AJH 1991; 4:183-188
Angiotensin-Induced Growth Related
Metabolism Is Activated in Cultured Smooth
Muscle Cells From Spontaneously
Hypertensive Rats and Wistar-Kyoto Rats
Timothy Scott-Burden, Thérèse J. Resink, Alfred W. A. Hahn, and Fritz R. Bühler
S m o o t h m u s c l e cells from s p o n t a n e o u s l y h y p e r t e n -s i v e r a t -s (SHR) p r o l i f e r a t e i n c u l t u r e fa-ster t h a n t h o s e i s o l a t e d from sex a n d a g e m a t c h e d W i s t a r -K y o t o (W-KY) a n i m a l s . T h e r e w a s n o difference i n t h e k i n e t i c s of S6 k i n a s e a c t i v a t i o n i n t h e t w o c u l -t u r e s , b u -t l a -t e r m e -t a b o l i c e v e n -t s a s s o c i a -t e d w i -t h p r o l i f e r a t i o n w e r e s t i m u l a t e d e a r l i e r i n S H R cells t h a n i n WKY, eg, a c t i v a t i o n of o r n i t h i n e d e c a r b o x y l a s e . B o t h cell t y p e s e l a b o r a t e d a n e x t e n s i v e e x t r a -c e l l u l a r m a t r i x i n -c u l t u r e -c o m p o s e d of a d i f f e r e n t b l e n d of c o n n e c t i v e t i s s u e m a c r o m o l e c u l e s . M a t r i x m a t e r i a l f r o m S H R cells w a s m o r e s t i m u l a t o r y t o g r o w t h of WKY c u l t u r e s t h a n t h e i r o w n m a t r i c e s . A n g i o t e n s i n s t i m u l a t e d t h e g r o w t h a n d s y n t h e s i s of e x t r a - c e l l u l a r m a t r i x m a t e r i a l i n S H R m o r e t h a n i n WKY d e r i v e d v a s c u l a r s m o o t h m u s c l e cell c u l -t u r e s . A m J H y p e r -t e n s 1991;4:183-188 KEY W O R D S : A n g i o t e n s i n , v a s c u l a r s m o o t h m u s c l e g r o w t h m e t a b o l i s m .
T
h e well established concepts e m b o d i e d in t h e" r e s p o n s e to injury t h e o r y , " as p r o p o s e d by
Ross a n d G l o m s e t ,1 h a s p r o v i d e d a m o d e l for
t h e s t u d y of t h e m a n y factors a n d their interac-tion responsible for t h e pathogenesis of vascular disease u n d e r situations w h e r e frank d e n u d a t i o n of t h e e n d o
-thelium h a s taken p l a c e .2 - 4 There is n o w evidence to
suggest t h a t b o t h h y p e r t r o p h y a n d hyperplasia can occur in t h e absence of a n y a p p a r e n t d a m a g e to t h e
endothelial l a y e r .5 - 7 For these processes to take place w e
n e e d to invoke t h e influences of c o m p o u n d s t h a t are normally present in t h e intact blood vessel wall.
C a n d i d a t e s for t h e processes m e n t i o n e d abor"*
in-From the Department of Research, University Hospital, Basel, Swit-zerland.
This study w a s supported by the Swiss National Fund N u m b e r 3.817.087.
Address correspondence and reprint requests to Professor F. R. Bühler, Department of Research, University Hospital, C h - 4 0 3 1 Basel, Switzerland.
