Review Article
Importance of the right ventricle in valvular heart disease
E. Nagel, M. Stuber* and O. M. Hess
Cardiology, University Hospital, Zurich, Switzerland and *Institute of Biomedical Engineering, Federal Institute of Technology, Zurich, Switzerland
The importance of the right ventricle as a determinant of clinical symptoms, exercise capacity, peri-operative survival and postoperative outcome has been underestimated for a long time. Right ventricular ejection fraction has been used as a measure of right ventricular function but has been found to be dependent on loading conditions, ventricular interaction as well as on myocardial structure. Altered left ventricular function in patients with valvular disease influences right ventricular performance mainly by changes in afterload but also by ventricular interaction. Right ventricular function and regional wall motion can be determined with right ventricular angiography, radio-nuclide ventriculography, two-dimensional
echocardiogra-phy or magnetic resonance imaging. However, the complex structure of the right ventricle and its pronounced trans-lational movements render quantification difficult. True regional wall motion analysis is, however, possible with myocardial tagging based on magnetic resonance tech-niques. With this technique a baso-apical shear motion of the right ventricle was observed which was enhanced in patients with aortic stenosis.
(Eur Heart J 1996; 17: 829-836)
Key Words: Right ventricle, ventricular function, valve disease, pulmonary artery pressure, ventricular interaction, postoperative changes, myocardial tagging.
Introduction
The importance of right ventricular function has been underestimated in the past, especially its role as a determinant of cardiac symptoms, exercise tolerance and survival in patients with valvular disease of the left heart. The pump function of the right ventricle has been thought not to be relevant for the overall function of the heart'1'21 and to improve if the cause for its dysfunction
is removed'3'. However, our understanding of the role of
the right ventricle in the maintenance of normal cardiac function has changed dramatically. It has been shown that right ventricular function is a major determinant of cardiac symptoms and exercise capacity in chronic heart failure'4'51. An increase in pulmonary wedge pressure as
a result of mitral or aortic valve disease is associated with a rise in mean pulmonary artery pressure. Thus, right ventricular afterload increases, as dilatation of the right ventricle develops with a resultant drop in the right
Submitted for publication 24 July 1995, and accepted 7 August 1995.
With support from the Swiss Cardiology Foundation.
Correspondence: Otto M. Hess, MD, Cardiology, University
Hospital, Ramistr. 100, 8091 Zurich, Switzerland.
ventricular ejection fraction. As a consequence, the tricuspid valve annulus dilates and may induce tricuspid regurgitation'6' with secondary right ventricular volume
overload.
Right heart failure is mainly a clinical diag-nosis and, thus, exact quantification is difficult. Early detection of right ventricular dysfunction is important and depends largely on the imaging technologies. Due to the complex structure and asymmetrical shape of the right ventricle, assessment of right ventricular function is often problematic. Echocardiography relies on geometrical assumptions or a three-dimensional analysis of the right ventricle which is not always sufficiently visualized'71. Radionuclide angiography
re-quires injection of radioactive markers and has a low spatial resolution. This technique suffers from attenu-ation artifacts and differentiattenu-ation between the right ventricle and right atrium may be difficult'8"101.
Con-trast angiography is invasive and requires conCon-trast injection in potentially haemodynamically unstable patients. Newer methods such as spiral computed tomography or magnetic resonance imaging1"1 allow
better analysis of right ventricular function than other imaging techniques, but they are not yet avail-able in all institutions. Most data on right ventricular function stem from clinical studies performed with
radionuclide angiography'12'131 or right ventricular
angiography*14'151.
Right ventricular ejection performance
in relation to pulmonary
haemodynamics
Right ventricular ejection fraction is dependent on right ventricular afterload and, thus, on left ventricular or left atrial filling pressures'16"191. An increase in left
ventricu-lar afterload is compensated for by an increase in mass to reduce left ventricular fibre stress. The right ventricle, however, is more sensitive to changes in load which is probably due to the smaller muscle mass and, thus, higher wall stress of a given load. Thus, a decrease in right ventricular ejection fraction with an increase in pulmonary artery pressure has been reported in patients with valvular or coronary artery disease1201 as well as
in patients with chronic obstructive pulmonary dis-ease121'221. A rapid improvement or normalisation of
right ventricular ejection fraction has been found after reduction of pulmonary artery pressure with nitro-glycerin'161, single lung transplantation'2'1 or left heart
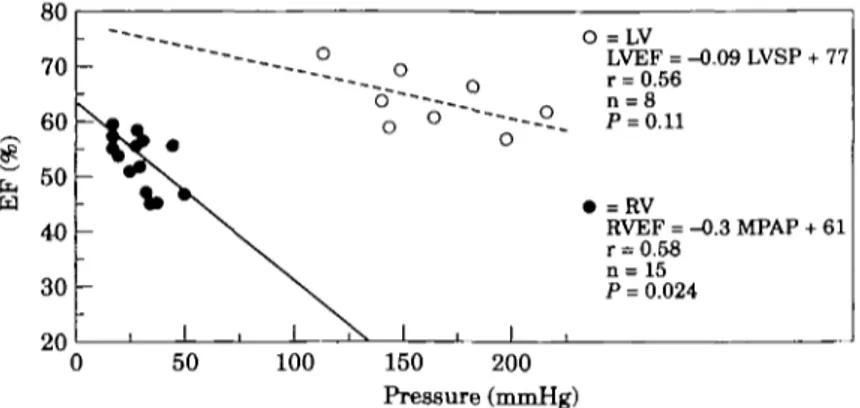
valve replacement. Right ventricular ejection fraction as a function of mean pulmonary arterial pressure has been plotted from several different studies (Fig. 1) and a close relationship between these two parameters documenting the load-sensitivity of the right ventricle has been observed. The steeper slope between mean pulmonary arterial pressure and right ventricular ejection fraction when compared to the slope between left ventricular systolic pressure and left ventricular ejection fraction (Fig. 2) demonstrates that the left ventricle can tolerate a higher load than the right. Right ventricular ejection fraction is reduced by approximately 10% (55 to 45%), when right ventricular afterload is doubled from 25 to 50 mmHg. However, a doubling of left ventricular after-load from 125 to 250 mmHg leads to a similar reduction in left ventricular ejection fraction from 70 to 60%.
