Publisher’s version / Version de l'éditeur:
Environmental Science & Technology, 47, 10, pp. 5193-5198, 2013-04-17
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1021/es4006652
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Joint photomicrobial process for the degradation of the insensitive
munition N-Guanylurea-dinitramide (FOX-12)
Perreault, Nancy N.; Halasz, Annamaria; Thiboutot, Sonia; Ampleman, Guy;
Hawari, Jalal
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=c58efae5-5ca4-41c4-b29f-3bf06a802562 https://publications-cnrc.canada.ca/fra/voir/objet/?id=c58efae5-5ca4-41c4-b29f-3bf06a802562
Joint Photomicrobial Process for the Degradation of the Insensitive
Munition N‑Guanylurea-dinitramide (FOX-12)
Nancy N. Perreault,
†Annamaria Halasz,
†Sonia Thiboutot,
‡Guy Ampleman,
‡and Jalal Hawari*
,††
National Research Council Canada, 6100 Royalmount Avenue, Montreal, Quebec H4P 2R2, Canada
‡
Defence Research Development Canada, Department of National Defence, Valcartier, Quebec G3J 1X5, Canada
ABSTRACT: N-Guanylurea-dinitramide (FOX-12) is a very insensitive energetic material intended to be used in the composition of next-generation insensitive munitions. To help predict the environmental behavior and fate of FOX-12, we conducted a study to determine its photodegradability and biodegradability. When dissolved in water, FOX-12, a guanylurea-dinitramide salt, also named GUDN, dissociated instantly to produce the dinitramide moiety and guanylurea, as demonstrated by high-performance liquid chromatography (HPLC) analysis. When an aqueous solution of FOX-12 was subjected to photolysis using a solar-simulated photoreactor, we found a rapid removal of the dinitramide with concurrent formation of N2O, NO2−, and NO3−. The second component,
guanylurea, was photostable. However, when FOX-12 was incubated aerobically with the soil isolate Variovorax strain VC1 and protected from light, the dinitramide component of FOX-12 was recalcitrant but guanylurea degraded effectively to
ammonia, guanidine, and presumably CO2. When FOX-12 was incubated with strain VC1 in the presence of light, both
components of FOX-12 degraded, giving similar products to those described above. We concluded that the new insensitive explosive FOX-12 can be effectively degraded by a joint photomicrobial process and, therefore, should not cause persistent contamination of surface waters.
■
INTRODUCTIONTraditionally, the defense industry has focused on the use of explosives, such as hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX), 2,4,6-trinitrotoluene (TNT), nitrocellulose (NC), and nitroglycerine (NG), but handling and use of these chemicals is often associated with a high risk of unwanted detonation.1 Efforts are now underway in North America, Europe, and Australia to develop insensitive materials to replace TNT, RDX, and HMX in the manufacturing of munition formulations. Examples of emerging insensitive explosives include dinitramidic acid, HN(NO2)2, and salts derived from
the acid, such as ammonium dinitramide.2 Because of their importance, several theoretical and experimental studies have been carried out to determine their physicochemical properties, thermal stability, and applications.2−6Recently, a Swedish team developed another nitramide salt, N-guanylurea-dinitramide (FOX-12), whose performance matches those of RDX and TNT but shows low sensitivity to impact and friction, thus allowing the chemical to be used as an ingredient in the composition of very insensitive high explosives and propellants and as a gas-generating material in automotive safety air bags.7,8 On the basis of past experience on the environmental risks associated with the use of the traditional explosives,9−12 it is imperative to understand the environmental impacts associated with the manufacturing and use of the new generation of insensitive munitions. In general, little information is available on the transformation pathways of these emerging explosives,
and no reports on the degradability of FOX-12 are currently available. A study by Mill et al.13 indicated that ammonium dinitramide can be rapidly photodegraded but is stable toward hydrolysis in water and recalcitrant to biotransformation in water and soil. To our knowledge, bacterial degradation of guanylurea has not yet been reported. However, we previously isolated a strain of Variovorax that degraded nitroguanidine and its degradation product nitrourea, as well as other guanidine derivatives.14 Variovorax species are ubiquitous in nature and degrade a diversity of pollutants, including the phenylurea herbicides linuron15and diuron.16Therefore, Variovorax strain VC1 was selected in this study to assess biodegradation of FOX-12.