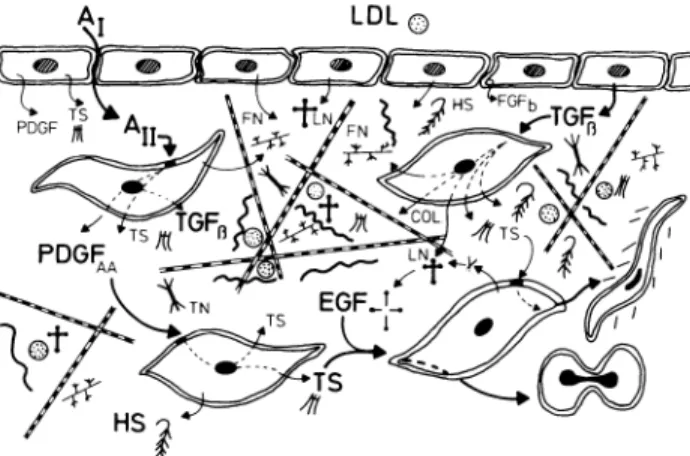
elude c o m p o u n d s t h a t h a v e b e e n characterized as g r o w t h factors, as well as a n u m b e r of molecules n o r -mally associated w i t h t h e structural c o m p o n e n t s of t h e vessel wall (see Figure 1). A m o n g these extracellular matrix c o m p o u n d s , t h r o m b o s p o n d i n (TS) h a s certain attributes w h i c h strongly suggest t h a t it could play a n i m p o r t a n t role in a variety of processes t h a t eventually
lead to t h e d e v e l o p m e n t of vascular d i s e a s e .8 - 1 0 T h e
vasoactive p e p t i d e h o r m o n e s are a n o t h e r g r o u p of m o l e -cules t h a t h a v e received attention w i t h r e g a r d to their p a t h o g e n i c potential. In this respect angiotensin II (Ang II) h a s b e e n suggested to i n d u c e irreversible vascular
c h a n g e s .1 1 1 2 Clearly, t h e latter is a c a n d i d a t e e m i n e n t l y
suited to playing a role in t h e chronic progressive i n d u c -tion of t h e vascular structural c h a n g e s t h a t are p a r t a n d parcel of essential h y p e r t e n s i o n (EHT), since m o r e t h a n t w o thirds of patients w i t h EHT exhibit elevated p l a s m a r e n i n / A n g II levels relative to t h e h e i g h t of their b l o o d
p r e s s u r e .1 3 1 4 Recent reports h a v e s h o w n t h a t i n d e e d
A n g II is capable of stimulating a n u m b e r of metabolic e v e n t s t h a t m a y lead to s m o o t h muscle proliferative
FIGURE 1. Schematic representation of vessel wall and some
components thereof which may contribute to the pathogenesis of vascular disease. Matrix molecules: fibronectin (FN); laminin (LN); thrombospondin (TS); tenascin (TN); collagen (COL); heparan sulfate/heparin and elastin (HS). Growth Factors/Stimulators: epidermal growth factor (EGF); basic fibroblast growth factor (FGFb); platelet derived growth factor (PDGF); transforming
growth factor β (TGFß); angiotensin (All) and low density lipopro
tein (LDL). Furthermore, within the matrix compounds such as LDL and basic fibroblast growth factor can be bound. In the course of the normal turnover of matrix molecules which accompanies the dynamic process of tissue remodelling, peptide fragments having EGF domains can be generated and some of the stimulatory proper ties of this growth factor may become manifest. The compounds known to possess such EGF domains include LN, TS, and TN, a recently discovered matrix glycoprotein also found in vascular tissue.39 Both endothelial and smooth muscle cells are capable of
secreting a number of growth factors without or with agonist stimulation, eg, Ang II. These events may lead to either hyper trophy or hyperplasia as well as the promotion of migration into the intimai space (see Discussion).
b e h a v i o r .1 1-1 2-1 5 The induction of c-fos, c-myc, a n d P D G F
A chain g e n e expression in cultures of rat s m o o t h m u s
cle cells b y A n g I I ,1 1'1 2 as well as its ability to stimulate
protein s y n t h e s i s ,1 5'1 6 strongly suggest t h a t chronic e x p o
sure of vascular s m o o t h muscle to this vasoactive p e p tide m a y h a v e pathologic consequences.
W e report h e r e o n our investigations w i t h A n g II using cultured s m o o t h muscle cells from s p o n t a n e o u s l y h y p e r t e n s i v e rats (SHR) a n d their n o r m o t e n s i v e Wistar-Kyoto (WKY) litter m a t e s . W e also report h e r e o n t h e u s e of p l a s m a - d e r i v e d s e r u m (PDS) in conjunction w i t h A n g II for t h e p r o l o n g e d p r o p a g a t i o n of cultured rat s m o o t h muscle cells. O u r rationale for t h e u s e of P D S is t h a t u n d e r n o r m a l physiologic conditions medial s m o o t h muscle cells w o u l d n o t come into contact w i t h s e r u m from w h o l e blood, b u t are r a t h e r " b a t h e d b y a
filtrate of p l a s m a , "1 7 essentially similar to t h e n o n
-mitogenic p r e p a r a t i o n s w e h a v e u s e d in s o m e of these studies.