The close correlation between right ventricular ejection fraction and mean pulmonary arterial pressure for patients with cardiac valve diseases indicates that right ventricular afterload rather than contractility is the major determinant of right ventricular ejection fraction. However, some studies in patients with chronic right ventricular pressure overload showed a reduced systolic ejection performance with a downward shift of the relationship between right ventricular ejection fraction and mean pulmonary arterial pressure below the 95% confidence limit (Fig. 1). The assessment of right ven-tricular contractility is difficult and has been attempted by right ventricular dp/dt, right ventricular end-systolic pressure/volume relationship, right ventricular maximal elastance or mean normalized systolic ejection rate'23'24'.
However, none of these parameters is very sensitive and, thus, quantification of right ventricular contractile function remains difficult.
In most patients with aortic valve disease, right ventricular ejection fraction is maintained, whereas it
70 60 50 40 30 20 Valvular disease y = -0.7 + 55 r = 070 P = 0.001 Controls • y = -0.7x + 63 10 15 20 25 30 35 40 45 MPAP (mmHg)
Figure 1 Relationship between radionuclide right
ven-tricular ejection fraction (EFRV) and mean pulmonary artery pressure (MPAP). Data are taken from the litera-ture. Control data (hatched area) are from two studies representing individual values of 18 patients'17'18'. The data points of the patients with valvular disease represent one study eachi1819-21-23-26-2^31". The solid line shows the correlation for patients with various valvular heart dis-ease; the dashed line shows the correlation for controls. • = mitral regurgitation; T = aortic regurgitation; 0 = mitral stenosis; O = mitral stenosis after replacement or valvuloplasty; +=controls; x = mitral regurgitation and aortic regurgitation.
is typically reduced in patients with mitral valve disease'19'25"291 (Fig. 3). Mitral valve disease generally
influences the right ventricle more than aortic valve disease'30'311. Left atrial volume overload in mitral
insuf-ficiency and pressure overload in mitral stenosis may cause an increase in pulmonary vascular resistance with an increase in the afterload of the right ventricle (Table 1, Fig. 4) and, thus, a decrease in right ventricular ejection fraction (Fig. 3). In contrast, left ventricular function is usually normal or even enhanced in mitral regurgitation due to low impedance leak'321. A mild
decrease of left ventricular function represents an early sign of myocardial dysfunction with a sudden worsening after valve replacement'331, whereas a decrease in right
ventricular ejection fraction usually represents a state of pressure overload hypertrophy.
Pulmonary artery pressure often decreases im-mediately after mitral valve surgery (Fig. 4) with a normalization of pulmonary pressure within 6 months. It may, however, stay elevated for more than 2 years in patients with severe pulmonary hypertension before operation (Fig. 5)'341. This can be explained by the
structural changes of the pulmonary vessels which may return only slowly after operation or may be irrevers-ible1351. A normalization of right ventricular ejection
fraction, concurrent with a normalization of pulmonary artery pressure, has been reported in most patients after mitral valve operation'28'29'. However, in some cases
right ventricular ejection fraction does not normalize but remains depressed. This is explained by the occurrence of myocardial dysfunction due to rheumatic heart dis-ease1361 or high wall stress, secondary to ventricular
dilatation'251. The extent of right ventricular dilatation
ou 70 60 50 40 30 on
-- *X
_
1 - - - 0 --- o - - - 0Oo °
-\ \ \ 1 X 1 O =LV LVEF = -0.09 LVSP + 77 r = 0.56 n = 8 ~~--- P - 0 11 • =RV RVEF = -0.3 MPAP + 61 r = 0.58 n = 15 P = 0.024 1 0 50 100 150 200 Pressure (mmHg)Figure 2 Relationship between angiographic RV ( • ) ejection fraction
(EFRV) and mean pulmonary artery pressure (MPAP) as well as left ventricular ( O ) ejection fraction and peak systolic pressure (LVSP). The slope of the regression line is steeper for the right ventricle than for the left ventricle, indicating a higher load-sensitivity of the right ventricle. Data are taken from the literature; each data point represents one different study116'17-30-37-38-40'411.
controls pre post pre post pre post pre MS MR AS AE
Figure 3 Mean radionuclide right ventricular ejection
fraction (EFRV) in pre- and postoperative patients with valvular disease of the left heartf12-17-19-21-26-28-31-39'. Please note that the right ventricular ejection fraction is abnormal in patients with mitral stenosis or regurgitation probably because of altered after-load condition, whereas right ventricular function is normal in aortic valve disease.
which no normalization of right ventricular ejection fraction can be achieved is, however, not known.
