection of VIM-2-MBL-producing Pseudomonas putida in Algeria through its
first isolation from hospital cockroaches of the Blattella germanica species.
Ten German cockroaches (B. germanica) were randomly caught from the burn unit
of Batna University Hospital, Algeria. Samples were subjected to the
bacterial-suspension preparation and preenrichment as previously described (5). Subsequently,
selective enrichment was performed in a vancomycin- and ertapenem-supplemented
brain heart infusion broth (BD, France) (5). One hundred microliters from each positive
tube was plated onto MacConkey agar plates (BD) supplemented with 64 mg/liter of
vancomycin and 1 mg/liter of imipenem. Representative colonies of nonfermenting
GNB were screened for carbapenemase production using the modified Carba NP test
(MCNP test). Only one positive MCNP test isolate was obtained from an external surface,
identified as P. putida by Vitek 2 (bioMérieux), and confirmed by matrix-assisted laser
desorption–ionization time of flight mass spectrometry (6). Susceptibility testing was
performed using a disk diffusion technique, and MICs were determined by Vitek 2.
Interpretations were made according to Comité de l’Antibiogramme de la Société
Française de Microbiologie (CA-SFM) breakpoints (7) (Table 1). The P. putida isolate was
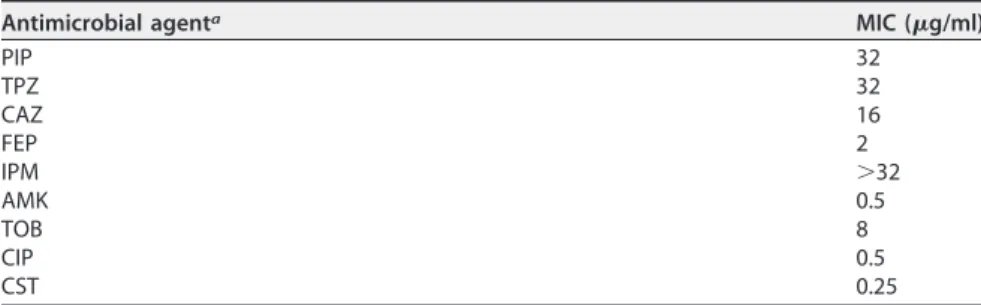
resistant to ticarcillin, ticarcillin-clavulanate, piperacillin, piperacillin-tazobactam,
cefta-zidime, imipenem, meropenem, gentamicin, and tobramycin, intermediate to
aztreo-nam, but susceptible to cefepime, ciprofloxacin, amikacin, and colistin. MBL production
was confirmed by the MBL double-sided Etest (imipenem and imipenem plus EDTA)
(bioMérieux). The isolate was screened using real-time PCR for the presence of the
following
-lactamase genes: bla
CTX-M, bla
TEM, bla
SHV, bla
KPC, bla
NDM, bla
VIM, and bla
IMP.
Accepted manuscript posted online 30 May
2017
Citation Loucif L, Cherak Z, Chamlal N,
Bendjama E, Gacemi-Kirane D, Grainat N, Rolain J-M. 2017. First detection of VIM-2 metallo-β-lactamase-producing
Pseudomonas putida in Blattella germanica
cockroaches in an Algerian hospital. Antimicrob Agents Chemother 61:e00357-17.https://doi.org/10.1128/AAC.00357-17.
Copyright © 2017 American Society for
Microbiology.All Rights Reserved. Address correspondence to Jean-Marc Rolain, jean-marc.rolain@univ-amu.fr.