M A T E R I A L S A N D M E T H O D S
All materials a n d m e d i a for tissue culture w e r e o b t a i n e d
from sources already d e s c r i b e d .1 8 T h e radioisotopes
u s e d in t h e studies described herein w e r e p u r c h a s e d from A m e r s h a m Radiochemical, A m e r s h a m , Bucking h a m s h i r e , England. Immunologic reagents w e r e o b tained from D a k o p a t t s , Glostrup, D e n m a r k . SHR a n d WKY rats w e r e supplied b y M a d ö r i n / B R L , F r e n k e n -dorf, Switzerland.
All cell culture techniques a n d details of p r o c e d u r e s e m p l o y e d for t h e isolation of vascular s m o o t h muscle cells (VSMC) from rats (SHR a n d WKY) h a v e b e e n r e p o r t e d before. Cell n u m b e r s (growth kinetics) w e r e d e t e r m i n e d o n cell s u s p e n s i o n s following e n z y m a t i c dis aggregation of cell layers a n d c o u n t i n g in a Coulter
(Hialeah, FL) c o u n t e r .1 8
P l a s m a - d e r i v e d s e r u m (PDS) w a s p r e p a r e d b y t h e
m e t h o d of Ross a n d K a r i y a2 2 a n d each b a t c h w a s as
sessed after sterilization b y filtration for its mitogenic potential. Samples ( 1 0 % final concentration) t h a t s t i m u
lated t h e incorporation of [3H ] - t h y m i d i n e into D N A to
levels greater t h a n 5 % of t h a t o b t a i n e d u s i n g 1 0 % w h o l e b l o o d s e r u m w e r e rejected; P D S w a s u s e d in experi m e n t s at a final concentration of 1%.
P r e p a r a t i o n of extracellular matrix (ECM)-coated plastic culture vessels a n d dishes, as well as all p r o c e d u r e s for S6 kinase activation of quiescent cultures,
w e r e d e s c r i b e d .1 8
T h e incorporation of [3H ] - t h y m i d i n e into D N A w a s
m e a s u r e d as described b e f o r e2 3 a n d t h e kinetics of [3H ]
-glycine incorporation into E C M protein w a s p e r f o r m e d
as described b y ourselves a n d o t h e r s .1 6'1 9 Assays for t h e
activation of o r n i t h i n e decarboxylase ( O D C ) w e r e
carried out as r e p o r t e d b y H o vis et a l .2 4
R E S U L T S
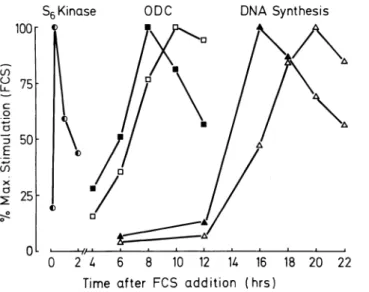
W h e n quiescent V S M C cultures (SHR a n d WKY) w e r e exposed to 1 0 % fetal calf s e r u m (FCS) t h e r e w a s a dif ferential t i m e - d e p e n d e n t activation of a n u m b e r of i m p o r t a n t g r o w t h associated metabolic e v e n t s w h i c h in cluded S6 kinase, o r n i t h i n e decarboxylase, D N A
synthesis, a n d [3H ] - t h y m i d i n e incorporation (Figure 2).
