Fetal liver organ cultures allow the
proliferative expansion of pre-B
receptor-expressing pre-B-II cells and the
differentiation of immature and mature B
cells
in vitro
Rhodri Ceredig
1,2, Edwin ten Boekel
1, Antonius Rolink
1, Fritz Melchers
1and
Jan Andersson
1,31Basel Institute for Immunology, Grenzacherstrasse 487, CH-4005 Basel, Switzerland 2U184 INSERM, LGME du CNRS, Faculte´ de Medecine, Strasbourg, France
3Department of Immunology, BMC, University of Uppsala, Uppsala, Sweden
Keywords: development, IL-7, IL-7 receptor,λ5, limiting dilution, lipopolysaccharide, ontogeny, RAG-2T
Abstract
We describe the phenotypic and functional properties of B lineage cells developing in fetal liver organ cultures (FLOC) of mouse embryos at day 14 or 15 of gestation which contain pro/pre-B-I cells. FLOC B cell development proceeds to mature IgMF, IgDFand CD23F lipopolysaccharide-reactive B cells within a culture period of 5–6 days. The phenotypes and relative proportions of pro/pre-B-I, pre-B-II, immature and mature B cells from FLOC were similar to that seen in livers freshly isolated from age-matched, i.e. newborn, mice. More importantly, the numbers of cells recovered in the different B lineage subpopulations from FLOC were close to those developed in vivo. Hence, in contrast to single-cell suspension cultures of fetal liver, FLOC allow the
proliferative expansion of pre-B cell receptor-expressing pre-B-II cells. FLOC from embryos of mice with targeted mutations in the RAG-2 andλ5genes, which cannot expand by proliferative
expansion of their pre-B-II compartmentin vivo because they cannot express a pre-B cell receptor on their surface, show this same defectin vitro. FLOC are accessible to the action of mAb and cytokines. Thus, addition of anti-IL-7 receptor mAb to FLOC of normal mice inhibits B cell
development at the transition of pro/pre-B-I to pre-B-II cells. This inhibition is reversed by addition of excess rIL-7. Addition of IL-7 alone stimulates the proliferation of pro/pre-B-I cells and inhibits their differentiation to pre-B-II and immature B cells, as it does in single-cell suspension cultures. FLOC should be useful to study the effects of other mAb, cytokines, ligands and other molecules on early B cell development.
Introduction
Our understanding of the differentiation pathway of mouse B cells has been derived from a combination of studies which defined B lineage subpopulations in the primary B-lymphoid organs by the differential expression of intracellular and surface bound molecules, the state of Ig gene rearrange-ments, and the growth and differentiation properties of these cells in tissue culture (1). These progenitor and precursor subpopulations were ordered in their sequence during devel-opment by a determination of the conformation of their Ig
Correspondence to: R. Ceredig
Transmitting editor: S.-I. Nishikawa Received 22 July 1997, accepted 30 September 1997 gene loci on the single-cell level (2,3). Thus, B lineage committed progenitors (herein called pro-B/pre-B-I cells) express the surface markers B220 (CD45), CD19 and c-kit, and have undergone DHto JHIg rearrangements at the heavy (H) chain locus. Pro-B cells with the same surface markers, but with all Ig gene loci in germline configuration, can be isolated from RAG-2-deficient (RAG-2T) mice (4). Such pro-B and pro/pre-pro-B-I cells isolated either from the fetal liver or the bone marrow of adult mice can be cloned and maintained
for long periods of time in vitro provided that they remain in contact with stromal cells and that the cultures are supple-mented with IL-7 (5). Such cells express the IL-7 receptor (IL-7R). Following VH to DHJH rearrangements at the heavy chain locus, cells progress to the pre-B-II stage. Pre-B-II cells express theµchains together with the surrogate light chains, composed of Vpre-Bandλ5,whenever the rearrangement has
been productive, i.e. in-frame. Pre-B-II cells have lost the expression of c-kit and have gained the expression of CD25. In the primary lymphoid organ in vivo, pre-B-II cells enter a phase of three to seven cell divisions, but only when they express the pre-B cell receptor on their surface.
VH to DHJH and VL to JL rearrangements can also be induced in vitro by the removal of IL-7 from the pro/pre-B-I cells, either previously grown on stromal cells in the presence of IL-7 in vitro or isolated as B2201 CD191 c-kit1 cells ex vivo. The cells differentiate apparently normally to CD251 pre-B-II cells and later to sIg1immature B cells but they do not enter the phase of proliferation, not even when they have succeeded to rearrange the IgH locus productively. It is evident that one of the main differences in the differentiation of normal B lineage cells between in vivo and single-cells in vitro conditions is the lack of proliferative expansion of pre-B cell receptor-expressing large, cycling c-kit–, CD251
pre-B-II cells in vitro.
The generation of B cells in the mouse fetal liver occurs as a seemingly synchronous wave of differentiation (6). This apparent synchrony is due to the fact that in the fetal liver at days 13–15, only pro-B cells are present, which increase in number during this time and then progress as a cohort through the successive B cell compartments. Mature B cells are first detected in the fetal liver at about day 18. In contrast to the apparent synchrony in the fetal liver, in bone marrow, from birth onwards, all subpopulations of developing B cells are present, thus making analysis of the transitions between the different B lineage compartments more difficult.
In addition to the stroma plus IL-7 culture system outlined above, other in vitro systems for studying B cell generation have been reported (7–10). All of them are less efficient in generating proper numbers of B lineage cells of the different stages of development when compared to the in vivo develop-ment. One reason for this inefficiency might be that the in vivo development of mature B cells from their precursors probably involves multiple cellular interactions which are disrupted in cell suspension cultures (11). Some time ago, Owen et al. described an in vitro organ culture system of mouse fetal liver fragments where B lymphopoiesis occurred (12). Here, we report the use of this fetal liver organ culture (FLOC) system with currently available tissue culture media to study B lymphopoiesis in normal as well as mutant mice with the sets of markers which have in the meantime been found to distinguish different B lineage cell subpopulations. Our results indicate that organ cultures allow an important step in B lineage development in vitro, which single-cell suspension cultures have been unable to support, i.e. the proliferative expansion of pre-B-II cells.
Targeted disruption of the RAG-2 gene (RAG-2T mice) and of theλ5gene (λ5T mice) leads to severe deficiencies in the development of B lymphocytes. In bone marrow of RAG-2T mice, development of B lineage cells proceeds to normal
numbers of c-kit1 B2201 CD191 cells which are normally responsive to the proliferation-inducing stimuli of stromal cells and IL-7 (4,13). Since the RAG-2T B lineage cells cannot make µH chains, they cannot form a pre-B cell receptor.
