CLINICAL ARTICLE - BRAIN INJURY
Validation of a new neurological score (FOUR Score)
in the assessment of neurosurgical patients with severely
impaired consciousness
Bixia Chen&Christoph Grothe&Karl Schaller
Received: 7 April 2013 / Accepted: 17 August 2013 / Published online: 8 September 2013 # Springer-Verlag Wien 2013
Abstract
Background The Glasgow coma scale (GCS) was introduced as a scoring system for patients with impaired consciousness after traumatic brain injury (TBI). Since, it has become the worldwide standard in TBI assessment. The GCS has repeat-edly been criticized for its several failures to reflect verbal reaction in intubated patients, and to test brain stem reflexes. Recently, the full outline of unresponsiveness (FOUR) score was introduced, which is composed of four clinically distinct categories of evaluation: eye reaction, motor function, brainstem reflexes and respiratory pattern. This study aims to validate the FOUR score in neurosurgical patients. Methods FOUR score and GCS were assessed in a consecu-tive series of neurosurgical patients with severely impaired consciousness (GCS<9). Their correlation with the 30-day Glasgow outcome score (GOS) was compared. Patients ad-mitted for TBI, spontaneous intracranial hemorrhage (intrace-rebral hemorrhage, aneurysmal subarachnoid hemorrhage,
cerebellar hemorrhage), or malignant middle cerebral artery infarction were included.
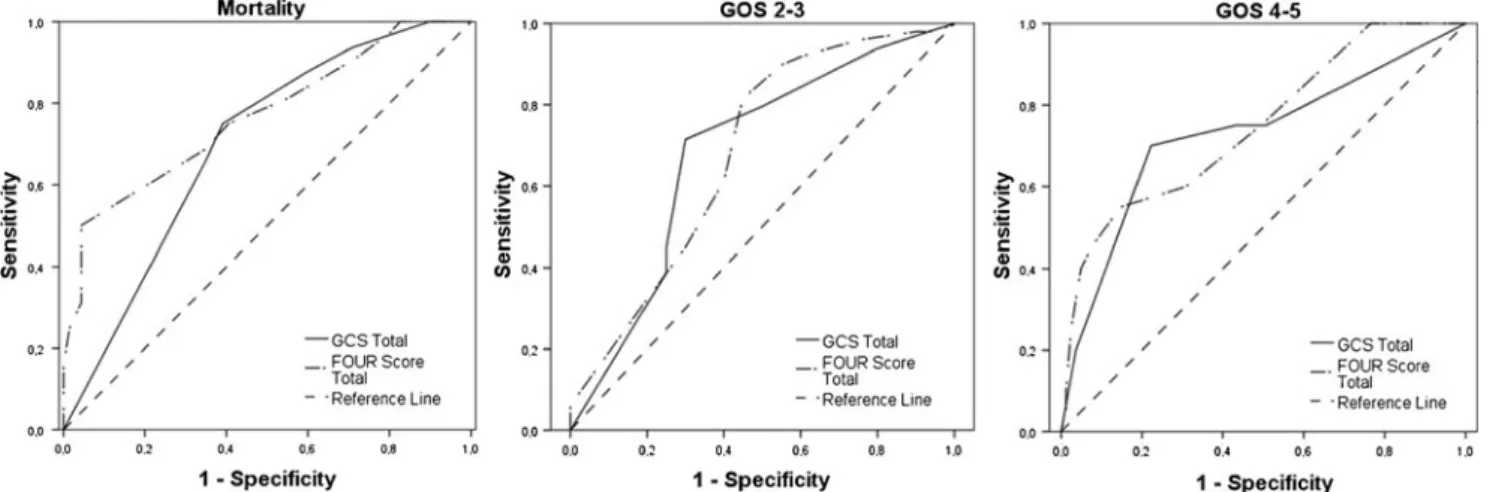
Results We assessed a total of 101 patients (mean age=64y, SD=36.1y). The area under the curve (AUC) for mortality was 0.768 (P =0.0001) for the FOUR Score, and 0.699 (P = 0.001) for the GCS. For poor outcome (GOS=2-3) the FOUR score AUC was 0.683 (P =0.018), the GCS AUC was 0.682 (P =0.019). The FOUR score value for favorable outcome (GOS=4-5) was 0.748 (P =0.001), the corresponding GCS value was 0.704 (P =0.002).
