Publisher’s version / Version de l'éditeur:
Journal of Colloid and Interface Science, 45, 1, pp. 154-169, 1973-10
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Phase transitions of adsorbates. V. Aqueous sodium chloride solutions
adsorbed of porous silica glass
Litvan, G. G.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=b49fba09-4700-471a-a188-e371e519e522 https://publications-cnrc.canada.ca/fra/voir/objet/?id=b49fba09-4700-471a-a188-e371e519e522
LA TRANSFORMATION UE:S P H A S E S JJES P R O U U I T S U'AUSORPTION. V. SOLUTIONS AQUEUSES U E C H L O R U R E DE S0I)IUM A U S O R B E E S
E N DU VE:RRE O E Q U A R T Z P O R E U X P a r G. G. L i t v a n ( N R C C 13437) SOMMAIRE L e s c h a n g e m e n t s d e d i m e n s i o n s e t d e t e n e u r e n c h a l e u r d e s t i l l o n s d e v e r r e c o m p l i t e m e n t s a t u r 6 s d ' e a u s o n t d b t e r m i n 6 s 2 t e r n p 6 r a t u r e d e n o n - 6 q u i l i b r e e n t r e t 5 e t - 8 0 ° C . L e s r 6 s u l t a t s a r l ' h y p o t h i s e q u e l e s p a r t i c u l a r i t k s p r o v i e n n e n t d e l a d i f f 6 r e n c e ion d e v a p e u r du p r o d u i t a d s o r b 6 s e m b l a b l e 2 un l i q u i d e et c e l l e i a n t . A u c u n e s e p a r a t i o n d e p h a s e n ' e s t o b s e r v d e d a n s l e s p o r e s .
.
. - - d i s t r i b u t i o n s e d e u x a s p e c t s : l ' e x ~ a n s i o n ' rles v i t e s s e s 3 r e s s i o n d e 1s a v e c d e sReprinted irom JOURNAL OF COLLOID :~SD IXTERF-ICE SCIENCE, Volume 4:, S o . 1, Oclober 1973 Copyright @ 1973 by .-lcademic Press, Inc. Priizted itz U.S.A.
Phase Transitions of Adsorbates
V. Aqueous Sodium Chloride Solutions Adsorbed
of Porous Silica Glass
Naliot~nl Rcscnrcl~ Co7ct1cil o/ C a n n d ~ l , Diai~iotz oJ Btrilditt~ Rejearclr, Ollazrc~, C'cltznda
Receivetl December 4, 1972; accepled Felxuary 21, 1973
.\clsorption isotherms for \vater on porous silica glass containing various anno~unls of NaCI, determined a t room temperature, can be superimposed if salt evclusion in the first non no la per ant1 relative pressure values based on esisting salt concentration are assumed T h e changes in clirnensions ancl heat content of the same glass speci~neiis fully saturated with water were deter- mined in nonequilibrium temperature cycles between +5 and -SO°C. T h e results are es- plicable by the hypothesis that peculiarities result from the difference between the vapor pres- sure of the liquid-like adsorbate and that of the solid in the surroundings. xo phase separation in the originally occupied pores was detected, b u t soliclilicntio~i of the solution after redistribu- tion proceeded in a may similar to t h a t of bull; two-co~nponen t systems. Observations following first freezing were in full harmony with those for the salt-free system. T w o aspects of the second freezing, ho~vever, mere unlike the previously studied case: the magnitude of espansion on cooling was larger a t slow rates than a t rapid rates and expansion instead of contraction occurred on n arming. Low vapor pressure, high viscosity and the presence of some ice in the larger pores are assunled to b e the source of these anomalies. Results ol,tainecl n ith ac1ueous glycer~nc solutions a s adsorbate appear to support t h i ~ vie\\.
'I'hc low-te~nperaturc behavior of porous bodies containing adsorbed water has been
described (1) and the processes leading to their
destruction h a m been cliscussccl (2). T h c 01)-
scrvations appe:lr to be csplicable b y a single theory containing no assumptions specilic to a particular sj,stcm and arc therefore expected to be of general validity. Inclectl, it is possible to interpret tllc results obtained with different
solids and adsorbates without introcl~icing new
assumptions.
Inclusion of aqueous solutions as ;~clsorbate seenis to be a logical extension of the inrrcstiga
'This paper is a contril~ution from the Division of Building Rcsearch, National Research Council of Canada, ant1 is pul)lisl~etl x i t h the approval of the Director of the Division.
'
Research Ofli~er, Division oi Building Resenrch, National Research Council oi Canada, Ottnn a, Canada. K l X O R 6 .tions. Bcsicles corroborating the proposed theory for consistency under these conditions,
the merit of such a s t u d - lies in potential
clarification of thc phase relations in acl- sorbatcs. 'There are scvcral areas whcrc an understanding of the freezing phenomeno~z of
solutions in the adsorbed state ~ ~ l o u l d be useful :
frost action in porous building materials i n the presence of de-icing salts, crjwpreser\ration of biological materials ant1 foodstul'fs, clesalina-
tion using porous i u c ~ ~ ~ h r a n c s , in~pregnation
of catalyst.
