Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez
pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the
first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Journal of Cerebral Blood Flow and Metabolism, 18, pp. 396-406, 1998-04-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=10063d1d-6355-44e5-9bd8-b8a9e349e82d
https://publications-cnrc.canada.ca/fra/voir/objet/?id=10063d1d-6355-44e5-9bd8-b8a9e349e82d
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. /
La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version
acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien
DOI ci-dessous.
https://doi.org/10.1097/00004647-199804000-00008
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Evidence that functional glutamate receptors are not expressed on rat
or human cerebromicrovascular endothelial cells
Morley, P.; Small, D.; Murray, C.; Mealing, Geoffrey; Poulter, M.; Durkin, J.;
Stanimirovic, Danica
Jounal of Cerebal Blood Flow and Metabolism
18:396406 © 1998 The Intenational Society of Cerebral Blood Flow and Metabolism Published by Lippincott-Raven Publishers. Philadelphia
Evidence That Functional Glutamate Receptors Are Not
Expressed on Rat or Human Cerebromicrovascular
Endothelial Cells
Paul Morley, Daniel L. Small, Christine L. Murray, Geoffrey A. Mealing, Michael O. Poulter,
Jon P. Durkin, and Danica B. Stanimirovic
Cellular Neurobiology GrouP. Institute for Biological Sciences. National Research Council of Canada, Ottawa, Ontario, Canada
Summary: Excitatory amino acids can modify the tone of ce rebral vessels and permeability of the blood-brain barrier (BBB) by acting directly on endothelial cells of cerebral vessels or indirectly by activating receptors expressed on other brain cells. In this study we examined whether rat or human cere bromicrovascular endothelial cells (CEC) express ionotropic and metabotropic glutamate receptors. Glutamate and the glu tamate receptor agonists N-methyl-d-aspartate (NMDA), l am in 0-3 -hydroxy -5 -meth y 1-i soxazole-4-propionic acid (AMPA), and kainate failed to increase [Ca2+li in either rat or human microvascular and capillary CEC but elicited robust responses in primary rat cortical neurons, as measured by fura-2 luorescence. The absence of NMDA and AMPA recep tors in rat and human CEC was further confirmed by the lack of immunocytochemical staining of cells by antibodies specific for the AMPA receptor subunits GluRI, GluR2/3, and GluR4 and the NMDA receptor subunits NRI, NR2A, and NR2B. We failed to detect mRNA expression of the AMP A receptor sub units GluR I to GluR4 or the NMDA receptor subunits NRI\xx, NRloxx' and NR2A to NR2C in both freshly isolated
Whereas the roles of glutamate in regulating physi ological responses (Collingridge and Singer, 1990) and mediating excitotoxic injury (Meldrum and Garthwaite, 1990; Small and Buchan, 1996) to neurons have been
Received February 3,1997; inal revision received October 9,1997; accepted October 14, 1997.
This project was approved by the McGill University and National Research Council (NRC) of Canada Human Subjects Research Ethics Committees and the NRC Animal Care Committee. This work was supported by the NRC and by a grant from the Heart and Stroke Foundation of Ontario (ST2717 to P. Morley); D.L. Small is supported by a fellowship from the Heart and Stroke Foundation of Ontario.
Address correspondence and reprint requests to Dr. Paul Morley, Cellular Neurobiology Group, Institute for Biological Sciences, Na tional Research Council of Canada, Bldg. M-S4, Montreal Road Cam pus, Ottawa, Ontario, Canada KIA OR6.
Abbreviations used: IS, 13R-trans-ACPD, l-amino-cyciopentyl-lS, 3R-dicorboxylate; AMP A, t -amino-3-hydroxy-S-methyl-isoxazole-4-propionic acid; BBB, blood-brain barrier; CEC, cerebromicrovascular endothelial cells; IP3, inositol trisphosphate; NMDA, N-methyl-D aspartate; PBS, phosphate-bufered saline; RT -PCR, reverse transcrip tase polymerase chain reaction.
396
rat and human microvessels and cultured CEC using reverse transcriptase polymerase chain reaction (RT-PCR). Cultured rat CEC expressed mRNA for KAI or KA2 and GluR5 subunits. Primary rat cortical neurons were found to express GluRI to GluR3 and NRI, NR2A, and NR2B by both immunocytochem istry and RT-PCR and KAI, KA2, GluR5, GluR6, and GluR7 by RT-PCR. Moreover, the metabotropic glutamate receptor agonist l-amino-cyclopentyl-IS, 3R-dicorboxylate (IS,3R tans-ACPD), while eliciting both inositol trisphosphate and [Ca2+li increases and inhibiting forskolin-stimulated cyclic AMP in cortical neurons, was unable to induce either of these responses in rat or human CEC. These results strongly suggest that both rat and human CEC do not express functional gluta mate receptors. Therefore, excitatory amino acid-induced changes in the cerebral microvascular tone and BBB perme ability must be affected indirectly, most likely by mediators released from the adjacent glutamate-responsive cells. Key Words: Cerebral endothelial cells-Glutamate receptors Ischemia-Calcium-Reverse transcriptase polymerase chain reaction.
established, the possible involvement of glutamate in modulating cerebrovascular functions is less clear. Nu merous reports have described various modes of vasoac tive action of glutamate agonists or antagonists in differ ent segments of the cerebral vasculature in vivo and on isolated vessels (Fukuda et aI., 1983; Cavazutti et aI., 1987; Busija and Leler, 1989; Perkins et aI., 1989; Faraci and Breese, 1993; Wendling et aI., 1994). Studies using isolated cerebral microvessels reported that bovine and ovine cerebral microvessels lack binding sites for the N-methyl-D-aspartate (NMDA) antagonists 2-amino-5-phosphonopentanoic acid, ketamine, MK-801, and dex trorphan (Beart et aI., 1988; Wendling et aI., 1996), whereas Koenig et aI. (1992) suggested that capillary NMDA receptors may regulate blood-brain barrier (BBB) breakdown through the activation of a polyamine cascade. However, none of these studies was able to resolve the issue of the primary site(s) of action of glu tamate in the cerebral vasculature.
GLUTAMATE RECEPTORS ON BRAIN ENDOTHELIAL LS 397
Due to the anatomical proximity of glutamate releasing neurons to the cerebral capillaries and mi crovessels, the presence of glutamate receptors on cere bromicrovascular endothelial cells (CEC) could signifi cantly affect vasomotor responses of the cerebral microvascular bed or the function of the BBB. Brain capillary and microvascular endothelial cells have been shown to express receptors for other neurotransmitters including noradrenaline (Bacic et aI., 1992), serotonin (Cohen et aI., 1995), acetylcholine (Linville et aI., 1995), and dopamine (Bacic et aI., 1991) as well as for the vasoactive mediators histamine (Stanimirovic et aI., 1994a), adenosine (Stanimirovic et aI., 1994a), endothe lins (Stanimirovic et aI., 1994b), bradykinin (Stan imirovic et aI., 1996b), and angiotensins (Stanimirovic et aI., 1996a). These neurotransmitters/mediators have also been shown to modulate both vascular tone (Luscher, 1990) and BBB permeability (Guillot and Audus, 1991; Stanimirovic et aI., 1996a) by acting on endothelium expressed receptors.