The present study thus aims at determining the photo-degradation of FOX-12 under solar-simulated conditions and its biotransformation using the soil bacterial isolate Variovorax strain VC1. Understanding the transformation pathways of FOX-12 will provide insight into the environmental fate of this new energetic material, which, in turn, should help site managers and environmental officers to manage their activities and training needs.
Received: February 11, 2013 Revised: April 16, 2013 Accepted: April 17, 2013 Published: April 17, 2013
pubs.acs.org/est
■
MATERIALS AND METHODSChemicals and Bacterial Strain.FOX-12 was provided by Defense Research and Development Canada (DRDC)-Valcartier (Quebec, Canada). Guanylurea was purchased from Sigma-Aldrich (Oakville, Ontario, Canada). Variovorax strain VC1 was previously isolated in our lab from a non-contaminated area adjacent to a military live-fire training range at DRDC-Valcartier.14 The mineral salts medium (MSMG; pH 7.0) consisted of (per liter of distilled water): 0.38 g of K2HPO4, 0.2 g of MgSO4·7H2O, 0.05 g of
FeCl3·6H2O, and 20 mM glucose.
Photolysis of FOX-12.Artificial sunlight generated from a SolSim solar-simulating photoreactor (Luzchem Research, Inc., Canada) was used to determine the photosensitivity of FOX-12 under solar-simulated conditions. The total power of the solar simulator output spectrum was calibrated to the best approximation of ASTM Air Mass 1.5 Global Tilt Standard in the 280−800 nm region, with a total irradiance of 590 000 W m−2. Irradiation assays were conducted in 20 mL quartz tubes
closed with a Mininerts valve for gas sampling, using 5 mL of FOX-12 (250 μM) in deionized water. Irradiation was run for 30 min and extended to 7 days at 25 °C. Samples were collected at selected time intervals for analysis. A control containing FOX-12 covered with aluminum foil was prepared. Growth of Variovorax Strain VC1 with FOX-12 as the Sole Nitrogen Source. Strain VC1, isolated from DRDC-Valcartier soil14was grown in 25 mL of MSMG supplemented with 424 or 957 μM FOX-12 in 250 mL Erlenmeyer flasks. Controls consisted of strain VC1 in MSMG without FOX-12 that ensured that growth was due to the presence of FOX-12 and inoculated flasks containing MSMG with FOX-12 (abiotic controls). Cultures, in triplicate, were incubated at ∼22 °C on a rotary shaker under visible light (desk lamp) or in the dark. Growth was monitored spectrophotometrically at 600 nm (OD600 nm), and the supernatant was centrifuged and used for
chemical analysis.
Biodegradation of FOX-12 and Guanylurea by Resting Cells of Strain VC1. Strain VC1 was grown until mid-log phase (OD600∼ 0.5) in MSMG supplemented with ∼1 mM of either FOX-12 or pure guanylurea. Cells were centrifuged, washed in distilled water, and resuspended at an OD600 nm of 2.0 in distilled water containing FOX-12 or pure
guanylurea at a concentration of 1050 μM. Each assay was prepared in triplicate in 10 mL vials and incubated at 22 °C in the dark. Controls with one of the substrates but in the absence
of cells and controls with cells in the absence of substrates were prepared.