Conclusions The FOUR score was more robust than the GCS in predicting mortality after 30 days in neurosurgical patients with severely impaired consciousness. There was no relevant difference in predicting poor and good outcome.
Keywords Assessment . Coma . Consciousness . FOUR score . Glasgow coma scale . Outcome
Introduction
Structured assessment of coma and impaired consciousness is essential in monitoring critically ill neurosurgical patients. For this purpose the Glasgow coma scale (GCS) is the most commonly used grading scheme [25]. The GCS was original developed for patients with TBI, but has been validated for outcome prediction in several other causes of coma [3,8,13,
18, 21], and evolved into a general tool for assessment of unconsciousness. There are, however, difficulties regarding its qualified application, especially in deeply unconscious pa-tients, and with implementation by inexperienced users [20], e.g., a noticeable number of such patients are intubated or suffer from additional facial trauma. In these cases the verbal score can only be guessed [17], thus rendering a reliable evaluation impossible [10].
Portions of this study were presented at the 58th Annual Meeting of the German Society of Neurosurgery, Leipzig, Germany, April 27, 2007. B. Chen
:
C. Grothe:
K. SchallerDepartment of Neurosurgery, University of Bonn Medical Center, Bonn, Germany
B. Chen
Department of Neurosurgery, University of Duisburg-Essen Medical Center, Essen, Germany
K. Schaller
Department of Neurosurgery, Geneva University Medical Center and Faculty of Medicine, Geneva, Switzerland
K. Schaller (*)
Department of Neurosurgery, University Medical Center, Faculty of Medicine, Rue Gabrielle-Perret-Gentil 4, 1211 Geneva 14, Switzerland
In addition, although the relevance of brainstem functions for the clinical prognosis is high, it is not taken into account by the GCS [6,11,26]. Furthermore, breathing patterns, which are also not part of the GCS, may be related to the depth of coma [19]. Thus, subtle neurological changes may be overlooked by the GCS in its current form.
Altogether, the shortcomings of the Glasgow coma scale have led to repeated suggestions and conceptions of modifi-cations [5], and of alternative coma scores. Because of lack of reliability or complicated handling, these scores were not widely accepted (e.g. Edinburgh-2 coma sScale [24], reaction level scale [23], comprehensive level of consciousness scale [22], Innsbruck coma scale [4])
The ease of use of the GCS may also have prevented other, possibly more detailed scales from introduction into daily routine.
Recently, the full outline of unresponsiveness (FOUR) score [28] was introduced by Wijdicks and colleagues in neurological patients with traumatic and nontraumatic disorders of the cen-tral nervous system and miscellaneous acute neurological con-ditions. This scale is supposed to take into account various levels of brain stem damage, and should thus allow for more precise clinical prognosis concerning patient outcome.
In the present study, we aimed at a validation of this new score in the assessment of newly admitted adult neurosurgical ICU-patients with severely impaired consciousness due to TBI, to spontaneous intracranial hemorrhage, or to malignant middle cerebral artery infarction.
Materials and methods
This consecutive prospective study involves 101 of initially 110 assessed patients from May 2006 until July 2007 who were all admitted to the neurological intensive care unit (ICU) of the University Medical Center department of neurosurgery, Bonn, Germany. This study follows the declaration of Helsin-ki as revised in Edinburgh in October 2000.
