Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Technical Translation (National Research Council of Canada), 1962
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC : https://nrc-publications.canada.ca/eng/view/object/?id=31ed151d-531a-497d-ad5f-a6c12c1be73d https://publications-cnrc.canada.ca/fra/voir/objet/?id=31ed151d-531a-497d-ad5f-a6c12c1be73d
NRC Publications Archive
Archives des publications du CNRC
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/20358582
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
The calculation of the amount of salt required to melt ice and snow on highways
Schneider, T. R.; National Research Council of Canada. Division of Building Research
PREFACE
Many millions of dollars are spent annually in Canada to remove snow and ice from roads, railways, runways and other facilities associated with transportation. Unfor- tunately, there is little information readily available that will guide those responsible for keeping transpor- tation facilities open in the winter to the most effec- tive means of doing this under given circumstances. It is one of the responsibilities of the Snow and Ice Sec- tion of the Division of Building Research to collect and digest such information and make it available in Canada.
A comprehensive study of snow and ice removal from
highways was recently undertaken by the Society of Swiss Highway Experts. The Swiss Snow and Avalanche Research
Institute, under the directorship of Dr. M. de Quervain,
undertook for this society a series of investigations on methods for removing snow and ice or controlling their disposition. This report contains the results of a pre-
liminary tl~eoretical investigation of the action of salts
on ice crusts. Because of its preliminary nature it
should not be considered as the final answer to the prac- tical problem. The project was financed by a federal fund established in Switzerland for highway research.
The Division of Building Research is privileged to have very close liaison with the Swiss Pederal Institute
for Snow and Avalanche Research. In 1948-49 Dr. de Quer-
vain accepted an invitation to visit Canada as a guest worker with.the Division of Building Research, National Research Council, for one year, giving the Division his expert guidance in formulating a programme of future re- search on problems involving snow and ice. During this visit he became thoroughly familiar with the serious problems that these materials create Tor Canadian trans- portation facilities and made significant recommendations as to how these problems should be studied. The National Research Council Is pleased indeed to have Dr. de Quervain again contribute in a very real way to these studies by his making available reports containing the results of the excellent investigations by Dr. T.R. Schneider. We wish to record our appreciation to the Society of Swiss Highway Experts as also to Dr. de Quervain for their permission to have these reports translated into English and published in the Technical Translation Series of the National Research Council.
Ottawa
January 1962
Robert F. Legget Director
T i t l e :
NATIONAL RESEARCH COUNCIL OF CANADA
T e c h n i c a l T r a n s l a t i o n 1004
The c a l c u l a t i o n of t h e amount of s a l t r e q u i r e d t o melt i c e and snow on highways
( ~ i e Berechnung d e r z u r ~ u f l b s u n g von Schnee- und E i s k r u s t e n notwendigen S a l z s t r e m e n g e n )
Author: T.R. Schneider
Reference : E i d g e n o s s i s c h e s I n s t i t u t f a r Schnee- und Lawinen- forschung, Weissfluhjoch-Davos, I n t e r n e r B e r i c h t N r . 328, 1960. 27p.
THE CALCULATION OF TI33 AMOUNT OF SALT REQUIRED TO MELT ICE AND SNOW ON HIGHWAYS
I. Introduction
The brief description of the action of salts used for melting
purposes contained in Internal Report no. 302 of June, 1959, is to
be enlarged on in the present one. The first part will deal with the theoretical principles, which in turn will enable us to draw
practical conclusions on the basis of the salts at present in general use. It will be possible to calculate the amounts that will have to
be spread in order to melt snow and ice crusts of various thickness
and at the same time to estimate the prospects of employing other substances.
It is doubtful whether it will be possible to translate the results obtained here directly into practice before certain relevant detailed investigations have been completed, since the theoretical calculations of all the chemical-physical reactions are based on the assumption of ideal mixtures. This is a condition which can scarcely be realized in actual operations on highways. The present report must therefore be.considered as laying the theoretical groundwork, and it will be the aim of future laboratory and field studies to de- termine what departures from these unrealizable theoretical assump- tions occur, and what are the effects of traffic and of various weather conditions.
11. Theoretical Principles
The description of the processes underlying the melting of ice by salts or other chemical compounds lies in general within the
province of physical chemistry and therefore the following exposition is based chiefly on Eucken's classic "Lehrbuch der chemischen Physik", 1930.
The basic research in this field was done in the second half of
the last century and the beginning of the present one, when it occu-
t i f i c t r e n d of t h e t i m e s a s atomic r e s e a r c h does i n contemporary s c i e n c e . It w a s a s s o c i a t e d w i t h names l i k e Kohlrausch, Arrhenius, Ostwald, N e r n s t , B j e r r u m , Debeye, ~ i f i c k e l , e t c . The c h i e f r e a s o n f o r t h e d i f f i c u l t i e s encountered i n t h e i n i t i a l i n v e s t i g a t i o n s
stemmed from t h e f r e q u e n t f a i l u r e of e x p e r i m e n t a l r e s u l t s t o a c c o r d w i t h t h e t h e o r e t i c a l p r e d i c t i o n s . T h i s was because no one as y e t had a c l e a r p i c t u r e o f t h e s t a t e of t h e p a r t i c l e s p r e s e n t i n t h e s o l v e n t s , f o r t h e r u l e s t h a t were i n i t i a l l y drawn up h e l d t r u e o n l y when t h e d i s s o l v e d s u b s t a n c e d i v i d e d i n t o e l e c t r i c a l l y n e u t r a l p a r - t i c l e s ( m o l e c u l e s ) . Only when i t was r e a l i z e d t h a t many s u b s t a n c e s s p l i t up on b e i n g d i s s o l v e d , e i t h e r p a r t i a l l y o r t o t a l l y , i n t o e l e c - t r i c a l l y p o s i t i v e and n e g a t i v e i o n s ( d i s s o c i a t i o n ) and a n a c c u r a t e knowledge o f t h e p r o p e r t i e s o f t h e s e i o n s was o b t a i n e d d i d i t become p o s s i b l e t o e x p l a i n t h e d e p a r t u r e s from t h e s t a n d a r d c a s e of d i s s o c - i a t i o n t o t h e molecule s t a g e o n l y .
