F. VERZAR, M.D., AND V. FREYDBERG, B.S.
(From the Physiological Laboratory of the University of Basel, Basel, Switzerland)
A
DECREASE of the activity of the thyroid inold age has often been mentioned in humans. Anatomic involution was described (5, 7, 10) and this was related to the decrease of basal metabolism in old age (2). Also a de-crease of plasma iodine (8) and an inde-crease of serum cholesterol (3) was observed. The uptake of I1" in the thyroid in old age has been studied by Quimby, Werner, and Schmidt (11) and this was related to the function of the kidney (1, 6, 9) while Skanse (12) found no significant differences.
Since all these, partly contradictory, works were performed on humans we have now studied the problem of whether the thyroid activity changes with age in rats, in the 'old age colony" of our laboratory, where all animals are of the same breed and are kept under identical conditions.
METHODS
Rats were injected intraperitoneally with 25 microcuries of Ilai (without carrier), dis-solved in 0.25 ml. of physiologic saline solu-tion. Eighteen to 24 hours later the thyroid region of the rats was tested for radioactivity by means of a TCG2 Geiger counter (Tracer-lab). The procedure was identical with that previously used in this laboratory (13, 14). The radiation values over the thyroid region were measured in series after 24, 61, 134, and
1.82 hours (1, 28, 58, and VA days) and in series II after 22-24, 69-71, 110-114 and 165 hours (1, 3, 4)2, and 7 days).
Immediately prior to the radioactivity meas-urements the animals were anesthetized with ether, using a glass bulb. The excitation pe-riod is very short when a massive dose is given. The anesthesia was continued during the estimation, which lasted about three min-utes. This procedure was then repeated every 24 hours, or in some instances after longer periods, until approximately the fourteenth day after the injection of F3\ at which time the radiation count approaches zero. It is
Submitted for publication April 6, 1955.
of course possible that the thyroid at the end of this period may still contain small amounts of I131 the radiation of which is too small to penetrate the skin. The values measured were always corrected for physical decay and for the background count. The reliability of this method for rats was proven in previous pub-lications (13, 14). The radiation values dur-ing a period of several days follow each other on a continuous line and single values are well reproduced in immediately following measurements. In former work, the uptake was studied from the first hours after injec-tion, but this was not necessary with regard to the present problem.
In a number of animals the excretion of iodine in the urine was also measured. For this purpose the rats were placed in individual glass cages. The urine was collected and the water used to wash the cage added to the sample. All urine samples were made up to 50 ml. with water and an aliquot used for radi-ation measurement.
The estimation was made by evaporating 1 ml. of the urine sample to dryness at 95 C. in a small planchet. The radiation was measured and compared with equal quanti-ties of the originally injected solution, which contained 25 microcuries in 0.25 ml. volume and expressed in percentage of the latter.
TABLE 1. COMPARISON OF I131
IN THE FIRST 24 HOUR URINE WITH TWO METHODS.
Animal No. Dry Method % of Originally Injected I131 31.6 17.3 35.3 31.3 25.6 Dip Method Radiation Values (c.p.m.) 1520 905 1445 1150 1289 53
Another procedure for measuring the urinary radioactivity was also employed. For this assay a dip Geiger counter (TCG5) was used on urine samples which had been diluted to 50 ml.; in this method only radiation values can be given. The range of measurements was the same for the two procedures; the dip counter method was found to have no advant-age over the dry method (table 1).
RESULTS
All the rats employed in the experiments were of the same breed and were kept under identical conditions. Fifty per cent of the rats of our colony survive the age of 23.5 months; we have therefore designated as old animals rats more than 20 months old. The groups of old rats included in the present series of experiments were between 20 and 29 months of age. Parallel to the studies on the old animals, experiments were conducted with young animals aged 7 to 9 months.
