HAL Id: hal-00783050
https://hal.archives-ouvertes.fr/hal-00783050
Submitted on 31 Jan 2013
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Elaboration of integrated microelectrodes for the
detection of antioxidant species
Céline Christophe, Fadhila Sekli-Belaidi, Jérôme Launay, Pierre Gros,
Emmanuel Questel, Pierre Temple-Boyer
To cite this version:
Céline Christophe, Fadhila Sekli-Belaidi, Jérôme Launay, Pierre Gros, Emmanuel Questel, et al..
Elab-oration of integrated microelectrodes for the detection of antioxidant species. Sensors and Actuators
B: Chemical, Elsevier, 2013, vol. 177, pp. 350-356. �10.1016/j.snb.2012.11.032�. �hal-00783050�
To link to this article
: DOI:10.1016/j.snb.2012.11.032
URL:
http://dx.doi.org/10.1016/j.snb.2012.11.032
This is an author-deposited version published in:
http://oatao.univ-toulouse.fr/
Eprints ID: 8030
To cite this version:
Christophe, Céline and Sekli-Belaidi, Fadhila and Launay, Jérôme and
Gros, Pierre and Questel, Emmanuel and Temple-Boyer, Pierre
Elaboration of integrated microelectrodes for the detection of antioxidant
species.
(2013) Sensors and Actuators B Chemical, vol. 177 . pp. 350-356.
ISSN 0925-4005
O
pen
A
rchive
T
oulouse
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers
and makes it freely available over the web where possible.
Any correspondence concerning this service should be sent to the repository
administrator:
staff-oatao@listes.diff.inp-toulouse.fr
!
Elaboration
of
integrated
microelectrodes
for
the
detection
of
antioxidant
species
C.
Christophe
a,b,d, F.
Sékli
Belaidi
a,b,c, J.
Launay
a,b, P.
Gros
c,
E.
Questel
d,
P.
Temple-Boyer
a,b,∗aCNRS,LAAS,7avenueducolonelRoche,F-31400Toulouse,France bUniversitédeToulouse,UPS,LAAS,F-31400Toulouse,France
cUniversitédeToulouse,LaboratoiredeGénieChimiqueUMRCNRS5503,UniversitéPaulSabatier,F-31062Toulouse,France dPierreFabreDermoCosmétique,CentredeRecherchesurlaPeau,31025Toulouse,France
Keywords: Thin-filmmicroelectrodes SU-8passivation Electrochemicalproperties Antioxidantspecies Oxidativestress
a
b
s
t
r
a
c
t
(Pt–Pt–Ag/AgCl)and (Au–Pt–Ag/AgCl)electrochemicalmicrocells(ElecCell) weredeveloped forthe detectionofredoxspeciesbycyclicvoltammetry.AspecialemphasiswasplacedontheSU-8 wafer-levelpassivationprocessinordertooptimizetheelectrochemicalpropertiesofthedifferent“thinfilm” metalliclayers,i.e.goldorplatinumfortheworkingelectrode,platinumforthecounterelectrodeand silver/silverchlorideforthereferenceelectrode.(Au–Pt–Ag/AgCl)microcellswereappliedforthe detec-tionofantioxidantspeciessuchasascorbicanduricacidsinphosphatebuffersolution,evidencinghigh sensitivitybutlowselectivity.Workswereextendedtoskinanalysis,demonstratingthatagood electri-calcontactwiththeskinhydrolipidicfilmallowedtheeffectiveevaluationoftheskinglobalantioxidant capacity.
1. Introduction
Electrochemistry provides an appropriate platform for the development of many analytical techniques in different fields including clinical biology, food industry or environment [1–5]. Indeed,electrochemicalproceduresprovidemanyadvantagessuch aseasyuse,lowcost,fastanalysis,aswellashighdetection sen-sitivityandselectivity[6].Researchworkswerethusdedicatedto developingtheelectrochemicaltheoryatamicroscalelevel, study-ingbioelectrochemicalsensitivelayerspropertiesandconstructing micro/nanoelectrodes. Due to theseworks, an improvement in electroanalyticalmethodswasachieves,consequentlyleadingto thedetectionofmanychemical,biochemicalandbiologicalredox species in complex media [7,8]. This is for instance the case for the evaluation of oxidative stress and the assay of specific antioxidantspeciesonskin.Oxidativestressisknowntobe respon-siblefortheproductionofreactiveoxygenspecies(ROS)[9–12]. Facetopro-oxidativeenvironments(atmosphere,pollutants,UV irradiations,pathogenicbacteria...),skinhasdevelopeddifferent strategies toprotect itself[9,13].Oneof them isrelated tothe productionofhydrophilicantioxidantspeciessuchasglutathione as well as ascorbic and uric acids. As a result, concerning the skinglobalantioxidantcapacity,thedetectionofthesedifferent moleculesbecameaveryimportantissuenotonlyforbiological
∗ Correspondingauthorat:CNRS,LAAS,7avenueducolonelRoche,F-31400 Toulouse,France.
E-mailaddress:temple@laas.fr(P.Temple-Boyer).
researchesbutalsoforroutineanalysis.Recently,non-integrated ultra-microelectrodes were developed and successfully applied to the skin analysis [14–16]. Nevertheless, in order to further improveskinelectrochemicalanalysis,microelectrodesfabrication processhad tobeimproved in ordertoensureminiaturization, massfabrication,reproducibility, reliability andeasiness ofuse. In order to obtain these specifications and develop integrated electrochemicalmicrocellscomprisedofathree-microelectrodes system,twodifferenttechnologieswereproposed.Firstly, screen-printing techniques were studied for the fabrication of “thick films”electrochemicalsensors[7,17].Unfortunately,theirspatial resolutionisnotefficientenoughtoreachmicroscalelevel. Sec-ondly,technologiesderivedfrommicroelectronicsweredeveloped [18]. By introducing photolithographic processes, silicon-based technologiesweresuccessfullyusedformassfabricationofthin metallic micro/nanoelectrodes and integration of electrochemi-calmicrocellsondifferentsubstrates(silicon,glass,polymers...) [19–23],dealingwithnumerousapplications:chemicaland bio-chemicaldetection[24–27],immunodetection[28],microfluidic electroanalysis [29–32], neurosciences [33,34],... Nevertheless, theso-fabricatedthinmetalliclayershavestilltobestudiedinorder tocontroltheirintrinsic electrochemicalcharacteristicsandthe relatedelectrochemicalmicrocellshavetobeoptimizedinorder tobefullycompatibleforskinelectrochemicalanalysis.