I n what f o l l o w s we s h a l l now p r e s e n t t h e most i m p o r t a n t formulae i n c o n j u n c t i o n w i t h v a r i o u s e x p e r i m e n t a l r e s u l t s s o f a r as t h e s e a r e needed f o r an u n d e r s t a n d i n g of t h e p r o c e s s e s o c c u r r i n g when s a l t i s s p r e a d on i c e . A . Lowering o f t h e F r e e z i n g P o i n t Lowering of t h e f r e e z i n g p o i n t can b e s t be i l l u s t r a t e d by t h e vapour p r e s s u r e
-
t e m p e r a t u r e diagram f o r t h e s t a n d a r d c a s e g i v e n i n F i g . 1. Considering f i r s t t h e p u r e s o l v e n t , i t s f r e e z i n g p o i n t Smust be, by d e f i n i t i o n , a t t h e p o i n t where t h e vapour p r e s s u r e c u r v e s of t h e s o l i d and l i q u i d phases i n t e r s e c t . It i s e a s y t o u n d e r s t a n d t h a t t h e vapour p r e s s u r e c u r v e o f t h e s o l i d s t a t e must f o l l o w a s t e e p - e r c o u r s e t h a n t h a t of t h e l i q u i d . When a s o l u b l e s u b s t a n c e i s added t h e vapour p r e s s u r e of t h e s o l u t i o n i s lowered, because t h e d i s s o l v e d p a r t i c l e s r e s t r i c t t h e n a t u r a l v i b r a t i o n s of t h e s o l v e n t molecules and c o n s e q u e n t l y a p a r t of t h e i r k i n e t i c energy i s consumed i n a d d i - t i o n a l c o l l i s i o n s w i t h t h e f o r e i g n p a r t i c l e s . The vapour p r e s s u r e curve of t h e s o l u t i o n t h u s l i e s below t h a t of t h e p u r e s o l v e n t . A s -
suming f o r t h e c a s e of i n t e r e s t h e r e , as h a s been confirmed by micro- s c o p i c s t u d i e s , t h a t i n t h e c o u r s e of f r e e z i n g pure s o l i d s o l v e n t
crystallizes out without forming mixed crystals with the salt, then the vapour pressure curve of the solid phase remains unchanged and
the solution has a freezing point S f , as Fig. 1 shows, that is lower
than that of the pure solvent by an amount AT
.
This lowering of theg
freezing point can be derived from the Clausius-Clapeyron equation and the laws of osmosis as follows:
where AT
-
lowering of freezing point (OK)g
T g
-
freezing point of pure solvent (OK)R
-
universal gas constant (= 1.985 cal/degree)le
-
latent heat of melting of the solvent (cal/mole)C t
-
molar concentration of the solution (moles/litre)n
-
molar count of the substance to be dissolvednL
-
molar count of the solventR
T~
The expression: ---& = E
1000 le €5
is called the molar lowering of the freezing point. It is the number of degrees by which the freezing point is lowered when one mole of a
substance is dissolved in 1000 g of a solvent (for water = 1 litre).
An essential point for the further considerations is that this quanti- ty depends only on the properties of the solvent, not on those of 'the
substance dissolved. The higher the freezing point and the smaller
the latent heat of melting, the greater the value of
E
.
For water,g
which is of special interest here, the following value can be calcu- lated from the above formula:
Because of the high latent heat of melting of ice in comparison with certain organic solvents this is a comparatively low value.
p o i n t , i t s v e r y r e s t r i c t e d a p p l i c a b i l i t y must be borne i n mind, f o r i t assumes t h a t t h e d i s s o l v e d s u b s t a n c e d i s s o c i a t e s o n l y i n t o i t s e l e c t r i c a l l y n e u t r a l molecules. However, t h i s c o n d i t i o n i s s a t i s - f i e d o n l y f o r a r a t h e r l a r g e number of o r g a n i c s u b s t a n c e s . The m a j o r i t y of i n o r g a n i c a c i d s , b a s e s and s a l t s b r e a k down i n s o l u t i o n n o t merely t o t h e molecule s t a g e , b u t t o a g r e a t e r o r l e s s e r d e g r e e a l s o i n t o t h e i r p o s i t i v e c a t i o n s and n e g a t i v e a n i o n s , a p r o c e s s mown as e l e c t r o l y t i c d i s s o c i a t i o n . On t h e one hand, t h e number of p a r t i c l e s p r e s e n t i n t h e s o l u t i o n i n c r e a s e s , and i n a d d i t i o n t h e s e a r e no l o n g e r e l e c t r i c a l l y n e u t r a l b u t c a r r y p o s i t i v e o r n e g a t i v e c h a r g e s . The r e s u l t i s t h a t t h e y a r e a b l e t o i n f l u e n c e each o t h e r r e c i p r o c a l l y i n t h e i r arrangement and i n t h e i r f o r c e e f f e c t s . Thus t h e l o w e r i n g of t h e f r e e z i n g p o i n t i s no l o n g e r a pure f u n c t i o n of t h e s o l v e n t , b u t i s a l s o i n f l u e n c e d b a s i c a l l y by t h e chemical s t r u c - t u r e of t h e d i s s o l v e d s u b s t a n c e .
The e x t e n t of d i s s o c i a t i o n of t h e n e u t r a l molecules i s c h a r a c - t e r i z e d by t h e d i s s o c i a t i o n l e v e l a of t h e s u b s t a n c e , which i s t h e
r a t i o of molecules t h a t have s p l i t i n t o i o n s t o t h e number of n e u t r a l molecules o r i g i n a l l y p r e s e n t . For example, i f a l l t h e molecules
have s p l i t , t h e n a = 1, i . e . t h e d i s s o c i a t i o n i s complete. I f t h e molar count b e f o r e d i s s o c i a t i o n i s no, t h e n a f t e r d i s s o c i a t i o n we o b t a i n t h e f o l l o w i n g v a l u e s : u n d i s s o c i a t e d molecules n = n u 0 (1
-
4
i o n s of one t y p e n = n - a i 0 a l l i o n s Eni = 2n ' a , o r 3no a 0depending on whether 2 ,
3
o r more i o n s o r i g i n a t e from one molecule. The t o t a l number of p a r t i c l e s p r e s e n t i n t h e s o l u t i o n i s given by( e . ~ . f o r ~ a ~ 1 )
( e . g . f o r MgC1, and c a c l , ) e t c . E l e c t r o l y t e s a r e weak o r s t r o n g depending on t h e d i s s o c i a t i o n l e v e l . With t h e e x c e p t i o n of a few compounds ( e . g . t h e h a l i d e s of antimony, t i n and mercury) a l l t h e n e u t r a l s a l t s of i n t e r e s t t o u s
h e r e belong t o t h e second group. The degree of d i s s o c i a t i o n i n t h e weak e l e c t r o l y t e s depends t o some e x t e n t on t h e c o n c e n t r a t i o n of t h e
s o l u t i o n . I n s t r o n g e l e c t r o l y t e s , however, complete d i s s o c i a t i o n may be expected up t o comparatively h i g h c o n c e n t r a t i o n s , as Rama-
k r i s h n a and Sambasiva (1936) were a b l e t o prove e x p e r i m e n t a l l y f o r aqueous s o l u t i o n s of N a C 1 , MgC1, and C a C l , , among o t h e r s , even f o r s o l u t i o n s of s e v e r a l M .
Thus t h e formula g i v e n above f o r t h e lowering of t h e f r e e z i n g p o i n t needs a c o r r e c t i o n which w i l l t a k e i n t o account t h e d i s s o c i a - t i o n l e v e l . T h i s i s accomplished by s u b s t i t u t i n g f o r n t h e e f f e c - ti1.e number of p a r t i c l e s ( e , g . no (1 i- a ) ) r e s u l t i n g frcm t h e d i s - s o c i a t i o n . The molecular lowering of t h e f r e e z i n g p o i n t of t h e e l e c t r o l y t e i s t h u s ( c t = 1; i . e . 1 mole s a l t p e r 1000 g s o l v e n t )
whence
The e l e c t r o s t a t i c charge of t h e i o n s i s t a k e n i n t o account i n a s i m i l a r manner, i . e . a c o r r e c t i o n f a c t o r f o (osmotic c o e f f i c i e n t ) i s i n t r o d u c e d , which i s d e f i n e d as t h e r a t i o of t h e t r u e osmotic p r e s s u r e P of t h e e l e c t r o l y t e s o l u t i o n t o t h e ( i d e a l ) p r e s s u r e P
r i
e x e r t e d by n e u t r a l p a r t i c l e s of t h e same c o n c e n t r a t i o n , i . e .
