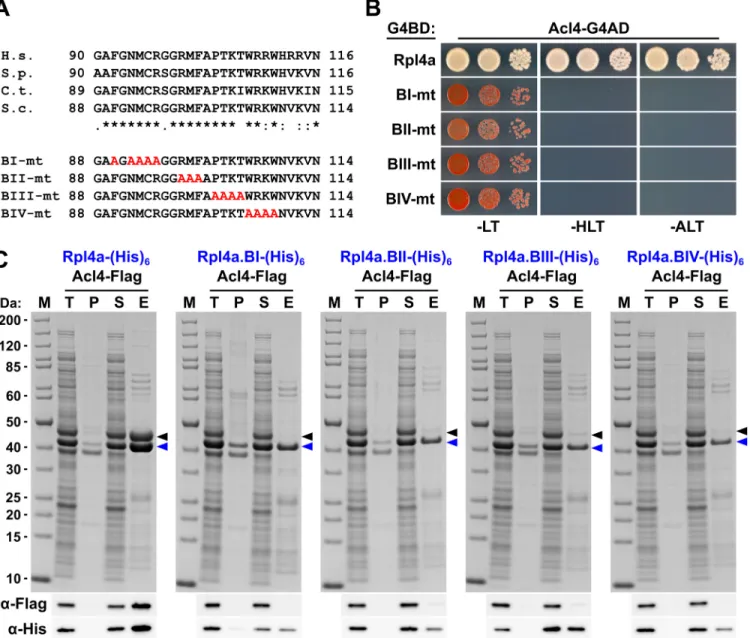
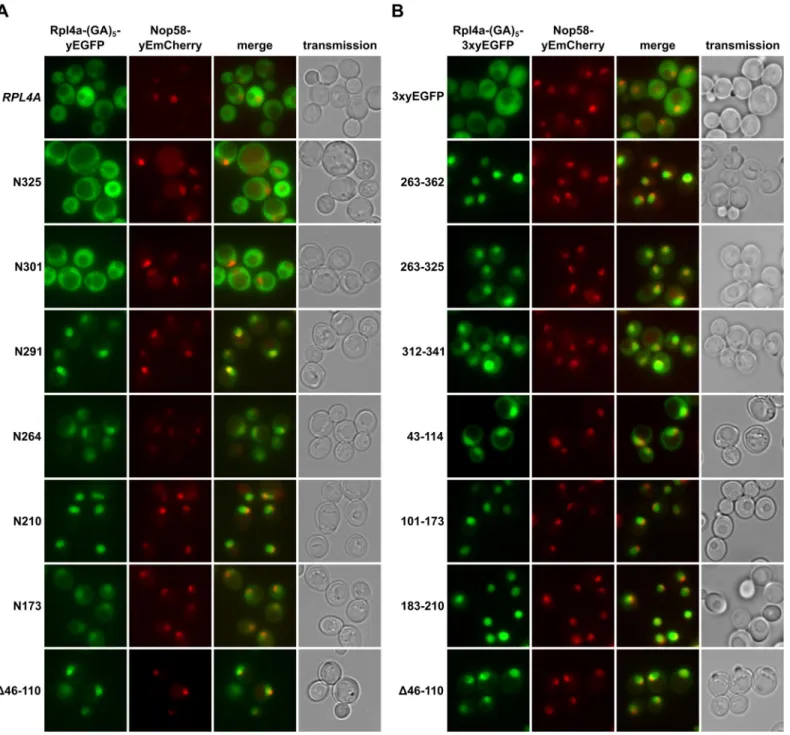
The dedicated chaperone Acl4 escorts ribosomal protein Rpl4 to its nuclear pre-60S assembly site
Texte intégral
Figure



Documents relatifs
We have proposed a method inspired by the NTFA (Nonlinear Transformation Field Analysis), which allows the model reduction of a complete elastic viscoplastic
Taken together, in vivo and in vitro analyses demonstrated that Syo1 can simultaneously bind Rpl5 and Rpl11 to form a heterotrimeric Syo1-Rpl5-Rpl11 complex.. To gain further
HeLa cells were permeabilized by digitonin and incubated with purified, Alexa488-labelled Syo1 or GST-SV40NLS-mRFP in transport buffer (C, upper panel), transport buffer
[r]
[r]
The results support Hypothesis 2: Coaches with higher preference for a systematic approach in coaching with measurable goals, scientifically supported methods and outcome evaluation
The vision of Ecumenopolis, resting on small-scale communities linked at the global dimension, calls for planned expansion of Man's use of land rather than waste- ful urban sprawl.
Since a large majority of food products contains proteins in consistent amount, proteins constitute privileged biopolymers for the design of delivery systems for improving