Newly admitted neurosurgical ICU patients older than 17 years with severely impaired consciousness defined by a GCS score under 9 were included. Nine patients in which the GOS after 30 days could not be determined by direct exami-nation or telephone interview were subsequently excluded from the study.
Clinical assessment
In all patients the Glasgow coma scale score and the full outline of unresponsiveness (FOUR) score (Table 1) were assessed on the day of admission by a board certified neurol-ogist (C. G.). The outcome was determined using the Glasgow outcome score (GOS)[14] at 30 days as part of the regular clinical outcome assessment.
The full outline of unresponsiveness (FOUR) score
The full outline of unresponsiveness score consists of four components: motor response, eye response, brain stem reflexes, and respiration. In every item the minimal score is zero, while the maximal score to reach is four. Thus, the total score ranges
Table 1 Comparison of the full outline of unresponsiveness score [28]a and the Glasgow coma scale [25]
Parameter Score
Full outline of unresponsiveness score Eye response (E)
Eyelids open or opened, tracking or blinking to command E4 Eyelids open but not tracking E3 Eyelids closed but open to loud voice E2 Eyelids closed but open to pain E1 Eyelids remain closed with pain E0 Motor response (M)
Thumbs-up, fist or peace sign M4
Localizing to pain M3
Flexion response to pain M2 Extension response to pain M1 No response to pain or generalized myoclonus status M0 Brainstem reflexes (B)
pupil and corneal reflexes present B4 one pupil wide and fixed B3 pupil or corneal reflexes absent B2 pupil and corneal reflexes absent B1 Absent pupil, corneal, and cough reflex B0 Respiration (R)
Not intubated, regular breathing patterns R4 Not intubated, Cheyne–Stokes breathing pattern R3 Not intubated, irregular breathing R2 Breathes above ventilator rate R1 Breathes at ventilator rate or apnea R0 Glasgow coma scale
Eye opening
Spontaneous eye opening 4
Eye opening response to speech 3 Eye opening response to pain 2
No eye opening 1
Motor response
Obeying commands 6
Localizing response to pain 5
Withdrawal from pain 4
Flexor response to pain 3
Extensor posturing to pain 2 No motor response to pain 1 Verbal response
from 0 as the worst to 16 as the best reaction. By suggestive naming the authors of the paper on the FOUR score aimed for the memorability of the new score. The FOUR score includes a motor and an eye movement category resembling the GCS sections. It does not contain the verbal reaction. Additionally, brainstem reflexes and breathing patterns are tested. The goal in this change was to withdraw information that does not increase the correlation with outcome and adding information which does. Altogether, adding selected information can lead to a better performance as well as leaving out selected information. The assessment of the motor and the eye responses is similar to the Glasgow coma scale, though with some excep-tions. The motor response category additionally contains the hand position test to detect subtle changes in alertness [29]. It also includes myoclonus, which is known to be associated with poor outcome [30]. Withdrawal and flexion response to pain are combined, as they may be mistaken or confused by inexperienced users.
Moreover, a locked-in syndrome can be detected, when a patient with opened eyes is not able to track the examiner’s finger [15].
Brain stem functions, such as the pupillary light reflex, the corneal reflex, and the cough reflex are tested, which refer to the function of mesencephalon, pons, medulla oblongata, and the oculomotor nerve, respectively.
The respiratory observations include Cheyne–Stokes res-piration, which is found to be in correlation to an intermediate prognosis, while other irregular breathing patterns and spon-taneous hyperventilation are associated with a poor prognosis [2, 19]. Another potential advantage is that alteration of breathing patterns can show indication for mechanical venti-lation [16]. In intubated patients, breathing over and breathing on ventilator rate or apnea are distinguished.
Statistical analysis
For every possible cut-off point, sensitivity and 1-specificity (false positive rate) were calculated. Out of these data sets, the coordinates of receiver operating characteristic curves (ROC) were generated. Accuracy of the scales was quantified by the area under the ROC curve (AUC). An AUC of 1.0 refers to a perfect test, while a perfectly inaccurate test has an AUC of 0.0. An area of 0.5 represents the chance diagonal, consequently a worthless test.
