Develop. Growth and Differ., 27 (a), 763-775 (1985)
Polysaccharide Complexes in Full-Grown
Oocytes
of
the Newt,
Pleurodeles,
and
Changes in their Distribution During
Progesterone-Induced Maturation
(glycoged granules/oocyte maturation/polysaccharide complexes/urodele amphibian) MICHEL CHARBONNEAU AND BERTRAND PICHERAL*
Laboratoire de Cytologic Expkrimentale L . A . C. N . R . S. 256 Universitk de RENNES I. FRANCE
Immature full-grown oocytes of Pleurodeles waltlii contain large amounts of small electron-dense polysaccharidic granules. These granules lack a limiting membrane, and have a dense but heter- ogeneous matrix and an apparent diameter of 24-36 nm. Their structure, organization and distribution strongly suggest that they are glycogen granules. On the other hand, mature oocytes both after oviposition or 22-24 hr after in vitro progesterone stimulation contain no polysaccharide granules or complexes. During the first 9-10 hr after hormonal stimulation, granules were abundant and present both individually and as large strands occupying most of the space between the organelles. Granules were frequeqtly found packed together and arranged in regularly arrayed stacks within large subcortical a n t cortical vacuoles. Between 4 and 6 hr after progesterone addi-
tion, oocytes released the contents of vacuoles to the outside. Between about 1 1 and 14 hr after
progesterone addition, oocytes still contained large amounts of polysaccharide complexes, but the vacuoles were empty. From about 15 hr after progesterone treatment until the end of maturation, the complexes progressively disappeared from the cytoplasm, coincident with the detachment of the follicle cell layer from the oocytes and a reduction in the number and size of microvilli.
Oocyte maturation is a complex process by which meiosis of full-grown oocytes is reinitiated starting from the stage of the first meiotic prophase and ending at the stage at which fertilization takes place, that is the second meiotic metaphase in amphibians. Full-grown urodele oocytes at stage VI of oogenesis can be induced to undergo maturation in vitro by progesterone (1-3).
The earliest response to progesterone so far reported is intracellular release of calcium ions (4).
Among the other most obvious early changes occurring during urodele maturation are the movement of the germinal vesicle toward the surface of the animal pole and its subsequent breakdown (3) and increase in the number of annulate lamellae until the stage of germinal vesicle breakdown (5). However, there is to date very little information available on urodele oocytes, and in fact there are onIy two reports describing and classifying the different steps of oogenesis, in Pleurodeles waltlii (6) and Triturus viridescens (7). Some particular features have been addressed, such as yolk formation (8, 9) and follicle-oocyte relations (7, 10) in developing oocytes of Triturus, annulate lamellae in developing oocytes (1 1) and in maturing oocytes
(9,
the cyto- skeleton of full-grown oocytes (12) and the organization of the germinal vesicle and of the nucleoli of maturing oocytes of Notophthalmus (3) and Cynops (13). In the present study, a* This work on the extracellular release of polysaccharides during urodele oocyte maturation was carried out in collaboration with Bertrand Picheral, who died in 1983. This paper is dedicated to his memory.
764 M. CHARBONNEAU AND B. PICHERAL
new cytological feature of full-grown oocytes of the urodele, Pleurodeles, is reported ; namely the presence of large amounts of polysaccharides. These polysaccharides are assembled in vacuoles at the periphery of oocytes and then released into the perivitelline space and through the follicle cells. The physiological significance of this loss of polysaccharides in relation with the events of oocyte maturation is discussed.
MATERIALS AND METHODS
Adult female Pleurodeles wultlii were reared in the laboratory and fed daily on commercial trout pellets and once a week on Chironomus larvae.