Hence, they cannot expand by proliferation to c-kit–CD251
large, and later, small pre-B-II cells and to sIg1 immature B cells. Although these mice have no mature sIg1 B cells (and no T cells), they are capable of differentiating, without proliferation, to mature CD231 MHC class II1 cells which have the capacity to undergo Sµ–Sε switching as in vitro stimulation of pro-B cells with CD40-specific mAb and IL-4 has shown (13).
In bone marrow ofλ5T mice development of B lineage cells,
again, proceeds to normal numbers of c-kit1B2201CD191 pre-B-I cells which are DHJH rearranged. Again, they are
normally responsive to the proliferation-inducing stimuli of stromal cells and IL-7 (14). They are capable of VH to
DHJH rearrangements and of µH chain production. In vitro
experiments have shown that sIg1 immature B cells are generated without proliferation at rates and in numbers which are indistinguishable betweenλ5T and normal B lineage cells
(14). In vivo, however, theλ5T pre-B-II cells cannot expand by proliferation since they cannot form a pre-B cell receptor. λ5T mice make small numbers of mature B cells because
their pre-B-I cells are capable of differentiating in vivo, though without proliferation, to sIg1B1 and conventional B cells (15). Since we find that FLOC of normal mice show the proliferative expansion in vitro, we can test whether the FLOC of RAG-2T and λ5T show the same defect in this proliferation in vitro
which they exhibit in vivo.
IL-7 and its receptor have been shown to be crucial for mouse B lymphopoiesis in vivo (16–18). IL-7R are expressed on pro/pre-B-I and large pre-B-II cells. In vitro IL-7 in co-operation with stromal cells stimulates the long-term prolifera-tion of pro/pre-B-I cells, and keeps these cells from differentiat-ing to pre-B-II and immature B cells, and also keeps pre-B-I cells alive in tissue culture, i.e. acts in an anti-apoptotic way. Removal of IL-7 from these cultures induces pro/pre-B-I cells to differentiate to short-lived pre-B-II and sIgM1 immature B cells.
In this paper, we test the actions of an IL-7R-specific mAb and of IL-7 on FLOC of normal and of mutant mice. The results indicate that the IL-7R-specific mAb as well as IL-7 can find their target cells in the organ cultures and influence B cell development in vitro as they do in vivo.
Methods
Mice
Normal C57Bl/6 (B6) mice and Lewis strain rats, all at 6–8 weeks of age, were purchased from Biological Research Laboratories (Fu¨llinsdorf, Switzerland). Homozygous Rag-2T mice (19), originally obtained as breeding pairs from Dr Fred Alt (The Children’s Hospital, Howard Hughes Medical Institute, Boston, MA), as well as homozygousλ5T mice (15) were bred
under pathogen-free conditions at the Basel Institute for Immunology.
FLOC
Fetal livers were obtained from normal (B6),λ5T and RAG-2T
of a vaginal plug being taken as day 0), and chopped with a scalpel. Organ cultures were set up as originally described by Owen et al. (12). Briefly, small explants (1–2 mm) were put onto 25 mm Nucleopore membranes (0.8µm polycarbonate; Costar, Cambridge, MA) floating on 5 ml IMDM medium (Gibco/BRL, Gaithersburg, MD), containing 5310–5M
2-mer-captoethanol, 13non-essential amino acids (Gibco/BRL), 0.03% primatone (Quest International, Naarden, The Netherlands) and 2% FCS (Gibco/BRL) (herein referred to as FLOC-medium). The individual membrane containing the explants from one of the four major fetal liver lobes was kept in six-well cluster plates (no. 3506; Costar) for 6 days, when not otherwise indicated, in a humidified incubator at 37°C in the presence of 10% CO2 in air. At harvest of FLOC, the
explants were flushed off the membranes with FLOC-medium using a 1 ml syringe equipped with a 26G needle. Single-cell suspensions were obtained by aspiration of the explants in medium through the needle. After passage of the cell suspension through gauze, the number of viable cells was determined in a Burker chamber using the Trypan blue dye exclusion test. Such cells were used without further washing for FACS analysis, cell sorting or further culture in limiting dilution analysis (LDA) assays (see below). Whenever recom-binant mouse IL-7 (rIL-7) was added to FLOC it was in the form of medium conditioned with the transfectant J558/L myeloma cell line stably transfected with a mouse IL-7 cDNA under the control of theκLchain promotor and Eµenhancer
of the vector pKm1 (20) (kindly given to us by Dr T. Winkler of this institute) which contained ~300 ng/ml IL-7. IL-7 concen-trations were determined by the growth of IL-7-dependent normal pro/pre-B-I cell lines on irradiated stromal cells as described (5), using purified recombinant IL-7 (PharMingen, San Diego, CA) as standard. In such assays, 1 µg of the monoclonal rat anti-mouse IL-7R α chain-specific antibody A7R34 (18) (kindly given to us by Dr S. Nishikawa, Kyoto University, Kyoto, Japan) inhibited the growth of pro-B cells in the presence of 1.2 ng rIL-7.
Antibodies
The FITC- and phycoerythrin (PE)-labeled mAb RA3 6B2 (anti-CD45R, B220), the biotin-conjugated mAb 7D4 (anti-CD25, IL-2R α chain, TAC) and mAb B3B4 (anti-CD23, IgE Fc receptor) were all obtained from PharMingen. The mAb ACK4
(anti-c-kit) (21), 1D3 (anti-CD19) (22,23), A7R34 (anti-IL-7R) (18), M41 (anti-µ,) (24), 1.19 (anti-δ) (25), 187.1 (anti-κLchain)
(26), M5-114 (anti-MHC class II) (27) and FGK 45 (anti-CD40) (13) are all IgG of rat origin and were purified from hybridoma culture supernatants on Protein G–Sepharose columns (Pharmacia, Uppsala, Sweden) as recommended by the supplier. Purified mAb were used unlabeled for coating ELISA plates or were conjugated with FITC, PE or biotin according to standard protocols. FITC-conjugated goat anti-mouse IgM and streptavidin–PE were purchased from Southern Biotech-nology Associates (Birmingham, AL).
Flow cytometry and cell sorting
Flow cytometric analyses were performed as described before (28). Single-cell suspensions in FACS buffer (PBS containing 1% FCS and 0.1% sodium azide) of fetal livers freshly isolated ex vivo or obtained from FLOC after culture periods specified
in the results section were briefly double stained with FITC-labeled anti-B220 and biotin-FITC-labeled other mAb for 30 min at 4°C. After washing of the cells, the biotin-labeled mAb were revealed by incubation for 30 min with streptavidin–PE. Stained cells were resuspended in FACS buffer containing 1 µg/ml propidium iodide (Sigma, St Louis, MO) so that dead cells could be excluded from analysis by gating in FL3. Analyses were performed on a FACScan (Becton Dickinson, Sunnyvale, CA) interfaced to a Hewlett-Packard computer (HP900) using the FACScan research software programs.