Analytical Methods. Guanylurea and dinitramide were analyzed using a high-performance liquid chromatography (HPLC) system equipped with a 600 pump (Waters), a 717 plus autosampler, and a 2996 photodiode array detector. Samples (50 μL) were separated on a Hypercarb column (150 × 4.6 mm, supplied by Cole-Parmer, Montreal, Quebec, Canada) at 35 °C. The mobile phase, composed of 90% of 0.5% trifluoroacetic acid in water (pH 1) and 10% acetonitrile, ran isocratically at 1 mL min−1. The detector was set to scan from
191 to 400 nm. Ammonium ion (NH4+) was analyzed by ion
chromatography, and nitrous oxide (N2O) was determined by
gas chromatography with an electron capture detector (GC/ ECD).14Guanidine was measured using HPLC from Waters (pump, model 600; autosampler, model 717 plus) equipped with an electrochemical detector (model 2465) with a gold cell in the pulse amperometric mode. The sample was injected on a cation-exchange column IonPac CS14 (4 × 250 mm) from Dionex. The mobile phase was 5 mM methanesulfonic acid at a flow rate of 1.25 mL min−1and 35 °C. The detection limit was
1 ppm. FOX-12 and its transformation products were analyzed using a Bruker MicroTOFQ mass analyzer attached to a HPLC system (Hewlett-Packard 1200 Series) equipped with a diode array detector (DAD). Samples (10 μL) were injected into a 2.5 μm pore size Synergi-Polar column (2 mm inner diameter ×100 mm; Phenomenex) at 25 °C. The solvent system was composed of CH3OH and HCOOH (0.05%) at a flow rate of
0.15 mL min−1using a gradient. For mass analysis, positive and
negative electrospray ionization mode was used to produce protonated [M + H]+ and deprotonated [M − H]−molecular
mass ions. The mass range was scanned from 40 to 500 Da.
■
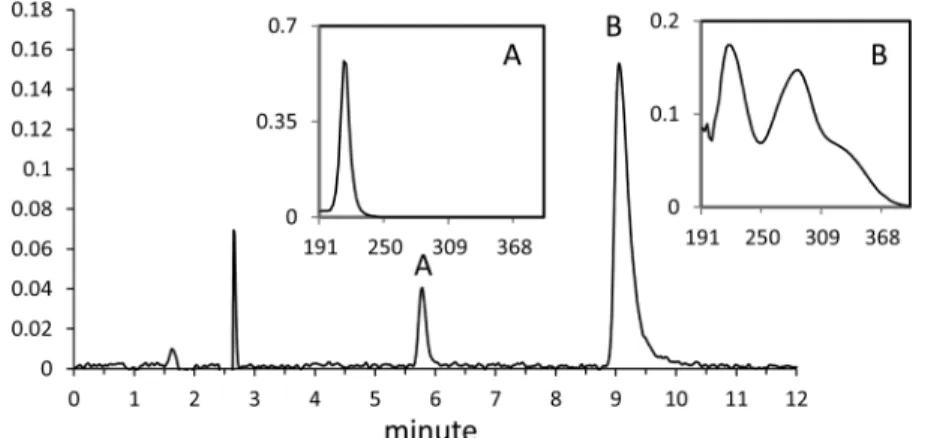
RESULTS AND DISCUSSIONDissociation of FOX-12 in Water. HPLC/ultraviolet (UV) analysis demonstrated that, in aqueous solution, the FOX-12 salt dissociates instantly to its two components, guanylurea and dinitramide. The HPLC chromatogram at 210 nm in Figure 1 clearly shows two peaks: one appearing at a retention time of 5.8 min identified as guanylurea by comparison to a reference standard material and another appearing at 9.1 min identified as dinitramide and confirmed by liquid chromatography−ultraviolet/mass spectrometry (LC− UV/MS) showing characteristic UV absorptions at 219 and 285 nm2,17,18and a deprotonated molecular mass ion [M − H] at 106 Da.
Figure 1.HPLC/UV chromatogram of an aqueous solution of FOX-12 (50 mg L−1) at 210 nm: (A) guanylurea and (B) dinitramide. (Insets)
Corresponding UV spectra.
Environmental Science & Technology Article
dx.doi.org/10.1021/es4006652 | Environ. Sci. Technol. 2013, 47, 5193−5198
Because the FOX-12 salt completely dissociates in water to guanylurea and dinitramide and each component is detected at a different retention time by HPLC, it was possible to look at the stability of each component separately. The following sections thus describe how the two components of FOX-12, guanylurea and dinitramide, behave in the presence of light and bacteria in aqueous solutions.