Thispaperdealswiththemassfabricationofintegrated, three-electrode (Pt–Pt–Ag/AgCl) and (Au–Pt–Ag/AgCl) electrochemical microcellsusingsilicon-basedmicrotechnologiesandtheir appli-cation in the detection of antioxidant species. To begin with, electrochemicalcharacteristicsofdifferentthinmetalliclayers,i.e.
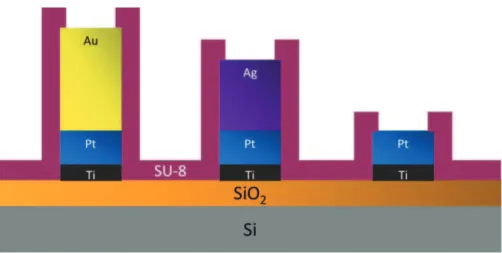
Fig.1.Schematicsofthe(Au–Pt–Ag)electrochemicalmicrocell.
gold,platinum and silver, were studied.Then, (Au–Pt–Ag/AgCl) electrochemicalmicrocellsareusedforthedetectionofascorbic anduricacidsinvarioussolutions.Finally,theiruseisextendedto theskinanalysis,focusingonthewafer-levelpassivationprocess improvementandexaminingtheskinglobalantioxidantcapacity measurement.
2. Experimental
2.1. Electrochemicalmicrocellsfabrication
(Pt–Pt–Ag/AgCl)and (Au–Pt–Ag/AgCl)electrochemical micro-cells (ElecCell) were fabricated on oxidized (oxide thickness: ∼1mm) silicon wafers. The different thin metallic layers were depositedbyevaporationinconventionalphysicalvapour depo-sition (PVD) equipments, and patterned using a bilayer lift-off processinordertoimprovefabricationreproducibility.ThreePVD processeswereperformedinarow:firstly,a200nmplatinumlayer wasdepositedona20nmtitaniumunderlayerinordertoensure platinum adhesiononsilicon oxide,followed by a800nmgold anda400nmsilverlayer.Thesedifferentthicknessesvalueswere choseninordertoenhanceadhesionpropertieswhilelimiting tech-nologicaldefectsrelatedtomechanicalstressandinter-diffusion phenomenaofmetallicatoms(Ti,Pt,AuandAg).
Finally,abiocompatibleSU-8passivationlayer(thickness:1.6 or3mm)wasdeposited atthewaferlevelusing photolithogra-phytechniques(Fig.1).SU-83005photoresist(purchasedfrom MicroChemCorporation)wasspin-coatedandasoftbakewasthen performedat95◦C.Thepolymercrosslinkingwasachieveddue toanultraviolet(UV)exposurewhileactivezonesweredefined usingaspecificphotolithographicmask.Apost-exposurebakewas performed at95◦C. Finally, after revealingthe different metal-licareas (Pt,Au,Ag)bythedevelopmentoftheactivezonesin
PGMEA(propylene glycol monomethyl ether acetate),an addi-tionalUVexposureandafinalhard-bakewereconductedtoensure theSU-8layercompletepolymerization.Thiswafer-level passiv-ationprocesswasusedtoinsulateelectricallythedifferentmetallic layersanddefinepreciselythedifferentactivesurfaces.Thegold orplatinumworkingmicroelectrodesweredefinedasdisksand theirelectroactiveareawasapproximately4.9×10−4mm2 (diam-eter:25mm).Incontrast,verylargesilver/silverchloridereference microelectrode(0.02mm2)andplatinumcountermicroelectrode (1mm2)werefabricated.(Pt–Pt–Ag)and(Au–Pt–Ag) electrochem-icalmicrodevicesweremanufacturedonsiliconchip(Fig.2).The wholechipwasthenplacedandgluedbyanepoxyinsulatingglue ona specificallycoated printed circuit, wire bondedand pack-aged at thesystem level using a silicone glop-top in order to befullycompatiblewithliquidphasemeasurement(Fig.2).For eachmicrodevice,thesilver/silverchlorideAg/AgClreferencewas finallyobtainedbyoxidizingthesilvermetalliclayerina0.01M KClsolution.Thisoxidationwasperformedbylinearvoltammetry (potentialscanrate:1mVs−1 between0.1and0.25V/SCE)using astandardsaturatedcalomelelectrode(SCE)Hg/Hg2Cl2/KClsatas reference.
2.2. Electrochemicalmicrocellscharacterization
All the electrochemical characterizations were held using a multi-channelVMPpotentiostatfromBiologic.Surfacesofthe“thin film” platinumand goldworkingmicroelectrodeswere electro-chemically characterized by cyclic voltammetryin a deaerated 0.5M H2SO4 solution (potential scan rate: 50mVs−1 between −0.25and+1.2V/SCE,andbetween−0.3and+1.6V/SCE respec-tively). Their active surface was then evaluated by plotting a current–potentialcurve(potentialscanrate:50mVs−1 between −0.5and+0.3V/SCE)inadeaerated,0.1M,pH7.0phosphatebuffer
Fig.3. Useofthe(Au–Pt–Ag/AgCl)electrochemicalmicrocellfortheskinanalysis.
solution(PBS)containing0.005MofferricyanideionsFe(CN)63−. Consideringasteady-stateregime,themicroelectroderadiuswas calculatedaccordingtotheSaïtorelation[35]:
Ilim=4nFDCr (1)
whereFistheFaradayconstant(F=96500Cmol−1),nisthe num-ber of electrons exchanged per mole (n=1), and C and D are theFe(CN)63− ionconcentrationand diffusioncoefficient,being estimated to5×10−3molL−1 and 7×10−6cm2s−1 respectively [36,37].