Pulmonary haemodynamics are usually normal, or only slightly altered, in aortic valve disease1341.
Equally symptomatic patients with aortic insufficiency have lower pulmonary artery pressures'271 and better
right ventricular ejection fraction than those with mitral valve disease1261 (Fig. 3). Similarly, the size of the right
coronary artery was normal in severe aortic valve dis-ease, whereas it was significantly increased in patients with mitral valve disease when compared to controls, indicating that secondary pulmonary hypertension is associated with right ventricular hypertrophy and en-largement of the right coronary artery137'381. Although
pulmonary haemodynamics are only slightly altered in patients with aortic valve disease, an increase of right ventricular ejection fraction and a reduction of
pulmonary blood volume and pulmonary capillary wedge pressure was found immediately after aortic valve replacement'391.
Right ventricular ejection performance
in chronic volume overload of the right
ventricle
Some authors have distinguished between patients with right ventricular pressure overload with and with-out tricuspid regurgitation'40""*21 and found higher mean
pulmonary artery pressures and lower right ventricular ejection fractions in those with tricuspid regurgi-tation'401, although there is a low impedance leak with
regurgitation into the right atrium. In some patients functional tricuspid regurgitation will normalize after left heart valve replacement when the right ventricle gets smaller and the tricuspid annulus shrinks to its normal size after correction or right ventricular pressure overload'3'431, whereas in others it may not'44'451. These
patients with significant tricuspid regurgitation after valvular surgery have a poor prognosis whether operated on or not'461. The severity of tricuspid
insuffi-ciency beyond which no normalization of right ventricu-lar dimensions will occur remains, however, unclear. The gold standard for the assessment of tricuspid insuf-ficiency is right ventricular angiography, but there are several drawbacks — such as catheter-induced extra-systoles, poor filling of the right ventricle and the impossibility of exactly quantifying the severity of the lesion'47'481. Newer, non-invasive methods, such as
con-trast echocardiography'49"5'1, colour-coded Doppler
echocardiography1521 or magnetic resonance imaging1531
allow semiquantitative evaluation of tricuspid insuf-ficiency, although exact quantification is also not possible. Doppler echocardiography allows us to detect minimal, so-called 'physiological' tricuspid regurgi-tation in almost all patients, especially the young154"56'.
Table 1 Data from the literature on mean pulmonary artery pressure (MPAP) pre- and postoperative right ventricular ejection fraction (R VEF) and left ventricular ejection fraction (L VEF) as well as NYHA classification
Author Morrison'171 Grose""! Nienaber"81 Unterberg14" Hirata"91 Mayer168' Iskandrian1251 Morrison'311 Unterberg1411 Niles126' Vassalli'38' Iskandrian'251 Cohen'28' Unterberg1411 Hirata"9' Burger129' Mayer168' Mornson'3 1' Unterberg1301 Niles1261 Villari1371 Trikas1271 Harpole1391 Villari1371 Year 1983 1983 1987 1989 1992 1994 1984 1985 1989 1990 1993 1984 1985 1989 1992 1993 1994 1985 1987 1990 1992 1994 1990 1992 Vulvc lesionT LX1 ¥ w i v v l V l l Controls Controls Controls Controls Controls Controls MR MR MR MR MR MS MS MS MS MS* MS* AR AR AR AR AR AS AS 9 8 10 18 9 10 21 12 26 18 10 22 8 35 11 11 15 9 23 15 7 19 11 8 Tech R A R A R A R R A R A R R A R T A R A R A R R A MPAP 15 ± 3 15±2 14±4 15±7 12±2 32 ±10 31 ± 10 23 ± 9 30 ± 12 25 ± 7 40 ±20 26 ± 7 25 ± 9 17±3 32 30 ± 11 19±8 18 ± 7 24 ± 12 21 ± 8 32 ± 2 20 ± 6 Pre-operative RVEF 55 ± 5 55 ± 4 52 ± 8 58 ± 7 45 ± 5 — 23 ± 10 39 ±10 51 ± 11 38 ± 9 — 29 ±12 32 ± 6 56± 13 39 ± 4 40 — 44±9 54±9 43 ± 8 — 54± 13 — LVEF 70 ± 9 71 ±8 66± 14 62 ± 7 63 ± 4 57 ± 15 49 ± 18 63 ±17 52 ± 10 62 ± 9 49 ± 14 48 ± 8 62 ±12 48 ± 10 — 56 ± 7 52 ± 11 57 ± 13 45 ± 12 59 ±8 — 52 ± 16 55 ±13 NYHA — — — — 2-4 — >2 2-4 — 2-8 — 2-4 2-3 2-1 1-8 — £ 2 1-8 2-5 3-4 1-7 Postoperative MPAP _ — — — — — — 16±3 — — 22 23 ± 10 — — 17±5 — 21 ± 6 RVEF — — — — — — — — — 37 ± 9 — 46±6 45 — — — — 58 ±8 —
n = Number of patients; Tech = technique; MR = mitral regurgitation; MS = mitral stenosis; AR = aortic regurgitation; AS = aortic stenosis; * = mitral valvuloplasty; A = angiography; R = radionuclide ventriculography; T=thermodilution.