The relation of the FOUR score and the GCS score to mortality (GOS 1), poor outcome (GOS 2–3), and favorable outcome (GOS 4–5) at 30 days was estimated. We performed all data analysis with PASW Statistics, Version 18.0 for Win-dows (SPSS Inc., Chicago, Illinois, USA).
Results
Patient characteristics
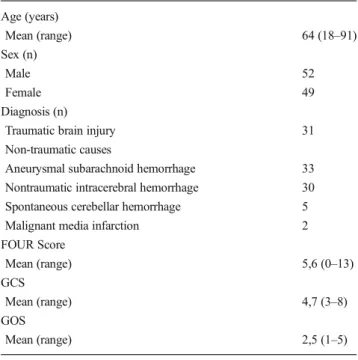
The 101 included patients suffered from traumatic brain injury (TBI) (n =31) and from non-traumatic brain damage (hyperten-sive intracerebral hemorrhage (ICH) (n =30), aneurysmal sub-arachnoid hemorrhage (SAH) (n =33), spontaneous cerebellar hemorrhage (n =5), and malignant middle cerebral artery in-farction (n =2)). Assessed FOUR scores ranged from 0 to 13, GCS scores had a range from 3 to 8 (Table2); 91 % (n =91) of the patients were intubated and sedated.
Scores and outcome
At 30 days, 32 (31.7 %) patients were deceased (GOS 1), 49 (48.5 %) had a poor outcome (GOS 2–3), and 19 patients (18.8 %) had a favorable outcome (GOS 4–5). Figure1shows the frequencies of the assessed total scores and subunit scores. The correlation between FOUR score, GCS score and the patient outcome at 30 days is displayed in Table3. The FOUR score had a slightly higher area under the curve (AUC) in predicting mortality with 0.768 (p <0.001; 95 % CI: 0.664–
Table 1 (continued) Parameter Score Confused conversation 4 Inappropriate speech 3 Incomprehensible speech 2 No verbal response 1
aFor the assessment of the eye reaction (E) make three attempts to figure
out the best reaction. A score of E4 accords to at least three tracking reactions with open or opened eyes (in patients who are not able to open their eyes independently e.g. because of facial oedema or trauma) or alternatively to two blinking reactions on command. E3 typifies open eyes without tracking. Eye opening to voice is rated with E2, to a pain stimulus with E1. E0 is assessed if the patient shows no eye opening upon a pain stimulus
The motor response (M) score is taken from the best reaction of the upper limbs. M4 requires the performance of at least one of the three hand positions from the hand position test [29] - thumbs up, fist and the victory sign. Whether the patient is not able to act on an instruction, the reaction to pain should be examined by setting a stimulus on the temporomandib-ular or supraorbital nerve. A localizing reaction M3 is rated if the patient touches the examiner’s hand whilst the stimulation. Flexion comes up to M2, an extension response to M1, no response to pain accords to M0 Within the category brain stem functions (B), the pupil and the corneal reflexes are tested; the best response is valued. The cough reflex is only examined in the case that both are absent. When pupil and corneal reflexes can be triggered on both sides, the reaction is rated with B4. Patients with one wide and fixed pupil receive B3 points. It is distinguished between absent pupil or corneal reflexes (B2) and the absence of both (B1). If in addition the cough reflex is missing, B0 is given
For respiration (R), the patient’s breathing pattern is observed. Breathing self-dependently in a normal pattern corresponds with R4. Cheyne– Stokes (R3) and other irregular breathing patterns (R2) are differentiated. Intubated patients are assessed with R1 for breathing over ventilator rate or R0 for breathing on ventilator rate or apnea
0.872) compared to GCS AUC of 0.699 (p =0.001; 95 % CI: 0.595–0.802). AUC for poor outcome (GOS 2–3) was lower and equal for both scales, 0.682 (p =0.019; 95 % CI: 0.531– 0.832) for FOUR score, and 0.683 (p =0.018; 95 % CI: 0.533– 0.832) for GCS. Both scales also had comparable results in predicting favorable outcome (GOS 4–5). AUC was 0.748 (p =0.001: 95 % CI: 0.624–0.871) for FOUR Score and 0.727 (p =0.002; 95 % CI: 0.588–0.865) for GCS (Fig.2).