The electrochemical characteristics of the “thin-film” silver microelectrodeswerestudiedbycyclicvoltammetryinadeaerated 0.1MKNO3acid(pH3.5)solution(potentialscanrate:50mVs−1 between−1and+0.5V/SCE).Finally,theefficiencyandstabilityof theresultingAg/AgClreferencemicroelectrodewereevaluatedby monitoringtheNernstpotentialatequilibriumina0.01KCl solu-tionandcomparedwithastandard saturatedcalomelelectrode (SCE).
AllthechemicalreagentswerepurchasedfromSigma. 2.3. Electrochemicalcharacterizationofantioxidantspeciesin solutionandontheskin
Thewhole(Au–Pt–Ag/AgCl)ElecCellmicrosensorsweretested in an autonomous way, i.e. using the “threeintegrated micro-electrodes” configuration, for the detection of two antioxidant molecules:ascorbic(AA)anduric(UA)acids.Thiswasperformed bycyclicvoltammetry(potentialscanrate:50mVs−1between−0.2 and+0.8V)inaPBSpH7.0containingascorbicacidand/oruricacid (concentration:10−3M,purchased fromSigma). Theiranalytical performanceswerecomparedwithstandard“non-integrated”gold microelectrodepresentedinpreviouspapers[14,16].Forabetter comparisonof thedifferentamperometricresponses,the resid-ualvoltammogrammsobtainedinPBSwerededucedfromthose obtainedwithAAand/orUAspecies.
Finally, electrochemical characterization of antioxidant molecules was extended to skin analysis by applying directly the(Au–Pt–Ag/AgCl) ElecCellmicrosensors ontheforearmskin surface(Fig.3).
3. Resultsanddiscussion
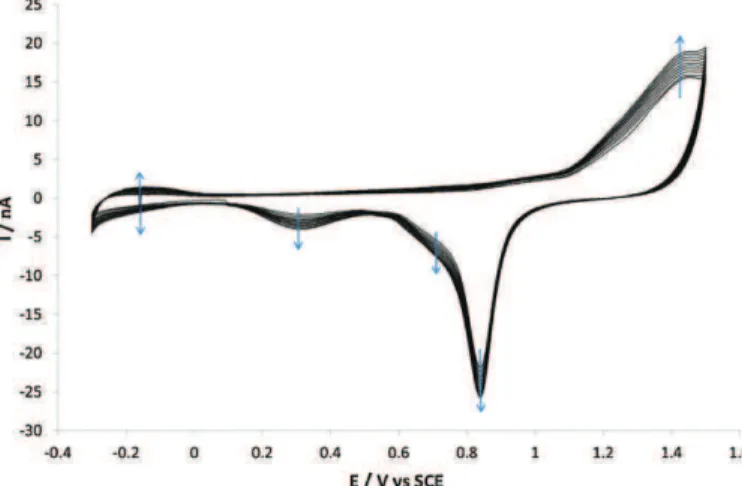
3.1. Electrochemicalcharacteristicsoftheplatinumthinfilm (Pt–Pt–Ag/AgCl) electrochemical microcells were first char-acterized by cyclic voltammetry. Fig. 4 shows the cyclic voltammogramtypicallyobtainedwiththe“thinfilm”platinum working microelectrode (SU-8 thickness: 1.6mm) in a 0.5M deaeratedH2SO4 solution.Thecurve shape isrepresentative of polycrystalline platinum [38–40]: the oxidation and reduction
Fig.4. Cyclicvoltammogramobtainedwiththe“thinfilm”platinumworking elec-trode(SU-8thickness:1.6mm)inadeaerated0.5MH2SO4solution(potentialscan
rate:50mVs−1).
peaksrelatedtothePtH2andPtHspeciesappearedinapotential rangebetween−0.25and0V.Theplatinumoxidationbeganaround 0.6Vandthesolventoxidationwasaround1.2V.Thepeakpotential correspondingtothereductionoftheplatinumoxidespreviously obtainedattheelectrodesurfacewasfoundaround0.45V.
Cyclicvoltammogramsrecordedwiththe“thinfilm”platinum workingmicroelectrodeina deaerated0.005MFe(CN)63− solu-tionyieldedasteady-statediffusion-limitedcurrentevenwithout stirring the solution and with a relatively high potential scan rateof 50mVs−1 (figure not shown). Accordingto Eq. (1), the Ilim experimental value was approximately34nA current. This valuecorrespondedtoanelectroactivesurfaceofapproximately 5×10−4mm2,i.e.toaradiusaround25mm,whichwasagreement withtheworkingmicroelectrodedimension.
Inconclusion,the“thinfilm”platinumlayerexhibited electro-chemicalpropertiessimilartothoseusuallydemonstratedonsolid platinumaswellaswell-definedelectroactivearea.
3.2. Electrochemicalcharacteristicsofthegoldthinfilm
Similarexperimentswereperformedforthe(Au–Pt–Ag/AgCl) electrochemical microcells and the associated “thin film” gold microelectrode. Fig. 5 shows successive cyclic voltammograms recordedinadeaerated0.5MH2SO4 solutionfora1.6mm-thick SU-8 passivationlayer. The curves demonstrated that the gold
Fig.5.Cyclicvoltammogramsobtainedwiththe“thinfilm”goldworkingelectrode (SU-8thickness:1.6mm)inadeaerated0.5MH2SO4solution(potentialscanrate:
Fig.6. Cyclicvoltammogramsobtainedwiththe“thinfilm”goldworkingelectrode (SU-8thickness:3mm)inadeaerated0.5MH2SO4solution(potentialscanrate:
50mVs−1).
oxidationwavebeganat1Vandthatpeakcorrespondingtogold oxidesreduction appearedcloseto0.85V [41,42].Nevertheless, someotheramperometricsignalsarealsoobservedat−0.2and 1.3Vintheanodicpartofthecurveaswellasat0.3and−0.2V duringthecathodicscan.Allthesesignalsincreasedwiththe num-berofpotentialcycles(seeevolutionarrowsonFig.5).Sincethese unexpectedcurves werenotevidenced forthe“thin-film” plat-inummicroelectrode(seeSection3.1),theycanbeattributedto theAu/SU-8interfacephenomena.Ontheonehand,non-adhesion oftheSU-8filmongoldaswellaspoorstepcoverageduetothe Ti/Pt/Austackinghigherthickness(∼1000nmratherthan∼200nm for the Ti/Pt one) is responsible to passivation defects, allow-ingthetitanium/platinumunderlayertobeincontact withthe electrolyticsolution[43].Ontheotherhand,thesulphur-based tri-arisulfoniumsaltsoftheSU-8resinareelectrochemicallyactive onthegold surface[44].Thiswas confirmedbycyclic voltam-metryperformed onSU-8/cyclopentanone solutions (figure not shown).