T h e t e m p o r a l relationship b e t w e e n these e v e n t s h a s
b e e n well d o c u m e n t e d2 5 a n d t h e time intervals
observed b e t w e e n t h e induction of, for e x a m p l e S6 ki n a s e a n d O D C reflect t h e proliferation rates of t h e s t i m u lated cultures. S H R - d e r i v e d cells exhibited shorter time intervals b e t w e e n t h e induction of S6 kinase a n d O D C t h a n cells from WKY sources (Figure 2). A l t h o u g h w e h a v e s h o w n previously t h a t A n g II differentially stimu lated S6 kinase activation in quiescent cultures of V S M C
from SHR a n d WKY s o u r c e s ,2 6 w e did n o t o b s e r v e a n y
H]-thymi-AJH-FEBRUARY 1991-VOL 4, NO. 2, PART 1 A N G I O T E N S I N - I N D U C E D M E T A B O L I S M I N SHR A N D WKY 185
S6 Kinase ODC DNA Synthesis
100 75 g ο 3 50 Ε ω χ Ö 25 0 2 k 6 8 10 12 U 16 18 20 22
Time after FCS addition (hrs)
FIGURE 2. Kinetics of activation of important metabolic events
leading to cell proliferation in VSMC from SHR and WKY rats. Quiescent cells were exposed to fetal-calf serum (10%) and the activity of S6 kinase, ODC, and the incorporation levels of [3
H]-thymidine into DNA determined (see Methods). The data repre sents the mean values from two experiments performed on a pair of SHR and WKY isolates at same passage number and at similar cell densities. Closed symbols represent data from SHR and open sym bols, WKY cultures.
dine incorporation b y this agonist w h e n a d d e d to serum-free m e d i u m .
T h e V S M C from SHR a n d WKY sources elaborated extracellular matrices d u r i n g long-term culture in p r e s ence of N a ascorbate, w h i c h consisted of a complex
b l e n d of connective m a c r o - m o l e c u l e s .2 7 Proliferation
rates of WKY cells w e r e differentially stimulated w h e n t h e y w e r e cultured u p o n matrices elaborated either b y themselves or SHR cells in previous passage (Figure 3). G r o w t h rates of SHR cells w e r e n o t significantly differ e n t w h e n t h e y w e r e plated u p o n matrices elaborated b y cells of either t y p e (Figure 3).
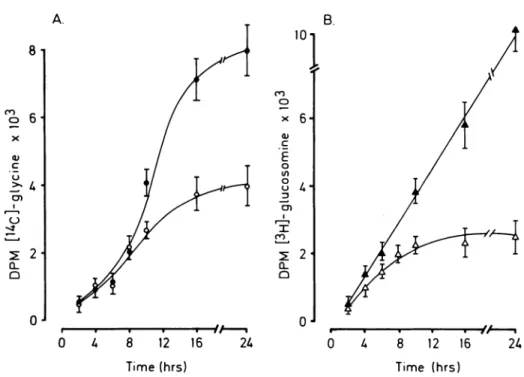
W h e n quiescent cultures of VSMC from SHR w e r e exposed to A n g II there w a s a t i m e - d e p e n d e n t stimula
tion of incorporation of [1 4C]-glycine a n d [3
H]-glucosa-m i n e into extracellular H]-glucosa-matrix H]-glucosa-material a b o v e levels o b served for serum-free m e d i u m controls (Figure 4). Similar findings w e r e obtained w i t h cells from n o r m o tensive animals (data n o t s h o w n ) . T h e data also sug gested a differential stimulation of glycoconjugate syn thesis b y A n g II since t h e ratios for t h e incorporation of t h e t w o isotopic matrix precursors w e r e significantly
(P < .05) different, ( [3H ] / [1 4C ] ; 1.17 ± 0.2 for A n g II ν
0.77 ± 0 . 2 for controls).
A l t h o u g h u n d e r serum-free conditions A n g II w a s incapable of stimulating mitogenic b e h a v i o r in V S M C , in t h e presence of 1% P D S cells from SHR exhibited a n e n h a n c e d responsiveness to t h e agonist leading to a
sig-g 4 0 0
ο
ο
LU > Ο3 0 0
ο
ι—I-J
ZDΣ
i—
CO Οer
ο
2 0 0
1 0 0
Ν =U
SHR MATRIX. WKY MATRIX.
FIGURE 3. Growth stimulation by extracellular matrix sub
strata. VSMC were plated onto either gelatin-washed or matrix-coated substrata18 and grown for 3 days with normal medium.