Analysis of fresh fetal liver cells was carried out following Ficoll-Paque (Pharmacia) density gradient purification accord-ing to the manufacturer. Control experiments of cell yield were carried out which showed that there was an at most 50% loss of CD191 B cells by this procedure. In order to calculate absolute numbers of CD191cells per fetal liver, the proportion of CD191 cells in the viable cell gate (propidium iodide negative) as determined by FACS analyses was multiplied by the number of viable (Trypan blue negative) cells.
Single cells from FLOC routinely kept in culture for 6 days were surface stained with FITC-labeled anti-CD19 (1D3) and biotin-labeled anti-CD23 (B3B4) (anti-IgE Fc receptor) revealed by PE-labeled streptavidin and subsequently sorted using a FACStar Plus (Becton Dickinson).
LDA
Determination of lipopolysaccharide (LPS) reactivity of B cells in fetal liver or FLOC was performed by LDA in Lewis rat thymus filler cells as described (29). One plate of 96 flat-bottom wells (Costar) each containing cultures of 0.2 ml FLOC culture medium containing 15–25µg/ml LPS and 33106rat
thymus filler cells/ml was set up for every concentration of indicated cells, ranging from 2000 down to 6 cells/well. LPS from Escherichia coli EH100 was kindly provided by Dr C. Galanos (Max Planck Institute for Immunobiology, Freiburg, Germany). The cultures were incubated for 9 days at 37°C in a humidified atmosphere containing 10% CO2in air. At the
end of culture, 50µl supernatant from each microwell was assayed for the presence of mouse IgM using ELISA (see below).
ELISA
Culture supernatant aliquots were assayed for total IgM content using the ELISA technique as described (30). ELISA plates (MaxiSorp F96; Nunc, Roskilde, Denmark) that were coated with 50µl of 2µg/ml of mAb M41 (rat anti-mouseµ -chain-specific antibody) in 0.1M carbonate buffer, pH 8.5, for 1 h at 37°C and then saturated with 4% BSA (Sigma) in PBS containing 0.05% Tween 20 (Fluka, Buchs, Switzerland) (PBS/ BSA/Tween) received 100 µl of culture supernatant diluted 1:1 in the same buffer. After incubation (4 h, 37°C) and washing, alkaline phosphatase-labeled goat anti-mouse IgM (µchain-specific; Southern Biotechnology Associates) diluted 1/1000 in PBS/BSA/Tween was added. After further incubation (1 h, 37°C) and washing, bound anti-IgM antibodies were revealed with the substrate 4-nitrophenyl phosphate (1 mg/ ml; Serva, Heidelberg, Germany) in 1 M diethanolamine buffer, pH 9.8. The alkaline phosphatase-driven conversion of substrate was stopped after 20 min by addition of 1 N NaOH and the absorbency read at 405 nm in a ThermoMax
microplate reader (Molecular Devices, Menlo Park, CA) equipped with the Softmax software (version 2.2) for Apple Macintosh computers. Cultures were scored positive when readings were above 1.5 times medium background. The data are presented as semi-logarithmic plots of the percent non-responding cultures recorded for each concentration of plated cells. The frequency (f) of LPS-responding cells is obtained from that plot at the intercept of 37% non-responding cultures, which according to Poisson’s distribution represents, on the average, the number of cells containing one responding cell.
Isolation of RNA and RT-PCR
Total RNA for cDNA synthesis was prepared from cells of freshly isolated day 16 fetal liver as well as 6 day FLOC or 6 day fetal thymus organ cultures (FTOC) (31) using RNAzol B (Tel-Test, Friendswood, TX) according to the manufacturer’s recommendations. RNA was reverse transcribed using 100 U Superscript II reverse transcriptase (Gibco/Life Technologies, Gaithersburg, MD), 40 U RNase inhibitor, 1 mM dNTPs, 1 µg random hexameric oligonucleotides (Pharmacia) and the supplied RT buffer. PCR assays were carried out using the following primer pairs: β-actin (32): 59-GAA GTC TAG
AGC AAC ATA GCA CAG CTT CTC-39, 59-GTG GGA ATT
CGT CAG AAG GAC TCC TAT GTG-39; TdT (33): 59-GAT TTC GAG ACT TGG TCC TCT TCA TTT TGG-39, 59-CAA GGA ATT CCC TCT GTG TCT TTC ATG CTG-39; IL-7 (34,35): 59-GAG AGT GTA CTG ATG ATC-39, 59-TAT ACT GCC CTT CAA AAT TT-39. Amplification of the cDNA was carried out with one cycle at 94°C for 30 s, followed by 32 cycles for theβ-actin RT-PCR and 35 cycles for the TdT and IL-7 RT-PCR at 94°C for 20 s, 55°C for 20 s and 72°C for 60 s. The expected sizes of the PCR products are 535 bp forβ-actin, 555 bp for TdT and 350 bp for IL-7. RT-PCR products from different dilutions of cDNA were subjected to electrophoresis in a 1% agarose gel and visualized by UV light after ethidium bromide staining.
Results
Development of B lineage cells in fetal liver of normal mice Fetal livers of day 13 of gestation and onwards were analyzed by flow cytometry using double staining with FITC-labeled antibodies for surface B220 and a panel of biotin-labeled mAb. The markers used were those previously shown to distinguish, among the total B lineage cells (CD19), the early pro/pre-B-I cells (c-kit, IL-7R) from later pre-B-II (CD25), immature (IgM) and mature (CD23, IgD) B cells (1).
Figure 1(A) shows a set of FACS profiles obtained with cells of a day 15 fetal liver from a normal C57Bl/6 embryo.
Figure 2 summarizes these analyses for fetal liver analyzed ex vivo at different times of gestation and for liver at different days after birth. Between day 13 and 15 of gestation, the majority of CD191 cells belong to the pro/pre-B-I subpopul-ation, and no immature (IgM1) and mature (CD231or IgD1) B cells can be detected. Such immature and mature B cells can only be detected in significant numbers by FACS analyses at day 18 and onwards. Staining for surface CD19 shows that not all B2201cells belong to the classical B lineage subset. The existence of CD19–B2201cells has been reported earlier
(23). Hence, we used the CD19-specific antibody as a marker for all subsequent experiments to estimate the total number of B lineage cells developing in vivo as well as in vitro.