Inordertoreducebothredoxphenomena,twodevelopments wereproposed.Firstly,theSU-8polymerizationprocesswas opti-mized in terms of exposure dose and annealing duration to counteractanyelectrochemicalinterference.Secondly, theSU-8 thicknesswasincreasedfrom1.6to3mmtoimprovestep cover-ageandavoidanycontactbetweenthemetallicunderlayersand theelectrolyticsolution.Fig.6showstheresultingcyclic voltam-mograminthesamedeaerated0.5MH2SO4solution.Thecurvewas verysimilartothattypicallyrecordedwithsolidgoldwhilethe cur-rentpeakscorrespondingtotheoxidationofgoldandthereduction ofgoldoxideswereonlyobservedinthiscase.ComparedtoFig.5, lowercurrentlevelswereevidenced,demonstratingthatabetter controlofthegoldmicroelectrodeelectrochemicalactiveareawas obtained.Thiswasconfirmedbycharacterizingbycyclic voltam-metrytheseoptimised“thinfilm”goldworkingmicroelectrodesin adeaerated0.005MFe(CN)63− solution(resultnotshown). Sim-ilarlytothe“thinfilm”platinummicroelectrode(Section3.1), a steady-statediffusion-limitedcurrentof32nAwasrecorded.As expected,accordingtoEq.(1),theworkingelectrodeelectroactive surfaceandradiuswereestimatedto4.3×10−4mm2and23.5
mm respectively.
So, by improving the SU-8 polymerisation process and by increasing the SU-8 passivation thickness from 1.6 to 3mm, “thinfilm”goldworkingmicroelectrodeswithgood electrochem-ical properties and well-defined active area, were successfully obtained.Finally,itshouldbementionedthattheincreaseinSU-8 thicknessfrom1.6to3micrometersdidnotinduceanydegradation
Fig.7.Cyclicvoltammogramofthe“thinfilm”silverelectrode(SU-8thickness: 3mm)inadeaerated0.1MKNO3solution(potentialscanrate:50mVs−1).
ofthe“thinfilm”platinumworkingmicroelectrodes(cf.Section 3.1).
3.3. StudyoftheAg/AgClreferencemicroelectrode
Cyclicvoltammogramstypicallyobtainedwiththe“thin-film” silver microelectrodes (SU-8 passivation thickness: 3mm)were recordedinadeaerated0.1MKNO3acid(pH3.5)solution(Fig.7). Typicalcurveswereobtained:thesilveroxidation startsaround 0.3V,theassociatedreductionwasclearlyshownaround0.4Vand thefinalprotonreductionisevidencedunder−0.5V.
Finally,thetemporalstabilityofthe“thinfilm”Ag/AgCl refer-encemicroelectrodewasstudiedbymeasuringitspotentialusing theSCE referenceelectrode in a 0.01 KClsolutionfor a 10min duration.TheAg/AgClmicroelectrodepotentialatequilibriumwas foundtobequitestablearound0.1225V(resultnotshown).This valueishigherthanthetheoreticalonegivenbytheNernstlaw,i.e. 0.098V.SuchdiscrepancywasrelatedtoAgCllayerdefectsandto chemicalactivitiesofchlorideionsCl−and/orAg-relatedspecies [45,46].Nevertheless,afteraninitialdecreasearound0.5mV dur-ing1min,thepotentialdriftoftheAg/AgClintegratedelectrode wasverylow(around1mVs−1),inagreementwithliterature[46]. 3.4. Detectionofantioxidantspecies
(Au–Pt–Ag/AgCl)electrochemicalmicrocells(SU-8passivation layerthickness:3mm)wereappliedforthedetectionof antioxi-dantmolecules,andmorepreciselyascorbicanduricacids(AAand UA),inpH7.0phosphatebuffersolution(PBS).Allthecyclic voltam-mogramswereobtainedinanautonomousway,i.e.usingthe“three integratedmicroelectrodes”configuration.Inallcases,no signifi-cantsignalwasobservedinPBSpH7.0exceptforthegoldoxidation starting around 0.8V. This PBS-related residual voltammogram wassystematicallydeducedfromthatobtainedwithantioxidant moleculesinordertoimproveresultsanalysis.
The ElecCellmicrosensorwasfirst usedfor thedetection of ascorbicacid(AA–0.001M)anduricacid(UA–0.001M) sepa-rately(Figs.8and9).Inbothcases,cyclicvoltammogramsevidence an oxidation wave starting around0V for theAA solution and around0.3VfortheUAone[47,48].Comparedtothesolidgold microelectrodeand whatever theantioxidant species, the Elec-Cellmicrosensorresponsesshowedsimilarhalf-wavepotentialE1/2 andhigherdiffusioncurrentdensityilim,i.e.thusprovidinghigher sensitivity(Table1).Suchenhancementshouldberelatedtothe electrocatalyticpropertiesofsolidgoldelectrodeandthinmetallic layerdepositedbyPVD,respectivelyandcouldresultfromchange
Fig.8. Cyclicvoltammogramofthe(Au–Pt–Ag/AgCl)electrochemicalmicrocell (solidline)andofthestandard“nonintegrated”goldelectrode(dottedline)ina PBSpH7.0solutioncontainingAA0.001M(potentialscanrate:50mVs−1).