The purpose of this study was to determine if CEC express glutamate receptors on their membranes and are therefore the primary site of action for modulation of BBB permeability and/or vascular tone by glutamate in the cerebromicrovascular bed. We studied the presence of functional glutamate receptors in both freshly isolated rat and human capillaries and microvessels and cultured rat and human CEC by measuring glutamate-induced
changes in [Ca2+]i' inositol tisphosphate IP3), and
cy clic AMP and the expression of glutamate receptor sub units by immunocytochemistry and reverse transcriptase polymerase chain reaction (RT-PCR). Our studies pro vide evidence that neither rat nor human CEC express functional glutamate receptors. Therefore, it appears that any microvessel vasomotor responses and/or BBB per meability changes effected by glutamate are endothelium independent and are likely elicited by glutamate actions on surrounding cells.MATERIALS AND METHODS Chemicals and reagents
All culture media and fetal bovine serum were obtained from GibcoBRL (Gaithersburg, MD, U.S.A.). Endothelial cell growth supplement, ITS Premix, human fibronectin, and rat tail type IV collagen were purchased from Collaborative Biomedi cal Products (Bedford, MA, U.S.A.). Human serum and gluta mate were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Mouse melanoma cells, Cloudman S91 (clone M-3), were obtained from American Type Culture Collection (Rock ville, MD, U.S.A.). NMDA, u-amino-3-hydroxy-5-methyl isoxazole-4-propionic acid (AMPA), kainate, l-amino cyclopentyl-IS 3R-dicorboxylate (lS,3R-trans-ACPD), and MK-801 were purchased from Research Biochemicals Intena tional (Natick, MA, U.S.A.). Fura-2 acetoxymethyl ester (fura 2-AM) was from Molecular Probes (Eugene, OR, U.S.A.).
TriReagent was obtained from Molecular Research Center (Cincinnati, OH, U.S.A.).
Freshly isolated cerebral microvessels
Rat brain microvessels and human brain capillaries or resis tance microvessels were isolated using a modification(s) of the procedures of Diglio et al. (1982) and Gerhart et al. (1988), respectively. Rat brain microvessels were isolated from cortical brain tissue of four to six adult rats. Human brain capillaries and microvessels were obtained from small samples of human temporal lobe excised surgically from patients treated for idio pathic epilepsy.
In both cases, the brain samples were cleaned of pia matter and associated surface vessels, and cortical gray matter was dissected. The tissue was minced with scissors and homog enized in a glass-telon homogenizer. The homogenate was passed once through 350-Lm and twice through 112-Lm mesh nylon nets (Nitex; Tetko, Elmsford, NY, U.S.A.) fitted in Mil lipore filter holders. Resistance micro vessels were dislodged from the 112-Lm mesh and collected in cold medium M199 (containing Earle's salts, 25 mmollL N-2-hydroxy ethylpiperazine-N' -2-ethanesulfonic acid, 4.35 gIL sodium bi carbonate, and 3 mmollL L-glutamine). Filtrates were subse quently centrifuged at 1,000 g for 10 minutes, and pellets were resuspended in 20 mL of 20% dextran (MW 70,000; Sigma) and centrifuged at 3,000 g for 15 minutes in a swinging bucket rotor. The pellets were dissolved in cold medium M199, and the microvessels and capillaries were collected on a 20-Lm mesh nylon net. Microvessels retained on the 20-Lm mesh were dislodged, washed three times in M199, and either used fresh or further processed to obtain endothelial cultures.
Cerebral endothelial cell cultures
Freshly isolated brain microvessels were enzymatically dis
sociated to obtain endothelial cell cultures as follows: The re
sistance vessels (112 to 350 Lm) and the capillaries and smaller microvessels (>20 Lm) derived from human brain samples were separately dissociated by exposure to 1 mg/mL type IV collagenase for 15 minutes at 37°C, centrifuged at 500 g for 5 minutes, resuspended in growth medium containing 65% me dium M199, 10% fetal calf serum, 5% human serum, 20% murine melanoma cell (mouse melanoma, Cloudman S91, clone M-3, melanin-producing cells)-conditioned medium, 5 Lg/mL insulin, 5 Lg!mL transferrin, 5 ng/mL selenium, and 10 Lg/mL endothelial cell growth supplement, seeded in 0.5% gelatin-coated plastic tissue culture dishes, and maintained at 37°C in an atmosphere of 5% CO2 in air. Microvessels of rat brain retained on the 20-Lm mesh were dissociated by a brief ( lO-min) exposure to type I collagenase (1 mg/mL, 37°C) and seeded in rat tail collagen-coated tissue culture flasks in me dium M199 supplemented with 1 % basal medium Eagle' s amino acids, 1 % basal medium Eagle's vitamins, antibiotic/ antimycotic mixture (100 units/mL penicillin G, 100 mg/mL streptomycin sulfate, 0.25 mg/mL amphotericin B), 1 % glu cose, 0.05% peptone, and 20% fetal bovine serum. Identified single endothelial cells or small groups of endothelial cells emerging from the microvessels 3 to 4 days after the seedings were isolated using cloning rings (Belleo Glass, Vineland, NJ, U.S.A.), and two to three of these cloned colonies were pooled and further passaged. Cells obtained from three diferent iso lations (passages 3 to 8) were used for the experiments in this study. The CEC cultures obtained from both rat brain microves sels and human brain resistance microvessels and capillaries were routinely characterized using indirect immunocytochem ical staining for von Will brandt factor (Factor VIII)-related antigen, incorporation of acetylated low-density lipoprotein
398 P. MORLEY ET AL.
conjugated to luorescent dye, and binding of luorescently labeled lectins Ricinus communis agglutinin I and concanavalin A, as described and published previously (Stanimirovic et a!., 1995, 1996a). More than 95% of the cells derived by the de scribed procedures were positive for these endothelial cell markers and free of glial contamination as ascertained by the absence of staining for glial fibrillary acidic protein. Both cap illary and resistance micro vessel-derived human CEC were h i g h l y e n r i c h e d i n t h e B B B - s p e c i f i c e n z y m e Y glutamyltranspeptidase (Stanimirovic et a!., I 996a,b ).
Rat cortical neurons
Rat cortical neurons were cultured by a previously described method (Durkin et a!., 1997). For RT-PCR and the determina tion of IP3 production, the cells were plated on polY-L-lysine coated, 35-mm tissue culture dishes (Dupont-Life Technolo gies, Burlington, Ontario, Canada). For immunohistochemistry and measurement of [Ca2+ji' cortical neurons were plated on polY-L-lysine-coated 12-mm glass coverslips (Belleo Glass) and incubated in 24-well tissue culture dishes (Dupont-Life Technologies) in 0.5 mL of plating medium. All cells were incubated at 37°C in an atmosphere of 5% CO2/95% air. Ex periments were performed on cultures following 14 to 18 days in vitro.