LETTER TO THE EDITOR
crossm
August 2017 Volume 61 Issue 8 e00357-17 Antimicrobial Agents and Chemotherapy aac.asm.org 1
on May 7, 2018 by Bibliotheque - Univ. de la Mediterranee
http://aac.asm.org/
It was positive only for the bla
VIMcarbapenemase gene. We searched for the antibiotic
resistance-encoding genes bla
PER, bla
VEB, bla
GES, bla
VIM, aac(3)-Ia, aac(6=)-Ib, aph(3=)-VI,
and armA by standard PCR and sequencing as previously described (8, 9). Sequencing
of the positive-standard PCR products showed that the P. putida isolate carried the
blaVIM-2
and aac(6=)-Ib genes. The transfer of the detected bla
VIM-2gene by both
conjugation and transformation experiments was unsuccessful. However, this does not
exclude a possible plasmid location of the bla
VIM-2gene, and further investigations are
required to determine its location. The presence of the class 1 integron was confirmed
by integrase gene PCR targeting the intI1 and intI2 integrase genes as previously
described (10). VIM-2-MBL-producing P. putida was first detected in Taiwan and Korea
(11, 12). Subsequently, nosocomial infections caused by VIM-2-producing P. putida have
occasionally been reported (13–15). In an earlier investigation, Saitou et al. searched for
MBL genes of the VIM-2 and IMP-1 types in 45 P. aeruginosa isolates from hospital
cockroaches in Japan. However, all the studied strains were negative for both MBL
genes (16). Moreover, the isolation of imipenem-resistant Pseudomonas fluorescens/
putida from hospital cockroaches has already been reported (17). In addition, the role
of P. putida as a reservoir of MBL genes that can be transferred to Pseudomonas
aeruginosa has already been demonstrated (18), which makes the presence of such
organisms in hospital cockroaches worrisome in spite of their low clinical importance.
To the best of our knowledge, these data document the first detection of
VIM-2-producing P. putida in cockroaches; therefore, these pests play a potential role in the
dissemination of multidrug-resistant bacteria.
ACKNOWLEDGMENTS
We are very grateful to Mohamed Islam Hatcha for his contribution and to Helis
Yassine (Laboratoire d’Analyses Médicales, LAM IBN ROCHD, Batna, Algeria) for Vitek 2
(bioMérieux) utilization.
This work was partly funded by the Centre National de la Recherche Scientifique
(CNRS 7278) and IHU Méditérranée Infection.
We declare no conflicts of interest.
REFERENCES
1. Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67:1597–1606.https://doi .org/10.1093/jac/dks121.
2. Gniadek TJ, Carroll KC, Simner PJ. 2016. Carbapenem-resistant non-glucose-fermenting Gram-negative bacilli: the missing piece to the puzzle. J Clin Microbiol 54:1700 –1710.https://doi.org/10.1128/JCM.03264-15.
3. Poirel L, Naas T, Nicolas D, Collet L, Bellais S, Cavallo JD, Nordmann P. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta-lactamase and its plasmid- and integron-borne gene from a
Pseu-domonas aeruginosa clinical isolate in France. Antimicrob Agents
Che-mother 44:891– 897.https://doi.org/10.1128/AAC.44.4.891-897.2000. 4. Diene SM, Rolain J-M. 2014. Carbapenemase genes and genetic
plat-forms in Gram-negative bacilli: Enterobacteriaceae, Pseudomonas and
Acinetobacter species. Clin Microbiol Infect 20:831– 838.https://doi.org/ 10.1111/1469-0691.12655.
5. Loucif L, Gacemi-Kirane D, Cherak Z, Chamlal N, Grainat N, Rolain J-M. 2016. First report of German cockroaches (Blattella germanica) as reser-voirs of CTX-M-15 extended-spectrum-beta-lactamase- and OXA-48 carbapenemase-producing Enterobacteriaceae in Batna University Hos-pital, Algeria. Antimicrob Agents Chemother 60:6377– 6380.https://doi .org/10.1128/AAC.00871-16.
6. Seng P, Drancourt M, Gouriet F, La Scola B, Fournier P-E, Rolain JM, Raoult D. 2009. Ongoing revolution in bacteriology: routine identifica-tion of bacteria by matrix-assisted laser desorpidentifica-tion ionizaidentifica-tion time-of-flight mass spectrometry. Clin Infect Dis 49:543–551.https://doi.org/10 .1086/600885.
TABLE 1 MICs of the P. putida isolate
Antimicrobial agenta MIC (g/ml)
PIP 32 TPZ 32 CAZ 16 FEP 2 IPM ⬎32 AMK 0.5 TOB 8 CIP 0.5 CST 0.25
aPIP, piperacillin; TPZ, piperacillin-tazobactam; CAZ, ceftazidime; FEP, cefepime; IPM, imipenem; AMK, amikacin; TOB, tobramycin; CIP, ciprofloxacin; CST, colistin.