Fig.9. Cyclicvoltammogramofthe(Au–Pt–Ag/AgCl)electrochemicalmicrocell (solidline)andofthestandard“nonintegrated”goldelectrode(dottedline)ina PBSpH7.0solutioncontainingUA0.001M(potentialscanrate:50mVs−1).
incrystallographicorientationorsurfaceroughness.Anyway,the useof(Au–Pt–Ag/AgCl)ElecCellmicrosensorsforthedetectionof ascorbicand/oruricacidsinliquidphasewassuccessfully demon-strated.
Finally, the ElecCell microsensor was studied in phosphate buffersolutioncontainingbothascorbicand uricacids(AA+UA – 0.001M(Fig.10).Asexpected and similarlyto thatobtained withthestandard“nonintegrated”goldelectrode,cyclic voltam-mograms evidenced two oxidation waves related to the two antioxidant species. Asbefore, slightly higher diffusion current Ilim,andthereforehigherdetectionsensitivity,wereobtainedwith theElecCellmicrosensor,resultinginhighersensitivity(Table1). Nevertheless,thesewavesarenotclearlydefinedandare there-foredifficulttoseparate.Half-wavepotentialE1/2 wereroughly defined,leadingtolowdetectionselectivitybetweenascorbicand uric acids[49,50].This problemcouldbesolved by developing
Fig.10.Cyclicvoltammogramofthe(Au–Pt–Ag/AgCl)electrochemicalmicrocell (solidline)andofthestandard“nonintegrated”goldelectrode(dottedline)ina PBSpH7.0solutioncontainingAAandUA0.001M(potentialscanrate:50mVs−1).
specifiedfunctionalizationbasedonelectroactivepolymerssuch aspoly(3,4-ethylenedioxythiophene)(PEDOT)[16].Worksarein progressinthisway.
3.5. Skinanalysisfortheantioxidantcapacitymeasurement The(Au–Pt–Ag/AgCl) electrochemicalmicrocells werefinally testedforskinanalysisinordertodeterminetheglobal antioxi-dantcapacitydetectionfeasibility.Theexperimentalprotocolwas quitesimplesincetheElecCellmicrodeviceswereapplieddirectly ontheforearmwithoutanypre-treatment(Fig.3).Themain prin-ciplewastousethe“stratumcorneum”naturalhydrolipidicfilmto guaranteetheelectricalcontactbetweentheworking,counterand referencemicroelectrodes[51].
Logically, the ElecCellmicrosensors with a 3mm-thick SU-8 passivationlayerwerefirststudied.Inthiscase,no amperomet-ric signal was evidenced on the skin while performing cyclic voltammetryexperiment.However,assoonasfewpH7.0 phos-phatebuffersolution(PBS)droplets weredepositedontheskin surface, thetypicalgold voltammogram wasfoundagain.Such resultdemonstratesthatthehydrolipidicfilmwasnotsufficient toprovidetheelectricalcontactbetweenthedifferent microelec-trodessinceitsthicknessisknowntobearoundonemicron[51,52]. Inordertotackleoffthisbottleneckandtoimprovetheelectrical contactontheskinsurface,themicroelectrodesrecessand there-foretheSU-8layerthicknessmustbedecreased.So,eveniftheir electrochemicalperformanceswerelower,theElecCell microsen-sorswitha 1.6mm-thickSU-8passivationlayerwerealsoused for theskinanalysis usingcyclic voltammetry(Fig.11). In this case,thankstothepassivationthicknessdecrease,agood electri-calcontactwasobtainedwiththehydrolipidicfilm.Furthermore, noelectrochemicalinterferencewasevidenced(cf.Section3.2). This improved electrical behaviour was related to the hydroli-pidicfilmphysicalproperties.Comparedtowater-basedsolutions, thisemulsion-like,two-dimensionalmediumischaracterizedby ahigherviscosity,lowerdiffusivitiesandahigherelectrical resis-tivity[51,52].Thus,obtainedvoltammogramsweretypicalofthe Table1
Characteristicsofthe(Au–Pt–Ag/AgCl)electrochemicalmicrosensorresponsesforthedetectionofascorbicand/oruricacids.
Ascorbicacid(AA) Uricacid(UA) Ascorbicanduricacids(AA+UA) ElecCellmicrosensor E1/2(V/SCE) 0.47±0.08 0.51±0.03 0.31±0.03/0.53±0.01
ilim(mAcm−2) 0.9±0.1 1.1±0.3 0.69±0.04/0.91±0.07
Standardgoldelectrode E1/2(V/SCE) 0.35±0.02 0.48±0.02 0.31±0.03/0.52±0.02 ilim(mAcm−2) 0.73±0.08 0.67±0.05 0.6±0.05/0.81±0.07
Fig.11.Cyclicvoltammogramofthe(Au–Pt–Ag/AgCl)electrochemicalmicrocellon theskinsurface(potentialscanrate:50mVs−1).
electrochemicalskinproperties[14,53].Theascorbicacidoxidation wasresponsibleforthefirstcurrentwavearound0.6V/SCE.Then, thesecondwavearound0.8V/SCEwasrelatedtouricacid, nicotin-amideadeninedinucleotidephosphate(NADPH)andcystein.The lastwavearound1.2V/SCEwasassociatedtotheglutathione oxi-dation.Finally,thereductionpeakobservedaround0.4V/SCEwas duetothegoldoxides.
Allinall,bydecreasingtheSU-8layerthicknessandtherefore themicroelectrodesrecessto1.6mm,theskinhydrolipidicfilmwas sufficienttoobtainagoodelectricalcontactbetweenthedifferent microelectrodesandtodetectelectrochemicallydifferent antioxi-dantspeciespresentontheskinsurface.
4. Conclusion
(Pt–Pt–Ag/AgCl)and (Au–Pt–Ag/AgCl)electrochemical micro-cells (ElecCell)werefabricatedusing silicon-based technologies derivedfrommicroelectronicsinordertodetectredoxspecies,and morespecificallyantioxidantmoleculessuch asascorbicand/or uricacidsinliquidphase.Whatismore,electrochemicalanalysisfor theskinglobalantioxidantcapacitymeasurementwasperformed. SpecificemphasiswasplacedontheSU-8wafer-levelpassivation process. In order tooptimize theelectrochemical properties of thedifferent“thinfilm”metalliclayers,i.e.goldfortheworking electrode,platinumforthecounterelectrodeandsilver/silver chlo-rideforthereferenceelectrode,itwasnecessarytoincreasethe SU-8layerthicknessupto3mm.Thus,theElecCellmicrosensors werestudiedforthedetectionofascorbicanduricacidsin phos-phatebuffersolution.Comparedtostandard“nonintegrated”gold electrodes,similarresponseswereshownbycyclicvoltammetry, evidencinghigherdetectionsensitivitybutlowdetection selectiv-ity.
