Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Research Paper (National Research Council of Canada. Division of Building
Research); no. DBR-RP-547, 1973-02
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC : https://nrc-publications.canada.ca/eng/view/object/?id=9ab8e36b-5f1a-4994-a4aa-acf0a894f08b https://publications-cnrc.canada.ca/fra/voir/objet/?id=9ab8e36b-5f1a-4994-a4aa-acf0a894f08b
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001748
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Identification of CIS-4, 5-Epoxy-2-Pentenal from pyrolysis of H[3]PO[4]
treated cellulose
NATIONAL RESEARCH COUNCIL OF CANADA
CONSEIL NATIONAL DE RECHERCHE ANADA
505i~9
lDEMTIFICATlOM OF CIS-4,
5-EPOXY-2-PEMTENAL FROM
PYROLYSIS OF
TREATE D CELLULOSE
1. K. M. Lam, D. P. C. Fung, Y. Tsuchiya and K. Sumi
Reprinted from
Journal of Applied Polymer Science Vol. 17, No. 2, February 1 973
p. 391-399
Research Paper No. 547 of the
Division of Building Research
OTTAWA February 1973
L'IDENTIFICATION DU CIS-4, 5-EPOXY-2-
PENTENAL RESULTANT
DE LA
PYROLYSE
DE
LA
CELLULOSE
TRAITEE
AU 13,P04
SOMMAIRE
Un produit important, C ~ H G O ~ , de la dkcomposition pyroly- tiquc de la ccllulo~c traitkc ii l'acide phosphoriquc a kt6 is016
et identiti6 comme cis-4, 5-kpoxy-2-pentknal. Les auteurs prkscntent une arlalyse d6taillke et un cxnmcn dcs donnbes de resonance nuclkaire magnktique e t clcs spectres infra- rouge, ultraviolet e t de masse du produit en cause. 11s prksentent kgalement uiie mkthode de prilparation du cis-4, a Ion. 5-kpoxy-2-pentbnal et proposent un mod&lc de sa form t'
JOURNAL O F APPLIED POLYMER SCIENCE VOL. 17, PP. 391-399 (1973)
Identification
of cis-4,5-Epoxy-2-Pentenal
from
Pyrolysis of W3P04,-Treated
Cellulose*
L. K. niI. LAM, Department of Chemistry, University of Winclsor, Windsor, Ontario, D. P. C. FUNG, Eastern Forest Products Laboratory, Department of
th.e Environment, Ottazoa 7, Canada, Y. TSUCHIYA and I<. SUMI, Division of Building Research, National Research Council of Canada,
Ottawa 7, Canacla
Synopsis
An important product, C6H602, from the pyrolytic decompositioil of phosphoric acid, treated cellulose was isolated and identified as cis-4,5-epoxy-2-pentenal. NMII, I R UV, and mass-spectral data of this product were analyzed and discussed. A method for the preparation of cis-4,5-epoxy-2-pentenal is presented, and a mechanism for its format,ion is proposed.
INTRODUCTION
The thermal decomposition of cellulose has been extensively studied, and work in this area has been reviewed by Brownc1 and by % I a ~ I < a y . ~ Re- cently, studies on the pyrolysis of cellulose treated with various fire rctar- dants were rep~rted.~-lO >lost of these investigations were directed toward the understanding of the nlechanism of fire retardance in the search for more effective fire retardant treatments of wood and cellulosc.
Several theories have becn advanced t o account for the mechanism of fire retardants on ccllulosc, among then1 t h e "levoglucosan theory."
This theory was proposed by Parks et al.1° who suggested that the first and rate-detcymining step during t h c pyrolysis of cellulosc was the depoly- mcrization step t o form flammablc tar, or lcvoglucosan (1,6-anhydro-P-D- glucopyranose). This study inlplicd t h a t thc prevention of levoglucosan formation by chemical means would decrease the flammability of the resultant cellulose derivative. Good fire retardants arc expccted t o reduce t h e amount of levoglucosan formation more than poor firc retardants c m . This theory was latcr supported by Schwenkcr and I ' a ~ s u ~ ~ 157110 cllcmically modified cellulose fabrics t o impart flame rctardance and glow resistance.