However, this regurgitation has no haemodynamic effects and thus no clinical consequences.
Right/left ventricular interaction
Left-to-right interaction
Apart from the influences of the left ventricle on the right ventricle via the pulmonary circulation, the left ventricle acts also on right ventricular function through the interventricular septum'57"591. In the experimental
animal, the exclusion of the right ventricle leads to an elevation of systemic venous pressure, a decline in pulmonary artery pressure, a reduction in cardiac output and a drop in arterial pressure'60'6''. However, scarring
of the right ventricular myocardium by cauterization'11
or the exchange of the right ventricle with a non-contractile pericardial patch'621 is associated with
reasonable right ventricular ejection fraction due to the function of the septum — which is able to maintain cardiac output as long as the right ventricle is not dilated. In a study of Hoffman et al.l62], left ventricular
contraction contributed 24% of its own stroke work to the generation of right ventricular stroke work via the interventricular septum. In pulmonary hypertension this contribution increased to 35%. A negative linear
correlation between right ventricular size and left or right ventricular stroke work was demonstrated. Thus, even though left-to-right ventricular interaction may compensate for a reduction in right ventricular function, in the presence of right ventricular pressure or volume overload it will not suffice to maintain adequate cardiac output without a sufficiently contracting right ventricle.
Right-to-left ventricular interaction
The filling state of the right ventricle influences the motion of the interventricular septum and thus, left ventricular performance'63641. Right ventricular volume
overload with dilatation of the right ventricle and right atrium causes an increase in intrapericardial pressure ( = pericardial constraint), reducing venous return, cardiac output and, thus, left ventricular func-tion'651. These effects can be significantly diminished by
opening or removing the pericardium. A recent study using magnetic resonance tagging in patients with right ventricular hypertrophy has shown an increased curva-ture of the septum and a decreased septal shortening1661.
Ventricular interaction through the interventricular sep-tum was reported to be less important than interaction through the pulmonary circulation but appeared to be of great importance for the balance of the right and left
controls pre post AR
Figure 4 Mean pulmonary artery pressure (MPAP) of
controls, patients with mitral regurgitation (MR), mitral stenosis (MS), aortic stenosis (AS) and aortic regurgi-tation (AR). Each data point represents one single study!16-19-21-25"30-37-38-68!. Pre- (pre) and post-operative (post) values are plotted; however, post-operative studies are rare and, thus, data points are limited. Most patients show normalization of MPAP after correction of the valvular lesion, except for mitral stenosis (see below).
(10) 213 (0.69) (0 69) n = 210 n - 207 n = 183 I I Pre-op 3 6 12 Months postop. 24 60
Figure 5 Mean pulmonary artery pressure early and late
after valve replacement in patients with increased pul-monary vascular resistance, before operation'34'. Please note that normalization of pulmonary artery pressure takes years after correction of the valvular lesion. Five years after operation of mean pulmonary artery pressure remained slightly elevated in these patients. (Published with the permission of the editors and authors of reference 34.)
the loss of right ventricular/left ventricular interaction is the mobilization of the subvalvular apparatus with an increased regional wall motion of the posterior wall.
Thus, right-to-left or left-to-right ventricular interaction is mediated through three mechanisms: (1) pulmonary circulation with changes in right ventricular loading conditions, (2) geometry and motion of the interventricular septum (e.g. by common myocardial fibres), and (3) pericardia! constraint.
Right ventricular regional wall motion
Regional wall motion of the right ventricle has been assessed with various techniques, including right ventricular angjography120-691, two-dimensional
echo-cardiography and radionuclide ventriculography as well as newer methods such as conventional magnetic resonance imaging and myocardial tagging. Most of these techniques have given conflicting results due to the complex geometry of the right ventricle and the pronounced translational movements of the heart.
Myocardial tagging170"721 allows the non-invasive
labelling of specific myocardial regions and, in contrast to most other techniques, the assessment of true regional function of the right ventricle'731. Conventional
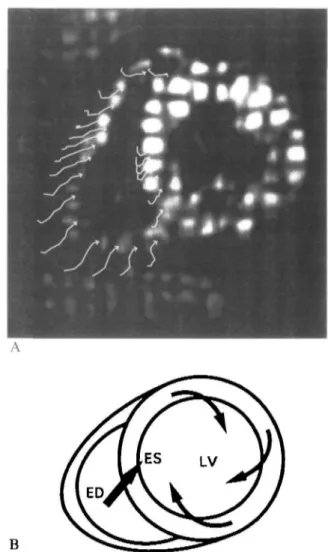
par-ameters for the description of regional right ventricular wall motion, such as radial shortening and regional area reduction, are dependent on the shape and motion of the right ventricle. Consequently, these parameters vary between different regions and planes. Regional circumferential shortening, which is dependent on the local shortening of the myocardium, is, however, more uniformly distributed in the different regions with a coefficient of variance which is significantly (P<00\) smaller than for radial shortening or regional area reduction. Using myocardial tagging, a baso-apical shear motion of the right ventricle was observed which is the result of the the 'wringing motion' of the left ventricle1731. This 'wringing motion' is characterized by a
clockwise rotation of the left ventricle at the base and a counterclockwise rotation at the apex which pulls the right ventricle in these directions through a network of common myocardial fibres between the two ventricles (Fig. 6). This baso-apical shear motion is significantly increased in patients with aortic stenosis when com-pared to controls (Fig. 7), probably as a result of the previously described left-to-right ventricular interaction.