Photodegradation of FOX-12. When we subjected an aqueous solution of FOX-12 (253.4 μM) to light from a SolSim photoreactor simulating solar irradiation, more than 96% (243.9 ± 0.3 μM) of the initial dinitramide from FOX-12 was photodegraded over 30 min of photolysis. Guanylurea stayed intact, indicating that only the dinitramide part of the FOX-12 salt was photoreactive (Figure 2). The UV absorption
of the two moieties of FOX-12, i.e., guanylurea (λmax of 210
nm) and dinitramide (λmax of 219 and 285 nm) shown in
Figure 1 clearly supports the photostability of guanylurea and the photodegradability of dinitramide using a solar simulator, whose emission spectrum and intensity match the standard solar AM1.5 (280−800 nm).
The disappearance of dinitramide was accompanied by the formation of NO2−(209.9 ± 1.0 μM), NO3−(146.9 ± 3.4 μM),
and N2O (51.2 ± 4.8 μM) (Figure 2), representing a nitrogen
recovery of 63.6%. After 5 min of irradiation, the NO2−/NO3−
ratio was relatively high, reaching 4.4, which is in line with the ratio of 4 obtained by Mill et al.13 during photolysis of ammonium dinitramide in water (pH 7). The NO2−/NO3−
ratio decreased to 0.37 after 7 days, indicating that NO2−might
have been photo-oxidized to NO3−.19 In an earlier study
involving photolysis of the nitramine RDX, (NO2−NCH2)3, we
found that NO2− formed from the photocleavage of N−NO2
can be photooxidized to NO3−.20 Earlier, Mill et al.13
investigated the hydrolytic stability of ammonium dinitramide in water and found the salt to be stable in the dark at 25 °C. However, the salt was rapidly photodegraded to NO2−, NO3−,
and N2O at wavelengths close to those naturally available from
the sun (June sunlight), with t1/2= 3.2 min and Φ = 0.1. They
reported a similar N balance from photolysis of potassium− dinitramide (61%) and ammonium−dinitramide (59%) in water. Guanylurea in FOX-12 (and as a control of guanylurea alone) was photostable under the same conditions, with no loss encountered over 7 days; likewise, photostability of guanylurea after 120 min of irradiation was previously reported.21Although it is known that, during photolysis of dinitramide several reactive free radicals can be produced, including•
NO, •
NO2,4
and •OH,19 none of these radicals was able to degrade
guanylurea. The potential participation of these radicals in other side reactions with dinitramide might have led to the formation of unidentified products that contributed to a non-stoichiometric N mass balance.
Aerobic Biotransformation of FOX-12 with Variovorax Strain VC1.We first determined that Variovorax strain VC1 grew in MSMG with FOX-12 as the sole N source. The cells reached an OD600of 0.68 ± 0.09 when grown with 424 μM
FOX-12 and 20 mM glucose (Figure 3). No lag time was
observed when acclimated VC1 cells (pre-grown on FOX-12) were used as inocula. Figure 3 also shows that strain VC1 degraded the guanylurea moiety of FOX-12 but did not attack dinitramide. This is in contrast to what we observed with light (Figure 2). Cometabolic biodegradation of dinitramide with glucose has been previously reported without mentioning any products;13 mineralization of ammonium dinitramide to ammonia in digested sewage sludge under strict anaerobic conditions was also reported.22A Bacillus soil isolate from our lab23also degraded dinitramide cometabolically in rich medium (Luria−Bertani broth) at a rate of 3.9 μM h−1 (data not
shown). These experimental findings indicate that biodegrada-tion of dinitramide is possible although not achieved by strain VC1. Degradation of guanylurea was accompanied by the formation of guanidine, which could not be quantified because of interfering substances in the culture supernatant.