'The present paper cleals with the stud>- of the
porous glass-~C~UCOUS XaC1 sj7stem; applica-
tion of the results to practical problenls will 11e the subject ol forthcoining publications.
ESI'ERII1EST;~L
Porous 96% silica glass (Corning f 930)
specin~ens, 30 by 8 by
5
Inn1 ancl having a BETJorlr rral o j Culloid nrrd Iirlo.juce Scir.ilcc, Vol. 45, So. 1, Oclober l 9 i 3 Co1)yright 0 l51i.3 11,. .\ca(lernic P r c ; ~ , Inc.
nitrogen surface area of 100.1 m y g , wcre used. Specimens werc heated in an oven a t 150°C to constant weight, then placed in individual glass cells and puinped down to 10-? Torr pressure. NaCl solution of known concentra- tion was injected into the cell by ineans of a hypodcrinic syringe through a rubber septum, thus maintaining vacuum intcgritj-. T h e amount of solution added to each cell was
detcrinined froin weight increase (&5X lo-' g).
Although separate experiments showed that there is no detectable change in the amount of salt contained in the glass after 6 hr, the speci- illens were iininersed in the solutions for a t least a month. Prior to the run each specinlen was renloved from the cell and again heated in an oven to 150°C until constant weight was at- tained. Salt deposit was not observed on the outer surface. The concentration of the NaC1 solutions was deternlined by titration, using 0.01 m AgK03 solutions according to Mohr's method. Saturation with glycerol solutions was carried out in a similar fashion, oinitting
heating after saturation. A 60% stock solution
was prepared gravimetrically from reagent grade glycerol, and individual solutions werc prepared b y volumetric dilution. Changes in dinlensions and thermal properties of thc sam-
ples during programmed temperature cycles
wcre deternlined simultaneouslj- (1).
&Idsorption isotherms of water on porous gldss containing various anlounts of S a c 1
werc obtained by the desiccator method. Weighing dishes containing impregnated glass samples were placed in a desiccator over saturated solutions of various salts, and to shorten equilibration tilne the desiccator uras de-aerated. I t was necessary to stir the salt solutions with a illagnctic stirrer and to use a thermostat to provide the required constancy of the relative pressure in the vessel. The speci- mens wcre conditioned over each solution until
the weight change in 24 hr was less than 2
mg/g or
lyO
of the adsorbate weight. At leastone week was required to achieve this state. S r W REJECT103 OF POROUS GLASS Using the total pore volume and amount of salt contained in the pores, i t is possible to calculate the concentration difference, if any, between adsorbed and iinpregnating liquid. (Pore volume was assessed from the water adsorption isotherin and salt content froin the weight increase due to impregnation dcter- mined after drying to constant weight a t 10joC. The estiinated error is between 3.0 and 0.08% pending on the concentration.) 'The
results for the six saiuples listed in Table 1
indicate that the concentration in the pores (Column 4) is less by approximately 10% (Col~unn 3) than that of the penetrating liquid (Column 1).
Salt rejection in adsorbed layers has been reported lor various systems such as porous
"Tola1 pore volurne 0.2366 ml/g, p satt = 2.163 g/ml (3). p,,,k, = 1.000 g/ml, monola) cr capacily 0.0290g/g. B I X area 100.1 m2/g, nrca o i water nioleculc 10.3 B?.
PHASE TRANSITIOSS OF ADSORBATES 156
glass (4-8) and haelnoglobin (9, 10). The
phenomenon has been explained by ion ex-
change and Donnan potential (4,
S),
but it hasalso been suggested that the water on the sur- face is structured and therefore unavailable to
the solute (8, 11, 12). To test the validity of
this hypothesis the concentration of the adsorbed liquid was calculated by assuming
that one i~~onon~olecular layer is coillpletely
free of salt (Colurnn 6, Table I). The values in
Column
7
(Table I ) indicate that the concen-tration of adsorbate, with the exception of the first layer, is essentially the same as that of the iinpregnating solution. This agreement sup- ports the concept of solute exclusion in layers of bound water (13).
factors affecting relative pressure will be considered first.
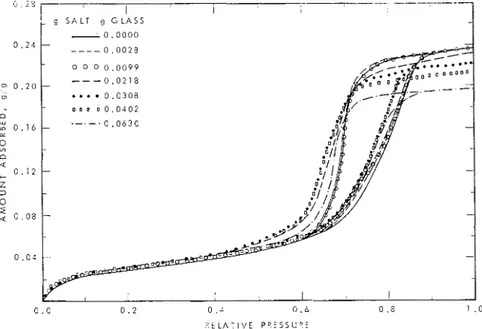
The ainount of salt in the speciinen is fixed in the course of the adsorption process, and the concentration of adsorbate varies as shown in Fig. 2. Three phases may be diflerentiated:
(I) at low relative pressures the ainount of water is insul'ricient to dissolve all the salt and both undissolved salt and saturated solution
are present ;
(2) all salt is dissolved and further uptake
of water results in dilution of the solution; and (3) when the available pore volume is coinpletely filled, concentration of the im- pregnating solution is achieved (disregarding salt rejection in the inonolayer).