In the subunits, the verbal response of the GCS showed the lowest correlation to outcome in this group of mostly intubated patients. The AUC for respiration patterns of the FOUR score was lower than the AUC of the total GCS and the AUC of the total FOUR score. The AUC for FOUR score brain stem reflexes exceeded the total GCS in predicting mortality, but not in predicting poor outcome and favorable outcome.
The maximum sum of sensitivity and specificity for mor-tality was at a total FOUR score of 4 (sensitivity=0.500; specificity=0.957) and a total GCS score of 5 (sensitivity= 0.750; specificity=0.609). In this collective of patients, no mortality at 30 days was observed at FOUR Scores>9 and GCS scores>7.
Although, the differences in AUC values did not reach statistical significance, it should be noted that the difference
Table 2 Characteristics of 101 patients with severely impaired consciousness Age (years) Mean (range) 64 (18–91) Sex (n) Male 52 Female 49 Diagnosis (n)
Traumatic brain injury 31 Non-traumatic causes
Aneurysmal subarachnoid hemorrhage 33 Nontraumatic intracerebral hemorrhage 30 Spontaneous cerebellar hemorrhage 5 Malignant media infarction 2 FOUR Score Mean (range) 5,6 (0–13) GCS Mean (range) 4,7 (3–8) GOS Mean (range) 2,5 (1–5)
in predictive power between GCS and FOUR score is partic-ularly larger in patients with very low scores. The rate of mortality in patients with the lowest FOUR score was higher than in patients with the lowest GCS score. There was a range of FOUR scores from 0 to 6 for patients with GCS scores of 3, thus indicating higher capacity for differentiation in investi-gation of this subgroup.
Discussion
The FOUR score proved to be a fast and reliable method for the examination of our cohort of patients suffering from severely impaired consciousness, regardless the origin of their stupor, or coma, respectively. Its capacity for the prediction of mortality it was superior to the GCS. Although the Glasgow coma scale is used worldwide for standardized evaluation of unconsciousness, major deficiencies remain. A number of attempts have been made to improve the predictive power regarding the outcome. Many of the developed scoring sys-tems were more complicated, and could not be established in
clinical routine. Nevertheless, the“gold standard” role of the GCS remains disputable.
The authors of the FOUR score have developed a new scale to overcome the shortcomings of the Glasgow coma scale. At the same time, it encompasses the minimal requirements for neurological testing in cases of impaired consciousness [28]. The FOUR Score does not contain a verbal score but provides testing of brain stem reflexes and assessment of respiratory patterns. The difference entails testability in intubated patients in contrast to the GCS, which is especially advantageous for the intensive care unit.
We have found that the FOUR Score is easily implementable in a neurosurgical setting. The assessment can be undertaken within few minutes and is practicable in daily routine.
Prediction of outcome
Total scores of FOUR score and GCS showed similar corre-lations in predicting poor outcome and favorable outcome. In prognosticating mortality, the FOUR Score exceeded the pre-dictive power of the GCS.