Reccntly, we quantitatively analyzed the pyrolysis products of un- treated cellulose and invcstigatcd the effect of elevcn inorganic firc rc- tardants (acidic, allialinc, and n e u t r d ) on thc forn~ation of thcse products * Based on a paper presented a t the 162nd ACS National Meeting, Ilivisidn of Cellu- lose, Wood and Fiber Chemistry Wnshington, D.C., September 13-17, 1071.
391 @ 1973 by John Wiley & Sons, Inc.
392 LAM ET AL.
from ~ c l l u l o s c . ~ The relationship between flammability (as nleasured by the oxygen index test)'? of t h e treated ccllulosc samples and the yield of levoglucosan from t h e pyrolysis of these samples was s t ~ d i c d . ~ I t nras found t h a t all fire retardants investigated in this study lo\vcrcd thc amount of lcvoglucosan formation. There was no direct correlation between t h e effcctivcncss of a fire retardant arid t h e amount of lcvoglucosan formation, however. Hence, t h e validity of t h e "levoglucosan theory" was qucs- tioned, and alternative mcchanisn~s were sought t o explain the action of fire retardants on ccllulosc.
Tsuchiya and Sumi4 reported t h e finding of a new pyrolysis product of cellulosc (designated compound I in t h e present papcr) and noticed t h a t the yield of this conlpound was increased substantially when ccllulosc was treated with either phosphoric acid or anlmonium dihydrogcn orthopl~os- phate prior t o pyrolysis. Potassium carbonate suppressed the formation of compound I completely. Thcsr results suggrst that pyrolytic mechnnism of cellulosc depends on t h e nature of t h e added fire retardant. The course of the reaction may be dependent on the chemical or physical association of the fire retardant n~olecules with the ccllulose. I t appears that con~pouncl I plays an important role in the pyrolysis of ccllulosc in the presence of acidic additives, and its identification may shed some light on the mechanism of decomposition of cellulose.
This paper describes the preparation and t h e idcritifici~tion of compound I and a proposed mechanism for its formation from the pyrolysis of ccllulosc treated with phosphoric acid.
EXPERIMENTAL
Treatment of Cellulose with H2PO.r
Whatman No. 1 filter paper (50.2 g) was cut into strips of ll/? in. by in. and dipped into 1% (v/v) solution of SS% H3P04 (500 n11) for about 2 min. T h e treated sample was blotted with papcr to\vels, dried a t 60°C (3.5 mm) for 24 hr, and air dried overnight. T h e final weight of the treated sample was 50.8 g.
Pyrolysis Procedure
Pyrolysis was carried out in a Pyrex tube (3 in. by 16 in.) with a side arm for evacuation. This side arm was connected t o a cold trap (-7S°C) in such a manner that t h e system could be cvacuatcd by a mechanical pump. The HaPO4-treated sample (50.8 g) was introduced into thc tube which us preheated t o t h e desired temperature (320°C). The pressure of t h e sys- tem ranged from 2 t o 3 mm during the period of pyrolysis (100 min).
T h e liquid pyrolyzate, solution I1 (approx. 15 ml), separated into two layers in t h e cold trap. The top laycr was colorless and thc bottom layer was brownish in color. After I1 was transferred into a 230-ml Erlcnmeycr flask, water (5 X 5 1nl) was used t o rinse t h c trap, and thc washings \ver(\ added t o solution I1 in the flask. After I1 \$.as rieutralixcd with 5yo
I13POd-TREATED CELLULOSE 393
NaHC03 solution (105 ml), it was filtered and the filtratc was extracted with ether (S X 25 ml). The ethereal extract was dried over anhydrous Na2S04, and the ether was evaporated. A brown liquid ( 5 g ; yield, 10%) was obtained. I t contained approximately goy0 of I . Compound I had a high boiling point, and attempts t o distil it in vacuo were not successful, probably because of its decomposition and rearrangement t o other products. Howcvcr, compound I could bc casily purified by preparative gas chroma- tography.