Workswereextendedtoskinanalysis.Inthiscase,the3 mm-thickSU-8layerwasresponsibleforaveryhighmicroelectrode recesstoprovideagoodelectricalcontactwiththeskin hydroli-pidic film. However, using a non-optimized 1.6mm-thick SU-8 layer,oxidationwavesrelatedtodifferentantioxidantspecieswere successfullyobtainedontheskin,demonstratingthatthestratum corneumglobalantioxidantcapacitycanbeeffectivelymeasured.
Inordertouse(Au–Pt–Ag/AgCl)ElecCellmicrosensorsforthe skinanalysis, a compromise hasto befoundon theSU-8 pas-sivation process.On theone handandas demonstratedforthe liquidphaseanalysis,ahighSU-8thickness,i.e.3mmandmore, isresponsibleforacorrectstepcoverageofthedifferentmetallic layersand,consequently,forgoodelectrochemicalpropertiesand
well-controlledactiveareaoftherelatedmicroelectrodes.Onthe otherhand,alowSU-8thickness,i.e.1.6mmorless,isstillrequired forobtainingalowmicroelectroderecessand,finally,toprovide thenecessaryelectricalcontactwiththeskinhydrolipidicfilm.This hastobefurtherstudiedintheframeofwafer-levelpassivation processes(basedonSU-8polymersand/orothersbiocompatible materials).
References
[1]R.Feeney,S.P.Kounaves,Microfabricationofultramicroelectrodearrays: devel-opments,advancesandapplicationsinenvironmentalanalysis,Electroanalysis 12(2000)677–684.
[2]J.Castillo,S.Gaspar,S.Leth,M.Niculescu,A.Mortari,I.Bontidean,V.Soukharev, S.A.Dorneanu,A.D.Ryabov,E.Csöregi,Biosensorsforlifequality– design, developmentandapplications,SensorsandActuatorsB102(2004)179–194. [3]I.J.Allan,B.Vrana,R.Greenwood,G.A.Milles,B.Roig,C.Gonzales,Atoolbox
forbiologicalandchemicalmonitoringrequirementsfortheEuropeanUnion’s waterframeworkdirective,Talanta69(2006)302–322.
[4] C.Spegel,A.Heiskanen,L.H.D.Skjolding,J.Emneus,Chipbasedelectroanalytical systemsforcellanalysis,Electroanalysis20(2008)680–702.
[5]I.E.Tothill,Biosensorsforcancermarkerdiagnosis,SeminarsinCell& Devel-opmentalBiology20(2009)55–62.
[6] S.A.Wring,J.P.Hart,Chemicallymodified,carbon-basedelectrodesandtheir applicationaselectrochemicalsensorsfortheanalysisofbiologicallyimportant compounds:areview,Analyst117(1992)1215–1229.
[7]S.Lashi,M.Mascini,Planarelectrochemicalsensorsforbiomedicalapplications, MedicalEngineeringandPhysics28(2006)934–943.
[8]J.Wang,AnalyticalElectrochemistry,thirdedition,JohnWiley&Sons,Inc, Hoboken,2006.
[9]R. Kohen,Skin antioxidants:their rolein aging and inoxidative stress, newapproachesfortheirevaluation,BiomedicalPharmacotherapy53(1999) 181–192.
[10]C.S.Sander,H.Chang,F.Hamm,P.Elsner,J.J.Thiele,Roleofoxidativestressand theantioxidantnetworkincoetaneouscarcinogenesis,InternationalJournalof Dermatology43(2004)326–335.
[11] S.Arbault,N.Sojic,D.Bruce,C.Amatore,A.Sarasin,M.Vuillaume, Oxida-tivestressincancerpronexerodermapigmentosumfibroblasts:real-timeand singlecellmonitoringofsuperoxideandnitricoxideproductionwith micro-electrodes,Carcinogenesis25(2004)509–515.
[12] D.R.Bickers,M.Athar,Oxidativestressinthepathogenesisofskindisease, JournalofInvestigativeDermatology126(2006)2565–2575.
[13]J.J.Thiele,L.Packer,Antioxidantsdefensesystemsinskin,JournalofToxicology: CutaneousandOcular21(2002)119–160.
[14] A. Ruffien-Ciszak, P.Gros, M.Comtat,A.M.Schmitt,E. Questel,C. Casas, D.Redoules,Explorationoftheglobalantioxidantcapacityofthestratum corneumbycyclicvoltammetry,JournalofPharmaceuticalandBiomedical Analysis40(2006)162–167.
[15] A.M.Bonastre,P.N.Bartlett,ElectrodepositionofPANIfilmsonplatinum nee-dletypemicroelectrodes.Applicationtotheoxidationofascorbateinhuman plasma,AnalyticaChimicaActa676(2010)1–8.
[16] F.SekliBelaidi,P.Temple-Boyer,P.Gros,Voltammetricmicrosensorusing PEDOTmodifiedgoldelectrodeforthesimultaneousassayofascorbicanduric acids,JournalofElectroanalyticalChemistry647(2010)159–168.
[17]X.Xu,S.Zhang,H.Chen,J.Kong,Integrationofelectrochemistryin micro-totalanalysissystemsforbiochemicalassays:recentdevelopments,Talanta 80(2009)8–18.
[18]G.C.Fiaccabrino,M.Koudelka-Hep.,Thin-filmmicrofabricationof electrochem-icaltransducers,Electroanalysis10(1998)217–222.
[19]J.W.Schultze,V.Tsakova,Electrochemicalmicrosystemtechnologies:from fundamentalresearchtotechnicalsystems,ElectrochimicaActa44(1999) 3605–3627.