Table 3 Correlation between initial score and outcome: area under the receiver operating characteristic curve
Mortality GOS 1 (95 % CI)
Poor outcome GOS 2–3 (95 % CI)
Favorable outcome GOS 4–5 (95 % CI)
FOUR score total 0.768 (0.664–0.872) 0.682 (0.531–0.832) 0.748 (0.624–0.871) FOUR Eye 0.590 (0.477–0.704) 0.558 (0.405–0.711) 0.588 (0.441–0.735) FOUR Motor 0.661 (0.552–0.770) 0.689 (0.542–0.837) 0.722 (0.585–0.860) FOUR Brainstem 0.743 (0.628–0.858) 0.526 (0.378–0.674) 0.619 (0.496–0.741) FOUR Respiration 0.576 (0.460–0.693) 0.634 (0.487–0.790) 0.648 (0.498–0.797) GCS total 0.699 (0.595–0.802) 0.683 (0.533–0.832) 0.727 (0.588–0.865) GCS Eye 0.611 (0.499–0.722) 0.547 (0.395–0.700) 0.587 (0.441–0.733) GCS Verbal 0.492 (0.369–0.614) 0.525 (0.371–0.679) 0.519 (0.374–0.664) GCS Motor 0.677 (0.571–0.782) 0.709 (0.563–0.855) 0.744 (0.608–0.881)
The verbal subscore of the GCS correlated least with good outcome, poor outcome as well as mortality, consistent with a rate of 90 % intubated patients and with the fact that verbal reaction cannot be evaluated in such a group of patients.
Appraisal of brain stem reflexes leads to more detailed information and is part of standardized evaluation of the neurological state in patients with impaired consciousness. In our collective, it allowed especially for further discrimina-tion of the relatively large patient group with GCS 3, who had FOUR Scores from 0 to 6, facilitating analysis of the time course of clinical evolution. Accordingly, the brain stem re-flexes subscore had the biggest AUC in predicting mortality.
Because most of our patients (90 %) were intubated at the time of assessment, the correlation of respiration patterns and outcome was narrowed. Capturing changes of respiratory patterns is not only an expedient way of predicting outcome [2,16], but it may also give evidence upon which therapeutic decisions may be based.
Clinical practice
We consider that the FOUR Score is a useful instrument for predicting outcome and for neurological screening in the neu-rosurgical ICU. The FOUR Score captures essential aspects of the neurological status. For making optimal decisions and predicting outcomes as precisely as possible, further techniques apart from pure clinical assessment should be included [27]. Studies on classification of TBI based on magnetic resonance imaging have shown significant correlation between the iden-tified locations of lesions and outcome of patients [12]. More-over, evoked potentials especially somatosensory evoked po-tentials correlate with neurological outcome: E.g. bilateral ab-sent somatosensory potentials correlate strongly with mortality [9, 32], and can be an additional tool for evaluation of unresponsive patients. Notwithstanding, one of the main objec-tives remains to appraise outcome based on an initial score.
Limitations of the study
As we had only one rater, we did not analyze interobserver reliability in neurosurgical staff, which has been reported to be comparable with the Glasgow coma scale’s [1,7]. In inexpe-rienced neurological users, an even better reliability was found [31].
The comprehensive relevance of evaluation of breathing parameters could not be satisfactorily tested in this study due to the fact that most patients were intubated at admission.
We focused on the initial hospital stay of our patient pop-ulation and assessed outcome at 30 days. For further valida-tion, studies on the long term outcome should follow. This is important insofar a high proportion of such patients may undergo rehabilitation and develop further secondary compli-cations affecting long-term clinical outcome.
Outcome was assessed according to the GOS and not to a more detailed scale. However, the GOS is an established and well validated scoring system, which provides the possibility of standardized discrimination between good and poor out-come and mortality, which we found was detailed enough for validating a coma score in deeply unconscious patients with-out providing much unused information.
Conclusions
The FOUR score is straightforward to apply and, at the same time, more refined in assessment of patients with severely im-paired consciousness. In this study, the predictive value of the FOUR score concerning 30-day mortality was slightly higher than that of the GCS. This needs to be corroborated, however, in larger studies on distinct pathologies requiring neurocritical care. It is more accurate for the evaluation of patients with very low GCS scores. The FOUR score also offers a wider range of scale points in patients of whom the verbal part of the GCS cannot be performed. This facilitates assessment of clinical evolution. In prediction of poor outcome and favorable outcome after 30 days, both scales showed similar performance.