Ovaries were initially removed from females that had received no hormonal pretreatment. However, the percentage of in viiro maturation obtained was very low, as previously reported for Notophthulmus (3). Since progesterone induced 50-80 % maturation of full grown oocytes from prestimulated females, mature females were given an intramuscular injection of 100-150 units of human chorionic gonadotropin (hCG, Sigma) and were anaesthetized and decapitated about 24 hr later. Small pieces of ovary were then removed and dissociated in OR, solution (14), with the following composition (mM): 82.5 NaCI, 2.5 KCI, 1 CaCI,, 1 MgCIz, 1 Na,HPO,,
5 Hepes, 3.8 NaOH, (pH 7.4). The largest oocytes (1.5-1.6 mm in diameter) were excised individually, with their
follicular layers intact, and equilibrated in OR, solution for 6 hr at 12°C. Samples of 20 to 50 oocytes were then transferred to glass dishes with 20 ml OR, solution containing 1 or 10 pg/ml progesterone at room temperature
(18-20'C). Stock solutions of progesterone were prepared in ethanol; the final cmcentration of the solvent in the physiological solution never exceeded 0.1 %. hogesterone was present thrdughout the maturation process; control oocytes were placed in OR, solution.
Under a stereoscopic microscope, the first external sign of maturation was seen as the appearance at the animal pole of a light brown spot, 6-7 hr after progesterone addition. This changed to a translucent light green spot limited by a dark brown areola 9-10 hr after addition of progesterone. These changes corresponded to the upward movement of the germinal vesicle and its subsequent breakdown, as shown by examination of animal- vegetal sections of oocytes previously boiled for 1-2 min in OR,. Maturation, judged by the appearance of a
typical maturation spot at the animal pole, was completed about 22-24 hr after progesterone addition.
Because of the inherent variability in the rate of maturation (15), we used a sampling strategy that allowed us t o select samples that were reasonably representative of the population from which they were drawn. Maturation cannot be observed externally during the first 6.5 hr after progesterone treatment. Therefore, the oocytes used for electron microscopy were taken randomly from the population. In the period from 6.5 to 12 hr after addition of progesterone, the percentage of visibly maturing oocytes in the test sample was matched with that of the oocyte population from which the sample was taken. Samples fixed during the period from 12 to 22 hr after addition of progesterone included only those of the population which showed external signs of maturation.
To obtain oviposited eggs, we injected 250-300 IU of hCG into mature females intramuscularly. This induced ovulation of mature, jellied eggs about 36 hr later.
Oocytes were fixed for about 6 hr at room temperature in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. They were then washed with the buffer, their cortical regions were trimmed with a razor blade, and they were post-fixed for 2 hr at room temperature in 1 % OsO, prepared in 0.1 M phosphate buffer, pH 7.4. This procedure was used to improve osmium penetration and selection of regions of oocytes from the animal or vegetal hemisphere. After the last wash, the samples were dehydrated in a graded acetone series and embedded in Epon-Araldite. Thin sections were observed with a Jeol 100 CX electron microscope. Polysaccharide complexes were detected in ultrathin sections by the periodic acid-thiocarbohydrazide-silver protein method (PATAG) (1 6).
POLYSACCHARIDES IN URODELE OOCYTES 765
RESULTS
General organization of immature and mature full-grown oocytes
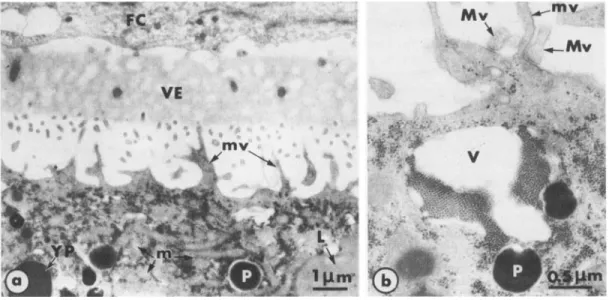
The main constituents of the periphery of immature oocytes are illustrated in Fig. 1. The cytoplasm is completely filled with yolk platelets, lipid vesicles, pigment granules and mito- chondria. The plasma membrane forms irregularly shaped expansions and microvilli that project into the perivitelline space and occasionally make contact with the macrovilli of follicle cells (Fig. lb). To exclude the possibility that prolonged incubation in saline induced mor- phological changes, we ran appropriate control experiments, in which nonstimulated oocytes were cultured in OR, for prolonged periods. No differences were observed in these oocytes (Fig. lc, d, e) from oocytes fixed just after removal from the ovary (Fig. la, b). A novel feature
of the urodele oocyte observed in the present study was the presence of large strands of small electron-dense granules (Fig. I).