cardiac output'631. An increase in right ventricular
vol-ume will affect not only left ventricular systolic function, but also left ventricular diastolic properties. In patients with pulmonary hypertension an abnormal septal motion and an increased chamber stiffness of the left ventricle has been reported'671. Right-to-left ventricular
interaction with a correlation between end-diastolic fill-ing pressure of the right and left ventricle (r=0-71) was observed in patients with mitral stenosis which was lost after mitral valvuloplasty1681. A possible explanation for
Importance of the right ventricle as a
prognostic factor in valve surgery
The importance of the right ventricle for peri-operative morbidity and mortality was recognized several years ago. Thus, the right ventricle plays an important role not only for survival but also for the postoperative course and functional recovery of the patient with valve disease of the left heart.
B
Figure 6 (A) Magnetic resonance myocardial tagging in a
volunteer. The right ventricle is shown in a basal short axis-view at endsystole. The arrows indicate the regional wall motion calculated from the intersection points of the rectangular grid. (B) In a schematic drawing the motion pattern of the left and right ventricle at the base is depicted and indicates the left ventricular clockwise rotation which pulls the right ventricle in this direction causing a baso-apical shear motion. ED = end-diastole; ES=endsystole.
Mitral valve disease
Generally, the prognosis of patients with mitral valve disease and depressed right ventricular function im-proves significantly after successful mitral valve replace-ment or repair1'9'361. However, according to Pinzani et
al.ll4\ in the presence of right ventricular failure,
mor-tality increases peri-operatively from 5 to 11% (/><002)
and, during follow-up, from 8 to 22% (P<00005). Hirata et al.[l9] reported, in patients with a pre-operative
right ventricular ejection fraction of less than 40%, no normalization of right ventricular ejection fraction after valve surgery. The persistence of symptoms after oper-ation was higher in patients with a pre-operative right ventricular ejection fraction of less than 30% compared to those with a right ventricular ejection fraction of more than 30%'36]. If right heart failure persisted after
'=P< 0.05
api equ bas api equ Controls AS
Figure 7 Shear angles of the centre of gravity at the
apex, equator and base. The baso-apicai shear motion is more pronounced in the hypertrophied than in the normal ventricle. AS = aortic stenosis; api = apical; equ = equatorial; bas=basal.
valve replacement, patients had a higher 5-year mor-tality (39%) than patients without right heart failure (4%; /><00001) after surgery1741.
A small number of patients developed right heart failure early after mitral valve surgery. These patients had a poor prognosis, with a mortality of 72% within 75 months of operation'741. The aetiology of
peri-operative right heart failure is multifactorial, and the pathophysiology is not yet fully understood.
Groves and coworkers[75] reported a significantly
lower exercise duration, a decreased maximal oxygen consumption and a lower anaerobic threshold in patients with than without tricuspid regurgitation late after successful mitral valve replacement. The presence of tricuspid insufficiency can explain the incomplete recovery from valvular surgery or the return of clinical symptoms in patients without prosthetic valve failure, persisting pulmonary hypertension or congestive heart failure. In the present study it was not possible to differentiate between (1) right ventricular pump failure with secondary tricuspid regurgitation and (2) tricuspid regurgitation with secondary right ventricular failure as the precipitating cause.
Aortic valve disease
Survival and postoperative symptoms in patients with aortic regurgitation are usually better than they are in mitral regurgitation'261. Patients with combined mitral
and aortic regurgitation had a poorer right and left ventricular ejection fraction than those with mitral re-gurgitation alone. The postoperative outcome of these patients with combined aortic and mitral regurgitation was less favourable than in those with isolated valvular lesions because four of eight patients died, and three of those surviving suffered asymptomatic deterioration within 72 months. However, these differences did not reach statistical significance owing to the small number of patients.
Conclusions
The assessment of right ventricular function is difficult because of its complex geometry and pronounced trans-lational motions. Several invasive and non-invasive tech-niques have been used for estimating right ventricular function. Right ventricular ejection fraction was found to be dependent on pulmonary circulation with alter-ations in right ventricular loading conditions, right ventricular geometry and motion of the interventricular septum (ventricular interaction) as well as myocardial structure. Pulmonary artery pressure is usually increased in patients with mitral valve disease but only slightly altered in patients with aortic valve disease. Irreversible damage to the right ventricle has been described in patients with advanced mitral valve disease but the point where no normalization after correction of the valve lesion occurs has yet to be determined.
References
[1] Starr I, Jeffers WA, Mead RH. The absence of conspicuous increments in venous pressure after severe damage to the right ventricle of the dog and a discussion of the relation between clinical congestive heart failure and heart disease. Am Heart J
1943; 26: 291-301.