[Ca2+]i measurement
To determine [Ca2+]i' human and rat CEC were cultured in complete medium on 24-mm glass coverslips coated with 10 .lg/mL human ibronectin and 0.5% rat tail collagen, respec tively, for 1 to 4 days, while primary fetal rat cortical neurons were grown for 15 days on polY-L-lysine-coated coverslips. [Ca2+ji was determined by measuring changes in the luores cence signal from the Ca2+-sensitive indicator fura-2, as de scribed previously (Durkin et a!., 1997). The figures shown are luorometric tracings depicting the ratio of the fura-2 intensity at 505 nm following excitation of the cells at 350 and 380 nm.
Inositol trisphosphate determination
To determine inositol phosphate formation, conluent rat CEC, human CEC, and primary cultures of rat cortical neurons grown in 35-mm petri dishes were prelabeled with
e
Hjmyo inositol (2.5 mCilmL) for 16 to 18 hours in serum- and inositol free medium M199. Unbound [3H]myo-inositol was removed by washing in M199, and the cells were then exposed to glu tamate and/or glutamate receptor agonists/antagonists in M199 in the presence of 20 mmollL LiCI (Berridge et a!., 1982) for 15 minutes. The reaction was stopped by replacing the medium with cold 0.3 mollL trichloroacetic acid, and the cells were scraped, briely sonicated, and sedimented by centrifugation. Aliquots of the supenatants were treated three times with an hydrous diethyl ether, the aqueous phases were separated by centrifugation, and the remaining ether was evaporated under nitrogen. The samples were neutralized with 6.25 mollL so dium tetraborate and applied to a 1-mL Dowex-AG lX8-formate form anion exchange column. Inositol phosphate frac tions were eluted from the columns and quantified as previ ously described (Stanimirovic et aI., 1994b). The IP3 fraction determined by this technique represents a mixture of IP3 iso mers.Cyclic AMP determination
To determine levels of cyclic AMP in rat and human CEC and rat cortical neurons, cells grown in 24-well tissue culture plates were treated with the metabotropic glutamate receptor agonist IS,3R-tans-ACPD, alone or in combination with for skolin, in phosphate-buffered saline (PBS) containing 0.2%
J Cereb Blood Flow Metab. Vol. 18. No.4. 1998
bovine serum albumin and 1 mmollL 3-isobutyl-l-methyl xanthine for 10 min. The reaction was stopped by removing the reaction mixture and adding 65% (vol/vol) ice-cold ethanol. The ethanol extraction was repeated twice, and the combined extracts were collected into test tubes and dried in a vacuum oven at 80°C. The cyclic AMP levels were determined using a commercial cyclic AMP enzyme immunoassay kit (Biotrak; Amersham). The assay is based upon the competition between unlabeled cyclic AMP and a fixed quantity of peroxidase labeled cyclic AMP for a limited number of binding sites on a cyclic AMP-speciic antibody.
Immunohistochemical detection of glutamate receptor subunits
Cells grown on glass coverslips were washed briely in PBS and then immersed in Lana' s ixative [4% paraformaldehyde and 14% (vol/vol) saturated picric acid in 0.25 mol/L NaH2P04, pH 6.9] for 1 hour at room temperature. The cells were then rinsed in PBS, permeabilized for 5 minutes in 0.25% Triton X-IOO in PBS, and then rinsed 2 x 5 min with PBS. Coverslips were inverted onto droplets of 5% normal donkey serum in 1 % bovine serum albumin (Sigma) in PBS for 40 minutes at room temperature to block nonspecific binding sites. The cells were then rinsed for 5 minutes in PBS and incubated with primary antibodies against the NMDA receptor subunits NR I, NR2A, and NR2B and the AMP A receptor subunits GluRl, GluR2/3, and GluR4 for 1 hour at room temperature. All antibodies were purchased from Upstate Biotechnology (Lake Placid, NY, U.S.A.). Antibodies were used at a concen tration of 10 or 2 .lglmL (for GluR2/3) diluted in 1 % bovine serum albuminlPBS. Control experiments were performed in which the primary antibodies were omitted. Following incuba tion with primary antibody, cells were washed 3 x 5 min in PBS and incubated with a CY3-conjugated donkey anti-rabbit IgG (secondary antibody), diluted 1:500 (Jackson, Bio/Can Scien tific, Mississauga, Ontario, Canada) in 1 % bovine serum albu minlPBS for 1 hour at room temperature. Covers lips were rinsed and mounted in Vectashield (Vector Laboratories, Bur lingame, CA, U.S.A.), viewed on an Olympus photomicro scope, and photographed using Ilford XP2 400 ASA film.
RT-PCR detection of glutamate receptor subunits
Reverse transcriptase polymerase chain reaction was used to determine the mRNA expression profile of the glutamate NMDA receptor subunits NR1, NR2A, NR2B, and NR2C, the AMPA receptor subunits GluRI, GluR2, GluR3, and GluR4, and the kainate receptor subunits KAI or KA2, GluR5, GluR6, and GluR7 in freshly isolated rat and human brain microves sels, cultured rat and human CEC, and cultured rat neurons. Total RNA was isolated from each culture dish [total cell num ber, -1.5 x 105 for human CEC, -5 x 105 for rat CEC, and -7.5 x 105 for rat neurons including a 20 to 35% glial component] using TriReagent. First-strand cDNA was made in 20 .ll, using Moloney murine leukemia virus RT (Perkin Elmer Cetus, NJ, U.S.A.) and primed with random hexamers. For the freshly isolated human microvessel preparations, 10 .lg of total RNA, digested with DNase I (Promega Corp., Madison, WI, U.S.A.), was reverse transcribed with avian myeloblastosis virus RT (Seikagaku America, Ijamsville, MD, U.S.A.) in 50 .ll and primed with oligo-dT (Perkin Elmer Cetus). First-strand cDNA (20-.lL cultures, 4 .lL fresh) was then used for PCR amplifi cation of NMDA, AMPA, and kainate receptor subunits. All PCR reactions were performed in a final volume of 100 .lL using the Perkin Elmer PCR kit, AmpliTaq DNA polymerase, and 50 pmol oligonucleotide primers. Following PCR amplifi cation, the product was electrophoresed on a 1.5% agarose gel
GLUTAMATE RECEPTORS ON BRAIN EDOTHELIAL CELLS 399
and visualized by staining with ethidium bromide. Serial dilu tions of copies of full-length clones of the various glutamate subunits were used as templates in the PCR reactions to assess the amplification efficiency of each of each reaction.
The oligonucleotide primers (Sheng et a!., 1994) used to amplify NRI receptor subunits were designed to lank the NRl's exon 5, resulting in two different-sized products (235 and 172 bp) corresponding to mRNAs containing (NR I\xx) or lacking (NRloxx) this exon. NR2 receptor subunits NR2A, NR2B, and NR2C were ampliied using oligonucleotide prim ers and the cycling paradigm as described by Audinat et a!. (1994) to produce products of all the same size (547 bp). To amplify the AMP A receptor subunits GluRl, GiuR2, GluR3, and GluR4, the oligonucleotide primers and cycling paradigm used were as described by Lambolez et a!. (1992) and produced products of all the same size (-750 bp). To amplify the kainate receptor subunits KAI or KA2, GiuR5, GiuR6, and GluR7, four sets of oligonucleotide primers and the cycling paradigm described by Ruano et al. (1995) were used and produced prod ucts of 512, 208, 259, and 421 bp, respectively.