Acknowledgments Thanks to Asita Sarrafzadeh-Khorrassani and Bawarjan Schatlo for their expertise and effort.
Conflicts of interest None.
References
1. Akavipat P (2009) Endorsement of the FOUR score for consciousness assessment in neurosurgical patients. Neurol Med Chir 49:565–571 2. Alexandre A, Colombo F, Curri D, Franciosi A, Volpin L (1982)
Breathing alterations in head injured patients. J Neurosurg Sci 26: 187–191
3. Benesch CG, McDaniel KD, Cox C, Hamill RW (1993) End-stage Alzheimer’s disease. Glasgow Coma Scale and the neurologic exam-ination. Arch Neurol 50:1309–1315
4. Benzer A, Mitterschiffthaler G, Marosi M, Luef G, Puhringer F, De La Renotiere K, Lehner H, Schmutzhard E (1991) Prediction of non-survival after trauma: Innsbruck Coma Scale. Lancet 338:977–978 5. Born JD (1988) The Glasgow-Liege Scale. Prognostic value and
evolution of motor response and brain stem reflexes after severe head injury. Acta Neurochir 91:1–11
6. Born JD, Albert A, Hans P, Bonnal J (1985) Relative prognostic value of best motor response and brain stem reflexes in patients with severe head injury. Neurosurgery 16:595–601
7. Bruno MA, Ledoux D, Lambermont B, Damas F, Schnakers C, Vanhaudenhuyse A, Gosseries O, Laureys S (2011) Comparison of the full outline of unresponsiveness and Glasgow Liege Scale/Glasgow Coma Scale in an intensive care unit population. Neurocrit Care 15: 447–453
8. Chan B, Gaudry P, Grattan-Smith TM, McNeil R (1993) The use of Glasgow Coma Scale in poisoning. J Emerg Med 11:579–582 9. de Sousa LC, Colli BO, Piza MR, da Costa SS, Ferez M, Lavrador M
with a score of 3 on the Glasgow Coma Scale. Otol Neurotol 28:426– 428
10. Diringer MN, Edwards DF (1997) Does modification of the Innsbruck and the Glasgow Coma Scales improve their ability to predict functional outcome? Arch Neurol 54:606–611
11. Firsching R, Woischneck D, Klein S, Ludwig K, Dohring W (2002) Brain stem lesions after head injury. Neurol Res 24:145–146 12. Firsching R, Woischneck D, Klein S, Reissberg S, Dohring W, Peters
B (2001) Classification of severe head injury based on magnetic resonance imaging. Acta Neurochir 143:263–271
13. Hirai S, Ono J, Yamaura A (1996) Clinical grading and outcome after early surgery in aneurysmal subarachnoid hemorrhage. Neurosurgery 39:441–446, discussion 446–447
14. Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484
15. Laureys S, Pellas F, Van Eeckhout P, Ghorbel S, Schnakers C, Perrin F, Berre J, Faymonville ME, Pantke KH, Damas F, Lamy M, Moonen G, Goldman S (2005) The locked-in syndrome : what is it like to be conscious but paralyzed and voiceless? Prog Brain Res 150:495–511 16. Mascia L, Zavala E, Bosma K, Pasero D, Decaroli D, Andrews P, Isnardi D, Davi A, Arguis MJ, Berardino M, Ducati A (2007) High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med 35:1815–1820
17. Meredith W, Rutledge R, Fakhry SM, Emery S, Kromhout-Schiro S (1998) The conundrum of the Glasgow Coma Scale in intubated patients: a linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J Trauma 44:839–844 18. Parry-Jones AR, Abid KA, Di Napoli M, Smith CJ, Vail A, Patel HC,
King AT, Tyrrell PJ (2013) Accuracy and Clinical Usefulness of Intracerebral Hemorrhage Grading Scores: A Direct Comparison in a UK Population. Stroke 44:1840–1845
19. Rout MW, Lane DJ, Wollner L (1971) Prognosis in acute cerebro-vascular accidents in relation to respiratory pattern and blood gas tensions. Br Med J 3:7–9
