Publisher’s version / Version de l'éditeur:
Transactions of the Engineering Institute of Canada, 3, 4, pp. 107-109,
1960-08-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
The Use of waste supphite liquor to reduce frost heaving in soils
Penner, E.; Robillard, P.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=726aaff1-f4c3-40a3-929d-f279467e208e https://publications-cnrc.canada.ca/fra/voir/objet/?id=726aaff1-f4c3-40a3-929d-f279467e208e
S
er
TH1
N21r2
no. 106
c.
2
NATIONAL RESEARCH COUNCIL
CANADA
DIVISION OF BUILDING RESEARCH
THE USE OF WASTE SULPHITE
LIQUOR TO REDUCE FROST
HEAVING IN SOILS
byE. Penner and P. Robillard
REPRINTED FROM
TRANSACTIONS OF THE ENGINEERING INSTITUTE OF CANADA
VOL.
3, NO.
4,DECEMBER 1959, P.107-109
PRICE 1 0 CENTS
RESEARCH PAPER NO. 1 0 6
OF THE
DIVISION OF BUILDING RESEARCH
OTTAWA
AUGUST 1 9 6 0
The
Use
of
Waste
Sulphite Liquor
to
Reduce Frost Heaving in Soils
E . Penner and P . RobillardT b e treatnzent of frost-susceptible soils w i t b wnste szilpbite liqzior bns been sbow?z t o be renso?zably szrccessfz~ll.~. Its effec- tive?zess bns been nttribzited t o the change i7z 71zoisnire penize- nbility l~rozigl.~r nbo~rt Dy, ( i ) a ,Treater viscosity i.12 t h e pore /Izrid nnd ( i i ) its dispersnnt action w h e n 771ixed w i t b soil w b i c b renrlts i77 a grenter density n77d hence n lower penllenbility.
Ice crystnllisntio?z ?izenszire711e?zrs in waste nllpbite liqrior ~olritio~zs reported i n the pnper slqozu t h e rate t o be grently rifected b y the concenrrntion of the solzuion. For exn?~zple, nt 10°C of szipercooling the rnte of ice crystnllizntio?~ in pzire wnter wns 116 ti?lles grenter tbnn i?z n SO per cent solzitio?z. T h e sztggestio?~ is t l ~ n t t h e redzlction i?z frost benz,ing reszilti?zg I1.0717 n wasre s~clpbite liqzior trent71zent cn?z be nttribzited portly t o the slower rnte of ice growth.
I
N R E C E N T years much interest has been focussed on the use of cheinical additives for the attenuation or prevention of frost heaving in soils for localized areas in roads, railroads, and airports that repeatedly sho\v destructive heaving characteristics. Among the many additives that have been studied is a waste product from the pulping of wood by the sulphite process, usually referred t o as waste or spent sulphite liquor. This inaterial consists largely of lignosulphonates but also contains sugars and acid degradation products of lignin and cellu- lose in s~llaller amounts.T h e effectiveness of waste sulphite liq~ior in reducing frost heaving both in the laboratory and under field conditions appcars t o be reasonably well established1.? but the processes underlying its use are not well under- stood. Lalnbe1 has attributed its beneficial effect t o its dispersant action when mixed with the soil which results in -greater densities and consequently lower perme- abilities. Hardy2 believes the higher viscosity of thc waste sulphitc liquor solution in the pore water, as con)- pared to thc nornlal soil moisture, t o be the major reason for the effectiveness of the treatment in reducing ice scgregation. Important as they may be it is believed that thcse processes do not conlpletely account for the very large reductions in frost heaving that are solnetiines reported.
Lusena3, from studies of ice crystallization rates in systcms of biological interest, showed the effectiveness of organic substances in reducing the rate of ice growth. T h e results of this work and the earlier work of Tammann aiid Eiichner' suggested that the effectiveness of n.aste sulphite liquor in reducing frost hcaving in soils might be partly due to this pl~enomenon. As a first step in in- vestigating this possibility, experilllents wcre undcrtalten to measure the rate of linear ice growth in waste sulphitc liquor solutions contained in sinall, glass capillary tubcs. Thcse rates, measured both as a function of supercooling and solute concentration, arc reported in this paper.
hf.inuscript received 30 April 1959.
Materials and Methods
Waste sulphite liquor designated as "Lignosol B" was diluted t o give concentrations close to 0.5, 1, 2, 1, 8, 16, 50 in per cent weight of solute t o weight of water.