RESULTS
Efects of glutamate agonists on [Ca2+]i' IP3, and cyclic AMP in rat and human CEC and rat cortical neurons
To probe for the presence of functional glutamate re ceptors in cultured rat and human CEC, we measured changes in [Ca2+]j and IP3 production in response to glutamate and glutamate receptor agonists. In Mg2+-free normal bufer solution containing 30 LmolL glycine, glutamate (100 LmolL; data not shown), NMDA [40 1molL; Fig. lA (rat) and B (human); n = 11], AMPA
[40 LmolL; Fig. IC (rat) and D (human); n = 7], and
kainate [40 LmolL; rat and human; data not shown; n =
7] did not affect [Ca2+]j in either rat or human CEC. In all experiments, these endothelial cells were capable of responding by increases in [Ca2+]j to a subsequent addi tion of endothelin-l (10 nmoL), angiotensin II (200 nmolL), or bradykinin (20 LmolL) (Fig. lA to D, F). Rat and human CEC exposed to very high concentrations of glutamate (l mmolL), NMDA (400 LmolL), and AMPA (400 LmolL) in the presence of the desensitiza tion blocker cyclothiazide (25 LmolL) (data not shown) and kainate (400 LmolL; rat; Fig. IF) also failed to show [Ca2+]j responses (data not shown). These findings re in sharp contrast to the ability of NMDA (40 LmolL; Fig. IE), glutamate, AMPA, and kainate (data not shown) to produce significant increases in [Ca2+]j in rat cortical neurons (Durkin et al., 1997). The results suggest that rat and human CEC in culture lack functional iono ropic glutamate NMDA, AMP A, and kainate receptors.
To test for metabotropic glutamate receptor expres sion, we determined the efects of the specific metabo tropic glutamate receptor agonist lS,3R-tans-ACPD on Ca2+ luxes, phospholipid hydrolysis, and adenylyl cy clase activity. IS,3R-trans-ACPD [300 LmolL; Fig. 2A (rat) and B (human); n = 4) did not affect [Ca2+]i in
either rat or human CEC. When added to either rat (Fig.
3A) or human (Fig. 3B) CEC cultures at 300 to 500
LmolL, lS,3R-tans-ACPD failed to induce phospho lipid hydrolysis and accumulation of IP 3' In the same cell cultures, the vasoactive peptides endothelin-l, previ ously shown to couple to phospholipase C in these cells (Stanimirovic et al., 1 994b, 1996a), induced a four- to fivefold augmentation of IP3 levels [Fig. 3A (rat) and B (human)]. By contrast, in rat cortical neuronal cultures, 300 LmolL IS,3R-tans-ACPD induced a twofold in crease in IP3 (Fig. 3C). The same concentrations of IS,3R-trans-ACPD have been shown to trigger a [Ca2+l rise in cortical neuronal cultures (Durkin et al., 1997). Both endothelin-l and angiotensin II-induced [Ca2+]i in creases in rat and human CEC (Fig.
I)
(Monette et al., 1996; Stanimirovic et al., 1996b) and IS,3R-trans ACPD-induced [Ca2+]i increases in cortical neurons (Durkin et al., 1997) were due to the release of Ca2+ from internal stores, since they were preserved in Ca2+-free medium.In addition to being inefective in activating phospho lipase C, IS,3R-trans-ACPD also failed to afect either basal or forskolin-stimulated cyclic AMP levels in rat or human CEC [Fig. 3D (human) and E (rat)]. In human CEC, previously shown to express the serotonin 5HT 1D
receptor (Cohen et al., 1995), 10 LmolL sumatriptan, a selective antagonist of 5HT lD&F receptors, reduced 1
LmolL forskolin-stimulated cyclic AMP by 40% (data not shown). However, in cultured rat neuronal cells, lS,3R-trans-ACPD reduced forskolin-stimulated cyclic AMP (Fig. 3F) in a concentration-dependent manner, suggesting that rat neurons express mGluR2 to mGluR4 and mGluR6 to mGluR8 metabotropic glutamate recep tors linked to the inhibition of adenylyl cyclase.
Collectively, these data indicate that there is an appar ent lack of functional metabotropic receptor expression in rat and human CEC as determined by the absence of signal transduction upon addition of receptor agonists to CEC.
Immunocytochemical detection of glutamate receptor subunits in cerebromicrovascular endothelial cells and rat neuronal cultures
To investigate whether the lack of signal transduction in response to glutamate agonists in rat and human CEC was due to the lack of one or more glutamate receptor subunits resulting in the nonsignaling receptor(s), we used indirect immunoluorescence microscopy to detect subunits of NMDA and AMP A receptors in CEC and rat cortical neuronal cultures. Both rat and human CEC failed to display any immunopositive reaction to the glu tamate receptor subunits tested. An example of the ab sence of immunoreactivity for the NMDA NRI subunit, shown to be essential for establishing a functional NMDA receptor (Ishii et aI., 1993), is shown in Fig. 4A. Similarly, both rat and human CEC lacked
400 P. MORLEY ET AL. o
l'
::C )0 00 C) )-1.0 oE
) C 0.5�O
00 :1M ::-ol'
::C 1.0 )0 lo eM� E
0.5 ) C�o
O) :I) ::-o 2.0l'
::c oA
i
i
NMDA ET-1
200c
i
j
A911
AMPA
o 200E
)0 lo c)�E
1.5 -) C�o
00 :I)j
NMDA
i
2+Mg
400 400 600 600::
- 1.0 -____ -____ -____ -, o 200 400TIME
(s)
600 1.08
0.5i
j
NMDA
ET-1
0.0 ---� o 200 400 600 1.0o
0.5i
ET-1
0.0 --....---, o 200 400 600 1.0F
0.5i
i
A
BK
0.0 -____ ..-____ -____ � o 200 400TIME
(s)
600FIG.
1. The effect of glutamate receptor agonists on [Ga2+1i in rat cerebromicrovascular endothelial cells (GEG)(A, C,
andF)
and cortical neurons(E)
and human GEG(8
andD).
Gerebromicrovascular endothelial cells were stimulated with 40 Jmol/L NMOA (A and B), 40 Jmol/L AMPA (G and0),
or 400 Jmol/L kainate(F)
in Mg2+-free normal buffer solution as described in Materials and Methods. Endothelin-1 (10 nmol/L; ET-1), angiotensin II (200 nmol/L; Ag II), or bradykinin (20 Jmol/L; BK) was added after the addition of glutamate receptor agonists to ensure that the cells were responsive. Rat cortical neurons were stimulated with 40 Jmol/L NMOA (E). The arrows indicate the times of addition of the specified agents. The results are tracings of typical responses replicated as described in the text. Also see text for abbreviations.tivity for NR2A and NR2B subunits (data not shown) or AMPA GluRl, GluR2/3, and GluR4 subunits (data not shown). By contrast, cortical neurons (14 to 18 days in culture) expressed strong immunoluorescent staining for NRl, NR2A, NR2B, GluRl, and GluR2/3 (Fig. 4) sub units associated predominately with the cell bodies and were negative for the GluR4 subunit (Fig. 4B). Negative results were also seen in control cells in which the pri mary antibody had been omitted (Fig. 4B). The results indicate that rat and human CEC, unlike rat neuronal
J Cereb Blood Flow Metab, Vol. 18, No.4. 1998
cultures, do not express immunoreactive NMDA and AMP A glutamate receptor subunits.