Following the growth period wells were trypsinized and cell num bers determined (see Methods). The data is expressed as the per centage of stimulation in growth (cell number) on the different matrix preparations (from SHR or WKY) relative to growth on gelatinized plastic (100°/o) and represents the means ± SD of re peated (n = 4) experiments. The hatched blocks represent data for SHR and * indicates the significance of difference (P > .001) in cell numbers for WKY when plated upon either SHR-derived ECM or their own.
nificant (P < .001) sustained increase in cell n u m b e r (Figure 5). W e also observed a m i n i m a l increase in prolif eration rates b y cells from WKY rats exposed to A n g II a n d 1% P D S , w h i c h only r e a c h e d significance (P < .01) after 9 d a y s in culture.
D I S C U S S I O N
V S M C from SHR sources exhibit elevated g r o w t h rates a n d e n h a n c e d r e s p o n s i v e n e s s to g r o w t h s t i m u l a n t s (growth factors, h o r m o n e s , a n d FCS) as c o m p a r e d to
cells from WKY s o u r c e s .1 8'2 3 2 8 - 3 0 W e h a v e also r e p o r t e d
o n t h e differential stimulation of S6 kinase in t h e t w o
FIGURE 4. Kinetics of incorpora
tion of radioactive matrix precursors into ECM macromolecules by VSMC from SHR. Quiescent cultures of SHR smooth muscle cells were ex posed to either [14C]-glycine or
[3H]-glucosamine in the presence or
absence of 100 nmol/L Ang II for the number of times shown. The determi nation of the levels of incorporation was performed as described under Methods and the data (mean values ± SD) is typical of that seen in a number of experiments (n = 4). Closed symbols represent cells maintained in the presence of Ang II and open, in its absence.
C O ο c CL Q 10 8 12 16 Time (hrs) 2U c o o E o O θ α 8 12 16 2k Time (hrs)
H o w e v e r , this agonist w a s u n a b l e to stimulate t h e acti vation of O D C or s u b s e q u e n t events leading to mitosis u n d e r serum-free conditions. We h a v e also s h o w n t h a t other potential g r o w t h e n h a n c e r s like l o w density lipo proteins (LDL) a n d t h r o m b o s p o n d i n (TS) b e h a v e in a
similar m a n n e r .2 0'2 1 FCS, w h i c h contains a n u m b e r of
mitogens for V S M C (ie, platelet derived g r o w t h factor
(PDGF), basic fibroblast g r o w t h factor ( F G Fb) , a n d epi
d e r m a l g r o w t h factor (EGF)) stimulated t h e induction of all three g r o w t h related metabolic events depicted in Figure 2. Cells from SHR r e s p o n d e d faster to FCS in
terms of O D C activation a n d [3H ] - t h y m i d i n e incorpora
tion t h a n did their n o r m o t e n s i v e (WKY) counterparts; this is a reflection of their faster g r o w t h rates in culture. A n g II stimulation of matrix glycoconjugate synthesis (Figure 4) m a y contribute to t h e structural c h a n g e s t h a t are part a n d parcel of vascular disease. T h e extracellular matrix plays a n i m p o r t a n t role in t h e m a i n t e n a n c e of
p h e n o t y p e ,1 0'3 1'3 2 a n d our observations t h a t matrix m a t e
rial from SHR stimulated t h e g r o w t h of WKY cells to a greater extent t h a n t h a t elaborated b y themselves serves to e m p h a s i z e t h e i m p o r t a n c e of this c o m p o n e n t of t h e vessel wall. We h a v e s u m m a r i z e d s o m e of t h e current k n o w l e d g e a n d s o m e of our o w n findings in regard to t h e interactions of n o r m a l vessel wall c o m p o n e n t s (Fig u r e 1). Central to these observations is t h e evidence t h a t A n g II is capable of stimulating t h e expression of a n u m ber of i m p o r t a n t c o m p o u n d s w h i c h include P D G F A
chain, TS, a n d T G F ^ .3 3 T h e induction of P D G F A chain
expression in VSMC exposed to A n g II h a s already b e e n
r e p o r t e d1 1 a n d it m a y account for our observation of