As can be seen from the data in Fig. 2(A), the absolute number of CD191cells per liver increases from 103 at day 13 to 106at birth (day 20). In the newborn liver the phenotype
of developing B cells have progressed to include CD251 pre-B-II cells, immature IgM1 and mature CD231/IgD1 cells (Fig. 1B). As reported earlier (36), pro-B/pre-B-I (c-kit1) cells are still detectable at this time.
Establishment of FLOC
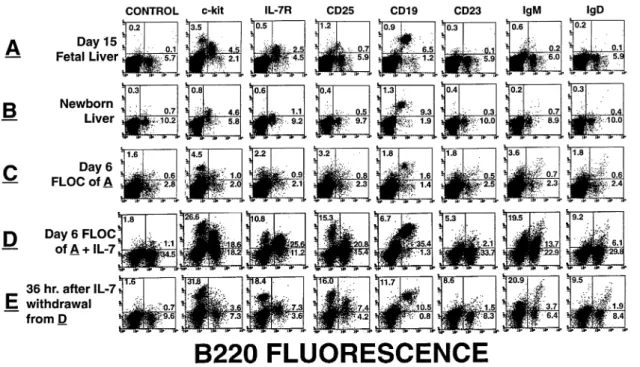
When day 15 fetal liver from normal B6 mice was cultured for 6 days as FLOC and the CD191 cells were counted at the end of the culture period, the number of cells (Fig. 2B) was close to that seen in the age-matched (i.e. newborn) liver developed in vivo (Fig. 2A). Phenotypic analyses of the FLOC (Fig. 1C) cells showed a progressive differentiation to imma-ture and maimma-ture B cells, similar in numbers of cells (Fig. 2B) to those determined in vivo in the newborn liver (Fig. 2A). The total number of pro/pre-B-I cells remained nearly the same during the 6 day FLOC. It was, therefore, lower (Fig. 2B) than the one developed in vivo, observed in the newborn liver (Fig. 2A).
A detailed phenotypic analyses of B lineage cells emerging with time of FLOC (Fig. 1C) revealed a progressive relative decrease of c-kit1pro-B/B-I cells and an increase of pre-B-II (CD251) and immature IgM1cells, so as to reach ~50% of the total B lineage cells. Concomitantly, there was a decrease in IL-7R expression, and increase in CD23 and IgD. Because of the weak CD25 staining in both fetal liver in vivo and in FLOC, the number of CD251 cells is probably an underestimate, thus giving the impression of simultaneous appearance of CD25 and IgM molecules on developing B cells. In a series of 10 different experiments, an average of 16% of the recovered cells were B2201 and, of these, 88% were CD191, 42% sIgM1and 19% sIgD1. Additional analyses using mAb to CD40, MHC class II and AA4.1, and Ig L chains, showed that the immature and mature B cells arising in FLOC were CD401, MHC class II1 and AA4.11, and showed the κ:λratio of peripheral B cells of adult mice (data not shown). One of the characteristics of fetal-derived B cells is their absence of N nucleotide additions at the heavy chain VDJ junctions, due to absence of the enzyme TdT (37) in B lineage cells at this time of development (38). This was confirmed by RT-PCR analyses of TdT transcripts in both day 16 fetal liver and FLOC derived thereof as shown in Fig. 3. As a positive control for the assay we used RNA from FTOC established from the same embryos, since it is known to express TdT (39). The results show that the B cells maturing in FLOC and emerging in the embryo are of embryonic origin and not from the mother. Additional experiments using embryos from RAG-2T mothers mated with normal males confirmed this conclusion (data not shown).
We conclude from these experiments that FLOC, like fetal liver single-cell suspension cultures studied previously (6), permit the differentiation to more mature forms of B lineage cells from earlier precursors, i.e. mature B cells, immature B cells and pre-B-II cells from pro/pre-B-I cells. This differenti-ation takes place with the same time schedule observed
Fig. 1.Characterization of FLOC from normal C57Bl/6 mice. FACS analyses of normal C57Bl/6 day 15 fetal liver (A), newborn liver (B) as well as day 6 FLOC of A (C). Each panel shows the cytogram displays of 20,000 gated events of cells stained with the indicated mAb. In each panel, the percentage of events in three of the four quadrants is indicated.
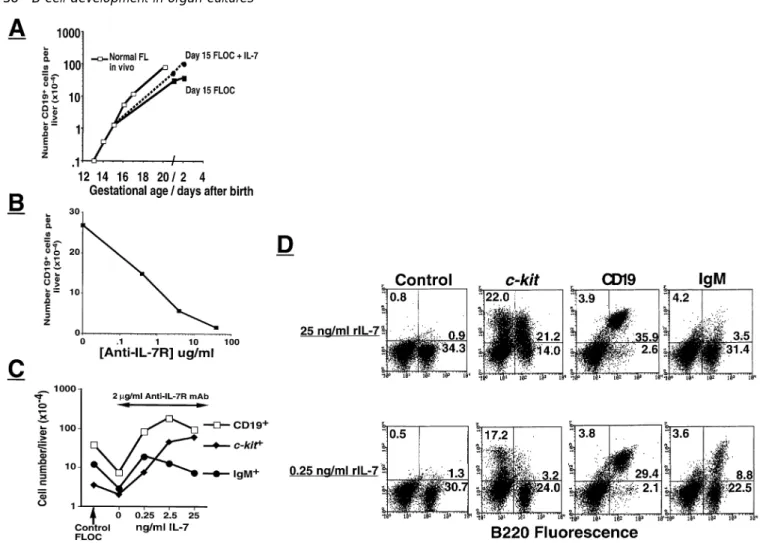
Fig. 2.Development of B cell subpopulations in fetal livers and FLOC of normal C57Bl/6 mice. Kinetics of increase in CD191, c-kit1, CD251, IL-7R1 and IgM1 cells (all expressing B220) in normal fetal liver in vivo at the indicated days of gestation (A) and in FLOC of day 15 normal fetal liver harvested at the indicated times of in vitro culture (B).
in vivo as an apparent single wave of cellular development. In contrast to previous single-cell cultures, FLOC have the added capacity of generating large numbers of more mature B lineage cells by proliferation and differentiation. In fact, the numbers of the different more mature B lineage cells resemble those generated during the equivalent time in vivo. The num-bers of pro/pre-B-I cells do not increase in FLOC as they do in vivo, presumably because the stromal cell compartments do not continue to grow in vitro.
Fig. 3. Transcripts for TdT and IL-7 in FLOC. RT-PCR analysis of transcripts for TdT, IL-7 and β-actin on RNA isolated from freshly isolated day 16 fetal liver, day 6 FLOC of day 16 fetal liver or day 6 FTOC from the same embryo as used for FLOC. Dilutions of cDNA used for the RT-PCR reaction (as described in Methods) are given at the top.