The animals were studied in two series, the first during the summer period (May-June, 1954) and the second during the winter sea-son (January, 1955): the results were simi-lar in both series. Series I comprised 10 young (7 month old) and 12 old (24 month
24 48 72 96 110 134 158 182 24 48 72 96 110 134 158 181 Hours
Fig. 1. Radiation measurements over the thyroid gland in rats following injections of 25 microcuries of Im at zero time. Series I. Observations during summer period.
old) rats; series II 20 young (7-9 month old) and 20 old (20-29 month old) animals.
The results of the radiation measurements over the thyroid gland were plotted in curves for each animal. The curves show individual differences which at first sight seem to raise considerable difficulties for comparing the I131 uptake and release. The comparison was sim-plified, however, when the values obtained on the same days after injection of I131 were correlated and the mean values computed. On the basis of single curves it was our impres-sion that old animals often release I"1 from the thyroid more slowly than the young rats, i.e., the curve becomes flat after a few days. However, not all the old animals reacted in the same way, and some of the young ani-mals also exhibited flat curves. Possibly such animals should have been considered abnor-mal and excluded from the comparisons.
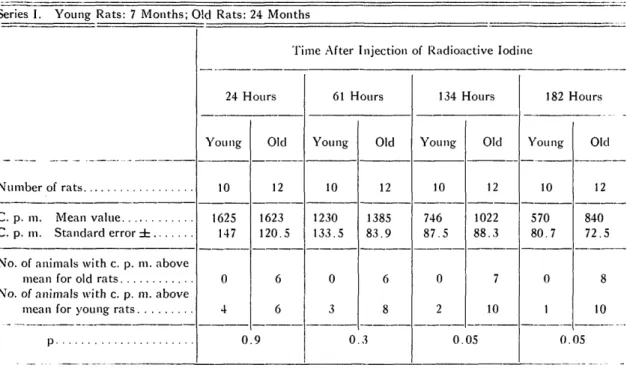
The mean values of the radioactivity meas-urements over the thyroid gland observed on the 1st, 3d, 5th, and 7th day after injec-tion of Im are presented in table 2. The table
Fig. 2. Radiation measurements over the thyroid gland in rats following injections at 25 microcuries of Im at zero time. Series II. Observations during winter period.
TABLE 2. RADIOACTIVITY MEASUREMENTS OVER THYROID GLAND OF RATS AT VARIOUS TIMES AFTER INJECTION OF RADIOACTIVE IODINE.
Series I. Young Rats: 7 Months; Old Rats: 24 Months
Number of rats C. p. m. Mean value C. p. m. Standard error db No. of animals with c. p. m. above
mean for old rats
No. of animals with c. p. m. above mean for young rats
P
Time After Injection of Radioactive Iodine
24 Hours Young 10 1625 147 Old 12 1623 120.5 0.9 61 Hours Young 10 1230 133.5 Old 12 1385 83.9 0.3 134 Hours Young 10 746 87.5 Old 12 1022 88.3 7 10 0.05 182 Hours Young 10 570 80.7 Old 12 840 72.5 10 0.05
Series II. Young Rats: 7-9 Months; Old Rats: 20-29 Months
Number of rats C. p. m. Mean value C. p. m. Standard error ± No. of animals with c. p. m. above
mean for old rats
No. of animals with c. p. m. above mean for young rats
P 22-24 Hours Young 20 1966 155 0 7 Old 22 2067 283 5 7 0.7 69-71 Hours Young 20 1052 85 0 9 Old 20 1216 83 9 14 0 . 1 110-114 Hours Young 20 672 66.5 0 9 Old 20 851 62 9 17 0.05 165 Hours Young 20 419 43.3 0 10 Old 20 657 66 8 17 0.01
further contains the calculated standard error and p values. Not all intermediate measure-ments had been included.
As seen from table 2 the mean values for the old animals recorded on the 5th and 7th day were found to be higher than those ob-served in the young rats. The calculated p values are 0.05 and 0.01, respectively, for the
groups in the two series of experiments. A listing has further been made in table 2 of the number of old animals which showed higher values than the mean value for the old group and the number of young rats exhibiting val-ues above the mean for the young group at the same time of estimation. If the val-ues for the 32 old animals in both series are
56
combined, it will be seen that 27, or 84 per cent, showed higher values on the 5th and 7th day than the average value for the young animals measured at the same time.