g r o w t h stimulation b y A n g II of cells from SHR in p r e s ence of 1% P D S (Figure 5). We h a v e f o u n d t h a t cell
isolates from SHR express a n d h a v e receptors for P D G F ^ h o m o d i m e r , w h e r e a s WKY-derived cells only express t h e g r o w t h factor b u t d o n o t r e s p o n d to it, since
t h e y lack A - t y p e cell surface r e c e p t o r s .3 4 T h e increase in
cell n u m b e r observed in cultures from h y p e r t e n s i v e a n imals m a i n t a i n e d in 1 % P D S m e d i u m alone m a y also b e a c c o u n t e d for b y their responsiveness to P D G F A-chain
h o m o d i m e r .3 4 T h e synthesis a n d secretion of t h e A A
h o m o d i m e r i c form of P D G F b y arterial s m o o t h muscle
cells h a s also b e e n observed b y o t h e r s .3 5 The combina
tion of vessel wall c o m p o n e n t s as depicted in the sche matic (Figure 1) can realistically lead to t h e initiation of neointimal formation w h i c h typifies vascular pathology in m a n a n d experimental animals. W e h a v e f o u n d that c o m p o u n d s such as TS, LDL, a n d A n g II are able, in cultured VSMC, of e n h a n c i n g metabolic events nor
mally associated w i t h p r o l i f e r a t i o n .1 6'2 0'2 1'2 8'2 9'3 6 TS
alone w a s n o t mitogenic to VSMC, but, w h e n adminis tered in combination w i t h EGF, its mitogenic potential
w a s considerably e n h a n c e d .8'3 6 A l t h o u g h there is n o evi
d e n c e as yet to suggest t h e presence of EGF (or its close
a n a l o g u e T G Fa) in t h e n o r m a l vessel wall, recent data
h a s s h o w n t h a t laminin, w h i c h is certainly present, p o s
sesses EGF-like sequences w i t h i n its s t r u c t u r e .3 7 It is
conceivable t h a t d u r i n g t h e course of n o r m a l matrix t u r n o v e r " E G F - l i k e " molecules m a y arise a n d t h a t these in combination w i t h TS could b e h a v e as a strong mito genic mixture. Additionally, t h e stimulation of TGF^ ex pression b y cultured s m o o t h muscle cells in r e s p o n s e to
A n g II e x p o s u r e3 3 m a y account for t h e influence of t h e
vasoactive p e p t i d e o n matrix synthesis, since TGF^ h a s b e e n s h o w n to n o t only m o d u l a t e t h e g r o w t h of s m o o t h muscle cells b u t also to e n h a n c e their synthesis of
extra-AJH-FEBRUARY 1991-VOL 4, NO. 2, PART 1 A N G I O T E N S I N - I N D U C E D M E T A B O L I S M I N SHR A N D WKY 187
Α. χ* B.
°J J
1 3 5 7 9 11 1 3 5 7 9 11
Days in Culture
FIGURE 5. Growth kinetics of VSMC from SHR and WKY in
presence and absence of Ang II. Cells were plated into 12-well multiwell plates (1.25 X 105 cells/well) in normal medium con
taining 10% whole blood serum. After 18 h medium was replaced with 1% PDS medium (see Methods) and some wells were further supplemented with Ang II (5X108 mol/L) on a daily basis. Me
dium (1% PDS) was changed every 48 h and cell numbers deter mined as described previously.18 Experiments (n = 3) were per
formed on cells from (A) SHR and (B) WKY and the data represent the means ± SD. Statistical significance was analyzed using Stu dent's t test for unpaired data and the levels of significance are indicated on the figure as *, Ρ < .01; **, Ρ < .001.
cellular matrix m a c r o m o l e c u l e s .3 8 In conclusion, it
w o u l d a p p e a r that w e n o longer h a v e to invoke d a m a g e to t h e e n d o t h e l i u m as t h e stimulus for t h e c h a n g e s t h a t lead to neointimal formation since m a n y c o m p o u n d s such as A n g II, alone or in combination w i t h n o r m a l constituents of t h e vessel wall, are potentially capable of p a t h o g e n i c m o d u l a t i o n of medial s m o o t h muscle cells leading to progressive structural h y p e r t r o p h y , as o b served in vascular tissue from h y p e r t e n s i v e a n i m a l s a n d m a n .