Mitogen reactivity of day 6 FLOC B cells
The functional capability of B cells emerging in FLOC was assessed by testing their reactivity to the B cell mitogen LPS (29). Cell suspensions of day 6 FLOC of fetal liver at day 15 of gestation were plated at graded numbers in the presence of rat thymus filler cells and LPS. Nine days later, IgM secretion
Fig. 4.LDA of LPS-responsive B cells of FLOC subpopulations. Day 6 FLOC from normal day 15 fetal liver was sorted as shown and the various fractions were analyzed for the frequencies (f) of LPS-reactive cells developing into IgM-secreting cells determined by LDA as described in Methods.
by developing clones of stimulated B cells was assessed by ELISA of the culture supernatants (see Methods). In a series of experiments, the frequency of LPS-reactive B cells among unfractionated FLOC cells ranged from 1/100 to 1/400. This frequency was approximately the same as the one measured with cells from newborn liver. To determine the frequency of LPS-reactive cells among different B lineage cells emerging in FLOC, we separated day 6 FLOC cells by FACS into CD191/CD231 and CD191/CD23– cells. As can be seen
from Fig. 4, whereas the frequency of responding cells in unseparated FLOC was 1/180 plated cells, the sorted mature CD191/CD231 B cells responded at a frequency of 1/4.5 plated cells. B lineage cells of the CD191/CD23–fraction (i.e. including pro-B, pre-B and immature B cells) responded at a 5-fold lower frequency. From these frequency data, we calcu-late that 46% of the total LPS-reactive B cells were found in the 2.8% CD191cells which are CD231and 53% of the LPS-reactive cells were in the 16% CD191/CD23–fraction. It cannot
be excluded that the low frequency of responding cells among the CD19–fraction was not due to contamination by CD191 cells. We conclude from these experiments that, in contrast
Fig. 5.Analysis of B cell development in RAG-2T (G2) fetal livers. FACS analysis of fresh day 15 RAG-2T fetal liver (A), newborn liver (B), day 6 FLOC of A (C) and day 6 FLOC in the presence of 25 ng/ ml of exogenous rIL-7 (D). Kinetics of CD191cells in FLOC from day 15 RAG-2T fetal liver as compared to their emergence in a normal C57Bl/6 and RAG-2T livers at various fetal/neonatal ages (E).
to single-cell suspension cultures, FLOC can develop mature, mitogen-reactive B cells.
FLOC of mutant mice
Since FLOC of normal mice apparently allowed the proliferat-ive expansion of B lineage cells, especially of pre-B-II cells, in vitro (Fig. 2B) we tested FLOC of RAG-2T andλ5T for their
capacity to generate B lineage cells in order to see whether their B lymphopoietic defects could also be seen in vitro.
At day 15 of development the number and phenotype of B lineage cells in the RAG-2T fetal liver is similar (Fig. 5) to that of age-matched normal animals (Fig 1). In FLOC of day 14 RAG-2T fetal liver, the number of recovered CD191 cells remained largely unchanged for 6 days but thereafter decreased significantly (Fig. 5E). In vivo, the number of CD191 cells continues to increase until day 17 and then
Fig. 6.Characterization of FLOC fromλ5T fetal livers. FACS analysis of fresh day 15 fetal liver (A), newborn liver (B), day 6 FLOC of A (C), day 6 FLOC cultured in the presence of 25 ng/ml rIL-7 (D) as well as D, 36 h after rIL-7 withdrawal (E).
shows a sharp decline due to failure of further development into more mature stages of B cell development. The majority of the CD191 cells in RAG-2T FLOC were and remained c-kit1, similar to the liver of RAG-2T newborns (Fig. 5B and C), i.e. were pro-B cells. We conclude that the RAG-2T defect observed in vivo is also manifest in vitro. Unlike single-cell suspension cultures of RAG-2T pro-B cells (4,13), the genera-tion of larger numbers of CD191 cells with more mature phenotypes does not occur in FLOC.
FLOC were also established from day 15 fetal livers ofλ5T
mice (Fig. 6). Total numbers of CD191cells in the fetal liver at the start of the culture was found to be close to those of fetal livers of normal mice (Fig. 7A). However, at day 6 of culture, the defect in B cell production due to theλ5mutation
became evident in a 10-fold decrease of the number of total CD191(Fig. 7A). sIgM1cells were hardly detected, i.e.,1%. This inefficient generation of B cells was further seen in a 10-fold lower frequency of LPS-reactive cells developing in the FLOC (Fig. 7B).
We conclude from these experiments that FLOC of normal, RAG-2T andλ5T mice enact B cell differentiation from early
progenitors and precursors more faithfully than single-cell suspension cultures do. In FLOC of normal mice, the proliferat-ive expansion of pre-B-II cells occurs, generating near normal in vivo numbers of B lineage cells with precursor, immature and mature phenotypes. FLOC of the immunodeficient RAG-2T and λ5T mice remain defective as in vivo, since they
do not generate large numbers of cells with more mature phenotypes.
Effects of IL-7R-specific mAb on FLOC
Control experiments (Fig. 8A) showed that addition of IL-7 to FLOC from normal mice did not dramatically alter the number
Fig. 7.Numbers of B cells and their function in livers ofλ5T embryos. Development of CD191cells in λ5T fetal livers of different times as compared to their development in FLOC with and without rIL-7. For comparison, we show data from a normal C57Bl/6 mouse as in Figs 2 and 5(E) (A). Frequency of LPS-reactive cells developing into IgM-secreting clones. Day 17 fetal livers from twoλ5T and one normal embryo after 6 days of FLOC were subjected to LPS LDA as described in Methods (B).
Fig. 8.Effect of exogenous rIL-7 and anti-IL-7R antibody on normal FLOC. Kinetics of increase in CD191cells in normal fetal liver in vivo and in FLOC grown in medium with and without 25 ng/ml rIL-7 (A). FLOC from day 15 fetal liver were cultured for 6 days in medium alone or containing the indicated concentrations of purified anti-IL-7R antibody (B). Reversal of anti-IL-7R mAb inhibition by exogenously added rIL-7 at the indicated concentrations (C). Phenotype of FLOC cultured in B in the presence of 25 ng/ml or 0.25 ng/ml of rIL-7 (D).
of CD191cells recovered. We then added to FLOC of normal mice a mAb specific for the IL-7R α chain. As shown in Fig. 8(B), a dose-dependent inhibition of the generation of CD191 cells occurred with a 50% reduction observed at 0.4µg mAb/ml. Phenotypic analyses revealed that the genera-tion of more mature cells, especially of IgM1 cells, was particularly affected (Fig. 8C and D).