ISOTHER>IS Because state (3) is achieved at relative
pressure less than unity (due to the lowering
Plotting water uptalie of the various i111-
eflect of dissolved salt on vapor pressure)
pregnated speciinens against relative pressure further increase in relative hulnidity results
of water results in a faillily ol curves not un- in surface condensation and dilution. ~h~
expectedly different from the isotherm of un- following inay serve to illustrate this
treated glass (Fig. 1). From among the pos- point: at 22OC the vapor pressure of a saturated
sible causes of distortion of the isotherms, the NaCl solution is 14.9 Torr, that of water
0 . 2 6 I SALT C O N T E N ' I I O F G L A S S , 9 g 0 0 0 0 0 / 0 0 . 2 0 . .: 0 . 6 0 .S 1 . 0 R F L A T I V E PPCSSUKE
I'rc. 1. Adsorption isotherms lor \\.ater on porous glass impregnatccl wilh KaC1 solulions ol various concentrations. Relative pressure is bascd on the saturation prcssure of pure water.
W A T E R A D S O R B E D , g ' g g S A L T ' g G L A S S - - - 0 . 0 0 2 8 o o 0 0 . 0 0 9 9
---
0 . 0 2 1 8 ' * * . 0 . 0 3 0 8 D O D O 0 , 0 4 0 2 0 . 0 6 2 0I:rc. 2. Conccr~Lralion of salt solulion ill the pores as a fuilclion of amount of \\.alcr adsorl~cd.
19.83 'rorr. I n a 1 0 0 ~ ~ relative humidit> en-
vironment the system containing salt is ex-
posed to a relative humiclity of 133% [(19.83/
14.9) X 1001. J t may be notecl also that a t
ally given relative humidity a porous body con-
taining a solilble substancc always has a higher
degree of saturation than one containing only water. This fact is most important from the frost damage point ol view.
Clearly, the water uptake of a salt-impreg- nnted solid is not governed by rclative pressure
e x ~ r e s s c d in terms of pure nlatcr but rather in
ternls ol the vapor pressure of a bull; soliltion
having concentration identical to that oi the pore liqilid a t the actual water content. 13c- cause the coilcentration of the liquid held in the capillr~rics varies during adsorption, relative
~ ~ C S S U ~ C has to be c~pi-cssecl i11 terms oi ap-
propriately changing saturation pressure ~ a l - ues. ilccordingly, the rcsillts ol Fig. 1 \\-ere
recalculated : the concentration ol adsorbed
liquid was csti~natetl froill the amount of salt and adsorbed water present; the capacity
ol the nlonolajwr, 0.0290 g/g, was subtracted
from the total water so that complete salt rejection in the first monolayer was assunled;
the appropriate saturation pressure
p0
for thegiven concentration was found b y interpolat- ing published d a t a (14) and the relative pres- sure computed. T h e replotted isotherms are
shon 11 in Fig. 3.
T h e isotherills arc very similar in shape but cannot be supcriulposed for two reasons: as
salt co~lccntration increases, ( 1 ) the horizoiltal
PHASE TRANSITIONS O F I\I)SORBATES g S A L T g G L A S S
i
~ o . o o o o - - - - 0 . 0 0 2 8 0 0 0 0 . 0 0 9 9 - - - 0 . 0 2 1 8. . . .
0 , 0 3 0 8 0 0 0 0 0 . 0 4 0 2 0 . 0 0 . 2 0 . 4 0 , & 0 .8 I .O R E L A T I V E PCESSUREFIG. 3. ilclsorption iso~herm lor \\,aLcr on porous glass imprcgnaLetl \I ill1 Sac1 soluLions of various
concentrations. Values of l.'ig. 1 rc~)loLLecl.
section of the curves a t high relativc pressures
shifts to lower watcr content values and (2)
the hysteresis loop occurs a t lower relative pressures. T h e reduced porc volume resulting fro111 the presence of salt may be the cause of
the first effect. Table I1 shows the computed
values of the volumes of the salt solutions plus
the salt-free monolayer (Column 4). KO trend
is apparent ancl the estiillated volunles closelj,
al>proach the total porc v o l u ~ n c obt:tined fro111
the water isotherm.
The second eficct, which changes the shape and position of the h!-steresis loop, maj- be attributed to a1tcr:~tions ol the morl)hology of the porous system by the undissolvccl salt. I t is not unrcaso~iable to assLuinc that porc dia~ileters are decreased by salt deposits and that increased surlace tension of the brine maj- also affect the hysteresis mechanism.
I n conclusion, it niaj- be stated that the adsorbed salt solution located outside the lirst salt-free illonolaycr has propcrties-vapor
pressure, density, solubility-that appear to
be similar to those in the bulk state. Unusual
features of the isotherms can be e ~ p l a i n e d b y
the physical presence of salt. T h e great
similarity of the isotherms (Fig. 3) nlakes it
improbable that the porous structure under the described conditions has been significantl~. altered by the presence ol salt, a phenomenon
reported in the literature (6, 5, 15).