Expression of glutamate receptor subunit mRNA in rat or human cerebromicrovascular endothelial cells and rat cortical neurons
To eliminate the possibility that the lack of immuno reactive glutamate receptor subunit expression in rat or human CEC is due to the inability of the antibodies to detect "aberrant" or translationally modified proteins,
GLUTAMATE RECEPTORS ON BRAIN ENDOTHELIAL CELLS 401 o
�E
::::)o
uO
::)
)
u E
l ::�o
0) ::S) L" 1.0 0.5 -0.0 0A
i
t
Ag II
ACPD
200 400TIME
(s)
1.0B
0.5i
i
ACPD ET-1
0.0 600 0 200 400 600TIME
(s)
FIG.
2. The effect of the metabotropic agonist 1 ,3R-tran�ACPD on [Ca2+1i in rat(A)
and human(8)
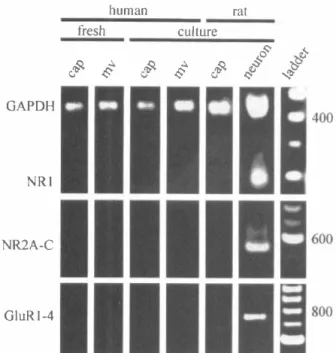
CEC. Cerebromicrovascular endothelial cells were stimulated with 300 Jmoi/L 1 S,3R-tran�ACPD in Mg2+-free normal buffer solution as described in Materials and Methods. Endothelin-1 (10 nmol/L; ET-1) or angiotensin II (200 nmol/L; Ag II) was added after the addition of 1 ,3R-tran�ACPD to ensure that the cells were responsive. The arrows indicate the times of addition of the specified agents. The results are tracings of typical responses replicated as described in the text. Also see text for abbreviations.we used the RT-PCR technique to assay for the presence of mRNA encoding various NMDA, AMP A, and kainate glutamate receptor subunits in rat or human CEC cul tures and rat neurons. We were not able to detect mRNA for either NMDA NRI1XX' NRloxx, NR2A, NR2B, or NR2C receptor subunits or AMPA GluRl, GluR2, GluR3, or GluR4 receptor subunits in any of the rat or human CEC cultures tested (Fig. 5). The lack of these glutamate receptor subunits was confirmed in both hu man (Fig. 5) and rat (not shown) CEC cultures derived from either resistance microvessels (112 to 350 LmolL) or capillaries (20 to 112 LmolL). All of the NMDA and AMPA receptor subunit mRNAs were also absent in pri mary cultures of CEC (data not shown), eliminating the possibility that the lack of glutamate receptor subunit mRNAs was due to cell passaging and dediferentiation in vitro. To address the contingency of receptors being lost due to the enzymatic dissociation of microvessels and culturing conditions, we analyzed freshly isolated microvessels prior to dissociation and plating. As in the cultures, in freshly isolated preparations, no message for NMDA or AMPA receptor subunits was detected (Fig. 5).
The sensitivity of the primer sets was assessed using a dilution series of the full-length clones for the various subunits. The sensitivity of the primer sets was such that the GluRl to GluR4 primer set detected as few as 103 copies, the NRI primer set detected as few as 104 copies, and the NR2A to NR2C primer set detected as few as 103 copies (data not shown). To establish whether our inabil ity to detect NMDA and AMPA receptor subunit mes sage in the CEC cultures was due to extracting insufi cient quantities of RNA from these preparations, each preparation was probed with a primer set specific for a ubiquitously expressed enzyme, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), using primers
GGAGATTGTTGCCATCAACGAC and ATGAGC CCTTCCACAATGCCAAAG. Each preparation probed was positive for GAPDH, suggesting that there was suf ficient RNA extracted from the cultures (Fig. 5, top).
Primary rat cortical neuronal cultures were probed for glutamate receptor subunit mRNAs as positive controls. The lack of NMDA and AMPA receptor expression ob served in the CEC cultures was in stark contrast to RT PCR assays performed on the neuronal cultures, in which both AMP A and NMDA receptor subunits were detected (Fig. 5). Although the NR11XX splice variants were de tected when probing message that had been pooled from four or more dishes of neurons (data not shown), the predominant splice variants expressed in the rat cortical neurons were those lacking the N-terminal NR10xx (Fig. 5, top). The mRNA encoding NR2 (Fig. 5, middle) and GluRl to GluR3 (Fig. 5, bottom) subunits was also abun dantly expressed in rat neuronal cultures. Control reac tions without RT product were always negative (data not shown). The results obtained using RT-PCR are in agree ment with the data obtained by immunocytochemistry showing expression of NMDA receptor NRl and NR2 subunits or AMPA receptor GluRI to GluR3 subunits in rat cortical neurons but not in rat or human CEC.
When probing for the presence of kainate receptor subunit mRNAs in rat CEC cultures and neurons, we detected mRNA for KAI or KA2 and GluR5 (Fig. 6) but not GluR6 or GluR7 (data not shown) in both microvas cular and capillary CEC. By comparison, KAI or KA2, GluR5 (Fig. 6), GluR6, and GluR7 (data not shown) subunit mRNA were all detected in rat cortical cultures. The specificity of the primers for rat kainate receptor subunits prevented probing human CEC, but the failure to observe any functional responses to very high concen trations of kainate or AMP A in either the rat or the human microvascular or capillary CEC (Fig. 1) suggests
402 P.
MORLEY
ETAL.
50 40-=
')..
E
30 -)�
S - 20 " -'.,
� -10 0 800 A ConT-
---300 lM t-ACPDI*
20nM ET-t B Con -300 lM t-ACPD 1* 20nM ET-1 C*
Con 300 lM t-ACPD 140 120 100-
= w , " 80 I 3 -Q 60 I..
0...
..
40g
20 0 350 D Eo Control
F•
1 lM forskolin 300=
600 ')e
)S
o 400S
e
�
- 200 o ---o 500 o 300 500 o 500* *
o 300 500 0 250 " >=
= 200 , = 3 0 ::: 150 Q 3 I..
0 100...
i.e
50 0 500 0 300 500 t-ACPD, lM t-ACPD, lM t-ACPD, lMFIG. 3.