Thus, f o r d i s s o c i a t i o n i n t o two i o n s o n l y ( e . g . N ~ C I ) , formula
( 3 )
f o r t h e molecular lowering of t h e f r e e z i n g p o i n t becomesIf the effect on the solvent, in the case of dissociation, is due to the increased number of particles which interfere with the natural movements of the solvent molecules, then this effect is
still further increased when the particles are electrostatically charged, because c>ach positively charged ion tends to attract nega- tively charged ones and vice versa, which in turn increases the
effective radius of action of the individual electrostatically charg- ed particles compared with neutral ones, whLch adopt a completely random arrangement.
There are two different ways of determining the osmotic coef- ficient. It can either be calculated by statistical computation of the electric component of the free energy of the ionic solution or it can be found experimentally from precision measurements of the lowering of the freezing point in solutions of known concentration.
The first method, assuming for monovalent, binary electrolytes (e.g. ~ a ~ 1 ) that the charges are lumped, leads to the equation:
where eo
-
electrostatic atomic charge NL-
Loschmidtlo numberD
-
dielectric constantk
--
Bolzmann constantT
-
freezing temperature of the solvent ( O K )Co
-
gross concentration of the salt (moles/litre)3
Leo
I
* T N ~ TThe term depends only on the kind of solvent 3 (D * k T ) ~ / ~
when e x p r e s s e d i n a b s o l u t e u n i t s and when t h e c o n c e n t r a t i o n Co i s g i v e n i n moles p e r l i t r e . F o r e l e c t r o l y t e s which s p l i t up i n t o a t o t a l o f Z i o n s a v a l e n - cy f a c t o r where Z
-
number o f i o n s}
of t y p e a and k n-
valency o f i o n smust be i n t r o d u c e d and t h e f a c t o r Z must r e p l a c e t h e 2 under t h e r o o t .
I n T a b l e I v a l u e s o f [ w ] and Z a r e g i v e n f o r a number o f f r e - q u e n t l y o c c u r r i n g t y p e s of' s a l t s :
T a b l e I
The f i n a l formula f o r aqueous s o l u t i o n s i s t h e r e f o r e
However, i f t h e i o n c h a r g e s c a n no l o n g e r be c o n s i d e r e d lumped Z 2
3
2 4 Type of s a l t K C 1 , N a C l C a C l , , MgC1, CaSO,
~ 1 ~ 1 , 't h e c a l c u l a t i o n i s more complicated and i t i s f i n a l l y found t h a t t h e
L w 1
1
2
* n =
2 . 8 3 4- 0
=8
3
* Q = 5.20f o r m u l a f o r lumped i o n s must be m u l t i p l i e d by a f'unctlon ( a ) o f t h e p r o d u c t
a [ ~ ] [ a = i o n r a d i u s ; [ K ] =
D ° K . T
which f o r s m a l l v a l u e s of same can be developed i n t o a converging s e r i e s
As these derivations for the general case show, the calcula- tions lead to rather complex formulae which, moreover, are quite difficult to evaluate numerically because some of the values ( e . g . the ion radius) cannot be determl.ned accurately. Furthermore, it was always assumed that these considerations involved dilute solu-
tions. Experimental testing of these mathematical derivations has
shown, however, that the assumption of lumped chnges is permissible only for concentratfons below 0.01, so that in investigations in- volving concentrations greater than this the assumption must be dropped, and hence, because of the uncertainties involved in the determination of certain coefficients, correction on the basis of
equation
(9)
no longer permits the calculation of absolute values.Consequently the uncertainties are usually avoided, especially when it is a question of comparatively high concentrations, by determin-
ing the coefficients experimentally. This too, of course, entails
great difficulties, because it is very hard to measure the lowering of the freezing point accurately, especially at smaller concentra- tions.
These determinations were carried out by Robinson and Stokes
(l848/49) for NaC1, MgCL, and CaC1,. The results are reproduced
in Fig. 2 and 3 for the osmotic coefficient fo under the assumption
of complete dissociation, which, as already mentioned, is justified.
The curve for NaCl shows a minimum at a concentration of 0.1~ (moles
per litre). For the other two salts this minimum must lie in the
vicinity of 0.05. This minimum of the osmotfc coefficient results
also in a minimum of the molar lowering of the freezing point, which is to say that the minimum effectiveness is attained at the corres- ponding concentration of the solutions. The continuous and ever
steeper rise of the curve after the minimum suggests that the effec- tiveness of the salt solutions improves continuously with increasing
conccntration, i .e. the rate of lowering of the freezing point in-
Plg. 4, in which the freezing point temperatures of salt solutions
according to data given in Landolt and ~8rnstein's tables (1923)
are shown, since here the curves become' steeper and steeper with increasing salt content (i.e. increasing concentration of the solu- tion).
B. The Phase Diagrams
In the foregoing the physical processes underlying the lower- ing of the freezing point of solution have been explained. We shall now consider the practical consequences to be drawn from these state- ments, restricting our investigations principally to the salts which are of special interest to us, namely NaC1, MgC1, and CaC1, and their solutions. The first aim is to construct the phase diagram of these
salt solutions ( ~ i g .
5),
which will serve as the starting point forfurther discussions. The curves of freezing point lowering (Fig.
4)
make an important contribution, because they can be used directly in plotting the left-hand side of the diagram down to the eutectic point. The right-hand side was completed from data found in Peeryvs "~hemical Engineers 11andbook" (1950)
.
These simply represent the solubilities of the salts at the corresponding temperatures. The diagrams therefore give information on the phase equilibria for various temperature-concentration conditions.In describing the processes, however, the effect of the water vapour must also be taken into account. That is to say, the curves
of the phase diagram simply represent the temperaturc-concentratlon
equilibrium conditions between the solution and ice (AE) on the one
hand, and between the solution and the salt (EB) on the other (Fig.
5a), which relate to the water vapour prcssure. Now, by definition the two phases must possess the same vapour pressure at O°C, the temperature which is realized by a mixture of water and ice which is in the state of equilibrium, because otherwise the vapour in the phase with the lower prcssure would condense and hence that with the higher pressure would vanish in the course of tlme. Therefore, our
curves must converge at this point ( A ) . If salt 1s added to the
water the solution will now show a lower vapour pressure compared with the pure solvent. The extent of the lowering wlll Incrlease
with increasing concentration in the manner shown in Fig.
6
for a temperature of 0°C. The ice also possesses a vapour pressure which depends on the temperature, as shown in Fig.7.