U.hcn water that has been adsorbecl in a
porous solid is cooled to below O°C, it remains
in a liciuid-like state and has n vapor pressure
similar to that of undercooled water unless rcarrangciiient ol the adsorbate occurs (16). Because the vapor pressure of undercooled water esceeds that of ice and because the es- ~ e r i i i ~ c n t a l l j - realizable pressure is limited to that of ice, a fully saturated sample is sub- jected, on cooling, to relative pressures less
than unity. A fraction of the adsorbate thus
beconies unstable, leaves the pore sj-stein, and freezes on the outside surlace, giving rise to the 1)henomenon rclcrred to as "freezing a t high tcmperaturcs" or "first freezing" (1).
T h e ice so fornled is in the bulk state and, ac- T;1BLE I1
cordingly, melts a t O°C. E s . r r h r . . i ~ a OF V O L U ~ I E O F LIQ[.TIES ADSOREED IN
Although constant cooling rate produces a V ~ ~ r ~ o u s ~ ' U L L Y SATI.K:YL.EU SPICCI~CENS
..-
unifor111 dtxrease of relative PressLlre, the rate - ."-a]~ collLr,lL \Vcigltt of \\,Iume
of \iolurnv of
of desorption may vary between zero and of glass I I a d r o r b e t ~ arlsorbcd brine
g,'g 6/g I)rir~e a r ~ d salt-free
infinit!- depending on the shape of the iso- n ~ i / g rnor~ola\.rr
ml/g therm. \\,'ith porous silica glass, water is
liberated in such larce cluantitics when the
-
A 0.0000 0.2360 0.0000 0.2360vertical section of the isotherm is traversecl 0 0028 0 2360 0 2208 0.219s
t h a t it is unable to leave the pore system be- 0 0099 0 2360 0.2101 0 2391
0 0218 0 2300 0 2079 0 2369
cause of high viscosity and the low permeability 0 0308 0 2260 0 2074 0 2361
of the adsorbent (decreased by the previously 0 0102 0 2180 0 2030 0.2320
formed ice crystals). This pheno~nenon is 0 0630 0.2050 0 1992 0.2282
- .- - -- -- -
called "freezing a t low temperatures" or "second freezing."
to 0.9 [Ref. ( I ) , see Fig. 41, the e\pected loss
Freezl ~cg n/ 111gl1 Tenzpera/z~res of w,Lter is 4 X 10-J g/g [lief. ( I ) , see Fig. 61
with a concomitant shrinlcage of Al/l = 40
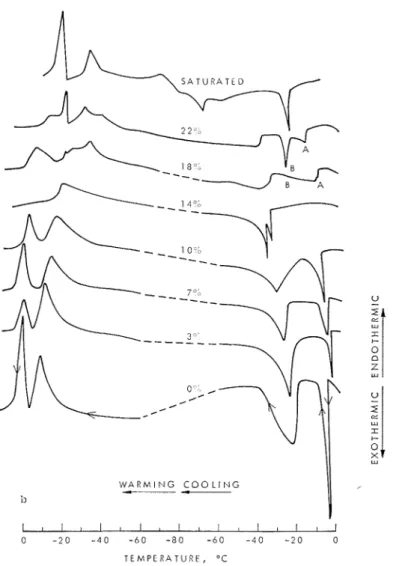
T h e estension diagrams (Fig. 4a) and dif-
ferential therillograills (Fig. 4b) of porous X 10-"Ref. ( I ) , see Fig. 91. This is in excellent
agreement with the e ~ t r a p o l a t e d length change
glass samples full)- saturated with salt solu-
in this region (Fig. -la). tions of various concentrations show, (1) on
T h e only novel feature of the length change cooling, anomalies 2 to 5 deg below the bull;
curves is the s ~ n a l l espansion t h a t occurs when
freezing point of the respective solutions, and
( 2 ) on heating, anoinalies a t a temperature samples are cooled with 10, 18 and 20%
solutions (Figs. 4 a and 6). J3ecausc, according closely approaching the bulk freezing point
to the model, some of the liquid must leave (Fig. 5).
the pores when the temperature is lowered, These results are consistent ~ v i t h the pro-
enclosure of the sample in an ice envelope posed model: on cooling, desorption takes
results in elpansion [Ref. ( I ) , see Fig. 31. 'I'he place prompted by the appearance of ice in
observed increase in length is attributed to the cell, followed by freezing on the surface;
inability of the liquid to tlow a t a suCficiently
on warming, melting occurs a t the bulk freez-
ing point. 'The double p e a l s in the thermo- high rate. Thus, the c ~ u d e d liquid solidifies,
perhaps a t the mouth of the pores, restricting grams for high salt concentration (18 and
227; and marbed A and J3 in Fig. 4b) are the flow of further amounts of liquid desorbed
caused by formation of ice in the undercooled a t lower temperatures. T h e rate dependence of
the phenomenon may be e\plained by this liquid and the eutectic solid, respectively.