In figures 1 and 2 these mean values to-gether with their standard errors are shown graphically for the two series of animals. It is evident from the figures that the release of I181 is slower in the old than in the young ani-mals.
In order to evaluate these findings a consid-eration of the renal excretion of I131 seemed of importance. Such measurements, which were carried out in 15 old animals and 12 young animals in Series II showed the following: During the first 24 hours after the intraperi-toneal injection of 25 microcuries of I131 very large quantities of iodine were excreted in the urine (table 3). Although some over-lapping of the values for the groups occurred it was found that the young animals excreted much more iodine on the first day than did the old rats, namely an average of 48.4 per cent (±3.56) of the injected quantity com-pared with 29.2 per cent (±2.27) in the old animals. On the second day after injection the excretion became more or less constant and was only slightly less on the 5th and 6th days. The excretion on the second day was found to value about 5 per cent of the injected quantity of iodine and on the third day about 3 per cent. No characteristic difference in this steady excretion was noted between the old and the young animals. The sum of all I131 excretion during the first three days fol-lowing the injection valued 57 per cent in the young and 38 per cent in the old animals (table 3). The total excretion over a period of 7 days was 68.1 per cent in the young and 50.9 per cent in the old rats.
DISCUSSION
The radioactive iodine, soon after it is con-centrated by the thyroid gland, becomes bound as organic iodine in the form of thyrox-ine and triiodotyrosin. These hormones are released from the thvroid during the follow-ing days, and the I131 activity of the gland thus becomes a measure of the rate of their release. The results of the present investiga-tion have revealed that the majority of old rats (84 per cent) exhibit a delayed release of PS1 from the thyroid gland as compared with young animals.
TABLE 3. URINARY EXCRETION OF RADIOACTIVE IODINE IN PERCENTAGE OF INJECTED DOSE OF
25 MICROCURIES.
Figures represent individual animals.
No. of Animals M e a n . . . . Standard Error. .. P First Young 30.4 44.5 42.6 43.2 51.0 51.8 69.5 70.5 39.0 51.5 52.8 34.0 12 48.4 ±3.56 0.01 Day Old 27.7 23.3 35.7 31.4 7.8 31.6 17.3 35.3 31.3 25.6 27.7 33.1 27.8 45.2 37.0 15 29.2 ±2.27 Second Day Young 3.9 5.1 4.1 1.9 5.63 4.40 3.78 6.31 7.44 9.16 5.70 5.18 12 5.22 ±0.54 0.6 Old 4.6 5.9 4.1 3.7 6.2 6.41 8.23 4.89 5.78 3.97 6.82 4.76 5.90 7.16 4.79 15 5.55 ±0.34 Third Young 3.0 3.1 3.0 4.1 2.53 4.07 3.51 4.71 5.23 4.11 2.92 2.24 12 3.54 ±0.26 0.4 Day Old 4.4 4.8 2.5 5.1 2.68 4.33 3.24 1.64 2.37 3.23 2.83 2.82 2.19 2.28 14 3.17 ±0.29
It was further found that old rats show a lower renal excretion of iodine during the first day after the iodine administration than do the young rats. This difference might be the result of an increased capacity of the thyroid of the old animals to retain iodine. The radio-activity measurements over the gland failed, however, to show an increased trapping of iodine by the thyroid in the old rats.
Another explanation of the lower iodine ex-cretion by the old rats might be a reduced ability of the kidneys to excrete iodine. The quantities of iodine which are excreted in the urine are so small, however, that it is dif-ficult to imagine that an impaired filtration by the kidneys could influence the excretion
sig-nificantly. It seems more likely that the de-creased excretion by the kidneys is also an expression of the diminished release of iodine from the thyroid gland in the aged animals; after the rapid fixation of the injected I131 by the thyroid a slow release will lead to a slower excretion of iodine in the urine.