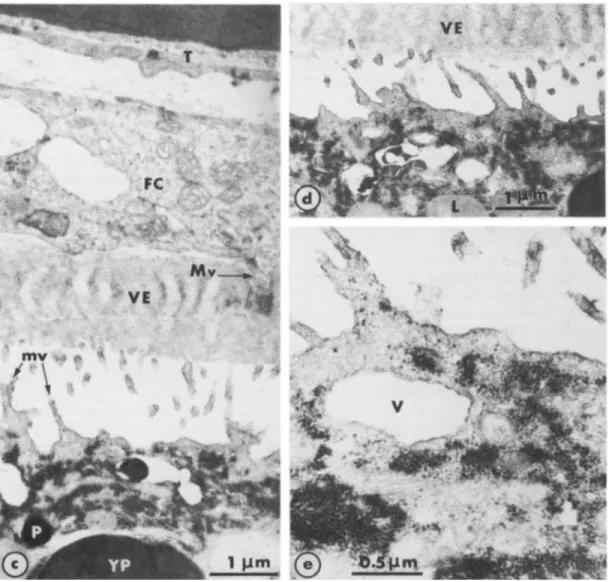
Fig. 2 shows the major features of the periphery of oviposited mature oocytes collected from a female induced to ovulate by hCG. The follicle cells have become detached from the oocyte. A marked reduction in the distance between the vitelline envelope and the plasma
membrane is also apparent. The number of microvilli has greatly diminished and microvilli that remain are very short (Fig. 2a). Most of the expansions of the plasma membrane consist of short, stubby protrusions (Fig. 2b). It is also appearent that the cytoplasm is devoid of the small electron-dense granules seen in immature oocytes.
Characterization of the electron-dense granule complexes
The structure and organisation of the granules observed in the cytoplasm of immature oocytes (see Fig. 1) closely resemble those of glycogen granules observed in other cell types (e.g. see 17). The PATAG method specifically detects polysaccharides (16). The results of this reaction are shown in fig. 3, where the electron dense precipitates of silver protein
166 M. CHARBONNEAU AND B. PICHERAL
Fig. 1. Immature full-grown oocytes (unstimulated). a. In spaces between organelles, the cytoplasm is completely filled with small electron-dense granules. FC: follicle cells; L: lipid vesicle; m: mitochondria; mv: microvilli; P: pigment granule; T: theca; VE: vitelline envelope; YP: yolk platelet. b. At
higher magnification, the small cytoplasmic granules appear to be membrane-free. Large numbers of granules are incorporated into large vacuoles (V) and become arranged into regularly arrayed and closely packed stacks. Macrovilli of the follicle cells (Mv) make junctions with the oocyte plasma membrane. c, d, e: Unstimulated oocytes cultured in OR, for 9 hr (d) and 15 hr (c, e). The characteristics of the oocytes and of the external envelopes, as well as their respective dispositions, remain unchanged after prolonged incubation in saline. In c and d, vacuoles are incorporating some electron-dense granules that then become regularly arranged ; e provides
a clear view of the granules individually or grouped in long and irregular strands. a : x 8,000; b: x 20,000; c:x16,000; d: ~ 1 4 , 0 0 0 ; e: ~33,000.
indicate the locations of polysaccharides. By comparing this pattern with that in Fig. 1
(and also all subsequent Figs.), it is clear that the polysaccharide precipitates detected by the PATAG method are numerous small electron-dense granules present throughout the per-
POLYSACCHARIDES IN URODELE OOCYTES 767
Fig. 2. Mature oocytes (oviposited). The cytoplasm is now completely free of the small electron-dense granules observed in immature oocytes (Fig. 1). The distace between the vitelline envelope and the plasma membrane has greatly diminished (in b, the vitelline envelope has been lost during sample processing). The surface of the oocyte forms large, stubby protrusions (a) on short finger-like microvilli (b). g: granules of the cortex; v: vesicles. a : ~ 9 , 0 0 0 ; b: ~14,000.