The effect of the metabotropic glutamate receptor agonist 1 S.3R-tran�ACPD on IP3(A
toC)
and cyclic AMP(0
toF)
levels in rat (A and D) and human(6,
E) CEC and rat cortical neurons (C andF)
in culture. To determine IP3 accumulation, rat or human CEC or rat cortical neurons were prelabeled with [3Hlmyo-inositol (20.5 Ci/mmol, 2.5 mCi/mL) for 18 hours as described in Materials and Methods. Drug solvent (Con) or 300 lmoi/L 1 .3R-tran�ACPD (t-ACPD) or 20 nmol/L endothelin-1 was added to the cultures for 15 minutes in the presence of 20 mmol/L LiCI2. IP3 was extracted and quantified as described in Materials and Methods. To measure cyclic AMP levels, rat or human CEC or rat cortical neurons were treated with the indicated concentrations of 1 S.3R-tran�ACPD with (shaded bars) or without (open bars) 1 lmoi/L forskolin for 10 minutes in the presence of 1 mmol/L 3-isobutyl-1-methylxanthine. Cyclic AMP levels were determined as described in Materials and Methods. Each bar represents the mean ± SO of four replicate dishes in one of threeexperiments yielding similar results. 'Significant differences (analysis of variance; P< 0.01) from control levels. #Significant difference as compared with forskolin (analysis of variance; P < 0.01) alone. See text for abbreviations.
that the KAl, KA2, and GluR5 subunit mRNA expres sion is either not translated or is posttranslationally modified to render nonfunctional receptors.
DISCUSSION
This study provides evidence that endothelial cells of cerebral microvessels and capillaries in rat or human do not express functional glutamate receptors. This conclu sion is corroborated by the following experimental
find-J Cereb Blood Flow Me/ab, Vol. 18, No.4, 1998
ings: (a) Neither rat nor human CEC responded to iono tropic glutamate receptor agonists by increasing [Ca2+]j; (b) none of the NMDA or AMP A receptor subunits tested (i.e., NRl, NR2A to NR2C, and GluRl to GluR4) was detected by either immunocytochemistry or RT PCR; (c) the metabotropic glutamate receptor agonist lS,3R-trans-ACPD was incapable of stimulating phos pholipid hydrolysis or Ca2+ release from internal stores or inhibiting adenylyl cyclase activity in CEC; and (d) even though the mRNA for KAl or KA2 and GluR5 was
GLUTAMATE RECEPTORS ON BN ENDOTHELIAL CELLS 403
FIG.
4. Immunocytochemical de tection of glutamate receptor sub units in rat and human GEG and rat cortical neurons in culture.(A)
Phase contrast images(top)
and immunocytochemical staining for NR1 subunit of NMDA receptor(bottom)
in rat cortical neurons (1), human GEG (2), and rat GEG (3) in culture. Whereas rat cortical neu rons show distinct immunofluores cent staining, both rat and human CEC were negative for NR1 recep tor subunit.(8)
Immunocytochemi cal staining of rat cortical neuronsA
for the NMDA receptor subunits
B
(top),
NR2A (1) and NR28 (2), andthe AMPA receptor subunits
(bot-tom)
GluR1 (1), GluR2/3 (2), and GluR4 (3). Cell fixation, antibodies, and procedures used were as de-scribed in Materials and Methods. Negative controls (top 83) were processed in the same way as probes, except that the primary an-tibody was omitted. The results pre-sented are representative of at least four experiments in different cell isolations. See text for abbrevia-tions.1
detected in rat CEC, kainate did not elicit measurable Ca2+ increases in either rat or human CEC at very high concentrations. We also demonstrated that the lack of NMDA and AMPA receptor expression in cultured CEC was not due to the enzymatic dissociation and culturing conditions, since mRNAs for NMDA and AMPA recep tor subunits were also not detected in freshly isolated microvessels. Moreover, our inability to reveal glutamate receptors or glutamate-induced signaling responses in CEC was not caused by the inadequacy of the method ological approaches used, since in parallel experiments we demonstrated glutamate receptor agonist-induced [Ca2+]j increases, activation of phospholipid tunover, and inhibition of adenylyl cyclase along with the
expres-2
3
sion of both protein and mRNAs for NMDA and AMPA glutamate receptor subunits in rat neurons in culture. To our knowledge, this is the first study assessing the ex pression of glutamate receptors in cerebral capillary and microvessel endothelial cells in culture, in isolation from other cellular elements forming the microvascular bed.
The finding that cerebral microvascular endothelial cells lack functional glutamate receptors has important ramifications. Previous studies using isolated cerebral ar teries and nicrovessels have sparked a significant con troversy as to what roles endothelial cell glutamate re ceptors may play in regulating vasomotor cerebrovascu lar responses. Some studies have shown that both glutamate and NMDA (Busija and Leler, 1989; Faraci
404 P.
MORLEY ET AL.
human ratresh
culture a;! � ..�
..�
..�
§
§
J'
;�
;�
... i GAPDH - - -•
•
-. '1 • NRI•
• -2A-C - . .t -GluRI-4 - - :1-�
FIG. 5.
RT-PCR products from mRNA extracted from freshly iso lated human capillaries (cap; 20 to 53 �mol/L) and microvessels (mv; 112 to 350 �mol/L), human capillary and microvessel, and rat capillary CEC in culture and fetal rat cortical neurons in culture using primers specific for GAPDH, NR1, NR2A, NR2B, NR2C, GluR1, GluR2, GluR3, and GluR4 receptor subunits. The DNA size marker was 100 bp ladder. The results presented are rep resentative of at least four experiments in different cell isolations. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.and Breese, 1993) and the noncompetitive NMDA re ceptor antagonists ketamine (Fukuda et aI., 1983; Cava zutti et aI., 1987; Wendling et aI., 1994) and MK-801 (Perkins et aI., 1989) are potent vasodilators of the ce rebral vasculature. However, while glutamate and NMDA dilated pial arterioles in situ (Busija and Leler, 1989; Faraci and Breese, 1993), they had no effect on isolated cerebral vessels in vitro (Fujiwara et aI., 1975; Swan et aI., 1988; Hardebo et aI., 1989; Takayasu and Dacey, 1989; Faraci and Breese, 1993; Wendling et aI., 1996), indicating that this vasomotor response was not induced by direct action of glutamate on vascular cellular elements. In support of this view, it has been suggested that the NMDA-induced dilation of cerebral vessels is mediated by nitric oxide released as a result of neuronal activation (Faraci and Breese, 1993; Meng et aI., 1995), since it could be inhibited by the nitric oxide synthase inhibitor P-nitro-L-arginine (Faraci and Brian, 1995; Meng et aI., 1995). Our observation that freshly isolated microvessels and cultured CEC of resistance microves sels do not express functional glutamate receptors sup ports the notion that glutamate-induced vasomotor re sponses in cerebral microvessels are endothelium inde pendent and are likely initiated by glutamate-induced production of vasoactive compounds, such as nitric ox ide, by other cells positioned in the vicinity of microves sels. In agreement with this conclusion are the findings
J Cereb Blood Flow Metab, Vol. 18, No.4, 1998
that isolated bovine (Wendling et al., 1996) and ovine cerebral microvessels (Beart et aI., 1988) lack binding sites for NMDA antagonists. However, we cannot ex clude the possibility that endothelium or other cellular elements of larger cerebral vessels may express gluta mate receptors, since porcine cerebral arteries have been found to express speciic radioligand binding sites for the noncompetitive NMDA receptor antagonist phencycli dine (Lu et aI., 1989).