[2] Sade RM, Castaneda AR. The dispensable right ventricle. Surgery 1975; 77: 624-31.
[3] Braunwald NS, Ross J Jr, Morrow AG. Conservative man-agement of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation 1967; 35: 163—9. [4] Engler R, Ray R, Higgins CB. Clinical assessment and follow
up of functional capacity in patients with chronic congestive cardiomyopathy. Am J Cardiol 1982; 49: 1832-7.
[5] Baker BJ, Wilen MM, Boyd CM, Dinh H, Francisa JA
Relation of right ventricular ejection fraction to exercise capacity of patients with chronic left ventricular failure. Am J Cardiol 1984; 54: 596-9.
[6] Ubago JL, Figueroa A, Ochoteco A, Colman T, Duran RM, Duran CG. Analysis of the amount of tricuspid valve annular dilatation required to produce functional tricuspid regurgi-tation. Am J Cardiol 1983; 52: 155-8.
[7] Levine RA, Gibson TC, Aretz T et al. Echocardiographic measurement of right ventricular volume. Circulation 1984; 69: 497-505.
[8] Marving J, Hoilund-Carlsen PF, Chraemmer-Jorgensen B, Gadsbell N. Are right and left ventricular ejection fractions equal?- ejection fractions in normal subjects and in patients with first acute myocardial infarction. Circulation 1985; 72: 502-14
[9] DelPItalia LJ, Starling MR, Walsh RA, Badke FR, Lasher JC, Blumhardt R. Validation of attenuation-corrected equi-librium radionuclide angiogaphic determinations of right ventricular volume: comparison with cast-validated biplane cineventriculography. Circulation 1985; 72: 317-26.
[10] Manno BV, Iskandrian AS, Hakki AH. Right ventricular function: methodologic and clinical condiderations in non-invasive scintigraphic assessment. J Am Coll Cardiol 1984; 3:
1072-81.
[ll]Sechtem U, Pflugfelder PW, Gould RG, Cassidy MM, Higgins CB. Measurement of right and left ventricular vol-umes in healthy individuals with cine MR imaging. Radiology 1987; 167:425-30.
[12] Maddahi J, Berman DS, Matsuoka DT el al. A new technique for assessing right ventricular ejection fraction using rapid multiple gated equilibrium cardiac blood pool scintigraphy. Circulation 1979; 60: 581-9.
[13] Goldberg HL, Herrold EM, Hochreiter C el al. Videodensi-tometric determination of right ventricular and left ventricular ejection fraction. Am J Noninv Cardiol 1987; 1: 18-23. [14] Goerke RJ, Carlsson E. Calculation of right and left
ventricu-lar volumes: method using computer equipment and biplane angiograms Invest Radiol 1967; 2: 360-7.
[15] Lange PE, Onnasch D, Farr FL, Heintzen PH. Angiocardio-graphic right ventricular volume determination. Accuracy, as determined from human casts and clinical applications. Eur J Cardiol 1978; 8: 477-501.
[16] Grose R, Strain J, Yipintosoi T. Right ventricular function in valvular heart disease: relation to pulmonary artery pressure. J Am Coll Cardiol 1983; 2: 225-32.
[17] Morrison DA, Goldman S, Wright AL et al. The effect of pulmonary hypertension on systolic function of the right ventricle. Chest 1983; 84: 250-7.
[18] Nienaber CA, Spielmann RP, Wasmus G, Montz R, Mathey DG, Bleifeld DG. Right ventricular ejection fraction from equilibrium krypton-81 m blood pool scans: a noninvasive predictor of pulmonary artery hypertension. Eur Heart J 1987; 8: 297-307.
[19] Hirata N, Sakakibara T, Shimazaki Y et al. PTeoperative and postoperative right ventricular junction during exercise in patients with mitral stenosis. J Thorac Cardiovasc Surg 1992; 104: 1029-34.
[20] Heywood JT, Grimm J, Hess OM, Jakob M, Krayenbuehl HP Right ventricular systolic function during exercise with and without significant coronary artery disease. Am J Cardiol 1991; 67: 681-6.
[21] Kramer MR, Valantine HA, Marshall SE, Starnes VA, Theodore J. Recovery of the right ventricle after single-lung transplantation in pulmonary hypertension. Am J Cardiol 1994; 73: 494-500.
[22] Lazar JM, Flores AR, Grandis DJ, One JE, Schulman DS Effects of chronic right ventricular pressure overload on left ventricular diastolic function. Am J Cardiol 1993; 72: 1179-82 [23] Finnegan P, Forbes MV, Bishop JM. Evaluation of
pressure-derived indices of right ventricular contractility. Eur J Cardiol 1977,6: 139-155.
[24] Niehues B, Schwanitz V, Hagemann K et al. Die Bedeutung der dp/dtmax fur die Beurteilung der Kontraktilitat des rechten Ventrikels. Z Kardiol 1973; 62: 1029^40.
[25] Iskandnan AS, Hakki A, Ren J et al. Correlation among right ventricular preload, afterload and ejection fraction in mitral valve disease: radionuclide, echocardiographic and hemodynamic evaluation. J Am Coll Cardiol 1984; 3: 1403-11.