We conclude that the IL-7Rαchain-specific mAb is capable of diffusing to its specific sites in the FLOC, competing there with endogenous IL-7 in the binding to the IL-7R. The effect of this competitive binding is an inhibition of the lymphopoiesis in FLOC. Endogenous production of IL-7 mRNA and, therefore, probably of IL-7 in FLOC was shown by RT-PCR analyses using primers specific for IL-7. As can be seen from Fig. 3, day 6 FLOC expressed transcripts for IL-7, although at a level considerably less than the control FTOC (thymus) from the same day 16 embryo.
In order to ascertain the IL-7R specificity of this inhibition and to compete with it, we added graded amounts of rIL-7 together with the IL-specific mAb to the FLOC. A dose-dependent reversal of the block was observed at,0.25 ng
rIL-7 added per milliliter of FLOC medium (Fig. 8B and C). Given the phenotypic recovery of FLOC at 0.25 ng/ml rIL-7, it can be estimated that the endogenous production of IL-7 or IL-7-like molecules in FLOC corresponds to this amount of recombinant protein. At higher concentrations of rIL-7 the recovery of CD191cells exceeds that of the control, unmanip-ulated FLOC by a factor of 2-to 3-fold. Thus, at 25 ng/ml of rIL-7 the proportion of pro/pre-B-I cells increases to constitute .50% of the recovered B lineage cells (Fig. 8C). Under the assumption that the IL-7R-specific mAb does not have a hidden cross-reactivity to IL-7, we can estimate from these experiments that 0.1–0.2 ng IL-7 can compete with 2000 ng of IL-7R-specific mAb to restore full capacity of B lymphopoiesis. Effects of exogenous IL-7 on FLOC
Addition of rIL-7 to pro/pre-B-I cells in suspension cultures on stromal cells stimulates the proliferation of c-kit1cells (5). Addition of exogenous rIL-7 to normal FLOC also resulted in an increase in the absolute number of c-kit1pro/pre-B-I cells as it did with FLOC from RAG-2T mice. As can be seen in Fig. 6(D and E), a 40-fold increase in total CD191 cells
recovered after 6 days in culture was recorded, which further increased upon prolonged culture in the presence of rIL-7. These cells remained largely c-kit1. This shows that pro-B cells from normal and from RAG-2T mice in their native environment are fully competent to respond to rIL-7.
Addition of IL-7 also induced proliferation of c-kit1 pro/pre-B-I cells inλ5T FLOC (Fig. 6D and 7A). The expansion of pro/
pre-B-I cells is dependent on the exogenous rIL-7 as shown by its withdrawal (Fig. 6E). Thus, 36 h after transferring rIL-7-treatedλ5T FLOC to medium without rIL-7, c-kit1pro/pre-B-I
cells disappear to reach numbers close to those found in the liver of normal mice at this time of development in vivo (newborn liver, see Fig 1B). Comparing the cell recovery (Fig. 7A) and phenotype (Fig. 6) of FLOC fromλ5T animals,
addition of IL-7 results in a ~10-fold increase in c-kit1CD191 pro/pre-B-I cells.
We conclude from these experiments that IL-7 acts to induce the FLOC-stroma-supported proliferation of a similar number of pro/pre-B-I cells in the FLOC of the normal and the immunodeficient mice to a similarly expanded number of CD191 with the early phenotype of the originally stimulated cells. It does not induce an increase of CD191 cells with a more differentiated phenotype. Removal of rIL-7 induces the differentiation to more mature types of B lineage cells.
Discussion
Mouse FLOC were first established by Owen et al. (12) who observed, with time, the development of sIg1B cells in these cultures. In those days, however, few other markers were known which could define the different stages of progenitors, precursors and immature B cells during this development; nor was much attention paid to the numbers of cells that could be generated in vitro, compared with the in vivo devel-opment. It was clear already then that there was a time schedule for B cell development in fetal liver which mimicked the development in vivo. Later, single-cell suspensions of fetal liver at different days of gestation, and of liver shortly after birth, were used to study B cell development (6). Again, a timed schedule of development in vivo could also be seen in vitro and this schedule appeared to involve the majority of all B lineage cells synchronously at a given time and stage. It was also evident from the single-cell cultures that the ~1000-fold expansion in the number of B lineage cells observed in vivo between day 14 and birth could not be generated in vitro. This had probably several reasons. For one, fetal liver stroma and, hence, the total size of the organ grows with time of gestation in vivo, but not in vitro. For another, it became apparent from studies of the continuous B cell generation in bone marrow that there was a proliferative expansion of precursor B cells at the transit from DHJH-rearranged,
c-kit1 pre-B-I cells to VHDHJH-rearranged, µH chain1, pre-B
receptor-expressing, c-kit– CD251 pre-B-II cells that could
not be seen in in vitro differentiating cultures of single pre-B-I cells from either fetal liver or bone marrow (14).
Our experiments with FLOC, aided by the experience with many B lineage-related markers, and by a better defined quantitation of the different compartments in B cell develop-ment of fetal liver and bone marrow, clearly show that FLOC allow the proper proliferative expansion of the pre-B-II
com-partments. It is rewarding to see the defects of the RAG-2T and λ5T mice in this proliferative expansion to be faithfully
retained in these FLOC. The phenotype and subpopulation distribution of cells from FLOC was similar to that seen in livers freshly isolated from age-matched animals (i.e. new born livers), thus arguing in favor of differentiation of B cells in FLOC and not outgrowth of rare subpopulations. More importantly, when the number of recovered B cells was determined, values obtained for the different subpopulations of developing B cells in FLOC were close to those obtained in vivo (Fig. 2A and B). It should be mentioned that cell recovery in FTOC (between 105and 53105cells/lobe) is often
far inferior to that of the age-matched in vivo newborn mouse thymus which can contain up to 53106cells (40,41). There-fore, the FLOC system, as presented herein, faithfully repro-duces normal B cell development seen in vivo. The advantage of the FLOC system is that, with an intact microenvironment, B lymphopoiesis can be manipulated by added reagents, i.e. cytokines and antibodies individually or in combinations (Fig. 8). We have used two such molecules to document this usefulness.
One is the cytokine IL-7. It expands by proliferation pro/ pre-B-I cells of normal, RAG-2T and λ5T FLOC at molar concentrations, where it will do the same in single-cell cultures with the support of a stromal cell layer. Since the development of more mature stages of B lineage cells does not occur in IL-7-stimulated FLOC of normal mice, it appears likely that further differentiation is, in fact, inhibited by IL-7 (5). This is in line with observations made in single-cell suspension cultures, in which IL-7 arrests pre-B-I cells to enter further differentiation, i.e. VHDHJHand VLJLrearrangements, as well as apoptosis (42).