11)-pothesis, assuming t h a t sufficient time is Esothermic eflect between the peaks is indica-
tion of continuous as elpectecl. avi~ilzble for solid formation a t a cooling rate
Significantly, if the impregnating solution is of ~ . ~ 4 1 f ° C I m i l l (Figs. 6~1) but not a t
saturated S a c 1 only a single peak occurs. 0.33"C/min. T h e increased viscosity a t these
T h e length change dii~grai~1 of the glass- low temperatures also hinders 1 1 0 ~ . Porous
water system lends further support to the sug- glass samples saturated wit11 aqueous glycerine
gestecl mechanism, according to which con- solutions often expand on first freezing (Fig. f ) ,
traction follow~ing the length anomaly is due an observation t h a t has never been made with
to desorption. If one considers the temperature pure water as adsorbate under siinilar condi-
region 0 t o - l l ° C , in which the n ~ a s i i n u n ~ tions and, verl- probably, due to the high
realizable vapor pressure decreases froin unity viscosity of the fluid.
I'HASE TRANSITIONS O F ADSOIiBllTES 160
Freezitzg at Lozu Temperatztres from 0.8 to 0.68, the equilibrium water con-
1. I n the temperature range -19 to -40°C
anonlalies were obtained in the dimensional changes and heat content of the waler- satured sample (Fig. 4). These are manifesta- tions of second ireezing (1) and arc assilined to result fro111 the following circumstances:
Maxinlunl relative pressure (based on vapor pressure of undercooled water), on cooling,
decreases continuously until a t -lO°C it
reaches a value of 0.8 [Ref. (I), see Fig. 41, the inception point of the steep, descending section of the desorption branch of the isotherill [Ref. (I), see Fig, 61. At constant rate of cooling the rate of desorption increases greatly in this region because, as the relative pressure changes
tent is reduced froin 0.24 to 0.08 g/g, the dif- fereilcc being equal to 65% of the ultinlate 111)t"li~ and 92% of the capillary-held water. The transfer of such an anlount of water causes significant disturbance even under equilibriunl conditions, conditions seldom established owing to the inadequate migration rate.
The dynainic character oi the phenomenon was de~llonstrated by very nluch reduced es- pansion at slow cooling rates compared with that observed a t higher rates [Ref. (I), see
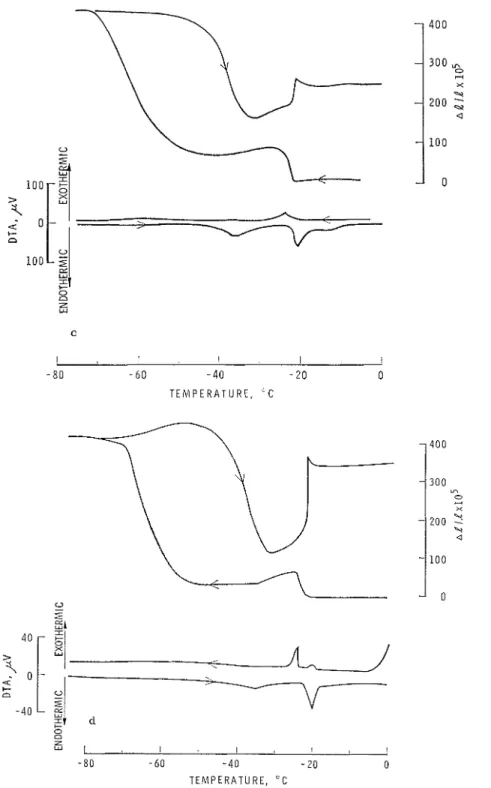
Figs. 5 and 81. I n another e~perirnent (Fig. S)
water-saturated glass was cooled a t a rate of 0.33"C/min until after the conlillenccnlent of freezing a t -lS°C, when (apart fro111 a drilt of approsinlately 1°C) constant telllpcrature
a W A R M I N G C O O L I N G
--- d
T E M P C R A T U R E , 'C
FIG. 1. l'orous glass saml~les saturated with NaC1 solutions ol various concentrations t l ~ ~ r i n f i tem- perature cycle (0.33°C/min). (a) Dimensional changes. (b) Difierential thernio,orams.
was maintained for 17 hr. I n this period,
following an initial 53 X 10-"elalive unit
expansion, the original dimensions were re- established. On resumption of cooling a t a ratc oi 0.33OC/min an overall espansion of
70 X 10-" was obtained, a value very sinlilar
to t h a t found a t a ratc of cooling of 0.041i°C/
min [Iief. (I), see Fig.
81;
this is in contrast\vith A l / l = 400 X 10-j expansion usually
occurring a t the higher rate of 0.33"C/min
(Fig. 4a). These results indicate t h a t expansion
is associated with nonequilibriunl conditions
caused by too-rapid cooling for a given per- meability and difiusion ratc.
2. If, instead o i water, S a C l solution is
used as adsorbate, icc crystals appear in the cell a t a lower teillperature ancl the deprcssioi~ of the second freezing temperature is propor-
tional to the salt concentration (Figs. 4 and 5 ) .