A Pcrlcin-Elmcr F-21 preparative chromatograph cquipped with a '/?-in. by 6-ft 5% Carbowax 20R'I on acid-washcd chromosorb stainless steel column, and with temperature programming in the rangc of 50"-lSO°C, was uscd t o purify compound I. The infrared spcctra were recorded on a Bcclcman IR-12 spcctrophotomcter, and NA4R spcctra were recorded on a Jcolco JNAI-cGOHL instrument in CDC13. Mass spectra were obtained on thc LI<B 9000 combined gas cllromatography mass spectrometer, and ultra- violet spcctra wcre obt:~incd on a Pcrlcin-Elmcr 350 UV spectrophotomcter.
RESULTS AND DISCUSSION
Wodley7 invcstigated the pyrolysis products of ccllulose and reported the finding of an unknown compound I11 having an empirical formula of C5H602. This compound gavc similar mass-spectral and gas-chromato- graphic data as compound I. Con~pound I11 was further examined by Lipslca and AIcCa~land,~ who tentatively assigncd a structurc of 1,5- anhydro-2,3-didcoxy-~-~-pent-2-enefuranose for I11 bascd on their inter- pretation of I R , NMR, and mass-spectral data. Both Wodley and Lipslca pointcd out thc irnportancc of 111 and suggcstcd that its identification might assist in the understanding of the mechanism of fire retardance on ccllulose:
Altllougl~ both compounds I and I11 wcre obtained from the sa111c source and have vcry similar fcaturcs in thcir mass-spcctral 2nd gas-chromato- graphic analyses, the structurc of I11 proposed by Lipska does not agrec with thc I11 and NAIIt data wc obtained for I . If I and 111 wcrc the samc compound, wc prcfcr thc structure of I .
Structure of Compound I
Con~pound I is n colorless liquid, and its nlolecular weight is 9S as deter- mined by the mass spcctromctcr (Table I). I t has a molecular formula of
394 LAM ET AL.
TABLE I
Mass Spectrum of Compound I
C5H602 and can be prepared readily from t h e pyrolysis of cellulose treated with lyo phosphoric acid a t 320°C. The yield of I ranged from 10% t o 15y0 by this method. T h e crude product from the reaction was extracted with ether and then purified by preparative gas chromatography.
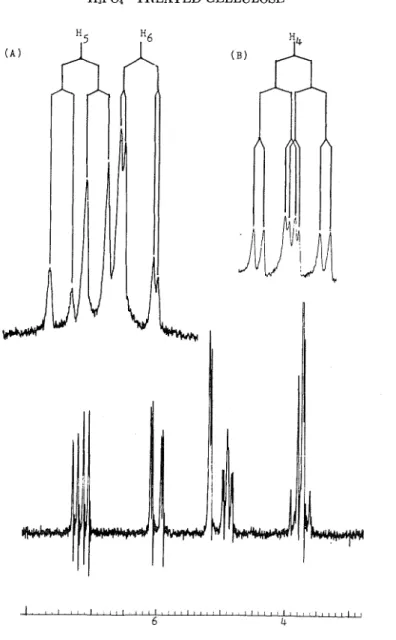
T h e Nn'lR spectrum of I is shown in Figure 1. Two quartets a t t h e low- field region are signals of the vinyl protons. T h e quartet a t 7.156 is lower than ordinary olefinic proton signals and is indicative of the 0-proton signal of an cc,P-unsaturated carbonyl compound. T h e coupling constant be- tween t h e vinyl protons being 10.1 Hz indicates t h a t they are cis t o each other. T h e two multiplets centred a t 4.906 and 3.756 are t h e protons of t h e epoxy ring. Insets in Figure 1 show t h e expanded scale of these multi- plets. It is clear from these spectra and from t h e results of doublc- irradiation work t h a t these protons are coupled t o each other and have t h e multiplicities and coupling constant expected of an epoxy ring. Table I1 shows t h e chemical shifts and coupling constants for all six nonequivalent protons in Compound I. T h e doublet a t 5.186 is assigned t o the aldehydic proton. While this value deviates considerably from the normal chemical shifts of aldehydic protons, we believe t h a t malcing such an assignment is justified for the following reasons. First, from the chemical as well as spectral d a t a we believe t h a t compound I is an a,p-unsaturated carbonyl
TABLE I1
Observed NMR. Values of Compound I
H3P04-TREATED CELLULOSE
Fig. 1. 60-MHz NMlt spectrum of compound I in CDC13, SW500: ( A ) 60-MHz spec-
trum of 136 and 136, SW100; (B) 100-MH7, spectrum of 1-14, SW5.0.