Accordingly for every ice of a certain temperature there is a solution concentration showing a vapour pressure equal to this due to the lowering of the vapour pressure. Now since, by definition, the freezing point is the point at which the vapour pressure is in a state of equilibrium between the solid and liquid phases, the values for the lowering of the freezing point found mathematically or experimentally in the previous section can be applied directly to the construction of the phase diagram.A
calculation of the relationship between ice at a certain tem- perature and the quantity of salt necessary to melt it by equaliza- tion or the vapour pressures cannot at present be carried out be- cause the necessary data for constructing the vapour pressure formu- lae of aqueous salt solutions as a function of the temperature are lacking for the general case. As in the case of the ice vapour pressure curve of Fig. 7, for which the empirical formula26911- .2
log p = 11.50406
-
0.4 log 'I'-
T
was very accurately determined by Scheel and Heuse (1909), corres- ponding investigations would have to be made for aqueous solutions of salts, and the formulae derived by similar empirical means. As the above considerations show, however, determining the lowering of the freezing point will accomplish the same purpose.
Let us now consider the phase diagram in Fig. 5b. Let a solu- tion of temperature t l and composition S 1 be slowly cooled. From this, in the diagram, we get the vertical straight line XC, because for the present nothing changes in the composition. At the tempera- ture t, the curve AE of the diagram is intersected, i.e. the freezing point of the solution of composition S, is reached and pure ice begins to crystallize out, so that thc salt concentration of the solution increases. The more ice that separates out wllth falling temperature
the more concentrated the solution becomes. The course.of this change is characterized by the curve CE. At the ternperature tE and the composition SE of the solution the so-called eutectic or cryo- hydratic point is reached at which salt as well as ice will begin to crystallize out, because here the solubility curve of salt is intersected and the solution solidifies to give an ice-salt mixture. Below the eutectic temperature, which is different but accurately defined for every kind of salt, no solutions can occur.
In considering the processes which go on during the melting of ice at a certain temperature by the scattering of salts the fol- lowing four cases relating to different salt-ice weight ratios must be distinguished ( ~ i g . 5b):
1. The eutectic mixture
( s ~ )
2. The mixture which, according to the phase diagram, will have a freezing temperature just equal to the initial ice temperature
(s,
)3.
Salt-ice ratio greater than the eutectic mixture( s ~ )
4. Salt-ice ratio smaller than that required to make the freez- ing temperature just equal to the initial temperature (S,)
.
The start of the reaction proceeds in the same way in all four cases. On the siuface of the salt crystal a film of solution begins to form involving the water vapour talcen hygroscopically from the air in combination with the moisture adhering to the ice. Thisfilm of solution becomes more and more concentrated. As soon as the solution in contact wlth the ice shows a lower vapour pressure than the ice at any given point (the concentration S, for the ice temper- ature t2 in Pig.
5b)
the ice beeins to melt. The liquefaction of the ice now dilutes the solution unless its concentration is main- tained by dissolving Inore salt. This reciprocal dissolving of salt and ice continues until one of the two substances is completely d i s -solved.
IIowever, in order to melt the ice, heat is required. This is obtained from the follol$rin@ sources: At t l ~ c first instant it is supplied by the icc itself as its tcrnperaturc is lovrercd by contact with the fllm of solution covcring it. The cutectic temperature
represents the lower limit of cooling, because the film of solution on the ice can cool only down to this temperature. This condition is reached shortly after the start of the reaction. The furnishing of the heat by the remaining ice is possible until the latter also reaches the eutectic temperature. Normally, however, this heat does not suffice to melt the ice completely and so the remaining heat required must be obtained from the environment, i.e. the sup- porting surface and the air. Depending on the kind of salt employed, its heat of dissolution, provided it is positive ( ~ g ~ 1 , - 6H20,
CaC1,- 2~,0), can also make a certain contribution. If the heat of dissolution is negative ( ~ a ~ 1 , CaC1,. 6H,O), however, additional heat must be acquired in order to dissolve the salt.
(1) The eutectic mixture
In the eutectic mixture exactly enaugh salt is present so that when the reciprocal dissolving of ice and salt is concluded both
substances are completely in solution. The reaction terminates as soon as sufficient heat has been drawn from the environment to melt the residual ice at the eutectic temperature. This occurs rather rapidly because a difference in temperature between the environment and the eutectic reaction mixture is maintained until the melting process is complete. Subsequent heat exchange will in time bring about an equalization of temperatures, bringing the solution back to the original temperature without involving any other processes.
For practical purposes, there is a certain advantage in this method in that only the quantity of ice to be dissolved, but not
its temperature, need be known. The disadvantage, however, consists in the comparatively large quantities of salt required.
( 2 ) The mixture which, according to the phase diagram will have a
freezing temperature just equal to the ice temperature
At this mixture ratio the salt is completely dissolved before all the ice is melted. After the salt has been dissolved a solution of eutectic concentration and temperature exists on top of the ice but a residual ice of the same temperature remains underneath. In order to melt this as well, the heat exchange with the environment
must continue u n t i l t h e e n t i r e system r e c o v e r s t h e o r i g i n a l tempera- t u r e . With r i s i n g temperature more and more i c e i s melted u n t i l , a t t h e conclusion of t h i s exchange, i t w i l l have vanished completely.
T h e o r e t i c a l l y , i t w i l l t a k e i n f i n i t e time t o r a i s e t h e tempera- t u r e of t h e s o l u t i o n t o t h a t of t h e environment. Using t h i s c r i t i c a l mixture i s t h e r e f o r e not very p r a c t i c a l , although e s p e c i a l l y a t high- e r temperatures complete m e l t i n g can be obtained w i t h c o n s i d e r a b l y s m a l l e r q u a n t i t i e s of s a l t t h a n a r e r e q u i r e d f o r t h e e u t e c t i c a p p l i - c a t i o n .
For a l l mixture r a t i o s between t h e s e two p o s s i b i l i t i e s t h a t have been d e s c r i b e d t h e same r e a c t i o n occurs; t h e time taken t o r e a c h com- p l e t e m e l t i n g of t h e i c e w i l l vary between t h e two extremes.
( 3 )
S a l t - i c e r a t i o g r e a t e r t h a n t h e e u t e c t i c mixtureThe r e a c t i o n h e r e follows e s s e n t i a 1 . j t h e same course as i n t h e e u t e c t i c c a s e , with t h e s i n g l e d i f f e r e n & t h a t a f t e r a l l t h e i c e has
been melted some undissolved s a l t ' w i l l remain. The method i s t h e r e - f o r e uneconomic and i l l o g i c a l because t h i s e x c e s s s a l t s e r v e s no pur- pose.
(4) S a l t - i c e r a t i o smaller t h a n t h a t r e q u i r e d t o make t h e f r e e z i n g temperature . j u s t equal t o t h e i n i t i a l temperature
Here t h e r e a c t i o n proceeds i n t h e same way a s i n c a s e 2 w i t h
t h e d i f f e r e n c e t h a t a f t e r conclusion of t h e h e a t exchange w i t h t h e environment a l l t h e i c e i s not melted.