20. Rowley G, Fielding K (1991) Reliability and accuracy of the Glasgow Coma Scale with experienced and inexperienced users. Lancet 337:535–538
21. Schefold JC, Storm C, Kruger A, Ploner CJ, Hasper D (2009) The Glasgow Coma Score is a predictor of good outcome in cardiac arrest patients treated with therapeutic hypothermia. Resuscitation 80:658– 661
22. Stanczak DE, White JG 3rd, Gouview WD, Moehle KA, Daniel M, Novack T, Long CJ (1984) Assessment of level of consciousness following severe neurological insult. A comparison of the psycho-metric qualities of the Glasgow Coma Scale and the Comprehensive Level of Consciousness Scale. J Neurosurg 60:955–960
23. Starmark JE, Stalhammar D, Holmgren E, Rosander B (1988) A comparison of the Glasgow Coma Scale and the Reaction Level Scale (RLS85). J Neurosurg 69:699–706
24. Sugiura K, Muraoka K, Chishiki T, Baba M (1983) The Edinburgh-2 coma scale: a new scale for assessing impaired consciousness. Neurosurgery 12:411–415
25. Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2:81–84
26. Tien HC, Cunha JR, Wu SN, Chughtai T, Tremblay LN, Brenneman FD, Rizoli SB (2006) Do trauma patients with a Glasgow Coma Scale score of 3 and bilateral fixed and dilated pupils have any chance of survival? J Trauma 60:274–278
27. Wijdicks EF (2010) The bare essentials: coma. Pract Neurol 10:51–60 28. Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL (2005) Validation of a new coma scale: The FOUR score. Ann Neurol 58:585–593
29. Wijdicks EF, Kokmen E, O’Brien PC (1998) Measurement of im-paired consciousness in the neurological intensive care unit: a new test. J Neurol Neurosurg Psychiatry 64:117–119
30. Wijdicks EF, Parisi JE, Sharbrough FW (1994) Prognostic value of myoclonus status in comatose survivors of cardiac arrest. Ann Neurol 35:239–243
31. Wolf CA, Wijdicks EF, Bamlet WR, McClelland RL (2007) Further validation of the FOUR score coma scale by intensive care nurses. Mayo Clin Proc 82:435–438
32. Zentner J, Rohde V (1994) SEP and MEP in comatose patients. Neurol Res 16:89–92
Comment
Chen and coworkers provide a prospective consecutive single center study comparing the FOUR (full outline of unresponsiveness) score with the GCS (Glasgow coma scale) score in 101 neurosurgical patients with severely impaired consciousness. They conclude that the FOUR score is more robust in predicting mortality after 30 days. No relevant difference was found in predicting poor and good outcome.
In my opinion, this is an interesting paper with relevant results. However, there are also some aspects of this paper which have especially to be considered:
The GSC was originally developed for patients with traumatic brain injury. Within the last years, however, also the outcome of other causes of coma has been assessed using the GSC—as the authors describe in this paper. This is important not only because of the different causes of coma included in the present paper, but also in daily practice of critical care.
Another aspect is that in the present paper outcome was assessed at 30 days. One has to keep in mind that—especially in traumatic brain injury patients—progresses sometimes will be seen only within 6 months.
Marcus Reinges Giessen, Germany
![Table 1 Comparison of the full outline of unresponsiveness score [28] a and the Glasgow coma scale [25]](https://thumb-eu.123doks.com/thumbv2/123doknet/14840011.624537/2.892.456.811.271.1097/table-comparison-outline-unresponsiveness-score-glasgow-coma-scale.webp)