The finding that capillary CEC do not express func tional glutamate receptors has implications beyond those concerned with the regulation of vascular tone in the microvascular bed, Capillary cerebral endothelial cells form the BBB, and glutamate released by neurons in the vicinity of cerebral capillaries during cerebral ischemia or seizures may significantly afect BBB integrity and the formation of vasogenic brain edema. The studies of Koenig et ai. (1992) using an in vivo model of vasogenic brain edema following cryogenic injury and an in vitro model of isolated cerebral micro vessels have suggested that glutamate regulates the breakdown of the BBB by triggering the activation of the ornithine decarboxylase/ polyamine cascade in cerebral endothelial cells. Further more, the same group implied that the activation of the polyamine cascade and BBB breakdown were mediated by a direct action of glutamate on endothelial NMDA receptors, since it was inhibited by the competitive NMDA receptor antagonist 2-amino-5-phosphono pentanoic acid and because their capillry preparations were reportedly devoid of glia, neurons, synaptosomes,
KAl/2
GluR5
FIG.
6. RT-PCR products from mRNA extracted from freshly iso lated rat capillaries (cap; 20 to 53 �mol/L) and microvessels (mv; 112 to 350 �mol/L) and rat capillary CEC in culture and fetal rat cortical neurons in culture using primers specific for KA 1 and KA2 and GluR5 receptor subunits. The DNA size marker was 100 bp ladder. The results presented are representative of at least four experiments in different cell isolations. See text for abbreviations.GLUTAMATE RECEPTORS ON BRAIN ENDOTHELIAL CELLS 405
and synaptic complexes (Koenig et aI., 1992). These studies were based largely on indirect evidence of glu tamate being able to activate a polyamine metabolic cas cade known to be stimulated by various other difusible biochemical stimuli including arachidonic acid (Chan et aI., 1983; Pappius and Wolfe, 1983), prostaglandins (Pappius and Wolfe, 1983), diacylglycerol (Politi et aI., 1985), and free oxygen radicals (Chan et aI., 1984). Therefore, these results are diicult to reconcile with the evidence presented in this study of the absence of NMDA and other glutamate receptors in both cultured endothelial cells and freshly isolated microvessels. The discrepancy between the two studies may be explained by a possible non-receptor-mediated action of glutamate or NMDA on CEC or by the unlikely presence of "atypi cal" NMDA receptors in CEC that we were not able to detect by the methods used. Nevertheless, based on our data, it appears highly unlikely that glutamate is capable of afecting BBB opening by acting directly on cerebral capillary endothelial cells. Therefore, it appears that glu tamate involvement in BBB opening during pathological events that stimulate glutamate release such as stroke, brain trauma, and epilepsy is largely caused by the para crine effects of secondary mediators stimulated and re leased by surrounding glutamate receptor-expressing cells. The nature of these secondary mediators is pres ently unknown but may include compounds such as re active oxygen species, eicosanoids, polyamines, and ni tric oxide, all of which have been shown to induce BBB opening in vivo and/or in vitro (Black, 1995).
In conclusion, our results demonstrate the absence of functional glutamate receptor expression in rat and hu man microvascular and capillary CEC and suggest that glutamate, even at the high extracellular concentrations found in cerebral ischemia (Hagberg et aI., 1987), is unlikely to affect microvessel tone or BBB permeability by acting directly on endothelial cells. Therefore, the neuroprotective effects of glutamate receptor antagonists developed for the clinical treatment of epilepsy, stroke, and head injury (Muir and Lees, 1995) are apparently not mediated through sites of action on cerebral microves sels.
Acknowledgments: The authors thank Ms. Rita Ball and Mr. Roger Tremblay for performing isolations of rat and human CEC and rat cortical neurons, respectively; Mr. Zvi Cohen for supplying the cDNA made from RNA extracted from the reshly isolated human microvessels; Dr. Suzanne Zukin for the NRlb (NRllQo) clone, Dr. Shigetada Nakanishi for the NR2A and C clones, and Dr. Stephen Heinemann for the NR2B, GluRl, GluR2, and GluR4 clones.
REFERENCES
Audinat E, Lambolez B, Rossier J, Crepel F (1994) Activity-dependent regulation of N-methyl-D-aspartate receptor subunit expression in rat cerebellar granule cells. Eur J Neurosci 6:1792-1800
Bacic F, Uematsu S, McCarron RM, Spatz M (1991) Dopaminergic receptors linked to adenylate cyclase in human cerebromicrovas cular endothelium. J Neurochem 57:1774-1780
Bacic M, McCaron RM, Uematsu S, Spatz M (1992) Adrenergic re ceptors coupled to adenylate cyclase in human cerebromicrovas cular endothelium. Metab Bain Dis 7: 125-137
Beart PM, Sheehan K-AM, Manallack DT (1988) Absence of N methyl-D-asparate receptors on ovine cerebral microvessels. J
Cereb Blood Flow Metab 8:879-882
Berridge JM, Downes P, Hanley RM (1982) Lithium amplifies agonist dependent phosphatdylinositol responses in brain and salivary glands. Biochem J 206:587-595
Black KL (1995) Biochemical opening of the blood brain barrier. Adv Drug Deliv Rev 15:37-52
Busija DW, Lefler CW (1989) Dilator effects of amino acid neuro transmitters on piglet pial arterioles. Am J Physiol 257:HI200-H1203
Cavazutti M, Porro CA, Biral GP, Benassi C, Barbieri GC (1987) Ketamine effects on local cerebral blood low and metabolism in the rat. J Cereb Blood Flow Metab 7:806-811
Chan PH, Langar S, Fidhman RA (1983) Phospholipid degradation and edema development in cold-injured rat brain. Brain Res 277:329-337
Chan PH, Schmidley JW, Fishman RA, Longar SM (1984) Brain in jury, edema, and vascular permeability changes induced by oxy gen-derived free radicals. Neurology 34:315-320
Cohen Z, Bouchelet I, Yong WV, Villemure J-G, Stanimirovic D, Hamel E (1995) Differential expression of serotonin receptors 5-HT1Da and 5HT2A mRNA in human brain vessels, vascular cells and astrocytes in culture. Soc Neurosci Abstr 21:1853
Collingridge GL, Singer W (1990) Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacal Sci 11:290-296
Diglio CA, Grammol-Las P, Giacomelli F, Wiener J (1982) Primary culture of rat cerebral microvascular endothelial cells. Isolation, growth, and characterization. Lab Invest 46:554-563
Durkin JP, Tremblay R, Chakravarthy B, Mealing G, Morley P, Small D, Song D (1997) Evidence that the early loss of membrane protein kinase C is a necessary step in the excitatory amino acid-induced death of primary cortical neurons. J Neurochem 68:1400-1412 Faraci FM, Breese KR (1993) Nitric oxide mediates vasodilation in
response to activation of N-methyl-D-aspartate receptors in brain. Cire Res 72:476-480
Faraci FM, Brian JE (1995) 7-Nitroindazole inhibits brain nitric oxide synthase and cerebral vasodilation in response to N-methyl-D aspartate. Stroke 26:2172-2176
Fujiwara M, Muramatsu I, Shibata S (1975) y-Amino-butyric acid receptor on vascular smooth muscle of dog cerebral arteries. Br J Pharmacal 55:561-562
Fukuda S, Murakawa T, Takeshita H, Toda N (1983) Direct effects of ketamine on isolated canine cerebral and mesenteric arteries. Anesth Analg 62:553-558
Gerhart DZ, Broderius MA, Drewse LR (1988) Cultured human and canine endothelial cells from brain microvessels. Brain Res Bull 21:785-793
Guillot FL, Audus KL (1991) Angiotensin peptide regulation of bovine brain micro vessel endothelial cell monolayer permeability. J Car diovasc Pharmacal 18:212-218
Hagberg HP, Andersson P, Kjellmer I, Thiringer K, Thordstein M (1987) Extracellular overflow of glutamate, aspartate, GABA and taurine in the cortex and basal ganglia of fetal lambs during hyp oxia-ischemia. Neurosci Lett 78:311-317
Hardebo JE, Wieloch T, Kahrstrom J (1989) Excitatory amino acids and cerebrovascular tone. Acta Physiol Scand 136:483-485 Ishii T, Moriyoshi K. Sugihara H, Sakurada K, Captain H, Yokoi M,
Azakawa C. Shigemoto R, Mizuno N, Masu M, Nakanishi S (1993) Molecular characterization of the family of the N-methyl D-aspartate receptor subunits. J Bioi Chem 268:283--2843 Koenig H, Trot JJ, Goldstone AD, Lu CY (1992) Capillary NMDA
receptors regulate blood-brain barrier function and breakdown. Brain Res 588:297-303
Lambolez B, Audinat E, Bochet P, Crepe1 F, Rossier J (1992) AMPA
receptor subunits
expressed
bysingle urinje cells.