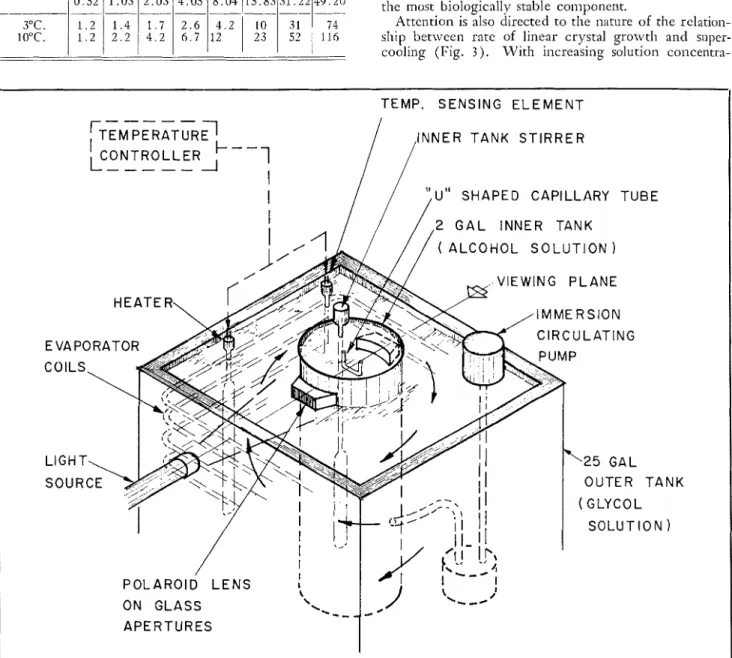
T h e freezing point depression determinations were carried out with the constant temperature apparatus shown in Fig. 1. A 20 C.C. thin-walled narrow-neck
glass bulb equipped with a mechanical stirrer and coppcr- constantan thcnliocouple was placed in the inner tanlt. T h e solution was cooled a t a rate less than O.l°C. per min. Crystallization was induced slightly below the freezing temperature of thc solution. At this point the stirrer in the inner tank was stopped t o reduce further the rate of cooling. T h e detcmlinations were repeated at least five times with different preparations of the solutions at each concentration. T h e mean values are shown in Fig. 2.
Crystallization rates were carried o u t according to thc method described by Lusena3. This consisted of deter- mining the time for t h e ice front to propagate between two fixed points 6 cm. apart along the bottom section of a U-shaped glass capillary tube 1.0 nim. I.D. and 0.5 mm. wall thickness. T h e tube was allowed t o establish tliern~al equilibrium with the bath before crystallization was induced. Ice propag.ition was viewed through crossed polaroids with the tubc submerged i n the inner tanlt of ethyl alcohol. Crystallization was induced wit11 a thin frosted wire at one end of the capillary tubc. T h e complete apparatus is shown in Fig. 1.
Timing was done with an electrical timer sensitive to 0.01 second. T h e tenlperatures were measured with a copper-constantan tllermocouple and a suitably pre- amplified emf. recorder previously calibrated ~vitli a high precision platinuni resistance thermometer. The re- liability of the temperature measurements for thesc ex~erinlents I was considered t o be within
*
0.02"C.T h e rate determinations were repeated at least fivc times with three different U-tubes filled with separate solutions of the same concentration. Each mean value reportcd at any particular supercooling was the average of 15 separate determinations.
Results and Discussions
T h e results sho~vn in Fig. 5 give the rate of linear ice
propagation as a function of supcrcooling for each con- centration. T h e ratcs for pure water shown were calcu- lated from the general equation v = (0.158
*
0.009)A
TI.""
oO.O"nm./sec. determined by Hillig and T u r n -bull" T h e points plotted about this curve are from determinations that were carried out in the DBR labora- tory.
T h e rate, as shown b y Fig. 3, decreases with concentra- tion at any particular degree of supercooling. To show this more clearly Table 1 ( a ) gives the pcr cent reduction cornpared with pure water at 3 and 10°C. supercooling. For examplc at 3°C. s~lpercooling the crystallization ratc has been reduced to .Z that of pure water a t a sulphite
liquor conccntration of 8 per cent. A t 10°C, of super- cooling t!lis same reduction has been achieved b y the 2 per cent soli~tion. By estending this trend t o srnaller amounts of supcrcooling, inore realistic under natural
TABLE I ( a ) . - Per cent Reduction in Ratc o f Linear Ice
Propagation in Solution
TABLE 1 ( b ) . - Ratio of Linear Crystallization Ratc i n
Pure Water to Solution I
I
Concentration \V/\V yoSuper- -- --
ccoling
/
0.52
I
1.03I
2.03I
1.05 18.04 115.83131.2?149.20conditions in the soil, the amount of solute needed to bring about this same percentage drop in rate would be in excess of 8 per cent.
Table l ( b ) gives the ratios of the rate o f ice growtll in pure water to that in solution and shows that i t is 116 times grcater in p u r e water than in a 50 per cent solution at 10°C. of supercooling.