The Thermal Balance Sheet
The mathematical d e s c r i p t i o n of t h e p r o c e s s e s d e s c r i b e d i n t h e f o r e g o i n g s e c t i o n i s important because i t w i l l permit u s t o a s s e s s t h e u s e f u l n e s s of t h e v a r i o u s t y p e s of s a l t and t h e v a r i o u s methods and t o e s t i m a t e t h e e f f e c t of t h e s u r f a c e and of t h e weather condi- t i o n s . C e r t a i n d i f f i c u l t i e s , however, a r i s e from t h e f a c t t h a t s e v e r a l assumptions and boundary c o n d i t i o n s have t o be introduced f o r a l l thermodynamic c o n s i d e r a t i o n s which a r e only r e a l i z e d i n n a t u r e t o a l i m i t e d e x t e n t .
following equation: SE =
%
+
+
$
t
LS ( f o r t = oOC) SE-
q u a n t i t y of h e a t r e q u i r e d t o melt a l l t h e i c e-
q u a n t i t y of h e a t d e r i v e d from t h e road s u r f a c e%
-
q u a n t i t y of h e a t d e r i v e d from t h e a i r$
-
q u a n t i t y of h e a t gained from t h e c o o l i n g of t h e i c e LS-
h e a t of s o l u t i o n of t h e s a l t These v a r i o u s q u a n t i t i e s can be c a l c u l a t e d a s f o l l o w s : ( a ) SE = m~ S~ rnE-
weight of i c e ( g ) s~-
l a t e n t h e a t of m e l t i n g of t h e i c eSE
-
depends on t h e temperature according t o Linke (1955):oOc
: 79.7 cal/g -10 : 74.5 I 1 -20 : 69.0"
-30 : 63.0"
-40
: 56.3 I1 -50 : 48.6 I1 (b)%
= VB dB cB ASB VB-
volume of s u r f a c e m a t e r i a l cooled (cm3) dB-
d e n s i t y of road s u r f a c e m a t e r i a l (g/cm3) cg-
s p e c i f i c h e a t of s u r f a c e m a t e r i a l c o n c r e t e : 0.21 cal/g degree a s p h a l t : 0.22 I I I I cement : 0.18 " 11 sandstone : 0.17 I 1 I I g r a n i t e : 0.18 " II s o i l : 0.44"
I I "B-
c o o l i n g of s u r f a c e ( O C )VL
-
volume of a i r (cm3) dL-
d e n s i t y of cooled a i r (g/m3) cL-
s p e c i f i c h e a t of a i r (0.24 cal/g d e g r e e ) A S L-
c o o l i n g o f a i r (OC) ( d l = VE * dE CE AitE VE-
volume o f i c e (cm3) dE-
d e n s i t y of i c e (g/cm3) c-
s p e c i f i c h e a t of i c e(0.5
c a l / g d e g r e e ) EAS^
-
t e m p e r a t u r e d i f f e r e n c e between s o l u t i o n and o r i g i n a l t e m p e r a t u r e o f i c e ( O C ) mS-
weight of s a l t ( g ) 1-
h e a t of s o l u t i o n of s a l t ( c a l / g ) s The v a r i o u s s a l t s have t h e f o l l o w i n g h e a t s of s o l u t i o n : T a b l e I1 These e q u a t i o n s g i v e no i n d i c a t i o n of t h e time r e q u i r e d f o r d i s s o l v i n g t h e i c e , a f a c t o r which i s e s s e n t i a l f o r comparing t h e methods, and t h e r e f o r e t h e c o n s i d e r a t i o n must be expanded. It i s c h i e f l y n e c e s s a r y t o determine t h e time i t w i l l t a k e t h e s u r f a c e and t h e a i r t o d e l i v e r t h e amount of h e a t r e q u i r e d t o d i s s o l v e t h e r e s i d - u a l i c e . The h e a t of s o l u t i o n of t h e s a l t and t h e h e a t a c q u i r e d by t h e c o o l i n g of t h e i c e need be t a k e n i n t o account o n l y i n s o f a r as t h e y a r e a b l e t o i n f l u e n c e t h e t o t a l q u a n t i t y of h e a t Q t o be d e r i v e d N a C 1 MgC1, 6H20 C a C 1 , 6H,O C a C l , 2H20 kcal/.g mole-
1 .164 i- 3.400-
4.118 i-12.5 c a l / g -19.9 i-16.7 -18.8 i-85from outside. In what follows it will be assumed that this influence has already been calculated, i.e. all that remains to be investigated are the laws which determine the heat obtained from the surface and from the air.
If the surface temperature of a (theoretically) infinitely thick, homogeneous surface of locally constant original temperature is chang-
ed suddenly by a value A 3 and is kept constant at the new temperature,
a heat conduction process will be initiated which during the time to will furnish to or withdraw from the surface (depending on whether the change of temperature is positive or negative) the following quantity of heat:
A 3
-
temperature difference1
b - 4 h - c a p = conductive capacity (kcal/m2 degree hZ)
b for concrete =
24.5
b " asphalt = 12.4 (applied here for the entire bituminous surface)
b
"
ice =9.3
to
-
time (h)The heat transfer from the air per unit surface area can be calculated by the following equation:
a
-
heat transfer coefficient (kcal/m2 h degree)Air: natural convection
3
-
20forced convection 10
-
100A 3
-
temperature difference between the air and the surface (assumed constant)For the sake of simplicity we must here neglect the radiation exchange, although it is recognized to be of great importance in nature and for practical application. Addition of the two equations gives the total heat transfer
Q:
The solution of this equation for the time to, which is the quantity we are mainly interested in here, is as follows:
We shall now illustrate these statements by the following practical example:
Question:
How long will it take to melt a layer of ice 1 mm thick on a surface of concrete and on one of black top by applying an eutectic quantity of NaCl?
Assumptions:
1. Air, ice and surface before spreading the salt have the same temperature (3 ) of -5OC.
1 2
2. There is little wind (approximately
0.5
m/sec): a =5
kcal/m h degree.3.
The heat conduction of the ice layer is neglected.4 . The boundary surfaces between ice and road surface material and
between ice and air are cooled rapidly to -21°C and retain this temperature until the ice is completely melted.
5.
The calculation is limited to a unit surface of 1 m2.For the sake of clarity the calculation is carried out in separate steps:
2 .
I n o r d e r t o melt
t h i samount of i c e t h e following q u a n t i t i e s
of heat
arer e q u i r e d
a t-21°C:
0.92
68.4
=62.8 kcal
3.