Fig. 3. PATAG method for polysaccharide detection in maturing oocytes, 4 hr (c) and 14 hr (a and b) after progesterone addition. The silver protein precipitates mark the sites of polysaccharides. The pattern of labelling is quite similar to the pattern of distribution of the small electron-dense granules shown in Figs. 1 and 4-6.
Polysaccharide granules can be observed throughout the peripheral cytoplasm either as individual granules or as large, irregular strands “flowing” between organelles. Granules can also be observed in the process of being packed into large vacuoles (a, as in Fig. la) or just after being released into the perivitelline space (c, and see Fig. 4). Uncontrasted sections, a : x 13,000; b: x 32,000; c: X20,OOO.
768 M. CHARBONNEAU AND B. PICHERAL
POLYSACCHARIDES IN URODELE OOCYTES 769
Structure and organization of polysaccharide complexes
At all stages in which they are present, the polysaccharide complexes have three main characteristics : (1) They are composed of individual membrane-free granules that have a very dense but, in most cases, heterogeneous matrix and an apparent diameter of 24 to 36 nm (Fig. 4a). (2) Although they can be found as individual granules, they tend to assemble into long, irregular strands filling the space between organelles (Fig. 4a). (3) In the cortical and subcortical (up to about 20 pm deep) cytoplasm, the granules seem to be present within relatively large vacuoles in which they are arranged in regular stacks. The polysaccharide contents of
these vacuoles are eventually released into the perivitelline space (Fig. 4b-f ).
Changes in the distribution of polysaccharide complexes during maturation
Polysaccharide complexes are present ‘throughout the peripheral cytoplasm of immature (Fig. 1) and maturing full-grown oocytes, but not mature oocytes (Fig. 2). No differences in their distributions in the oocytes could be detected, but in this study, only the outer 100,um thick region of the cytoplasm was examined.
Pronounced changes in the distribution of polysaccharide occur during maturation leading to the complete disappearance of polymer complexes from mature oocytes (Fig. 2).
In the early stage of maturation, from 0 to about 9 hr after addition of progesterone, there was no change in the distribution of the granules in the cytoplasm. Vacuoles containing polysaccharide were frequently found in the subcortex, or close to the plasma membrane or opening to the outside, releasing polysaccharide stacks (Fig. 4). The general pattern of dis- tribution of polysaccharides in oocytes did not seem to differ in different stages after addition of
progesterone. However, in the period from about 4 to 6 hr after progesterone addition poly- saccharide stacks moved toward the vitelline envelope, the space between the vitelline envelope and the follicle cell layer, the intercellular spaces between follicle cells and the external side of
the follicle cell layer (Fig. 5). Before and after this 4-6 hr period, no polysaccharide complexes could be observed further outside than the perivitelline space (Fig. 5).
At 12,13 and 14 hr after progesterone treatment, polysaccharide complexes were still present in the peripheral cytoplasm, but the vacuoles, present in the cytoplasm or opening to the outside, were empty (Fig. 6).