con~pound. Talting the resonance signals of othcr protons of the molecules into account, togcthcr with the IR data and thc molecular formula, i t is rcasonable t h a t thc doublet a t 5.186 bc assigncd t o the aldehydic proton. Secondly, construction of a molecular modcl of I showcd t h a t the most preferred conformation of the compound has the carbonyl group trans t o the doublc bond and thc planc of the cpoxy ring pcrpcndicular t o the plane containing the two unsaturated bonds. This places the aldehydic proton directly abovc the planc of t h c epoxy ring. Thus, t h e aldehydic proton can be expected t o be strongly shielded by the epoxy ring. Consideration
396 LAM ET AL.
of the Ni\lIR spectrum of I in this manner not only can derive the structure of I but also can gain some insight into the conformation of the molecule. As far as we know, this is the first reported instance in which an aldehydic proton signal is considerably shifted upficld.
The C-13 spectra of compound I confirm the presence of a carbonyl car- bon, a carbon-carbon double bond conjugated with the carbonyl carbon and an allylic carbon atom. The a,Bunsaturated carbonyl system in I mas also confirmed by its UV absorption a t A,,, 234 mp in ethanol.
Figure 2 shows the infrared spectrum of I . The absorption band a t GSO cm-' is the bending frequency of C-H of the olefin, and the band 1610 cm-' is the C=C stretching frequency of the olefin. The absorption bands a t 830 cm-' and 1260 cm-' arc due t o the C-0-C stretching frequencies of the epoxy ring. The twin bands a t 1705 cm-' and 1725 cm-' are charac- teristic of a,B-unsaturated carbonyl compounds. The band a t 2900 cm-I is assigned t o the C-H stretching frequency of the aldehydic hydrogen. The band a t 2250 cm-' is due t o CDC13 solvent.
The P-1 peal< m/e 97 of the mass spectrum in Table I gives further sup-
port t o the presence of an aldehydic function in compound I . The other prominent peaks a t wnz/e 96, 70, 68, 53, 42, 40, and 39 from I can be ratio- nalized as follows. Pirlcle and Dines have found similar mass-spectral re- sults for 2-pyrone13:
On the basis of our interpretation of the data obtained by UV, NA'IR, I R , and MS, we have assigned the structure of cis-4,s-epoxy-2-pcntenal t o com- pound I .
LAM ET AL.