Theq u a n t i t y of
s a l t(Table
VI) i s :f o r
100g i c e 30.4
gs a l t ; f o r 920 g i c e
t h i scomes t o
J o m 4
920
=280 g s a l t
100
4 . The heat of s o l u t i o n of t h e s a l t
i s :280
( - )19.9
=5600 c a l
=5.6 k c a l
I n
t h ep r e s e n t case ( n e g a t i v e heat of s o l u t i o n )
t h i smust
be addedt o t h e l a t e n t heat of melting:
62.8
+
5.6
=68.4 k c a l
5. From t h e cooling of t h e I c e t o t h e e u t e c t i c temperature
w eob-
t a i n e d
0.92
0 . 5 (21-5)
7.4 k c a l
6 . The following q u a n t i t y of h e a t must be furnished
by t h e a i rand
by t h e s u r f a c e :
Q = Q l
-
QE =68.4
-
7.4
=61.0 k c a l
7 . The various h e a t t r a n s f e r equations a r e :
( a )
A i r : QL = a(aE
-
5)
t o= 5 - 1 6 - t o
(b) Concrete surface:
(c) Bituminous surface:
Q,
= 1.13-
)
bK
o= 1.13 16 14.4
* E
o&E
= 224 o8. This yields the following heat transfer equations:
(a) Concrete surface:
Q
=80
to+
443
0
(b) Bituminous surface:
9 . After substitution of x
=6
we get the following equation0
for
x:
(a)
Concrete surface:2
x
= K ;
t = x = (0.13)~ = 0.0169 h - 1 mino0 sec 0 0 (b) Bituminous surface: x =K ;
t =x2
= (0.25) = 0.0625 h-
3
min45
sec 0 0When these calculations are carried out for ice thicknesses of 0.1, 1 and 10 rnrn, and temperatures -1,
-5
and -10°C for NaCl and MgCIP* 6Hz0 we get the results given in Table 111, as minimum times without taking into account the rate at which the salt can dissolve.Table 111 Temperature OC -1
-5
Thickness of ice mm 0.1 1 10 0.1 1 Salts NaC 1 M ~ C 1,6%
o
NaC 1 M ~ C & -6%0 NaCl MgC&=6%O NaCl MgC1, -6$O NaCl MgC1,
61-b
0 -10 NaC 1 MgC1,*6$0
NaC 1 MgC 1,.
6H2
O NaCl MgC 1, 6H, 0 NaCl lJie;Cl,.6%o
10 0.1 1 10 Concrete surface < I s G ~ S 4 7 s3
s 5 0 m 4 2 s5
m58
s C l s<<
1 s l m 0 0 s6
s Bituminous surf ace 1 s<<
1 s 2 m 1 0 s15
s 1 h 2 5 m17
m 31 s 3 S ( c l s 3 m 4 5 s 23 s l h 2 0 m8
m 13 s 1 s << 1 s 2 m 2 4 s9
s 2 h 3 0 m13
m 15 s 2 h 5 2 m24
m 29 s6
s t l s ' ( m 4 6 s32
s 5 h 0 1 m37
m27
sFor CaC1; 2H20 a certain difference is encountered in that because of the great positive heat of solution the amount of heat required for dissolution can be furnished by the process of salt dissolution itself, so that no acquisition of heat from outside is necessary. For the case of CaC1; 2H20, therefore, the above
example appears as follows:
The amount of heat SE required for melting 1 rnm of ice
SE '
mss~(-510)
= 0.9248
= 44.2 kcalThe amount of heat required to melt 100 g ice
a able
VI)
is67.3
g.Therefore, for 920 g ice, 920 = 619 g.
100
The latent heat of solution of the salt is: LS = 619 (+)
85
=
52600
cal= 52.6 kcal
SE
-
LS =44.2
-
52.6
=-8.4
kcali.e. the temperature of -51°C is not reached at all.
From these calculations the following rules can be derived for practical application to ice on roads:
1. Because of the smaller heat conductive capacity, the melting process on a bitumihous surface (using, as already mentioned, the coefficient of asphalt for lack of more accurate values)
takes about 1.5 to
4
times longer than on a concrete surface.Actually, the difference is somewhat less.
2. The positive heat of solution and the greater temperature change required to reach the eutectic temperature yields considerably shorter melting times for MgC1,- 6H20. In particular, the great- er quantities of salts spread in conjunction with the posi.tive heat of solution give more favourable conditions.
3.
Since the influence of the air enters into the denominator inthe equation the melting times become shorter with stronger air
circulation (greater a). For a = 20, for example, the above
for the concrete surface
57
sec instead of 1.00 rninfor the bituminous surface 2 min and
37
sec instead of3
min45
sec,4.
Especially at lower temperatures, with thicker layers of ice, the melting times are the order of hours on both types of sur- face with NaCl and on black top surfaces with MgC1; 6H20.5.
For thin coatings of ice and high temperatures, however, the melting takes only a matter of seconds.A part that still has to be investigated is the assumption that the surface can be considered a semi-infinite body. For this it is necessary to consider the temperature variations in the course of time. According to ~r8ber, Erk and Grigull (1955) the equation
is as follows: A Y i.e. Ox is a function G of
(2)
J-,
G t Ox x at 1-
= G(-) or with a conversion - =-
OO' x tx
"
ltz=
3,
-
temperature at the point xa 0
-
initial temperature of the upper surfacea
-
temperature conduction coefficient of the surface naterial concrete: 0.0025 (m "/h)asphalt : 0.0013 (m2/h)
t
-
duration of heat emission (h).-2
5-
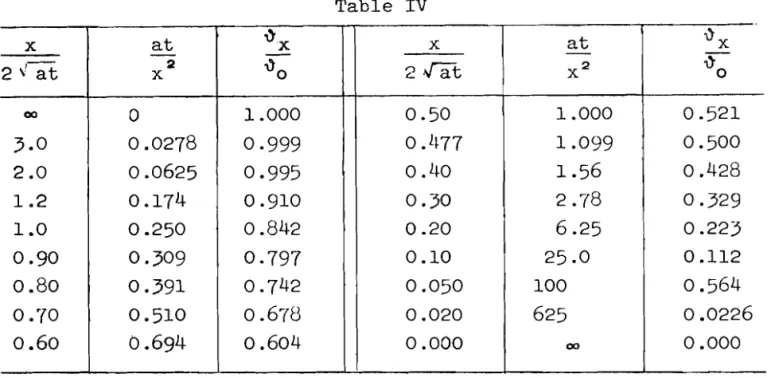
Table IV
It is now possible to assess the conditions by determining the percentage of surface temperature difference contributed by the bot- tom of the surfacing material. Using equation (15) these percentage differences were calculated for the standard contemporary road sur- face thicknesses of 22 cm concrete and
8
cm bituminous material.Table V
- -
In order for equation (15) (the assumption of an infinite half Dissolving time 6 h 4 h
3
h 2 h l h30
rn 10 m Concrete surfaceAS(%)
20 127
2 0-
-
Bituminous surface ~3(%) 5242
35
25
10 2 0s p a c e ) t o be v a l i d , t h i s t e m p e r a t u r e d i f f e r e n c e s h o u l d n o t exceed 10% of t h a t a t t h e t o p s u r f a c e .
For c o n c r e t e , t h e r e f o r e , t h e l i m l t i n g t i m e i s between t h r e e and f o u r h o u r s , and f o r t h e bituminous s u r f a c e i t i s 1 h o u r . There- f o r e , a l l t h e t i m e s g i v e n i n T a b l e I11 which exceed t h i s l i m i t i n g v a l u e can o n l y be r e g a r d e d a s approximate, g i v i n g t h e o r d e r of mag- n i t u d e . The d e p a r t u r e s , however, w i l l n o t be v e r y g r e a t . The sup- p o r t i n g s t r a t a u n d e r n e a t h t h e s u r f a c e m a t e r i a l can a l s o be t a k e n i n t o a c c o u n t f o r t h e t h e r m a l e m i s s i o n , b u t of c o u r s e w i t h somewhat d i f f e r e n t c o e f f i c i e n t
.