The other is an antibody specific for theαchain of the IL-7R. It is capable of competing with the action of endogenously produced IL-7, inhibiting B lymphopoiesis in FLOC of normal mice. Hence, not only IL-7 with a mol. wt of 15 kDa, but also antibodies with 10-fold that mol. wt are capable of reaching their targets in the FLOC. On a molar ratio, .100 mAb molecules are needed in the same volume of FLOC to compete fully with the action of one IL-7 molecule, as the relief of the mAb-mediated inhibition of B lymphopoiesis by exogenously added IL-7 shows. This result in vitro should be compared with in vivo experiments where multiple injections of milligram quantities of the same anti-IL-7R mAb were required to inhibit B cell development either in normal adult mice or developing embryos whose mothers were injected with antibody (18). Given the prolonged half-life of rat Ig molecules in mice, we estimate that in these in vivo experi-ments, mAb concentrations were well in excess of those used herein. In experiments where IL-7 was infused (17), or anti-IL-7 mAb administered (43), it is difficult to estimate the final ligand concentration reached. Importantly, however, our in vitro experiments (Fig. 8) demonstrate that IL-7, or IL-7-like molecules whose effects are dependent upon interaction with the IL-7R, such as thymic stromal lymphopoietin (44), are playing a role in fetal liver B lymphopoiesis. Hence, while the effectiveness of diffusion of some molecules may not yet be optimal, our experiments nevertheless document that FLOC should be the system of choice to study the influence of
molecules on B cell development from the earliest pro/pre-B-I to immature (and maybe, mature) B cells.
The phenotypic and functional analyses reported herein demonstrate the undoubted capacity of the fetal liver to be a primary lymphoid organ. Recently developed molecular and cellular techniques can be applied to the FLOC system to address important issues regarding B lymphopoiesis. The microenvironment required for efficient B lymphopoiesis is present in FLOC and can be manipulated. This could be directly studied using immuno-histochemical and in situ hybridization approaches. The system could allow a rapid screening procedure for compounds affecting B lymphopo-iesis. As shown by results with the anti-IL-7R mAb, this could result in short-circuiting gene knockout approaches. Importantly, as has been amply demonstrated for T cells in the FTOC system (45), using currently available Ig and antigen transgenic mice, the mechanisms of positive and negative selection for B cells could also be studied. Finally, the full potential of this system will be achieved when reconstitution experiments are performed using combinations of geno-typically distinguishable precursor and stromal elements.
Acknowledgements
The Basel Institute was founded and is supported by F. Hoffmann-La Roche Ltd, Basel, Switzerland. R. C. thanks INSERM for support. J. A. is partly supported by The Swedish Medical Research Council. We thank Ms Andrea Groenewegen and Ms Nadja Straube for excellent technical assistance, Ernst Wagner and Werner Metzger for animal breeding, and Marc Dessing for expert cell sorting. We also thank Dr F. Alt for the RAG-2T mice, Dr S. Nishikawa for the anti-IL-7R mAb and Dr T. Winkler for the IL-7-producing J558/L transfectant. Drs H. J. Fehling, W. Hein, K. Karjalainen and H.-R. Rodewald are gratefully acknowledged for useful comments and discussion following their critical reading of this manuscript.
Abbreviations
FTOC fetal thymic organ culture FLOC fetal liver organ culture IL-7R interleukin-7 receptor LPS lipopolysaccharide LDA limiting dilution analysis
PE phycoerythrin
References
1 Melchers, F., Rolink, A., Grawunder, U., Winkler, T. H., Karasuyama, H., Ghia, P. and Andersson, J. 1995. Positive and negative selection events during B lymphopoiesis. Curr. Opin. Immunol. 7:214.
2 Ehlich, A., Matin, V., Mu¨ller, W. and Rajewsky, K. 1994. Analysis of the B-cell progenitor compartment at the level of single cells. Curr. Biol. 4:573.
3 Ten Boekel, E., Melchers, F. and Rolink, A. 1995. The status of Ig loci rearrangements in single cells from different stages of B cell development. Int. Immunol. 7:1013.
4 Grawunder, U., Rolink, A. and Melchers, F. 1995. Induction of sterile transcription from the κL chain gene locus in V(D)J-recombinase deficient progenitor B cells. Int. Immunol. 7:1915. 5 Rolink, A., Kudo, A., Karasuyama, H., Kikuchi, Y. and Melchers,
F. 1991. Long-term proliferating early pre-B cell lines and clones with the potential to develop to surface-Ig positive mitogen-reactive B cells ‘in vitro’ and ‘in vivo’. EMBO J. 10:327.
6 Melchers, F. 1977. B lymphocyte development in fetal liver. I.
Development of reactivities to B cell mitogens ‘in vivo’ and ‘in vitro’. Eur. J. Immunol. 7:476.
7 Dexter, T. M., Allen, T. D. and Lajtha, L. G. 1977. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J. Cell. Physiol. 91:335.
8 Cumano, A., Dieterlen-Lievre, F. and Godin, I. 1996. Lymphoid potential, probed before circulation in the mouse, is restricted to caudal intraembryonic splanchnopleura. Cell 86:907.
9 Nishikawa, S., Ogawa, M., Nishikawa, S., Kunisada, T. and Kodama, H. 1988. B lymphopoiesis on stromal cell clone: stromal cell clones acting on different stages of B cell differentiation. Eur. J. Immunol. 18:1767.
10 Whitlock, C. A. and Witte, O. N. 1982. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc. Natl Acad. Sci. USA 79:3608.
11 Kincade, P. W., Medina, K., Pietrangeli, C. E., Hayashi, S. I. and Namen, A. E. 1991. Stromal cell lines which support lymphocyte growth. II. Characteristics of a suppressive subclone. Adv. Exp. Med. Biol. 292:227.
12 Owen, J. J. T., Cooper, M. D. and Raff, M. C. 1974. In vitro generation of B lymphocytes in mouse fetal liver, a mammalian ‘bursa equivalent’. Nature 249:361.
13 Rolink, A. G., Melchers, F. and Andersson, J. 1996. The SCID but not the RAG-2 gene product is required for Sµ–Sε-heavy chain class switching. Immunity 5:433.
14 Rolink, A., Karasuyama, H., Grawunder, U., Haasner, D., Kudo, A. and Melchers, F. 1993. B cell development in mice with a defectiveλ5gene. Eur. J. Immunol. 23:1284.
15 Kitamura, D., Kudo, A., Schaal, S., Muller, W., Melchers, F. and Rajewsky, K. 1992. A critical role of lambda 5 protein in B cell development. Cell 69:823.