To further assess biotransformation of the guanylurea moiety in FOX-12, resting cells of VC1 were incubated with either FOX-12 or pure guanylurea (from Sigma) as substrates (Figure 4). VC1 mostly transformed guanylurea from FOX-12 to guanidine and ammonia; dinitramide remained intact (Figure
Figure 2. Effect of simulated sunlight irradiation on dinitramide
(DNA-FOX12) and guanylurea (GUU-FOX12) moieties of FOX-12. Figure 3.Cells growth (OD600) and guanylurea (GUU) degradation
by Variovorax strain VC1 with FOX-12 as the sole N source in dark. DNA-FOX12, dinitramide in FOX-12; GUU-FOX12, guanylurea in FOX-12.
4a). The disappearance of guanylurea was accompanied by the concurrent formation of guanidine, followed by the appearance of ammonia that was only detected after 6.5 h of incubation (Figure 4a). After 26 h, 0.70 ± 0.11 mol of guanidine and 0.74 ± 0.02 mol of ammonia were produced for each mole of guanylurea degraded, corresponding to a N mass balance of 71%. Neither nitrite, nitrate, nor N2O were observed, consistent
with the fact that dinitramide was not degraded.
Biotransformation of pure guanylurea (from Sigma) by VC1 was faster than biotransformation of guanylurea (from FOX-12) (Figure 4b). Removal of guanylurea (from Sigma) was accompanied by the formation of guanidine and ammonia. After 26 h, for each disappearing mole of guanylurea, 0.56 ± 0.06 mol of guanidine and 0.94 ± 0.04 mol of ammonia were formed, representing a N mass balance of 65%. Seemingly, dinitramide had an inhibitory effect on the degradation of guanylurea. From Figure 4b, we also observed that, in contrast to Figure 4a, the formation of ammonia was concurrent with the formation of guanidine. The presence of dinitramide appeared to affect the formation of ammonia.
The missing N from both panels a and b of Figure 4 could be attributed to several products that we detected by LC−MS and that were tentatively identified as secondary product(s) of guanidine. One of the products showed a protonated molar mass ion [M + H]+ at m/z 102 Da, matching a molecular
formula of C2H7N5, and presumed to be biguanide, NH2
C-(NH)NHC(NH)NH2, a dimeric condensation product of
guanidine. We detected another compound with [M + H]+at
m/z 112 Da, matching a molecular formula of C3H5N5that we
tentatively identified as the heterocyclic compound formoguan-amine.24It is also known that guanidine is a reactive molecule and condenses in the presence of an amine to form oligomers and polymers.25,26All of these products taken together can lead to a reduction in the N mass balance. These products were not detected in the controls of VC1 cells in the absence of substrate.
Joint Photomicrobial Transformation of FOX-12. We combined bacteria and light to achieve degradation of both components of FOX-12. Strain VC1 was allowed to grow in MSMG supplemented with FOX-12 and exposed to room lighting. Under these conditions, guanylurea and dinitramide degraded simultaneously with almost similar rates, i.e., 15.9 and 16.9 μmol L−1h−1, respectively (Figure 5). After 2 days, 1.74 ±
0.18 mol of nitrite ion was formed for each mole of dinitramide degraded. Part of the anion was later consumed by the microorganisms (Figure 5). Nitrous oxide was formed at 0.170 ±0.002 mol per mole of dinitramide. A small amount of NO3−
was also detected and supposed to originate from dinitramide degradation (data not shown).
Dinitramide degradation in the combined photomicrobial system was significantly decelerated in comparison to what was observed in the solar simulator (Figure 2). The kinetic retardation can be explained by the fact that, in contrast to photolysis in the solar simulator, the combined assay (Figure 5) was performed under suboptimal conditions using Erlenmeyer flasks exposed to a 13 W fluorescent tube and in the presence of bacterial cells that interfered during irradiation.