The final stages of oocyte maturation (16-22 hr after progesterone) were characterized by detachment of the follicle cell layer from the oocytes and reduction in the number and size of the microvilli (Fig. 7). In addition, vacuoles that had released their polysaccharide contents started to disappear about 16 hr after progesterone stimulation (Fig. 7a). Coincidently, the number of polysaccharide granules present in the cytoplasm decreased greatly, and so the
Fig. 4. Characteristics of the polysaccharide granules and complexes. a, b, c : unstimulated immature oocytes,
~ 2 2 , 0 0 0 , x18,OOO, x15,OOO; d: 4hr after progesterone addition, x12,OOO; e: 5 hr after progesterone, addition, x 21,000; f: 6 hr after progesterone addition, X21,OOO. a: typical distribution of individual polysac- charide granules; b, c: individual granules are incorporated in vacuoles at the sites shown by arrows and closely pack together to form regularly arrayed stacks. d, e, f : during the period from 4 to 6 hr after progesterone addition, some of the polysaccharide complexes are released into the perivitelline space, probably by simple opening of the vacuoles containing granules to the outside (e, f). At this time, polysaccharides are also present inside oocytes as individual granules or enclosed in cortical vacuoles.
770 M. CHARBONNEAU AND B. PICHERAL
POLYSACCHARIDES IN URODELE OOCYTES 77 1
Fig. 6. Maturing oocytes fixed 12 (a), 13 (c) and 14 hr (b) after progesterone addition. At these stages, polysac- charide granules and strands are still present in large amounts in the peripheral cytoplasm, but are no longer observable on the outside of oocytes. a : x 9,000; b: x 7,000; c : x 18,000.
cytoplasm became much less dense. A feature common to this stage and the previous ones,
however, was the presence of numerous, long microvilli (Fig. 7a). The stage 18 hr after proges- terone addition was essentially the same as that 16 hr after progesterone addition with regard to the density and distribution of polysaccharide granules, although their density tended to decrease
Fig. 5 . Release of polysaccharide complexes from oocyte cortical vacuoles in to the perivitelline space 4 and 6 hr after progesterone treatment, (bottom left of a and b). These complexes then migrate through the vitelline envelope and can be observed in the space between the vitelline envelope and the follicle layer (arrows in a and b). In c, one of them is present inside an intercellular space between two follicle cells (arrows); in d, three are located on the external side of the follicle layer (arrows); T : theca. a : 6 hr after progesterone addition, ~25,000; b: 4 hr after progesterone addition, x 17,000; c : 6 hr after progesterone addition, x 19,000; d : 4 hr after progesterone addition, ~ 2 2 , 0 0 0 .
712 M. CHARBONNEAU AND B. PICHERAL
POLYSACCHARIDES IN URODELE OOCYTES 773
(Fig. 7b). At this time the vacuoles had completely disappeared and the microvilli started to become reduced in size and number (Fig. 7b).
In mature oocytes (about 22 hr after progesterone addition) (Fig. 7c) or oviposited (in
vivo matured) oocytes (Fig. 2), polysaccharide complexes were almost completely absent. Although still visible in the spaces between organelles, the granules were much more sparsely distributed than in the stages 16 and 18 hr after progesterone addition. This final stage, and the previous one to a lesser extent, were also characterized by the appearance of numerous small cytoplasmic vesicles (Fig.
7).
The kinetics of oocyte maturation, including the phenomena observed at the ultrastructural level, were similar with 1 and 10 pg/ml progesterone.
DISCUSSION
In the present study, large amounts of polymerized electron-dense granules were seen in the peripheral cytoplasm of full-grown immature and maturing oocytes of the urodele amphibian,
Pleurodeles. The cytochemical reaction used revealed that these granules contained poly- saccharides, while ultrastructural observations indicated that the polysaccharide was glycogen. In many cells, large individual molecules of glycogen appear as granules of 10 to 40 nm diameter, which is consistent with the 25-35nm range observed in this work. Moreover, the poly- saccharide granules of Pleurodeles oocytes and their organization in large pools are very similar to the glycogen granules and pools observed in other cells, such as hepatocytes (17-19). None of the changes in the distribution of polysaccharide complexes observed during oocyte maturation appear to be due to the culture conditions. The culture periods required for completion of maturation were long, but we found that the ultrastructure of immature oocytes (unstimulated) remained unchanged after different times of culture in vitro.