3. With forination of ice crystals in the cell, a vapor-pressure diflerence is created between the solid outside ancl the undcrcooled liquid inside the porous systein. Whereas in pure licluids equilibri~uil condition can be restored
PIIXSE ?'R:\SSITIOWS 01: I\I)SOI<BI\TES
only b) desorption, in solutions the possibility exists, a t least theoretically, that the vapor pressure dificrcncc can be eliminated by in- creasing the concentration of the solution through ice formation, with no need to leave the originally occupied capillaries.
Esothermic heat effect and cspansion, in- dicating second freezing, were found to occur
a ~ p r o s i m a t e l y 20°C below the bulk frcezi~lg
point of the solutions (Fig. 5 ) ) in good agree-
ment with the value obtained for watcr (19 C:
deg). T h e inciting points of the adsorbed solu-
tions shifted to apl~rosimately 13 C dcg below
the corresponding bull; value, also in agreement
in ~ ) O K ) L I S glass.
with the value obtained for water. T h e great similarity between the behavior of water and solution adsorbates suggests t h a t the mech- anism is the same in all cases. Phase separa- tion, however, takes place in neither water nor salt solutions while held in the originally oc- cupiecl capillaries. 'Thus, the mechanisnl de-
scribed in the previous paragraph is a mere
110ssibilit)r and not a reality.
1. Having concluded that adsorbate is un-
able to solidify, even partially, in the original
I I O ~ C S and that it migrates out of them, the
cluestion arises whether its free energy can be lowered by solidification in the larger pores or
it has to leave the porous system before it can freeze. T h e results of the present study suggest the fornler alternative.
The cndotherrnic heat effect a t -34"C:, in-
dicated by the differential thermogra~n in cases
of concentrations above 14%, may be assumed to result froill melting of the capillary-held eutectic mixture. This is suggested b y the value and the invariance of the tenlperaturc a t which the anonlaly occurs and t h a t it is
TAB1,E III
~ ) T I ~ P E R I I N C E IN LICSG.I.II UETIVEES L~Lrx~aru.zr O N Coor- JNG A F T E R FIRST FREEZING AND >I:ZSI~~UM BEFORE
Low T E M ~ E R ~ T U R E 'I'I~AIVIXG 01.. .2 POROUS
GLASS SAMPLE SATURATED WIT11 20% NaCl
So1,u.rro~ A l / l X 103
Coolir~g mte. 1Varming mtc, 'C/min OC/min
0.0.i 17 0.33
---
---
concentration dependent. T h e peak occurs es-
actly 21 C: deg below the melting point of
ci~pillary-held ice ( - 1 3 C ) , and this tempera- the warnling was 0~041ioChlill,
ture difference is equal to the freezing point overall expansion was less a t the higher warm-
lowering of the eutectic misture. ing rate of 0.33"C/min.
5. 'I'he behavior of systems containing salt
differs in a t least two respects fro111 t h a t of systerns containing pure water as adsorbate:
( I ) the magnitude of espansion on low tem- perature freezing is greater on slow cooling than on rapid cooling (Figs. 6a and 6b), in direct contrast with the one-component case; and (2) instead of maintaining constant length or contracting, there is substantial expansion (-45°C) a t low tenlperatures during warnliilg of systelns containing salt solutions of high concentration, the completely saturated solu- tion (Fig. 4) being a n esception.
Phenon~enologically, it be interpreted
t h a t a t a cooling rate of 0.33"C/min espansion a t low temperatures cannot be completed, be- comes "frozen in," and that the system will continue to expand after the same teillperature region has been attained on warming. 'The
values shown in Table TI1 indicate that overall
espansion was reduced b y the combination of rapid cooling and rapid warming, presunlablj- because the shorter time period available on warming was less than that required for com- pletion of the espansion prematurely ter- nlinated on cooling.
'I'he observations related to these phenomena
Although the inforination available is in-
may be summarizecl as follows :
sufficient to identify the physical causes of
1. Esl~ansion occurs a t temperatures below the phenomena, certain factors regarding the
the second melting point and reaches its maxi- behavior of salt solution are potentially
nlum just prior to the nlasimum endothernlic siznificallt
-
:heat effect. 'l'he minimum length, on warming,
1. The vapor pressure of the adsorbed salt is attained during the endotherillic heat effect.
solution is very low owing to low temperatures 2. Expansion on warming is obtained, re-
gardless of heating rate, if the cooling rate is ancl the effect of the solute. Redistribution
through transfer in the vapor phase is es- 0.33"C/min. The corollary of this statement
pected to be negligible. was also true; regardless of heating rate, no
2. 'I'he viscosity of the salt solutions is very
espansion took place if the cooling rate was
0.041 i0C:/n1in (Fig. 6). high, again due to low tenlperatures but also
to the presence of slllall ice crystals following 3. The magnitude of espansion on warming
is inversely proportional to t h a t of espansion phase separation in the larger voids. This effect
on second freezing ( ~ i ~ . 9). ~h~ difference in may retard or even inhibit flow of liquid.
length between the lllinilllunl on cooling and 3. T h e formation of a solid phase in the
the lllaxilnunl on before second pores probably leads nlechanical strength and
thawing, was the same a t both cooling rates if rigidity to the pore structure.