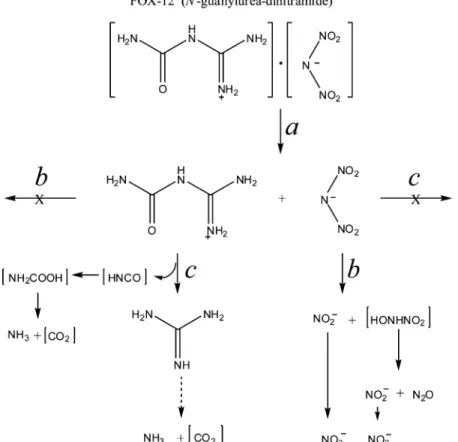
Insights into the Photomicrobial Degradation Path-ways of FOX-12. Using time course studies and product distribution from the photodegradation (Figure 2), biotrans-formation (Figure 3 and panels a and b of Figure 4), and joint photodegradation−biotransformation experiments (Figure 5), we constructed a degradation pathway that can best describe FOX-12 photobiodegradation, as shown in Figure 6 (routes a, b, and c). The pathway consisted of a primary route that first lead to the complete dissociation of FOX-12 to its two acid/ base components, dinitramide and guanylurea (route a in Figure 6). When the dissociated aqueous solution of FOX-12 was subjected to light, dinitramide degraded to NO2−, NO3−,
Figure 4.Transformation of (a) FOX-12 and (b) guanylurea (GUU) with resting cells of Variovorax strain VC1 and the concomitant formation of guanidine and ammonia. DNA-FOX12, dinitramide in
FOX-12; GUU-FOX12, guanylurea in FOX-12. Figure 5.Guanylurea and dinitramide degradation by growing cells of Variovorax strain VC1 with FOX-12 as the sole N source in the presence of light. DNA-FOX12, dinitramide in FOX-12; GUU-FOX12, guanylurea in FOX-12.
Environmental Science & Technology Article
dx.doi.org/10.1021/es4006652 | Environ. Sci. Technol. 2013, 47, 5193−5198
and N2O, leaving guanylurea intact (route b in Figure 6). In this
context, dinitramide would first undergo photodenitration to initially produce NO2−and the hydroxyl-nitramide,
NH(OH)-NO2. The latter being unstable should undergo spontaneous
decomposition to give NO2−, N2O, and water.13It is known
that photolysis of NO2−can lead to NO3−.19,20
When another FOX-12 aqueous solution was incubated with the bacterium Variovorax strain VC1 without light, guanylurea degraded to guanidine, ammonia, and presumably CO2but, this
time, leaving dinitramide intact (route c in Figure 6). We presume that the primary step in the biodegradation of guanylurea involved the loss of the cyanic acid, HCNO, a precursor of the carbamic acid, NH2COOH, which, upon
decomposition in water, would produce NH3and CO2(route c
in Figure 6). The lag time in the ammonia production during guanylurea (from FOX-12) incubation with VC1 (Figure 4a) suggests that HCNO was formed prior to the formation of the unstable carbamic acid, NH2COOH. Cyanic acid intermediate
have been detected during hydrolytic decomposition of urea by urease.27As we mentioned earlier, the lag time in the ammonia production was not observed during incubation of pure guanylurea with VC1 (Figure 4b), suggesting that dinitramide might have stabilized the precursor intermediate, leading to the formation of ammonia.
Guanidine can then be transformed to ammonia and CO2
(Figure 6). Many guanidine derivatives were shown to be mineralized by microorganisms in soil and water.28,29 Mineralization of another guanidine derivative, nitroguanidine, by the same strain was reported to give the same products NH3
and CO2.14
The present study demonstrates that, by combining photolytic and microbial processes, the new insensitive
explosive FOX-12 can be effectively degraded to nontoxic products. This first assessment of the degradation of FOX-12 suggests that dinitramide would be broken down quite rapidly by sunlight in the upper layers of surface waters. In the absence of light in subsurface environments, dinitramide may persist significantly longer, particularly in oligotrophic ecosystems. Studies to date only revealed cometabolic degradation of dinitramide. Likewise, degradation of guanylurea by VC1 was achieved with glucose, and its remediation without organic carbon may be limiting in practice. On the other hand, guanylurea with its four amino groups serves as a valuable N source for microbial growth and eventually should be completely mineralized. Therefore, in addition to its insensitivity to impact and friction, FOX-12 is more environ-mentally friendly than traditional explosives.