16 Grabstein, K. H., Waldschmidt, T. J., Finkelman, F. D., Hess, B. W., Alpert, A. R., Boiani, N. E., Namen, A. E. and Morrissey, P. J. 1993. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J. Exp. Med. 178:257. 17 Morrissey, P. J., Conlon, P., Charrier, K., Braddy, S., Alpert, A.,
Williams, D., Namen, A. E. and Mochizuki, D. 1991. Administration of IL-7 to normal mice stimulates B-lymphopoiesis and peripheral lymphadenopathy. J. Immunol. 147:561.
18 Sudo, T., Nishikawa, S., Ohno, N., Akiyama, N., Tamakoshi, M., Yoshida, H. and Nishikawa, S. 1993. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl Acad. Sci. USA 90:9125.
19 Shinkai, Y., Rathbun, G., Lam, K. P., Oltz, E. M., Stewart, V., Mendelsohn, M., Charron, J., Datta, M., Young, F., Stall, A. M. and Alt, F. W. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855. 20 Traunecker, A., Dolder, B. and Karjalainen, K. 1986. A novel
approach for preparing anti-T cell receptor constant region antibodies. Eur. J. Immunol. 16:851.
21 Ogawa, M., Matzusaki, Y., Nishikawa, S., Hayashi, S. I., Kunisada, T., Sudo, T., Kina, T., Nakauchi, H. and Nishikawa, S. I. 1991. Expression and function of c-kit in hemopoietic progenitor cells. J. Exp. Med. 174:63.
22 Krop, I., de Fougerolles, A. R., Hardy, R. R., Allison, M., Schlissel, M. S. and Fearon, D. T. 1996. Self-renewal of B-1 lymphocytes is dependent on CD19. Eur. J. Immunol. 26:238.
23 Rolink, A., Ten Boekel, E., Melchers, F., Fearon, D. T., Krop, I. and Andersson, J. 1996. A sub-population of B2201 cells in murine bone marrow does not express CD19 and contains NK cell-progenitors. J. Exp. Med. 183:187.
24 Leptin, M. 1985. Monoclonal antibodies specific for murine IgM. II. Activation of B lymphocytes by monoclonal antibodies specific for the four constant domains of IgM. Eur. J. Immunol. 15:131. 25 Parkhouse, R. M. E., Preece, G., Sutton, R., Cordell, J. L. and
Mason, D. Y. 1992. Relative expression of surface IgM, IgD and the Ig-associatingα(mb-1) andβ(B-29) polypeptide chains. Immunology 76:535.
26 Yelton, D. E., Desaymard, C. and Scharff, M. D. 1981. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma 1:5.
27 Bhattacharya, A., Dorf, M. E. and Springer, T. A. 1981. A shared alloantigenic determinant on Ia antigens encoded by the I-A
and I-E subregions: evidence for I region gene duplication. J. Immunol. 127:2488.
28 Andersson, J., Melchers, F. and Rolink, A. 1995. Stimulation by T cell independent antigens can relieve the arrest of differentiation of immature auto-reactive B cells in the bone marrow. Scand. J. Immunol. 42:21.
29 Andersson, J., Coutinho, A., Lernhardt, W. and Melchers, F. 1977. Clonal growth and maturation to immunoglobulin secretion in vitro of every growth-inducible B lymphocyte. Cell 10:27.
30 Grandien, A., Coutinho, A. and Anderson, J. 1990. Selective peripheral expansion and activation of B cells expressing endogeneous immunoglobulin in mu-transgenic mice. Eur. J. Immunol. 20:991.
31 Jenkinson, E. J., Anderson, G. and Owen, J. J. T. 1992. Studies on T-cell maturation on defined thymic stromal cell populations in vitro. J. Exp. Med. 176:845.
32 Tokunaga, K., Taniguchi, H., Yoda, K., Shimizu, M. and Sakiyama, S. 1986. Nucleotide sequence of a full-length cDNA for mouse cytoskeletalβ-actin mRNA. Nucleic Acids Res. 14:2829. 33 Koiwai, O., Yokota, T., Kageyama, T., Hirose, T., Yoshida, S. and
Arai, K. 1986. Isolation and characterization of bovine and mouse terminal deoxynucleotidyltransferase cDNAs expressible in mammalian cells. Nucleic Acids Res. 14:5777.
34 Namen, A. E., Lupton, S., Hjerrild, K., Wignall, J., Mochizuki, D. Y., Schmierer, A., Mosley, B., March, C. J., Urdal, D. and Gillis, S. 1988. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature 333:571.
35 Mertsching, E., Burdet, C. and Ceredig, R. 1995. IL-7 transgenic mice: analysis of the role of IL-7 in the differentiation of thymocytes in vivo and in vitro. Int. Immunol. 7:401.
36 Rolink, A., Haasner, D., Nishikawa, S. I. and Melchers, F. 1993.
Changes in frequencies of clonable pre-B cells during life in different lymphoid organs of mice. Blood 81:2290.
37 Gregoire, K. E., Goldschneider, I., Barton, R. W. and Bollum, F. J. 1979. Ontogeny of terminal deoxynucleotidyl-transferase positive cells in lymphohemopoietic tissues of rat and mouse. J. Immunol. 123:1347.
38 Feeney, A. 1990. Lack of N regions in fetal and neonatal mouse immunoglobulin V–D–J junctional sequences. J. Exp. Med. 172:1377.
39 Rothenberg, E. and Triglia, D. 1983. Clonal proliferation unlinked to terminal deoxynucliotidyl transferase synthesis in thymocytes of young mice. J. Immunol. 130:1627.
40 Ceredig, R., MacDonald, H. R. and Jenkinson, E. J. 1983. Flow microfluorometric analysis of mouse thymus development in vivo and in vitro. Eur. J. Immunol. 13:185.
41 Ceredig, R. 1988. Differentiation potential of 14-day fetal mouse thymocytes in organ culture: Analysis of CD4/CD8-defined single positive and double negative cells. J. Immunol. 141:355. 42 Rolink, A., Grawunder, U., Haasner, D., Strasser, A. and Melchers,
F. 1993. Immature surface Ig1B cells can continue to rearrange
κandλL chain gene loci. J. Exp. Med. 178:1263.
43 Bhatia, S. K., Tygrett, L. T., Hogge, D. E., Fatur-Saunders, D., Takei, F. and Humphries, R. K. 1995. The effect of in vivo IL-7 deprivation on T cell maturation. J. Exp. Med. 181:1399. 44 Ray, R. J., Furlonger, C., Williams, D. E. and Paige, C. J. 1996.
Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur. J. Immunol. 26:10. 45 Andersson, G., Owen, J. J. T., Moore, N. C. and Jenkins, E.
J. 1994. Thymic epithelial cells provide unique signals for positive selection of CD41CD81 thymocytes in vitro. J. Exp. Med. 179:2027.