111. C a l c u l a t i o n o f Q u a n t i t i e s t h a t Must be Applied t o t h e Road
The q u a n t i t i e s t h a t must be s c a t t e r e d on a g i v e n q u a n t i t y of i c e o r snow of' g i v e n t e m p e r a t u r e can be e a s i l y c a l c u l a t e d from a phase diagram, s i n c e t h e p e r c e n t by weight o f t h e q u a n t i t i e s of s a l t needed t o d i s s o l v e t h e i c e c a n be r e a d d i r e c t l y . It must be k e p t i n mind, however, t h a t t h e e x p e r i m e n t a l i n v e s t i g a t i o n s were concerned w i t h anhydrous s a l t s c o n t a i n i n g no w a t e r of c r y s t a l l i z a t i o n . A s h a s a l s o been e x p l a i n e d , f o r any g i v e n q u a n t i t y of i c e t h e r e i s fundamen- t a l l y a n u n l i m i t e d number o f p o s s i b l e d o s e s , depending on t h e t i m e t h a t ' can be allowed t o e l a p s e b e f o r e a l l t h e i c e i s m e l t e d . Prom t h e l o g i c a l and economic p o i n t of view t h e extreme c a s e s a r e t h e dose which, a c c o r d i n g t o t h e phase diagram, i s r e q u i r e d j u s t t o melt a l l t h e i c e and produce a m i x t u r e whose f r e e z i n g t e m p e r a t u r e i s e q u a l t o t h e i n i t i a l i c e t e m p e r a t u r e , and t h e dose r e q u i r e d t o produce a e u t e c t i c m i x t u r e . The g r a p h i c a l e v a l u a t i o n s of t h e s e c a l c u l a t i o n s a r e g i v e n i n F i g . 8a-h f o r t h e f i r s t phase and i n F i g . 9a,b f o r t h e second. They r e f e r t o t h e s t a n d a r d s u r f a c e a r e a of 1 m 2 . The t h i c k - n e s s of t h e c o a t i n g t o be melted was t a k e n a s t h e v a r i a b l e . S i n c e t h e s e c o a t i n g s can be v e r y d i f f e r e n t i n form, two extreme c a s e s more o r l e s s embracing a l l t h e n a t u r a l phenomena a r e g i v e n , namely, com- p a c t i c e ( d = 0 . 9 c/cm3) and f r e s h snow ( d = 0 . 1 g/cm3). The t h i c k - n e s s v a r i e s between t h e f o l l o w i n g v a l u e s :
Ice : 0.01 rnm to 10 cm
Snow: 0.1 cm to 100 cm
Exact specifications of the temperatures to be considered for practical application areat present not possible. In first approxi- mation, however, the air temperature can be used.
IV. Comparison between the Three Salts
For this comparison we may use the parts by weight of anhydrous
salt required for the melting ( ~ i g .
5).
At the same time we mustdraw two temperature houndaries at -3°C and -11.5O~. Between O O C
and -3°C NaCl is superior to the other two salts. Next best is
MgCl,, which is decidedly better than CaCl~right down to its lower
effective boundary. Below -3OC to -11.5OC NaCl and MgC1, exchange places and the latter shows increasingly favourable efficiency with decreasing temperature compared to the other two salts. For -1l.5OC the conditions for NaCl and CaC1, approach one another more and more and finally become more favourable for CaC12.
However, as Table VI shows, conditions favour NaCl very strongly when we take into account the water of crystallization in ordinary
commercial MgC12 .and CaC12. With NaCl generally better than
50%
lesssalt is required in order to melt e.g. 100 g ice at the corresponding
temperatures.
There is less difference between Mg~1,*6~,0 and CaC1,*6H20, but
in every case MgC1, *6H20 is more advantageous.
The strong influence of water of crystallization is evident in the case of CaC1,*2H20, which corresponds more or less to ordinary
commercial
77
-
80%
salt, for here already less salt is needed thanfor MgC12=6H20. If we take into account further the strongly posi- tive heat of solution together with the large temperature gap down to the eutectic temperature, then this salt becomes superior to all the others with respect to the speed of melting.
Since decreasing amounts of water of crystallization generally affect the heat of solution favourably, we may say that salts con- taining less water of crystallization are more advantageous with respect to speed of reaction and quantities required.
Table V I Temperature
-7
-8
-9
-10-15
-20-25
Eutectlc -g salt for melting 100 g Ice CaCl, -6H20
NaC 1 CaCl, -2H20
(77-80%)
M~CI.,
-611.0
For comparatively thick coatings of ice the melting time is so great, especially for NaC1, that the salt appears ineffective. Be- cause of the greater temperature change to reach the eutectic value and the partially positive heat of solution, the conditions are con- siderably better in this respect for the other salts. If we take
30
minutes, for example, as the maximum permissable melting time weget the following ice thicknesses that NaCl can melt at the indicated
temperatures
a able
VII and Fig. 10).Table VII Temperature
"
C -1- -
Thickness of ice crust (rnm)
V . Summary
Supplementing t h e remarks made i n Report No. 302 ( p . 71-72), t h e f o l l o w i n g a d d i t i o n a l c o n c l u s i o n s , drawn from t h e above e x p o s i - t i o n can be reached w i t h r e f e r e n c e t o t h e p r a c t i c a l a p p l i c a t i o n of s a l t s s c a t t e r e d on roads:
1. Within c e r t a i n l i m i t s NaCl i s s u p e r i o r a s f a r a s t h e amounts r e - q u i r e d a r e concerned t o t h e o t h e r t h r e e s a l t s MgC1;6H20, C a C 1 2 *
6H,O and CaC1,*2H20. CaC1,*2H20 must be c o n s i d e r e d t h e n e x t b e s t
s a l t . I f N a C l cannot be used because of t h e low t e m p e r a t u r e s , t h e n CaC1,-2H,O i s s u p e r i o r t o t h e o t h e r two s a l t s down t o i t s
lower e f f e c t i v e boundary. The next b e s t i s MgC1, *6H20.
The s u p e r i o r i t y of N a C l from t h e economic s t a n d p o i n t i s f u r - t h e r r e i n f o r c e d i n Switzerland by t h e f a c t t h a t i t s purchase p r i c e i s only about one-half t h a t of t h e o t h e r s a l t s , which a r e now a v a i l a b l e a t approximately e q u a l p r i c e s . I n t h e U . S . A . t h e r e i s a c e r t a i n d i f f e r e n c e i n t h i s r e s p e c t , because t h e r e t h e pur- chase p r i c e f o r NaCl and CaC1, s a l t s a r e approximately e q u a l . A s
a consequence, CaC1, i s used on t h e r o a d s much more f r e q u e n t l y t h e r e t h a n h e r e .
2 . For speed of r e a c t i o n CaC1;2H,O i s e n t i r e l y s u p e r i o r t o t h e o t h e r s a l t s . I n t h i s r e s p e c t N a C l i s t h e l e a s t f a v o u r a b l e .
3 . The q u a n t i t i e s g i v e n f o r c e r t a i n t h i c k n e s s e s of i c e and snow i n - d i c a t e t h e l i m i t s w i t h i n which a complete m e l t i n g of t h e i c e i s p o s s i b l e . The u s e of e i t h e r t o o much o r t o o l i t t l e s a l t l e a d s t o u n d e s i r a b l e phenomena. I n t h e f i r s t c a s e t h e e x c e s s s a l t does n o t undergo r e a c t i o n and i n t h e s e c o n d c a s e n o t a l l t h e i c e i s m e l t e d . It i s t h e r e f o r e a b s o l u t e l y e s s e n t i a l t o determine t h e n a t u r e o f t h e i c e t o be d e a l t w i t h as a c c u r a t e l y a s p o s s i b l e .