The delayed release of the thyroid hormone in the old animals, as measured by I131, may be due primarily to a decreased metabolism of the body cells in the aged animals. Con-sequently, the thyroid would release less of its hormone, which increases the oxidation processes. Such explanation would agree with the well-known facts that in old age oxida-tion processes (2), body temperature and the upkeep of body temperature in cold sur-roundings (4), and the reaction of body temperature to diminished oxygen pressure (15) decrease. It may be of course that the decrease of cell metabolism is not the cause but rather the result of a decreased release of thyroid hormone. At present it seems hardly possible to decide definitely between these two possibilities.
SUMMARY
1. The activity of the thyroid gland was compared in 32 old (20-29 month) and 30 young (7-9 month) rats by measurement of the radioactivity of the gland following in-jection of F8\
2. The release of I131 from the gland was in general found to be slower in the old than in the young animals. It was possible to demonstrate such difference in 84 per cent of the old rats on the fifth and seventh day after the injection.
3. The excretion of I131 in the urine on the first day after injection valued 48.4 per cent of the injected quantity in the young rats and only 29.2 per cent in the old rats. The most probable explanation of this difference seems to be that the decreased release of I131 from the thyroid in the old rats is the cause of the lower urinary iodine excretion.
REFERENCES
1. Ackermann, P. G., and Iversen, K.: Radio-Iodine Excretion in the Aged. J. Gerontol., 8: 458-464, 1953.
2. Boothby, W., Berkson, J., and Dunn, H.: Stu-dies of the Energy of Metabolism of Normal Individuals: A Standard for Basal Metabolism, with a Nomogram for Clinical Application. Am. /. Pkysiol, 116: 468-484, 1936.
3. Bonati, B., Salvi, A., and Rancati, G.: Sulla Funzionalita della Tiroide Senile. Gior. di Ger-ontol, 2: 182, 1954.
4. Cannon, W. B.: Problems of Ageing, 2nd ed. E. V. Cowdry, ed. William and Wilkins, Balti-more, 1942.
5. Cooper, E.R.H.: Histology of Endocrine Organs at Various Ages. Oxford University Press, Ox-ford, 1929.
6. Davies, D. F., and Shock, N. W.: Age Change* in Glomerular Filtration Rate, Effective Renal Plasma Flow, and Tubular Excretory Capacity in Adult Males. /. Clin. Investigation, 29: 496-507, 1950.
7. Kimble, S. T., and Stieglitz, E. J.: Hypothyroid-ism—a Geriatric Problem. Geriatrics, 7: 20-31, 1952.
8. Kountz, W. B., Chieffi, M., and Kirk, J. E.: Serum Protein Bound Iodine and Age. J. Ger-ontol, 4: 132-135, 1949.
9. Lewis, W. H., and Alving, A. S.: Changes With Age in the Renal Function in Adult Men. Am. J. Physiol, 123: 500-510, 1938.
10. Mustacchi, P. O., and Loewenhaupt, E.: Senile Changes in the Histological Structure of the Thyroid Gland. Geriatrics, 5: 268-273, 1950. 11. Quimby, E. H., Werner, S. C , and Schmidt, C : Influence of Age, Sex, and Season Upon Radio-iodine Uptake by the Human Thyroid. Proc. Soc. Exper. Biol Med., 75: 537-540, 1950. 12. Skanse, B.: Radioactive Iodine in the
Diag-nosis of Thyroid Disease. Ada med. Scandinav. Suppl. 235, 136: 1-186, 1949.
13. Verzar, F.: Control of Thyroid Activity with, I131 in the Rat. Radioisotope Technique, vol. I. Oxford University Press, Oxford, 1951, 1953. 14. Verzar, F., Sailer, E., and Vidovic, V.: Changes
in Thyroid Activity at Low Atmospheric Pres-sures and at High Altitudes, as Tested with 13
T. /. Endocrinol, 8: 308-320, 1952. 15. Verzar, F., and Fliickiger, E.: Lack of
Adapta-tion to Low Oxygen Pressure in Aged Animals. /. Gerontol, 10: 306-311, 1955.