[20]K.Stulik,C.Amatore,K.Kolub,V.Marecek,W.Kutner,Microelectrodes: defini-tions,characterizationandapplications,PureandAppliedChemistry72(2000) 1483–1492.
[21]H.Suzuki,Advancesinthemicrofabricationofelectrochemicalsensorsand systems,Electroanalysis12(2000)703–715.
[22]B.Lakard,J.C.Jeannot,M.Spajer,G.Herlem,M.deLabachelerie,P.Blind,B. Fahys,Fabricationofaminiaturizedcellusingmicrosystemtechnologiesfor electrochemicalapplications,ElectrochimicaActa50(2005)1863–1869. [23]Y.P.Chen,Y.Zhao,J.Chu,S.Y.Liu,W.W.Li,G.Liu,Y.C.Tian,Y.Xiong,H.Q.Yu,
Fabricationandcharacterizationofaninnovativesolid-statemicroelectrode, ElectrochimicaActa55(2010)5984–5989.
[24]A.Schwake,B.Ross,K.Camman,Chronoamperometricdeterminationof hydro-genperoxideinswimmingpoolwaterusinganultra-microelectrodearray, SensorsandActuatorsB46(1998)242–248.
[25]K.Morimoto,S.Upadhyay,T.Higashiyama,N.Ohgami,H.Kusakabe,J.Fukuda, H.Suzuki,Electrochemicalmicrosystemwithporousmatrixpacked-bedsfor enzymeanalysis,SensorsandActuatorsB124(2007)477–485.
[26] M.Miyashita,N.Ito,S.Ikeda,T.Murayama,K.Oguma,J.Kimura, Develop-mentofurineglucosemeterbasedonmicro-planaramperometricbiosensor anditsclinicalapplicationforself-monitoringofurineglucose,Biosensorsand Bioelectronics24(2009)1336–1340.
[27]D.Kim,I.B.Goldberg,J.W.Judy,Microfabricatedelectrochemicalnitratesensor usingdouble-potential-stepchronocoulometry,SensorsandActuatorsB135 (2009)618–624.
[28]C.O.Parker,Y.H.Lanyon,M.Manning,D.W.M.Arrigan,I.E.Tothill, Electrochem-icalimmunochipsensorforaflatoxinM1detection,AnalyticalChemistry81 (2009)5291–5298.
[29]N.Triroj,M.A.Lapierre-Devlin,S.O.Kelley,R.Beresford,Microfluidic three-electrodecellarrayforlow-currentelectrochemicaldetection,IEEESensors Journal6(2006)1395–1402.
[30] R.Popovtzer,T.Neufeld,A.Popovtzer,I.Rivkin, R. Margalit,D.Engel,A. Nudelman, A.Raphaeli,J. Rishpon,Y. Shacham-Diamand.,Electrochemical labonachipforhigh-throughputanalysisofanticancerdrugsefficiency, Nanomedecine:NBM4(2008)121–126.
[31] N.Pereira-Rodriguez,Y.Sakai,T.Fujii,Cell-basedmicrofluidicbiochipforthe electrochemicalreal-timemonitoringofglucoseandoxygen,Sensorsand Actu-atorsB132(2008)608–613.
[32]R.S. Pai, K.M. Walsh, M.M. Crain,T.J. Roussel, D.J.Jackson, R.P. Baldwin, R.S.Keynton,J.F.Naber,Fullyintegrated three-dimensionalelectrodesfor electrochemicaldetection inmicrochips: fabrication,characterization and applications,AnalyticalChemistry81(2009)4762–4769.
[33] O.Frey,P.D.vanderWal,S.Spieth,O.Brett,K.Seidl,O.Paul,P.Ruther,R. Zengerle,N.F.deRooij,Biosensormicroprobeswithintegratedmicrofluidic channelsforbi-directionalneurochemicaldetection,JournalofNeural Engi-neering8(2011)1–9.
[34]A.Altuna,L.MenendezdelaPrida,E.Bellistri,G.Gabriel,A.Guilera,J.Berganzo, R.Vila,L.J.Fernandez,SU-8basedmicroprobeswithintegratedplanar elec-trodesforenhancedneuraldepthrecording,BiosensorsandBioelectronics37 (2012)1–5.
[35]A.Saito,Atheoreticalstudyofthediffusioncurrentatthestationaryelectrodes ofcircularandnarrowbandtypes,ReviewofPolarography15(1968)177–187. [36]S.J.Konopka,B.McDuffie.,Diffusioncoefficientsofferri-andferro-cyanideions inaqueousmediausingtwin-electrodethin-layerelectrochemistry,Analytical Chemistry42(1970)1741–1745.
[37]J.Legrand,E.Dumont,J.Comiti,F.Fayolle,Diffusioncoefficientsofferricyanide ionsinpolymericsolutions–comparisonofdifferentexperimentalmethods, ElectrochimicaActa45(2000)1791–1803.
[38]H.Angerstein-Kozlowska,B.E.Conway,W.B.A.Sharp,Therealconditionof oxi-dizedPtelectrodes:partI,JournalofElectroanalyticalChemistry43(1973) 9–36.
[39] B.V.Tilak,B.E.Conway,H.Angerstein-Kozlowska.,Therealconditionof oxi-dizedPtelectrodes:partIII,JournalofElectroanalyticalChemistry48(1973) 1–23.
[40]J.Barber,S.Morin,B.E.Conway,SpecificityofthekineticsofH2evolutiontothe
structureofsingle-crystalPtsurfaces,JournalofElectroanalyticalChemistry 446(1998)125–138.
[41]H.Angerstein-Kozlowska,B.E.Conway,B.Barnett,J.Mozota,Theroleofion adsorptioninsurfaceoxideformationandreductionatnoblemetals: gen-eralfeaturesofthesurfaceprocess,JournalofElectroanalyticalChemistry100 (1979)417–446.
[42]A.Berduque,B.Y.H.Layon,V.beni,G.Herzog,Y.E.Watson,K.Rodgers,F.Stam, J.Alderman,D.W.M.Arrigan,Voltammetriccharacterizationofsilicon-based microelectrodearraysandtheirapplicationtomercury-freestripping voltam-metryofcopperions,Talanta71(2007)1022–1030.