Letter to the Editor Antimicrobial Agents and Chemotherapy
August 2017 Volume 61 Issue 8 e00357-17 aac.asm.org 2
on May 7, 2018 by Bibliotheque - Univ. de la Mediterranee
http://aac.asm.org/
7. EUCAST. 2016. Comité de l’Antibiogramme de la Société Française de Microbiologie. Recommandations 2016, v.1.0 Février. Société Française de Microbiologie, Paris, France.http://www.sfm-microbiologie.org/UserFiles/ files/casfm/CASFM2016_V1_0_FEVRIER.pdf.
8. Mesli E, Berrazeg M, Drissi M, Bekkhoucha SN, Rolain J-M. 2013. Preva-lence of carbapenemase-encoding genes including New Delhi metallo-beta-lactamase in Acinetobacter species, Algeria. Int J Infect Dis 17: e739 – e743.https://doi.org/10.1016/j.ijid.2013.02.024.
9. Mellouk FZ, Bakour S, Meradji S, Al-Bayssari C, Bentakouk MC, Zouyed F, Djahoudi A, Boutefnouchet N, Rolain JM. 17 June 2016. First detection of VIM-4-producing Pseudomonas aeruginosa and OXA-48-producing
Kleb-siella pneumoniae in northeastern (Annaba, Skikda) Algeria. Microb Drug
Resisthttps://doi.org/10.1089/mdr.2016.0032.
10. Koeleman JG, Stoof J, Van Der Bijl MW, Vandenbroucke-Grauls CM, Savelkoul PH. 2001. Identification of epidemic strains of Acinetobacter
baumannii by integrase gene PCR. J Clin Microbiol 39:8 –13.https://doi .org/10.1128/JCM.39.1.8-13.2001.
11. Yan JJ, Hsueh PR, Ko WC, Luh KT, Tsai SH, Wu HM, Wu JJ. 2001. Metallo-beta-lactamases in clinical Pseudomonas isolates in Taiwan and identification of VIM-3, a novel variant of the VIM-2 enzyme. Antimicrob Agents Chemother 45:2224 –2228. https://doi.org/10.1128/AAC.45.8 .2224-2228.2001.
12. Lee K, Lim JB, Yum JH, Yong D, Chong Y, Kim JM, Livermore DM. 2002.
bla(VIM-2)cassette-containing novel integrons in
metallo-beta-lactamase-producing Pseudomonas aeruginosa and Pseudomonas putida isolates
disseminated in a Korean hospital. Antimicrob Agents Chemother 46: 1053–1058.https://doi.org/10.1128/AAC.46.4.1053-1058.2002. 13. Trevino M, Moldes L, Hernandez M, Martinez-Lamas L, Garcia-Riestra C,
Regueiro BJ. 2010. Nosocomial infection by VIM-2 metallo-beta-lactamase-producing Pseudomonas putida. J Med Microbiol 59:853– 855.
https://doi.org/10.1099/jmm.0.018036-0.
14. Marchiaro P, Viale AM, Ballerini V, Rossignol G, Vila AJ, Limansky A. 2010. First report of a Tn402-like class 1 integron carrying blaVIM-2 in
Pseu-domonas putida from Argentina. J Infect Dev Ctries 4:412– 416.
15. Almuzara M, Radice M, de Garate N, Kossman A, Cuirolo A, Santella G, Famiglietti A, Gutkind G, Vay V. 2007. VIM-2-producing Pseudomonas
putida, Buenos Aires. Emerg Infect Dis 13:668 – 669.https://doi.org/10 .3201/eid1304.061083.
16. Saitou K, Furuhata K, Kawakami Y, Fukuyama M. 2009. Isolation of
Pseudomonas aeruginosa from cockroaches captured in hospitals in
Japan, and their antibiotic susceptibility. Biocontrol Sci 14:155–159.
https://doi.org/10.4265/bio.14.155.
17. Pai H-H. 2013. Multidrug resistant bacteria isolated from cockroaches in long-term care facilities and nursing homes. Acta Trop 125:18 –22.
https://doi.org/10.1016/j.actatropica.2012.08.016.
18. Juan C, Zamorano L, Mena A, Alberti S, Pérez JL, Oliver A. 2010. Metallo--lactamase-producing Pseudomonas putida as a reservoir of multidrug resistance elements that can be transferred to successful Pseudomonas
aeruginosa clones. J Antimicrob Chemother 65:474 – 478.https://doi.org/ 10.1093/jac/dkp491.
Letter to the Editor Antimicrobial Agents and Chemotherapy
August 2017 Volume 61 Issue 8 e00357-17 aac.asm.org 3