REFERENCES
1. Ross R, Glomset JA: The pathogenesis of atherosclerosis. Ν Engl J Med 1976;295:369-377, 420-425.
2. Baumgartner HR, Haundenschild C: Adhesion of plate
lets to subendothelium. Ann NY Acad Sei 1972;201:22-36.
3. Austin GE, Ratliff NB, Hollman J, et al: J Am Coll Cardiol 1985;6:369-375.
4. Clowes AW, Collazzo RE, Karnovsky MJ: A morphologic and permeability study of luminal smooth muscle cells after arterial injury in the rat. Lab Invest 1978;39:141-150.
5. Reidy MA: A reassessment of endothelial injury and arte rial lesion formation. Lab Invest 1985;53:513-520.
6. Poole JCF, Florey HW: Changes in the endothelium of the aorta and the behavior of macrophages in experimen tal atheroma of rabbits. J Pathos Bacteriol 1958,75:245-251.
7. Booth RGF, Martin JF, Honey AC, et al: Rapid develop ment of atherosclerotic lesions in the rabbit carotid artery induced by perivascular manipulation. Atherosclerosis 1989;76:257-268.
8. Majack RA, Cook SC, Bornstein Ρ: Control of smooth muscle cell growth by components of the extracellular matrix: Autocrine role for thrombospondin. Proc Natl Acad USA 1986;83:9050-9054.
9. Majack RA, Goodman LV, Dixit VM: Cell surface throm bospondin is functionally essential for smooth muscle cell proliferation. J Cell Biol 1988;106:415-422. 10. Scott-Burden T, Bühler FR: Regulation of smooth muscle
proliferative phenotype by heparinoid-matrix interac tions. Trends Pharmacol Sei 1988;9:94-98.
11. Naftilan AJ, Pratt RE, Dzau VJ: Induction of platelet-derived growth factor Α-chain and c-myc gene expres sion by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest 1989;83:1419-1424. 12. Kawahara Y, Sunako M, Tsuda T, et al: Angiotensin II
induces expression of the c-fos gene though protein ki nase C activation and Calcium ion mobilization in cul tured vascular smooth muscle cells. Biochem Biophys Res Commun 1988;150:52-59.
13. Bühler FR, Laragh JH, Baer L, et al: Propranolol inhibi tion of renin secretion. A specific approach to diagnosis and treatment of renin-dependent hypertensive dis eases. Ν Engl J Med 1972;287:1209-1214.
14. Laragh JH: The meaning of plasma renin measurements: renin and sodium-volume-mediated (low renin) form of vasoconstriction in experimental and human hyperten sion and in the oedematous states of nephrosis and heart failure. J Hypertens 1984;2(suppl 1):141-150.
15. Scott-Burden T, Resink TJ, Baur U, et al: Amiloride sensi tive activation of S6 Kinase by angiotensin II in cultured vascular smooth muscle cells. Biochem Biophys Res Commun 1988,151:583-589.
16. Berk BC, Vekstein V, Gordon HM, Tsuda T: Angiotensin II-stimulated protein synthesis in cultured vascular smooth muscle cells. Hypertension 1989;13:305-304. 17. Campbell JH, Campbell GR: in Campbell JH, Campbell
GR (eds): Vascular Smooth Muscle in Culture, Vol. 1. Boca Raton, Florida, CRC Press, 1987, pp 1 5 - 2 1 . 18. Scott-Burden T, Resink TJ, Baur U, et al: Response to
epidermal growth factor by cultured smooth muscle cells from hypertensive rats. Hypertension 1989; 13:295-304.
19. Jones PA, Scott-Burden T, Gevers W: Glycoprotein elas tin and collagen secretion by rat smooth muscle cells. Proc Natl Acad Sei USA 1979;76:353-357.
20. Scott-Burden T, Resink TJ, Baur U, et al: Activation of S6 kinase in cultured vascular smooth muscle cells by sub-mitogenic levels of thrombospondin. Biochem Biophys Res Commun 1988;150:278-286.
21. Scott-Burden T, Resink TJ, Hahn AW A, et al: Induction of growth related metabolism in human vascular smooth muscle cells by low density lipoprotein. J Biol Chem 1989;264:12582-12589.