[43]H.Möller,P.C.Postorius,Theelectrochemistryofgold-platinumalloys,Journal ofElectroanalyticalChemistry570(2004)243–255.
[44]S.P.Pappas,Photoinitiationofcationicandconcurrentradicalcationic poly-merization,ProcessinOrganicCoatings13(1985)35–64.
[45]B.J.Polk,A.Stelzenmuller,G.Mijares,W.MacCrehan,M.Gaitan,Ag/AgCl micro-electrodeswithimprovedstabilityformicrofluidics,SensorsandActuatorsB 114(2006)239–247.
[46]M.W.Shinwari,D.Zhitomirsky,I.A.Deen,P.R.Selvaganapathy,M.J.Deen,D. Landheer,Microfabricatedreferenceelectrodesandtheirbiosensing applica-tions,Sensors10(2010)1679–1715.
[47] A.M.Sullivan,P.A.Kohl,Electro-oxidationofascorbicacidinanaqueouscitrate buffersolution,PlatingandSurfaceFinishing85(1998)56–60.
[48]Y.C.Luo,J.S.Do,C.C.Liu,Anamperometricuricacidbiosensorbasedonmodified Ir–Celectrode,BiosensorsandBioelectronics22(2006)482–488.
[49]Y. Zhao, J. Bai, L. Wang,X.E.P. Huang,H. Wang, L.Zhang,Simultaneous electrochemicaldeterminationofuricacidandascorbicacidusingl-cystein self-assembledgoldelectrode,InternationalJournalofElectrochemicalScience 1(2006)363–371.
[50]L.Wang,J.Y.Bai,P.F.Huang,H.J.Wang,X.W.Wu,Y.Q.Zhao,Selective deter-minationofuricacidinthepresenceofascorbicacidusingapenicillamine self-assembledgoldelectrode,MicrochimicaActa158(2007)73–78. [51] H.M.Sheu,S.C.Chao,T.W.Wong,J.Y.Y.Lee,J.C.P.Tsai,Humanskinsurfacelipid
film:anultrastructuralstudyandinteractionwithcorneocytesand intercellu-larlipidlamellaeofthestratumcorneum,BritishJournalofDermatology140 (1999)385–391.
[52] C.Pailer-Mattei,S.Nicoli,R.Pirot,R.Vargiolu,H.Zahouani,Anewapproachto describetheskinsurfacephysicalpropertiesinvivo,ColloidsandSurfacesB68 (2009)200–206.
[53] R.Kohen,E.Vellaichamy,J.Hrbac,I.Gati,O.Tirosh,Quantificationoftheoverall reactiveoxygenspeciesscavengingcapacityofbiologicalfluidsandtissues, FreeRadicalBiologyandMedicine28(2000)871–879.
Biographies
C.ChristophewasbornonDecember16,1981.ShereceivedherEngineer’sDegree inmaterialssciencefromthe“InstitutNationalPolytechniquedeToulouse”(France) in2006.Shejoinedthe“Laboratoired’Architectureetd’AnalysedesSystèmes”from theFrench“CentreNationaldelaRechercheScientifique”(LAAS-CNRS)in2007.She isworkingonthedevelopmentofelectrochemicalmicrosensorsforchemicaland biochemicaldetection.
F.SékliBelaidiwasbornonFebruary22,1980.ShereceivedherMaster’sDegree inprocessandenvironmentalengineeringfromthe“InstitutNationaldesSciences AppliquéesdeToulouse”(France)in2006.Shejoinedthe“LaboratoiredeGénie Chimique”(LGC)fromtheUniversityofToulouse(France)in2007.Sheisworking onthedevelopmentofelectrochemicalmicrosensorsforchemicalandbiochemical detection.
J.LaunaywasbornonMarch11,1975.Hereceivedthedegreeinelectronic engi-neeringfromtheInstitutNationaldesSciencesAppliquéesdeToulouse”(France)in 1998.Hejoinedthe“Laboratoired’Architectureetd’AnalysedesSystèmes”from theFrench“CentreNationaldelaRechercheScientifique”(LAAS-CNRS)in1998 andreceivedthePhDdegreefromthe“InstitutNationaldesSciencesAppliquées deToulouse”(France)in2001.In2002,hebecamelecturerattheUniversityof Toulouse(France).Hisresearchactivitiesincludethedevelopmentof electrochem-icalmicrosensorsforthedetectioninliquidphase.
P.Groswasbornin1970.HegraduatedinPhysicalChemistryin1992andreceived hisPhDdegreeinChemicalEngineeringin1996attheUniversityPaulSabatierin Toulouse.HeisnowProfessorinElectroanalyticalEngineeringintheChemical Engi-neeringLaboratory(Toulouse-France).Heiscurrentlyworkingonthedevelopment ofelectrochemical(bio)sensors.
E.QuestelwasbornonOctober16,1965.HereceivedhisEngineer’sDegreein molecularandsupramolecularchemistryfromtheUniversityofToulouse(France)in 1992.HejoinedtheFrench“GroupedeRecherchesurl’Oncogénèse,lesUltraviolets etlaPigmentationCutanée”in1994.In1997,hejoinedtheFrench“Laboratoire PierreFABREDermocosmetique”companyinToulouse.Since2004,heisincharge ofthe“SkinPhotobiologydepartment”.Heisworkingonclinicalstudiesonhealthy volunteers.
P.Temple-Boyer was born onOctober 25, 1966. Hereceived his Engineer’s Degreeinelectronicengineeringfromthe“EcoleSupérieured’Electricité”(Paris– France)in1990andhisMaster’sDegreeinmicroelectronicsfromtheUniversityof Toulouse(France)in1992.Hejoinedthe“Laboratoired’Architectureetd’Analyse desSystèmes”(LAAS)fromtheFrench“CentreNationaldelaRecherche Scien-tifique”(CNRS)in1992andreceivedthePhDdegreefromthe“InstitutNational desSciencesAppliquéesdeToulouse”(France)in1995.Sincethen,asaCNRS researcher,hehasworkedatLAASonthedevelopmentofphysicalandchemical microsensors.