Y
PH
c e l l u l o s eFig. 3. Proposed mechanism for the formation of cis-4,5-epoxy-2-pentenal. Mechanism for the Formation of Compound I
A mechanism for the formation of I from thc pyrolysis of phosphoric acid- trcated cellulose can be postulated as shown in Figurc 3. I t is assumed t h a t cellulose phosphate is formed after the treatment of cellulose wit11 pllos- phoric a c i ~ l ~ , ' ~ and t h a t the e.xtcnt of phosphorylation depends on the trcat- ing conditions. At temperatures bclow 320°C, ccllulose phosphate de- composes t o form intermediate compound IV by bond-breaking and bond- forming processes. Renrrangcment of IV yields I. It is t o be notcd t h a t t h e untreated cellulose could also undergo similar proccsscs l o give I, but its yield would be lower than t h a t from t,hc treated material.4
Another possibility is t'llat phosphoric acid-t'reated cellulose may de- grade t o compound I via t'lic formntiorl of levoglucosan which disappears ?s
HjPOd-TREATED CELLULOSE 399
a result of secondary pyrolysis. If this is t h e case, one would expect t o find a large amount of I from the pyrolysis of both levoglucosan and phosphoric acid treated levoglucosan. This, however, was not observed in our pre- vious i n v e ~ t i g a t i o n . ~ Since phosphoric acid-treated cellulose degrades t o yield much larger amounts of cis-4,5-epoxy-2-pentenal than t h e untreated material, it appears t h a t I plays an important role in the flame retardance of cellulose treated with phosphoric acid.
The authors thank Dr. G. W. Buchanan and Dr. J. B. Stothers for running the 100-MHz proton and carbon 13 NMR spectra, and Dr. 0 . Mamer for the mass spectral analyses. The financial support from the National Research Council of Canada to one of us (L. K . M. Lam) is gratefully acknowledged.
References
1. F . L. Browne, U.S. Dept. of Agricullure Forest Prod. Lab. Report, 2136 (1958). 2. G. D. $1. MacKay, Wood, Fire Behauiour and Fire Retardant Treatment, Canadian Wood Council, Ottawa, Canada, 1966.
3. D. P. C.Fung, Tappi, 52,319 (1969).
4. Y. Tsuchiyn and K. Sumi, J . Appl. Polym. Sci., 14, 2003 (1970). 5. D. P. C. Fung, Y. Tsuchiya, and K . Sumi, J . Wood Sci., 5,3S (1972). 6 . J. W. Lyons, J . Fire Flamm., 1 , 302 (1970).
7. A. F . Wodley, J . Appl. Pol?jm. Sci., 15, 835 (1971).
8. A. E. Lipska and G. E. McCasland, J. Appl. Polynz. Sci., 15,419 (1971). 9. D. F. Arseneau, Can. J . Chem., 49,632 (1971).
10. W. G. Parks, R. M. Steve, Jr., M. H. Gollis, R . Guercia, and A. Petrarca. Paper No. 15, Division of Cellulose Chemistry, Abstracts, 127th ACS Meeting, Cincinnati, Ohio, 1955.
11. R. F . Schwenker, Jr., and E. Pacsu, Ind. Eng. Chena., 50,91 (1958). 12. C. P. Fenimore, and F . J . Martin, Mod. Plas., No. 11, 141 (1966). 13. W. H. Pirkle and M. Dines, J . Amer. Ciaem. Soc., 90,2318 (196s).
14. A. C. Nuessle, F. $1. Ford, W. P. I-Iall, and A. L. Lippert, Text. Res. J., 26, 32 (1956).
Received May 3, 1972 Revised August 2,1972
This publication is being distributcd by t h e Division of Building Rcscnrch of thc National ltescarch Council of Canada. It should not be reproduced in 1vhole or in part without permission of thc original publisher. T h c Division ~vould be glad t o bc of assistance in obtaining such per- mission.
Publications of the Division may be obtaincd by mailing thc appropriate rcmittance (a Ba~lli, Express, or Post Ofice I l o n c y Ordcr, or a cheque, made payable t o the Receiver Gcneral of Canada, credit NRC) to the National Rcsearch Council of Canada, Ottawa. lilAOR6. Stainps arc not acceptable.
A list of all publications of the Division is available and may be obtained from thc Publications Section, Division of Building Research, National Research Council of Canada, Ottawa. ICIAORB.