■
AUTHOR INFORMATIONCorresponding Author
*Telephone: 514-496-6267. Fax: 514-496-6265. E-mail: jalal. hawari@cnrc-nrc.gc.ca.
Notes
The authors declare no competing financial interest.
■
ACKNOWLEDGMENTSThis work was supported by Defence Research Development Canada, Department of National Defence. We thank L. Paquet and C. Beaulieu for their analytical support.
■
REFERENCES(1) Beauregard, R. L. The History of Insensitive Munitions; http:// www.insensitivemunitions.org.
Figure 6.Proposed photobiodegradation routes of FOX-12 in water (a) using light (b) and Variovorax strain VC1 (c). Compounds in parentheses were not detected.
(2) Bottaro, J. C.; Penwell, P. E.; Schmitt, R. J. 1,1,3,3-Tetraoxo-1,2,3-triazapropene anion, a new oxy anion of nitrogen: The dinitramide anion and its salts. J. Am. Chem. Soc. 1997, 119, 9405−9410.
(3) Brill, T. B.; Brush, P. J.; Patil, D. G. Thermal decomposition of energetic material 58. Chemistry of ammonium nitrate and ammonium dinitramide near the burning surface temperature. Combust. Flame 1993, 92, 178−186.
(4) Pace, M. D. Spin trapping of nitrogen dioxide from photolysis of sodium nitrite, ammonium nitrate, ammonium dinitramide, and cyclic nitramines. J. Phys. Chem. 1994, 98, 6251−6257.
(5) Rossi, M. J.; Bottaro, J. C.; McMillen, D. F. The thermal decomposition of the new energetic material ammoniumdinitramide (NH4N(NO2)2) in relation to nitramide (NH2NO2) and NH4NO3.
Int. J. Chem. Kinet. 1993, 25, 549−570.
(6) Vyazovkin, S.; Wight, A. C. Ammonium dinitramide: Kinetics and mechanism of thermal decomposition. J. Phys. Chem. 1997, 101, 5653−5658.
(7) EURENCO Bofors AB. Dinitramide News; EURENCO Bofors AB: Karlskoga, Sweden, Oct 2004; http://www.eurenco.com/en/ high_explosives/newsletters/Dinitramide_oct_2004.pdf.
(8) Östmark, H.; Bemm, U.; Bergman, H.; Langlet, A. N-guanylurea-dinitramide: A new energetic material with low sensitivity for propellants and explosives applications. Thermochim. Acta 2002, 384, 253−259.
(9) Gong, P.; Hawari, J.; Thiboutot, S.; Ampleman, G.; Sunahara, G. I. Ecotoxicological effects of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) on soil microbial activities. Environ. Toxicol. Chem. 2001, 20, 947−951.
(10) Lachance, B.; Robidoux, P. Y.; Hawari, J. A.; Ampleman, G.; Thiboutot, S.; Sunahara, G. I. Cytotoxic and genotoxic effects of energetic compounds on bacterial and mammalian cells in vitro. Mutat. Res. 1999, 444, 25−39.
(11) Robidoux, P. Y.; Hawari, J.; Thiboutot, S.; Ampleman, G.; Sunahara, G. I. Chronic toxicity of energetic compounds in soil determined using the earthworm (Eisenia andrei) reproduction test. Environ. Toxicol. Chem. 2000, 19, 1764−1773.
(12) Robidoux, P. Y.; Hawari, J.; Bardai, G.; Paquet, L.; Ampleman, G.; Thiboutot, S.; Sunahara, G. I. TNT, RDX and HMX decrease earthworm (Esenia andrei) life-cycle responses in a spiked natural forest soil. Arch. Environ. Contam. Toxicol. 2002, 43, 379−388.
(13) Mill, T.; Su, M.; Spanggord, R. Fate Assessment of New Air Force Chemicals, Final Report; SRI International: Menlo Park, CA, 1997.