[26] Niles N, Borer JS, Kamen M, Hochreiter C, Devereux R, Roman M. Preoperative left and right ventricular perform-ance in combined aortic and mitral regurgitation: comparison with isolated aortic or mitral regurgitation. Am J Cardiol 1990; 65: 1372-8.
[27] Trikas A, Papadopoulos P, Triposkiadis F el al. Factors affecting the postoperative exercise capacity of patients with mitral stenosis and aortic regurgitation. Cardiology 1994; 85: 201-6.
[28] Cohen M, Horowitz SF, Machac J, Mindich BP, Fuster V. Response of the right ventricle to exercise in isolated mitral stenosis. Am J Cardiol 1985; 55: 1054-8.
[29] Burger W, Kneissl GD, Kober G, Schrader R. Effect of balloon valvuloplasty for mitral stenosis on right ventricular function. Am J Cardiol 1993; 71: 994-6.
[30] Unterberg R, Rommich P, Volker W, Mauser M, Karsch KR. EinfluB der Volumenbelastung des linken Ventrikels bei Aorten- und Mitralinsuffizienz auf die Geometrie und Funktion der rechten Kammer. Z Kardiol 1987; 76: 761-9. [31] Morrison DA, Lancaster L, Henry R, Goldman S, Turgeon J.
Right ventricular function at rest and during exercise in aortic and mitral valve disease. J Am Coll Cardiol 1985, 5: 21-8. [32] Wisenbaugh T, Spann JF, Carabello BA. Differences in
myocardial performance and load between patients with similar amounts of chronic aortic versus chronic mitral regurgitation. J Am Coll Cardiol 1984; 3: 916-23.
[33] Conn WJ, Monrad WS, Murakami T, Nonogi H, Hess OM, Kiayenbuehl HP. The relationship of afterload to ejection performance in chronic mitral regurgitation Circulation 1987; 76: 59-67.
[34] Horstkotte D, Niehues R, Schulte HD, Strauer BE. Belast-barkeit nach Herzklappenersatz. Z Kardiol 1994; 83 (Suppl 3): 111-20.
[35] Harris P, Heath D (eds). The human pulmonary circulation. Edinburgh. Churchill Livingstone, 1977.
[36] Borer JS, Hochreiter C, Rosen S. Right ventricular function in severe non-ischaemic mitral insufficiency. Eur Heart J 1991; 12 (Suppl B): 22-5.
[37] Villari B, Hess OM, Meier C et al Regression of coronary artery dimensions after successful aortic valve replacement. Circulation 1992; 85: 972-8.
[38] Vassalli G, Hess OM, Krogmann ON et al. Coronary artery size in mitral regurgitation and its regression after mitral valve surgery Am Heart J 1993; 126: 1091-8.
[39] Harpole DH, Jones RH. Serial assessment of ventricular performance after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 1990; 99: 645-50.
[40] Brilla C, Konz KH, Karsch KR, Seipel L. Right ventricular function in mitraVaortic valve disease with and without tncuspid incompetence. Z Kardiol 1986; 75 (Suppl 2): 114-6 [41] Unterberg R, Konig S, Volker W, Piesch-Breifeld B, Karsch KR. Funktion des rechten Ventrikels bei Patinten mit Mitralvitien. Z Kardiol 1989; 78: 386-93.
[42] Morrison DA, Ovitt T, Hammermeister KE. Functional tricuspid regurgitation and right ventricular dysfunction in pulmonary hypertension. Am J Cardiol 1988; 62: 108-12. [43] Simon R, Oelert H, Borst HG, Lichtlen PR. Influence of
mitral valve surgery on tncuspid incompetence concomitant with mitral valve disease. Circulation 1980, 62. 1152-7. [44] Duran CMG, Pomar JL, Colman T, Figueroa A, Revuelta
JM, Ubago JL. Is tricuspid valve repair necessary? J Thorac Cardiovasc Surg 1980; 80: 849-60.
[45] Breyer RH, McClenathan JH, Michaelis LL, Mclntosch CL, Morrow AG. Tricuspid regurgitation. A comparison of nonoperative management, tricuspid annuloplasty and tri-cuspid valve replacement. J Thorac Cardiovasc Surg 1976; 72: 867-74.
[46] King RM, Schaff HV, Danielson GK el al Surgery for tricuspid regurgitation late after mitral valve replacement. Circulation 1984; 70 (Suppl I): 1193-7.
[47] Cairns KB, KJoster FE, Bristow JD, Lees MH, Griswold HE. Problems in the hemodynamic diagnosis of tricuspid insufficiency. Am Heart J 1968; 75: 173-5.
[48] Stewart D, Leman RB, Kaiser J, Mann DL. Catheter-induced tricuspid regurgitation: incidence and clinical significance. Chest 1991; 99: 651-5.
[49] Curtius JM, Thyssen M, Breuer HM, Loogen F. Doppler versus contrast echocardiography for diagnosis of tricuspid regurgitation. Am J Cardiol 1985; 56; 333-6.
[50] Jaksch R, Karsch KR, Seipel L. Accuracy in detection and quantification of tricuspid regurgitation by contrast and Doppler echocardiography. Z Kardiol 1986; 75 (Suppl 2): 33-4.