The presence of polysaccharides in oocytes cannot be directly correlated with progesterone stimulation, for even unstimulated full-grown oocytes have such complexes. However, the absence of granules in mature oocytes, unlike in unstimulated controls, seems to indicate that only those oocytes which are undergoing maturation eventually lose their polysaccharide contents. The kinetics of polysaccharide depletion show a peak roughly coincident with germinal vesicle breakdown ; although the precise correlation between these two events could not be established. Our results indicate a gradual decrease in the amount of cytoplasmic polysaccharides from the time of their external release until the final stage of maturation. Con- current with this disappearance of polysaccharide, a number of other changes take place; namely, detachment of follicle cells from the oocyte, progressive decrease in the number and size of oocyte microvilli, and the appearance of numerous small vesicles in the cytoplasm. Further studies are necessary on the relations of these diverse events.
Fig. 7. Maturing oocytes fixed 16 (a, b) and 18 hr (c, d) and mature oocyte fixed 22 hr (e, f ) after progesterone addition. During these final stages of oocyte maturation, the follicle cell layer has detached completely from the oocyte; remnants are still visible in a. The end of maturation is also characteriied by progressive decreases in both the number of polysaccharide granules and the number and size of microvilli. The progressive decrease in the number of polysaccharide granules from 16 to 22 hr after stimulation is best seen at high magnification (b, d, f). In mature oocytes (e, f), polysaccharides were seen only as individual granules. However, they were clearer than when much more numerous and packed together (a-d), because the cytoplasm was less dense in mature oocytes. a, c, e: ~ 9 , 0 0 0 ; b: ~21,000; d, f: x19,OOO.
774 M. CHARBONNEAU AND B. PICHERAL
The presence of glycogen in the cytoplasm of amphibian oocytes has been known for a long time from light microscopic studies (20-22). Recently, the presence of glycogen particles of the /3-type according to DROCHMANS (19) in the cytoplasm of immature oocytes of the anuran
Rana esculenta and the urodele Pleurodeles waltlii, has been observed at the ultrastructural level (23). However, the report on Pleurodeles by FAVARD and FAVARD-SBRBNO (23) was confined to yolk platelets and did not mention the distribution or abundance of glycogen particles in the cytoplasm or the presence of polysaccharide complexes packed into vacuoles.
A recent study of oogenesis in Pleurodeles waltlii (6) did not report the presence of any of the large granule complexes observed in the present study. Polysaccharides have also been detected in the vitelline envelope that surrounds amphibian oocytes (23-29), in the cortical granules of anuran oocytes (23, 26-28, 30, 31) and in yolk platelets of anuran and urodele oocytes (23, 32-34).
The poly- saccharides found in primordial germ cells of many vertebrates are gradually lost as the pri- mordial germ cells mature and migrate towards the gonad area (see references in 35). Maturation of early germ cells in the human fetal testis is also associated with loss of poly- saccharides (36-37). The reason for polysaccharide extrusion from Pleurodeles oocytes during maturation remains unexplained.
Similar loss of polysaccharide has been observed in at least two other cases.
M.C. thanks Dr. D. J. Webb for valuable discussion and comments, Mrs A. Cavalier for block sectioning, Mrs M. Mathelier for typing the manuscript and Mr B. Morille for assistance in preparing illustration. This work was supported in part by grant no 135025 from the Institut National de la Santd et de la Recherche Mddicale, France, PRC, Reproduction et Wveloppement.
REFERENCES
1. BARSACCHI, G. and A. A. HUMPHRIES, Jr., 1970. Association Southeastern Biologists Bull., 17,30.
2. BARSACCHI, G., 1971. Bull. Zool., 38,491.
3. BARSACCHI, G., G. PILONE and A. A. HUMPHRIES, Jr., 1975. J. Embryol. exp. Morphol., 34,451-466.
4. MOREAU, M., J. P. VILAIN and P. GUERRIER, 1980. Develop. Biol., 78, 201-214. 5. IMOH, H., 1982. J. Embryol. exp. Morphol., 70, 153-169.