(14) Perreault, N. N.; Halasz, A.; Manno, D.; Thiboutot, S.; Ampleman, G.; Hawari, J. Aerobic mineralization of nitroguanidine by Variovorax strain VC1 isolated from soil. Environ. Sci. Technol. 2012, 46, 6035−6040.
(15) Dejonghe, W.; Berteloot, E.; Goris, J.; Boon, N.; Crul, K.; Maertens, S.; Höfte, M.; De Vos, P.; Verstraete, W.; Top, E. M. Synergistic degradation of linuron by a bacterial consortium and isolation of a single linuron-degrading Variovorax strain. Appl. Environ. Microbiol. 2003, 69, 1532−1541.
(16) Sørensen, S. R.; Albers, C. N.; Aamand, J. Rapid mineralization of the phenylurea herbicide diuron by Variovorax sp. strain SRS16 in pure culture and within a two-member consortium. Appl. Environ. Microbiol. 2008, 74, 2332−2340.
(17) Oehrle, S. A. Analysis of anionic impurities in ammonium dinitramide (ADN), Part I: Ion chromatographic analysis. J. Energ. Mater. 1996, 14, 27−36.
(18) Shlyapochnikov, V. A.; Cherskaya, N. O.; Luk’yanov, O. A.; Gorelik, V. P.; Tartakovsky, V. A. Dinitramide and its salts 4. Molecular structure of dinitramide. Russ. Chem. Bull. 1994, 43, 1522− 1525.
(19) Fischer, M.; Warneck, P. Photodecomposition of nitrite and undissociated nitrous acid in aqueous solution. J. Phys. Chem. 1996, 100, 18749−18756.
(20) Hawari, J.; Halasz, A.; Groom, C.; Deschamps, S.; Paquet, L.; Beaulieu, C.; Corriveau, A. Photodegradation of RDX in aqueous solution: A mechanistic probe for biodegradation with Rhodococcus sp. Environ. Sci. Technol. 2002, 36, 5117−5123.
(21) Trautwein, C.; Kummerer, K. Incomplete aerobic degradation of the antidiabetic drug Metformin and identification of the bacterial dead-end transformation product Guanylurea. Chemosphere 2011, 85, 765−773.
(22) Kwon, S.-H. Degradation of ammonium dinitramide (ADN) in digested sewage sludge under strict anaerobic conditions. Toxicol. Environ. Chem. 2000, 75, 35−42.
(23) Perreault, N. N.; Manno, D.; Halasz, A.; Thiboutot, S.; Ampleman, G.; Hawari, J. Aerobic biotransformation of 2,4-dinitroanisole in soil and soil Bacillus sp. Biodegradation 2012, 23, 287−295.
(24) Shirai, K.; Odo, K.; Sugino, K. Cyanamide derivatives (LIV). Cyanoformamidine and its reactions. J. Org. Chem. 1958, 23, 100−101. (25) Mattheis, C.; Schwarzer, M. C.; Frenking, G.; Agarwal, S. Phantom ring-closing condensation polymerization: Towards anti-bacterial oligoguanidines. Macromol. Rapid Commun. 2011, 32, 994− 999.
(26) Mattheis, C.; Wang, H.; Schwarzer, M. C.; Frenking, G.; Agarwal, S. Exploring suitable oligoamines for phantom ring-closing condensation polymerization with guanidine hydrochloride. Polym. Chem. 2013, 4, 707−716.
(27) Estiu, G.; Merz, K. M., Jr. Competitive hydrolytic and elimination mechanisms in the urease catalyzed decomposition of urea. J. Phys. Chem. B 2007, 111, 10263−10274.
(28) Ebisuno, T.; Takimoto, M. Examination of biodegradability of guanyl compounds. Eisei Kagaku 1981, 27, 156−162.
(29) Mitchell, W. R. Microbial degradation of guanidium ion. Chemosphere 1987, 16, 1071−1086.
Environmental Science & Technology Article
dx.doi.org/10.1021/es4006652 | Environ. Sci. Technol. 2013, 47, 5193−5198