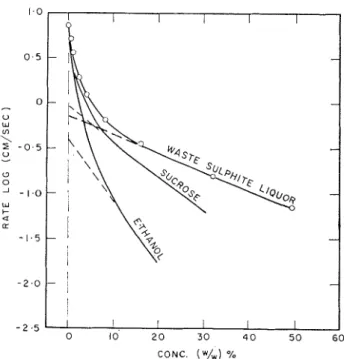
Luscna3 has plotted his results in the f o r m (log cin./scc. versus ccncentration) as a nleans of comparing t h e effcc- tiveness of the various solutes. T w o of tllese have bccn plotted in Fig. 4 together \\,it11 the results of t h e luastc sulphite liquor. It may be s e c ~ l that the nature of the effect is similar t o some other organic substances a l t l ~ o u g l ~ the magnitude of the effect is a little smaller.
In o r d e r t o determine what component ill the liquor is mainly responsible f o r t h e reduction in rate, a n d any interaction effect, further esperin~ellts are being planned. T h i s is of some importance wllen considering t h e per- manence of the treatment in t h e biologically active soil. T h i s n ~ o u l d be quite apart froill the leaching o u t of the material with time. It would be satisfactory if t h e main reduction i n crystallization rate could be attributed t o the most biologically stable component.
Attention is also directed t o the nature of the rclatioli- ship bctmecii rate of linear crystal growth a n d super- cooling (Fig. 3 ) . W i t h increasing solution concentra-
Fig. 1. Constant tenlperaturc liquid bath appamtus f o ~ . both freezing point tlep~.cssion ancl crystallization rate detern~inations.
Fig. 2. Freezing point depression vs. sulphite lic~nor concentration.
tion the inflection in the curves occurs a t a smaller super- cooling. In the case of the 32 and 50 per cent solution the inflection is n o t evident f r o m Fig. 3. In plots drawn t o a larger scale this effect does sllow up. T h e r e also appears t o be some evidence of an inflection f o r pure water. In carlier results b y T a m m a n n and Biichncr' the inflection f o r p u r e water occurs a t about 8°C. supercooling. These phenon~ena arc, ho\\,cver, of acadcmic intercst only. Conclusion
T h e rate of ice crystallization in waste sulphice liquor is greatly reduced below the valucs f o r p u r e water at t h e sarne degree of supercooling. T h e greatest reduction per unit concentration occurs in the most dilute solutions. A t high concentrations and large supercoolings t h e effect becomes logarithmic with concentration.
A l t h o u ~ h D the conditions of cr\zstallization as carried o u t in these experiments are n o t entirely similar to the g r o w t h of ice lenses i n soil, it is believed this pheno- menon accounts, in part, f o r the reduced heaving ob- served in soils treated with waste sulphite liquor. I t does not eliminate, however, the importance of the pro- cesses of dispersion and viscosity describcd by Lambe and Hardv.',? i
In a broader sense, all solutes tend t o reduce the rate of ice crystallization below that of pure water. T h i s cfiect does n o t appear t o have been previously considered in frost action research.
ACKNOWLEDGMENT
T h e authors are indebted t o their colleague Mr. Lorne
IT.
G o l d f o r his many useful comlnents; i t was at hissuggestion that t h e experiments were initiated. T h i s is a contribution f r o m the Division of Building Research, National Research Council of Canada, and is published with the approval of the Director of t h e Division.
REFERENCES
1. I , ~ ~ n b c , 7'. W. "Alodification of I:ro\t IHc,lr ing of So115
\I lth Additives". Hzghwny Iiesenrch Bonrd Bulletin 135,
pub. 425, pp. 1-23, 1956.
2. Hardy, R. A?. "Prelention of Frost IHea\-ing by Injection
of Spent Solphite Liquor". Proceedings 3rd Internati?nal Conference on Soil h/Ieclranics and Foundation Engineering,
vol. 11, pp. 103-106, 1953.
;. Lusena, C. V. "Ice Propagation in Systcms of Biological Interest. ILI. Effect of Solutcs on Xucleation and Growth of Ice Crvstals". Archivex o f Biochc7/tistrv nnd Bioahvsics. r - ,
vol. 27, no. 2, ~ p . 277-284, 1955.
4. l'ammann, G . and A. Biicllner. "Die Lineare ICristallisa- t~ons-ncscli\vindinkeit dcs Eises aus ncwohnliche~n und sch\l~el<rn ass;". Zeitscl~ri/t fiir un~~orgn~ziscl:e zrnti nllgevteh7e Chcnzie, Band 221, pp. 12-16, 1935.
Fig. 3. Rate of lincar iee crystallization vs. supercooling at different concentrations of s u l p l ~ i t e liquor.
C O N C . ("'/,I %
Fig. 4. Effect of solute coneentration o n rote of linear iee-crystal growth at 10°C. super-cooling. (Results for
sucrose and e t l ~ a n o l fro111 Lusena3.)
5 . I-Lillig, \V. 13. 2nd D. Turnbull. "Theory of Crystal
Gro\vtli in Undercooled Liquids". Jozlt~/nl of Che/t7. Physics vol. 24, p. 914, 1956.