4 .
The advantage of u s i n g e u t e c t i c p r o p e r t i e s l i e s i n t h e f a c t t h a t t h e p r e v a i l i n g t e m p e r a t u r e need n o t be t a k e n i n t o account and m e l t i n g t a k e s p l a c e a t maximum s p e e d . However, t h e c o m p a r a t i v e l y l a r g e d o s e s r e q u i r e d a r e e x p e n s i v e .5 .
The m e l t i n g p r o c e s s i s q u i c k e r on c o n c r e t e s u r f a c e s t h a n on b l a c k t o p , because t h e y g i v e up t h e n e c e s s a r y h e a t more r a p i d l y .0 . Any moisture in the salt reduces the actual percentage of pure
salt in an undesirable manner, the salt should be as dry as poa- sible when used. For this reason the use of sprayed-on salt solution for any sort of road ice melting Is uneconomic.
7.
In order to ensure intimate contact between the salt and the iceto be melted the salt should be as fine-grained as possible and should be spread uniformly. This also increases the surface of the salt and thus intensifies the hygroscopic attraction of water vapour and accelerates the entire melting process.
8.
The choice of salts used today is determined principally by theirpurchase price. It is entirely possible, however, that in future
I I
other salts or an organic compound occuring as a less valuable" by-product of a technolgoical process, could take the place of the chemicals being used at present. In assessing such a com- pound the following points, permitting an estimate of its effi- ciency, would have to be considered:
(a) Eutectic polnt: A eutectic temperature as low as possible
for a small percentage salt content is desirable.
(b) Water content: Since any water content of a salt has an unfavouraBle effect on the weight ratio of a pure salt sol- vent, dry, slightly hygroscopic salts without water of crys- tallization would be preferable.
(c) Heat of solution: A positive heat of solution on the part of the salt facilitates and accelerates the melting process, because a part of the heat needed to melt the ice does not have to be derived from the environment, but may come from the dissolving process.
(d) Dissociation: As complete a decomposition of the dissolved substances as possible is desirable, because the greater num- ber of ions formed yields a greater molar lowering of the freezing point.
(e) Number of ions per molecule formed in the course of decompo- sition: Because of the favourable effect on the molar lower- ing of the freezing point, polyionic salts are more advan- tageous.
(f) Osmotic coefficient: A high osmotic coefficient produces a favourable molar lowering of the freezing point.
(g) In addition to these factors, which affect the efficiency, a number of other points must of course be considered. They are merely listed below:
1. Harmful effects on humans, animals and plants
2. Corrosive effects
3.
Damage to surfaces, materials, leather, soils4.
Ease of handling5.
Tendency to produce skids6.
Storage properties.Literature
Eucken, A . Lehrbuch der chemischen Physik. Akademische Verlags- gesellschaft M.B.H. Leipzig, 1930.
~rbber, Erk and Grigull. Die Grundgesetze der Warmefibertragung. Springer-Verlag. Berlin, ~bttingen, Heidelberg, 1955.
Landolt, Bbrnstein, Roth and Scheel. Physikalische-Chemische Tabellen, Band I und 11, 1923.
Linke's meteorologisches Taschenbuch, neue Ausgabe, Band 11.
Herausgegeben von F. Baur. Akademische Verlagsgesellschaft Geest und Portig. K.-G., Leipzig, 1953.
Peery, J.H. Chemical Engineer's Handbook. McGraw-Hill Book Company, Inc. New York, Toronto, London, 1950.
Ramakrishna, R. arid Sambasiva, R. Dissociation of strong electro- lytes in controlled solutions. Nature, 137: 1936.
Robinson, R.A. and Stokes, R.H. Tables of osmotic and activity coefficients of electrolytes in aqueous solutions. Trans. Faraday Soc.
44
and 45, 1948/49.Scheel, K. and Heuse, W. Bestimmung des SHttigungsdruckes von Wasserdampf unter O°C. Ann. Physik, 29 (4), 1909.
Fig. 1 SOLVENT (SOLID) [ I C E ] f , OSMOTIC C O E F F I C I E N T S G
-
2-
-
t NaCt 11 -r? 13 - 14 1 5 - 1 6 - CONCENTRATION ( ~ o 1 / 1 0 0 0 ~ HzO) P1
Fig. 2 A Tg T P- T- DIAGRAM I L L U S T R A T I N G LOWERING OF F R E E Z I N G POINTSOLVENT (LIQUID) [ WATER ] S O L U T I O N [ WATER
+
S A L T ] /i
/ I I I . 5-
PHASE DIAGRAM OF NaCl ,MgC12 und CaC12
-
-30--
--- CaC12 l l S l l l l l l l , , l l l , l , l 10 20 30 40$ BY WT. OF DRY ANHYDROUS SALT
ADlOUNTS OF SALT NEEDED
TO THAW SNOW COVERS MCl Cli6H20
NIOUNT OF
SALT PEX M2
0 rn rrrn 0- (D
-g
0 "c i 10 100 CI- u,lg/cm3AMOUNTS
OF
SALTNEEDED FOR
TKE
THAWING OF
ICE
CRUSTS Ca Ch.6 H,OI h
MvIOUNTS.
OF
SALT PERid
Fig. 2e
AT4OUNTS OF SALT NEEDED
TO THA';l SNOW COVERS Ca C12.6 H20
AI4OUNT OF SALT PER
M~
3
d
-
0,l g/cm~ i g . 8f Fig. 8q
AMOUNTS OF SALT NEEDED FOR THE THAWING OF ICE CRUSTS
Ca C12 . 2 Hfl(77-80 '/,I
AMOUNTS OF SALT PER bI2
2 2 m o
s
w-9-
z:
vl h C 8
6
V 02
'Y wB
3
a
vl!!i
f3
3 f90 4 0 4 a - 58
iz
m -!s
3
*
3
8
2
m 5w g g z " .
U z
m 0 z H k U H (Y f E m X E 0 u Y ?2;:
g
9 E 2 Lg
bl0,d~
oz 0'1 CU r.ti
PC vl3
F4 0e
-Ln 3 . - P--
Ln cl-g
0 -m 6 0 9 OOE 0 001F i g . gb
AMOUNTS O F S A L T I N EUTECTIC
M I X T U R E S NEEDED T O THAW SNOW COVERS
AMOUNT O F S A L T PER M~ r Pl


![Fig. 1 SOLVENT (SOLID) [ I C E ] f , OSMOTIC C O E F F I C I E N T S G - 2 - - t NaCt 11 -r? 13 - 14 1 5 - 1 6 - CONCENTRATION ( ~ o 1 / 1 0 0 0 ~ HzO) P 1 Fig](https://thumb-eu.123doks.com/thumbv2/123doknet/14478148.523557/33.921.167.798.141.1114/fig-solvent-solid-osmotic-nact-concentration-hzo-fig.webp)