PHXSE TRANSJ'rIONS 01' ADSORBATES 164 T E M P E R A T U R E . 'C - Z L L I I 8 0 - 6 0 4 0 20 0 T C M P E R A T U R E C
- 80 - 6 0 - 4 0 - 20 0
T E M P E R A T U R E , " C
FIG. 6. Changes in dimensions and heat rontent of porous silica glass saturatecl with 20' NaCl solu- tion during t e ~ ~ ~ p c r a t u r e cycle. (a) Cooling ant1 heating rates, 0.33"C/min. (b) Cooling rate 0,33'C/rnin, h c a t i ~ ~ g rate 0 O4li0C/min (c) Cooling rate, 0.04lfoC/min, heating rate 0.33'C/min. (cl) Cooling and heatin:: rates 0 C417°C/n~in.
5'0
-
-
-
-
-z C WARMING COOLING".
"
--
U TEMPERATURE, OC-
,
.
_
o
l
-
J
j O >-v---
/- - -
2 O"dl;
WARMING C O O L I N G x b 1- - Y TEMPERATURE, "C 166LITVAN
---
1 7 H O U R S-1
200.33°1hil'N C O O L I N G R A T E
V
0.33'lhllN ,PIG. S. Changes in dinlensions and heat content of porous silica glass saluratccl with ijXaler during tcnlperature cycle: cooling rate 0.33°C/min I~elween 0 and -18°C; 0.12"C/niin betiveen -18 and 21°C; arrest for 17 hr a t -21°C; 0.33°C/n~in rate in rclnaindcr of the cycle.
There is little doubt about the csistence and relevance of the listed effects, but further work is recluired before a detailed illechanislll can be suggested. The tindings of the esperinlents with
aqueous glyceriilc solutions (Fig. 7) may be ac-
cepted as evidence of the cfiect oi the high viscosity of the solutions. Particularly in-
structive is the case of the 20% solution where,
presumably because of the high viscosity after the appearance of ice in the system, no desorp- tion took place and the loclted-in licluid caused a vcry large expansion on first freezing. With the pore system scaled, the sccoild freezing also resulted in a vcry large e\pansion (A1/1
= 1700 X 10-j). E ~ p a n s i o n was obtained also
on warining. The absence of significant changes in the dinlensions of the higher concentrations may be attributed to their glass-forming capability on solidification, in which case no din'erence exists in the vapor pressure oi the bullc solid and undercooled liquid. The crjo-
protective action of glycerine in biological systeills has been explained by this illechanism
(17).
MECHANICAL FAILUItE
Frost action, i.e., the impairinent oi me- chanical strength, is the most inlportant practi- cal aspect of the low teinperature behavior of porous systems. As this subject will be dis- cussed in a fortl~coming publication on the
study of cement paste containiilg K:LC~ solution
as adsorbate, only a icur conllllcnts will be made.
It appears that the systc~u sufiers mechanical
damage in a temperature cycle ii the redistribu- tion of adsorbate cannot take place a t an adccl~iate rate. I n this respect certain condi- tions specific to two-component sj-stems are significant. The gradual freezing of such solu- tions and the widening oi the tclllpcrature range of the phase change are beneficial.
I:~G. 7. Porous gl:rss samples saluralctl n i t h aqueous glycerine solulions of various concentralions during temperature cycle (0.33°C/min). (a) 1)imensional ~ h : ~ n g e s . (I,) Differential thcrmogmms.
PIIASE TRANSITIONS OF ADSORBrWES 168
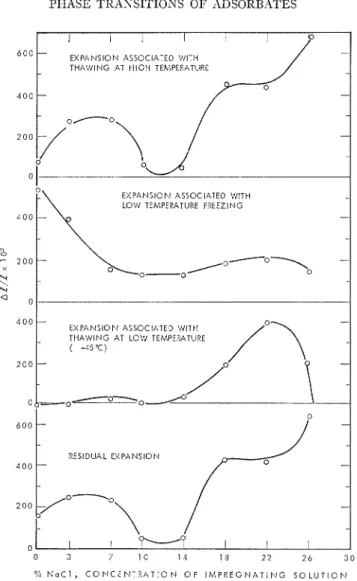
EXPANSION ASSOCIATED '//ITH LOVJ TLMPEPATURE FRCEZING
0 -? -% a opp 9 ; N o C 1 , C O N C E N T R A T I O N O F I M P R E G N A T I N G S O L U T I O N
FIG. 9. I'arnmctcrs of Lhc cstcnsion curves of 1;ig. 4a as a functioll of salt concentration.