6. BONNENFANT-JAIS, M. L. and P. MENTRE, 1983. J. Submicrosc. Cytol., 15, 453478.
7. HOPE, J., A. A. HUMPHRIES, Jr. and G. H. BOURNE, 1963. J. Ultrastruct. Res., 9, 302-324. 8. HOPE, J., A. A. HUMPHRIES, Jr. and G. H. BOURNE, 1964. J. Ultrastruct. Res., 10, 547-556. 9. SPORTNITZ, U. M. and A. KRESS, 1973. Z. Zellforsch. Mikrosk. Anat., 143, 387407. 10. HUMEAU, C. and P. SENTEIN, 1968. C. R. Soc. Biol., 162,2181-2184.
11. HUMEAU, C. and D. TEMPLE, 1969. C. R. Soc. Biol., 163, 1898-1902.
12. FRANKE, W. W., P. C. RATHKE, E. SEIB, M. F. TRENDELENBURG, M. 0s BORN and K. WEBER., 1976. Cytobiologie, 14, 11 1-130.
13. IMOH, H., 1981. Develop. Growth Differ., 23, 33-39.
14. WALLACE, R. A., D. W. JARED, J. N. DUMONT and M. W. SEGA, 1973. J. Exp. Zool., 184, 321-334.
15. MASUI, Y. and H. J. CLARKE, 1979. Int. Rev. Cytol., 57, 185-282.
16. THIERY, J. P., 1967. J. Microscop., 6, 987-1018.
17. PORTER, K. R. and M. A. BONNEVILLE, 1968. An Introduction to the Fine Structure of Cells and Tissues. Eds., Led and Febiger, Philadelphia.
18. FAWCETT, D. W., 1955. J. Nat. Cancer Inst., 15, 1475. 19. DROCHMANS, P., 1962. J. Ultrastruct. Res., 6, 141-163. 20. BRACHET, J. and J. NEEDHAM, 1935. Arch. Biol., 46, 821-835.
POLYSACCHARIDES IN URODELE OOCYTES 775 21. FITCH, K. L. and H. W. MERRICK, 1958. Exp. Cell Res., 14, 389-397.
22. SENTEIN, P., 1963. Bull. Assoc. Anat., Ilk rkunion, 389-397.
23. FAVARD, P. and C. FAVARD-SERENO, 1969. J. Submicrosc. Cytol., 1, 91-111. 24. Voss, H. and H. WARTENBERG, 1955.
25. WARTENBERG, H., 1956. Acta Histochem., 3, 25-71. 26. WARTENBERG, H., 1959. Acta Histochem., 8,458-477. 27. WARTENBERG, H., 1962. Z. Zellforsch., 58,427-486. 28. ROSENBAUM, R. M., 1958. Quart. J. Micr. Sci., 99, 159-169.
29. WARTENBERG, H. and W. SCHMIDT, 1961. Z. Zellforsch., 54, 118-146. 30. KATAGIRI, C., 1960. J. Fac. Sci. Hokkaido Univ., 14, 166-174.
31. KEMP, N. E. and N. L. ISTOCK, 1967. J. Cell Biol., 34, 111-122. 32. RINGLE, D. A. and P. R. GROSS, 1962. Biol. Bull., 122, 263-280.
33. OHNO, J., S . KARASAKI and K. TAKATA, 1964. Exp. Cell Res., 33, 31G318. 34. TANDLER, C. J. and J. L. LA TORRE, 1967. Exp. Cell Res., 45,491494.
35. HARDISTY, M. W., 1978. “The Vertebrate Ovary: Comparative Biology and Evolution”. Ed. R. E. Jones, Plenum Press, New York and London.
36. WARTENBERG, H., A. F. HOLSTEIN and J. VOSSMEYER, 1971. Z. Anat. Entwicklungsgesch., 134,165-185. 37. FUKUDA, T., C. HEDINGER and P. GROSCURTH, 1975.
Wiss. Z . Univ. lena, 4,413417.
Cell Tissue Res., 161, 55-70. (Received May 16, 1985; accepted July 8, 1985)