Neuron 9:247-258
406 P. MORLEY ET AL.
Linville DG, Cohen Z, Olivier A, Yang WV, Stanimirovic 0, Hamel E (1995) Expression of muscarinic M3 receptors mRNA in human brain microvascular fractions, astrocytes and vascular cells in cul ture. Soc Neurosci Abstr 21 :2042
Lu YF, Sun FY, Zhang LM, Zhang AZ ( 1 989) Phencyclidine receptors in porcine cerebral arteries. Acta Pharmacol Sin 1 0:508-511 Luscher TF (1990) Endothelial control of vascular tone and growth.
Clin Exp Hypertens Theoy Pract Al 2:897-902
Meldrum B, Garthwaite J (1990) Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci 11:379-387 Meng W, Tobin JR, Busija OW (1995) Glutamate-induced cerebral vasodilation is mediated by nitric oxide through N-methyl-D aspartate receptors. Stroke 26: 857 -863
Monette R, Stanimirovic 0, Ball R, Hamel E, Chaundy K, Morley P (1996) Distinct expression of receptors linked to Ca2+ signalling in rat and human cerebromicrovascular endothelial cells. Soc Neuro sci Abstr 22:308.2
Muir KW, Lees KR (1995) Clinical experience with excitatory amino acid antagonist drugs. Stroke 26:503-513
Pappius HM, Wolfe LS (1983) Functional disturbances in brain fol lowing injury: search for underlying mechanisms. Neurochem Res 8:63-72
Perkins WJ, Lanier WL, Karlsson BR, Milde JH, Michenfelder JD (1989) The effect of the excitatory amino acid receptor antagonist dizocilipine maleate (MK-801) on hemispheric cerebral blood low and metabolism in dogs: modification by prior complete cerebral ischemia. Brain Res 498:3444
Politi LE, Rodriguez de Turco EB, Bazan NG ( 1 985) Dexamethasone effect on free fatty acid and diacylglycerol accumulation during experimentally induced vasogenic brain edema. Neurochem Pathol 3:249-269
Ruano 0, Lambolez B, Rossier J, Paternain AV, Lerma J ( 1 995) Kain ate receptor subunits expressed in single cultured hippocampal neurons: molecular and functional variants by RNA editing. Neu ron 14: 1009-10 7
Sheng M, Cummings J, Roldan LA, Jan YN, Jan L Y ( 1 994) Changing subunit composition of heteromeric NMDA receptors during de velopment of rat cortex. Nature 368: 1 44-147
J Cereb Blood Flow Metab. Vol. I!, No. 4, 1 998
Small DL, Buchan AMB (1996) Mechanisms of cerebral ischemia: intracellular cascades and therapeutic interventions . I Cardiotho rac Vase Anesthesiol 10: 139-146
Stanimirovic DB, Bertrand N, Merkel N, Bembry J, Spatz M (1994a) Interaction between histamine and adenosine in human cerebromi crovascular endothelium: modulation of second messengers. Metab Brain Dis 9:275-289
Stanimirovic DB, Yamamoto T, Uematsu S, Spatz M ( I 994b) Endo thelin-l receptor binding and cellular signal transduction in cul tured human brain endothelial cells . I Neurochem 62:592-60 I Stanimirovic DB, Wong J, Ball R, Durkin JP (1995) Free radical
induced membrane dysfunction at the site of blood-brain barrier: relationship between lipid peroxidation, Na,K-ATPase activity and 5 l Cr release. Neurochem Res 20:1417-1427
Stanimirovic DB, Morley P, Ball R, Hamel E, Mealing G, Durkin JP ( l 996a) Angiotensin II-induced luid phase endocytosis in human cerebromicrovascular endothelial cells is regulated by the inositol phosphate signalling pathway . I Cell Physiol 169:455-467 Stanimirovic DB, Morley P, Hamel E, Ball R, Mealing G, Durkin JP
( 1 996b) Calcium and protein kinase C signalling in response to vasoactive peptides in human cerebromicrovascular endothelial cells. In: Advances in Experimental Biology and Medicine, Biology and Physiology of the Blood-Brain Barrier (Couraud PO, Scher man 0, eds), New York, Plenum Press, pp 221-227
Swan JH, Evans MC, Meldrum BS (1988) Long-term development of selective neuronal loss and the mechanism of protection of 2-amino-7 -phosphonoheptanoate in a rat model of incomplete fore brain ischaemia . I Cereb Blood Flow Metab 8:64-78
Takayasu M, Dacey RG Jr (1989) Effects of inhibitory and excitatory amino acid neurotransmitters on isolated cerebral parenchymal ar terioles. Baill Res 482:393-396
Wendling WW, Daniels FB, Chen 0, Harakal C, Carlsson C (1994) Ketamine directly dilates bovine cerebral arteries by acting as a calcium entry blocker. I Neurosurg Anesthesiol 6:186-192 Wendling WW, Chen 0, Daniels FB, Monteforte MR, Fischer MB,
Harakall C, Carlsson C (1996) The effects of N-methyl-D-aspartate agonists on isolated bovine cerebral arteries. Anesth Analg 82:264-268