Figure 10 s h o ~ ~ s the amount of ice formed in
bull< 9 a C 1 solutions of various concentrations per Centigrade degree. I t may be seen that a t intermediate concentrations the rate of ice Sorlnation is least nonuniform ancl that, ac- cordingly, length changes of the sj.stem with 10 and 19% solutions are comparatively small.
r 7
I he other factor assunled to have bearing on
the behavior of the system is low teml>erature and its consecluences, i.e., high viscosity, low vapor pressure, etc. One of the most significant observations of the present esl>eriment is the fact that the temperature cycle destroyed every sample containing glycerine solution of less than 60% concentration, whereas with
pure water as adsorbate under sinlilar cir- culnstances this never happened.
CONCLUSIONS
I t is the major conclusion of the present work that the low telnperature behavior of the porous glass-water systenl in thc presence of S a C l is, mutatis
-__-
_-_.--
n?utandis, si~nilar to that in its absence. I t is suggested that the primary cause of peculiarities is the vapor pressure dif- ference betwee11 the bulk solid without ancl the undercooled liquid-like adsorbate within thepores. Confirnlation of this 11) pothesis has
been obtained regarding the phenomenon re- ferred to as first freezing. \Yith regard to the Jorrrjiol o/ Colloid a?id I~rlei./irce Scicacc, Vol. 45, No. 1. October 1973
T E M P E R A T U R E , "C
I;IG. 10. il m o u n ~ of icc, esprcsscd in weight pcr ccnt, formctl in hTaC1 solutions of various conccntra- Lio~is per tlcgrcc Cclsius
second freezing phenomenon, the modifying effect of very low temperatures, high viscosity, low vapor pressure and the freezing n~echanisin of two-component solutions is tentatively as-
~ ~ l l t t c d to be responsible for the divergence in
some of the features of the two systems. ACKSO\\:LEDGL4FCN'TS
T h e author is grcatly indcl~tcd to 11. Schultz lor carrying oul Ihc csperimcntal worl;, Lo R. Myers for surlacc area tlctcrminatiou, and to I-[. S. Slatle for assislancc in Lhc construction of Lhc apl~aratus.
IZEI:EI<ENCES
1. I,~Tv.IN, G. G., J. Colloid ItllerJ~rce S c i . 38, 75 (1972).
2. LITV~Z~', G. G., J. :1111er. C F Y U I J I . SOC. 5.5, 38 (1972).
3. K ~ U P X A X N , D. W. (Ed.), "Sodium Clllor de. T h c I'roduction nr~tl Propertics of Salt and-Brine," 1). 592. Amcr. Chen). Soc. Monograph Series. 4. R ~ : \ u s , K . A., hi,\~<crso\vs~\-, A. E., Jorrxsow,
J. S., AND SIIOK, A. J., Scict~ce 151, 194 (1966). 5. i\r.acc, T., ax11 HAIR, M. I,., J. Plrys. Cllcn~. 72,
,599 (1968).
6. B.\LI.ou, E . V., LEI:;\N, ;\'I. I., .\XI) WYDEVES, T., :vi~lr~re Plrysicnl Sciet~ce 229, 123 (197 1).
7. U.ir.r.oo, E . V., I\SD \VYUEVEN, T., J:'ir:,irolr. S C ~ .
l'cclir~ol. 1032 (1971).
8. Scr~u~:rz, R. I>., ANI) :\susar..\~, S. R., i l l '.I<ccent
Progress in Surfacc Science"
(5.
I.'. I)nniclli, K . G. A. P a n l t h ~ ~ r s t , and r\. C. Riddifortl, Etls.), Vol. 3, 13. 291. Xcadcmic Press, ITcw Yorlt, 1970.9. PI~.KUTZ, L'I., [)kc. P ~ I I . O ~ ( I ; V S O C . 42B2 187 (1946). 10. GAKY U O I : ~ , C. l I . , J. ( ; P I / . Plrj'siol. 50, 2547
(1967).
11. I-~ORNE, I<. :I.,
r)..~\,,
A. I:.) \-OI;XG, I:, I),, ZYXDYu, K,'. T., J~lrrlrocl~it~a. ~ l c l n 13, 397 (1968). 12. S ~ U H I K ~ \ J . ~ N , S., Itld. 1i11g. Clierir. Prcrrdntt~~~trlirls 2,
51 (1963).
13. LLNG, G . S. it6 "\Yatcr and ;\clucous Solutions"
(1:. ;\. Horne, Ed.) p. 684, \Vilcy-Tntersciencc, New Yorli, 19i2.
14. "International Critical Tablcs," Vol. 111, 1). 3i0. hlcGm\\. Hill, Nc\v S70rk, 1928.
15. G o ~ u o s - K ~ s o u , J., I ~ R ; \ X L - O I S - ~ < ~ ~ S E T T I , J., :\XU
TVELII<, Ii., Brrll. Soc. Cl~ittr. l?ri~)rce 4, 816 (1962). 16. S~ur..~~o,r.roa~, E. \V., 4x11 Lrrv,\s, G. G., Trnlrs.
I J a l . ~ ~ d i l ~ ~ S O C . 67, 2726 (1 9il).
17. L r r v a ~ , G. G., Cryobio1o:y 9, 182 (1972).