22. Ross R, Kariya B: Morphogenesis of vascular smooth
muscle in atherosclerosis and cell culture, in Bohr D, Somlyo AP, Sparks HV Jr (eds), Handbook of Physiol ogy, section 2: The Cardiovascular System, Vol. 2. Wash ington, DC, American Physiological Society, 1980, p. 69. 23. Scott-Burden T, Resink TR, Hahn AWA, Bühler FR: Dif
ferential Stimulation of growth related metabolism in cultured smooth muscle cells from SHR and WKY rats by combinations of EGF and LDL. Biochem Biophys Res Commun 1989;159:624-632.
24. Ho vis JG, Stumpo DJ, Halsey DL, Blackshear PJ: Effects of mitogens on ornithine decarboxylase activity and messenger RNA levels in normal and protein kinase C-deficient NIH-3T3 fibroblasts. J Biol Chem 1986; 261:10380-10386.
25. Chambard J-C, Pouyssegur J: TGF/? inhibits growth fac tor-induced DNA synthesis in hamster fibroblasts with out affecting the early mitogenic events. J Cell Physiol 1988;135:101-107.
26. Resink TJ, Scott-Burden T, Baur U, et al: Enhanced re sponsiveness to angiotensin II in vascular smooth muscle cells from spontaneously hypertensive rats is not asso ciated with alterations in protein kinase C. Hypertension 1989;14:293-303.
27. Scott-Burden T, Resink TJ, Bürgin M, Bühler FR: Extra cellular matrix: differential influence on growth and bio synthesis patterns of vascular smooth muscle cells from SHR and WKY rats. J Cell Physiol 1990;141:267-274. 28. Scott-Burden T, Resink TJ, Bühl er FR: Enhanced growth
and growth factor responsiveness of vascular smooth muscle cells from hypertensive rats. Cardiovascular Pharmacol 1989;14:S16-S21.
29. Yamori Y, Igawa T, Kanbe T, et al: Mechanism of struc tural vascular changes in genetic hypertension: analysis on cultured vascular smooth muscle cells from spontane ously hypertensive rats. Clin Sei 1981;61:121s-123s.
30. Haudenschild CC, Grunwald J, Chobanian AV: Effects of hypertension on migration and proliferation of smooth muscle in culture. Hypertension 1985;7:(suppl 1)101-104.
31. Castellot JJ, Wright TC, Karnovsky MJ: Regulation of smooth muscle cell growth by heparin and heparin sul fates. Sem Throm Hem 1987;13:487-503.
32. Herman IM, Castellot JJ: Regulation of smooth muscle cell growth by endothelial-synthesized extracellular ma trices. Arteriosclerosis 1987;7:463-469.
33. Hahn AWA, Scott-Burden T, Resink TJ, et al: Angioten sin II induction of PDGF A chain expression in cultured rat vascular smooth muscle cells is preceeded by throm-bospondin gene transcription. Eur J Biochem (in press). 34. Resink TJ, Scott-Burden T, Hahn AWA, et al: Specific
growth stimulation of cultured smooth muscle cells from spontaneously hypertensive rats by platelet-derived growth factor A chain homodimer. Cell Reg (in press). 35. Sjolund M, Hedin U, Sejersen T, Heldin CH: Arterial
smooth muscle cells express platelet-derived growth fac tor (PDGF) A chain mRNA, secrete a PDGF-like mitogen and bind exogenous PDGF in a phenotype- and growth state-dependent manner. J Cell Biol 1988;106:403-413. 36. Scott-Burden T, Resink TJ, Bühler FR: Growth regulation in smooth muscle cells from normal and hypertensive rats. J Cardiovasc Pharmacol 1988;12:sl24-sl27. 37.
38.
Panayotou G, End P, Aumailley M, et al: Domains of laminin with growth-factor activity. Cell 1989;56:93-101.
Penttinen RT, Kobayashi S, Bornstein Ρ: Transforming growth factor β increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sei USA 1988;85:1105-1108. 39. Mackie EJ, Halfter W, Liverani D: Induction of tenascin in healing wounds. J Cell Biol 1988;107:2757-2767.