[51] Ruffmann K, Hess OM, Krayenbuhl HP. Diagnostik der Trikuspidalinsuffizienz anhand von klinischen Zeichen und Kontrast-Echokardiographie. Schweiz Med Wschr 1983; 113: 1999-2003.
[52] Suzuki Y, Kambara H, Kadota K et al. Detection and evaluation of tricuspid regurgitation using a real-time two-dimensional color-coded Doppler flow imaging system: comparison with contrast two-dimensional echocardiography and right ventriculography. Am J Cardiol 1986; 57: 811-5. [53] Nagel E, Jungehiilsing M, Smolarz K et al. Diagnose und
Quantifizierung der Trikuspidalinsuffizienz mit dynamischer Magnetresonanztomographie: Vergleich mit rechtsven-trikularer Angiographie. Z Kardiol 1991; 80 561-8.
[54] Berger M, Hecht SR, van Tosh A, Lingam U. Pulsed and continuous wave Doppler echocardiogaphic assessment of valvular regurgitation in normal subects. J Am Coll Cardiol 1989; 13: 1540-5.
[55] Kostucki W, Vandenbossche JL, Friart A, Englert M. Pulsed Doppler regurgitant flow patterns of normal valves. Am J Card 1986; 58. 309-13.
[56] Yoshida K, Yoshikawa J, Shakudo M et al Color Doppler evaluation of valvular regurgitation in normal subjects. Circulation 1988; 78: 840-7.
[57] Elzinga G, van Grondelle R, Westerhof N, van den Bos GC. Ventricular interference. Am J Physiol 1974; 226: 941-7. [58] Feneley MP, Gavaghan TP, Baron DW, J A. B, Roy PR,
Morgan JJ. Contribution of left ventncular contraction to the generation of right ventricular systolic pressure in the human heart. Circulation 1985, 71: 473-80.
[59] Weber KT, Janicki JS, Shroff S, Fishman AP. Contractile mechanics and interaction of the right and left ventncles Am J Cardiol 1981; 47. 686-92.
[60] Rose JC, Cosimano SJJ, Hufnagel CA, Massullo EA. The effects of exclusion of the right ventricle from the circulation in dogs. J Clin Invest 1955; 34: 1625.
[61] Furey SAI, Zieske HA, Levy MN. The essential function of the right ventricle. Am Heart J 1984; 107: 404.
[62] Hoffman D, Sisto D, Frater RWM, Nikolic SD. Left-to-right ventricular interaction with a noncontracting right ventricle. J Thorac Cardiovasc Surg 1994; 107: 1496-502.
[63] Slinker BK, Glantz SA. End-systolic and end-diastolic ventncular interaction. Am J Physiol 1986; 251: H1062-75. [64] Weyman AE, Wann S, Feigenbaum H, Dillon JC. Mechanism
of abnormal septal motion in patients with right ventncular volume overload: a cross-sectional echocardiographic study. Circulation 1976; 54: 179-86.
[65] Hess OM, Bhargava V, Ross J Jr, Shabetai R. The role of the pericardium in interactions between the cardiac chambers. Am Heart J 1983; 106: 1377-83.
[66] Dong SJ, Crawley AP, MacGregor JH et al. Regional left ventncular systolic function in relation to the cavity geometry in patients with chronic right ventricular pressure overload. Circulation 1995; 91: 2359-70.
[67] Krayenbuehl HP, Turina J, Hess OM. Left ventricular func-tion in chronic pulmonary hypertension. Am J Cardiol 1978; 41: 1150.
[68] Mayer I, Jakob M, Suetsch G, Lattmann J, Vassalli G, Hess OM. Reversal of increased diastolic stiffness in mitral stenosis after successful valvuloplasty. Circulation 1994; 90 (Suppl 2): 1-483 (Abstr.).
[69] Unterberg R, Plesak L, Voelker W, Karsch KR. Quantitative segmentale Wandfunktionsanalyse der rechten Kammer bei Herzgesunden. Z Kardiol 1988; 77: 120-24.
[70] Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology 1989; 171: 841-5. [71] Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP.
Human heart: tagging with MR imaging: a method for noninvasive assessment of myocardial motion. Radiology
1988; 169: 59-63.
[72] Fischer SE, McKinnon GC, Maier SE, Boesiger P. Improved myocardial tagging contrast. Mag Resonance Med 1993; 30: 191-200.
[73] Nagel E, Stuber M, Fischer SE, Boesiger P, Simon R, Hess OM. Assessment of nght ventricular motion with magnetic resonance tissue tagging and slice following technique. J Nuclear Cardiology 1995; 2 (Suppl): 42 (Abstr).
[74] Pinzani A, de Gevigney G, Pinzani V, Milon H, Delahaye JP. L'insuffisance cardiaque droite pre- et postoperatoire des mitraux et mitro-aortiques. Arch Mai Coeur 1993; 86: 27-34. [75] Groves PH, Lewis NP, Ikram S, Maire R, Hall RJC. Reduced exercise capacity in patients with tricuspid regurgitation after successful mitral valve replacement for rheumatic mitral valve disease. Br Heart J 1991; 66: 295-301.