HAL Id: hal-01278898
https://hal.archives-ouvertes.fr/hal-01278898
Submitted on 25 Feb 2016
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Influence of electrode material and roughness on iron
electrodeposits dispersion by ultrasonification
Audrey Iranzo, Fabien Chauvet, Théodore Tzedakis
To cite this version:
Audrey Iranzo, Fabien Chauvet, Théodore Tzedakis. Influence of electrode material and roughness on
iron electrodeposits dispersion by ultrasonification. Electrochimica Acta, Elsevier, 2015, vol. 184, pp.
436-451. �10.1016/j.electacta.2015.10.052�. �hal-01278898�
O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 15089
To link to this article : DOI:10.1016/j.electacta.2015.10.052
URL :
http://dx.doi.org/10.1016/j.electacta.2015.10.052
To cite this version :
Iranzo, Audrey and Chauvet, Fabien and
Tzedakis, Théodore Influence of electrode material and roughness
on iron electrodeposits dispersion by ultrasonification. (2015)
Electrochimica Acta, vol. 184. pp. 436-451. ISSN
0013-4686
Any correspondence concerning this service should be sent to the repository
administrator:
staff-oatao@listes-diff.inp-toulouse.fr
Influence
of
electrode
material
and
roughness
on
iron
electrodeposits
dispersion
by
ultrasonification
A.
Iranzo
a,b,
F.
Chauvet
a,b,*
,
T.
Tzedakis
a,b,**
aUniversitédeToulouse,INPT,UPS,LaboratoiredeGénieChimique,118RoutedeNarbonne,F-31062Toulouse,France bCNRS,UMR5503,F-31062Toulouse,France
Keywords:
zero-valentironelectrodeposition sonoelectrochemistry
adhesionenergy surfaceroughness electrodepositsdispersion
ABSTRACT
This studyrelates thesonoelectrochemical productionof metallicparticles andnanoparticles. The emphasis is on the influence of electrode material and roughness on the morphology of iron electrodepositsandtheirdispersionfromtheelectrodebyultrasonification.Ultrasonificationiseither appliedduringcyclicvoltammetrieswithsolutionstirringoraftergalvanostaticironelectrodeposition; nodispersionwasobservedwhenusingagoldelectrode,whereasdispersionwasalwaysobservedwhen usingvitreouscarbon(VC) substrates.ScanningElectronMicroscopy (SEM)imagingofthe electro-depositsshowshigherironcoverageongoldthanonVCelectrodes.IronspreadsmoreongoldthanonVC. Thevaluesofboththeinterfacialenergyoftheiron/electrodeinterfaceandtheworkofadhesionofiron ontheelectrodeareinagreementwiththepreviousobservations.DispersionkineticsonVCwerefound tobedependentontheelectrodesurfaceroughness.Resultssuggestthatdispersionfollowsafirstorder kinetics,whichiscoherentwiththeconstantactionofcavitationbubblesinthevicinityoftheelectrode surface.Enhancementofmass-transferbyultrasoundhasalsobeenobserved.
1. Introduction
Iron-based nanoparticles (Fe3O4,
g
-Fe3O4, nickel-cobalt-ironalloy)exhibitinterestingmagneticpropertiesinthemedicalfield, suchascontrastagentsforMagneticResonanceImaging[1,2]andfor treatment of tumors by hyperthermia [3,2]. Recent studies [4] indicatethatzero-valentironnanoparticles(ZVI-nP)showbetter magnetic performancethanironoxidesformedicalapplications. ZVI-nPcanalsobeusedforvariousotherapplicationssuchasan effective reducing agent: i) in water treatment [5], ii) for dechlorination[6,7],iii)nitrateremoval[7–9],andiv)for destruction ofvariousotherpollutants(see[5]foranextensivereview).
ZVI-nPcanbesynthetizedbyseveraltechniques(see[10]and [5] for an exhaustive list):ball milling [11], thermal reduction (reductionofironsalts[12]andreductionofoxide[13]),ironsalts wet-chemical reduction [6,14] (using borohydride salt as a
reducing agent) and direct electrochemical reduction [15,16,9]. Themostwidelyusedtechniqueisthewet-chemicalmethod.
Scaling-upofwet-chemicalmethod,inordertoproducelarge quantities of ZVI-nP, requires expensive reagents and specific securityconditionsbecauseofthegaseoushydrogenproduction [12].
DirectelectrochemicalZVI-nPsynthesisappearsasapromising techniqueforeconomicandsafeproductionprocesses,especially atalargescale;thereducingreagentisreplacedbyelectricityand under controlled conditions hydrogen production is avoided. Nevertheless, metallic iron, produced at the cathodic surface, mustberemovedfromtheelectrodeanddispersedintheliquidat therequiredsize.Variousworksinvolveultrasonicdispersionof electrodepositediron, simultaneouslyor sequentiallywith iron-precursorsreduction,allowingtherenewalofthecathodesurface. Generally,powerultrasounds(!20kHz,usinganultrasonichorn orbath)areusedtogeneratecavitationbubblesthat,duringtheir violentcollapses,createfluidmotionwhichremovessoliddeposits fromtheelectrodesurface.During ironelectrodeposition,under sequential pulses of the appliedcurrent, Delplancke et al. [15] applyshiftedpulsesof ultrasounds,usinga titaniumultrasonic horn (20kHz, 50W/cm2), also used as the polarized cathode.
Nanoparticles(6nm-100nm)ofpureFeandalloysofFe/Ni/Cohave been successfully synthetized in aqueous solutions; partial ‘chemicaloxidation’ofpureironparticleshasbeenobserved.In
* Correspondingauthorat:LaboratoiredeGénieChimique,UMRCNRS5503, UniversitédeToulouseIII-PS, INPT,118routedeNarbonne,F-31062Toulouse, France.Tel.:+33561557468.
**Correspondingauthorat:LaboratoiredeGénieChimique,UMRCNRS5503, UniversitédeToulouseIII-PS, INPT,118routedeNarbonne,F-31062Toulouse, France.Tel.:+33561558302.
E-mailaddresses:chauvet@chimie.ups-tlse.fr(F. Chauvet),
anotherstudy[16],cathodeisassembled bothwithahigh (0.2-2MHz, 5W/cm2) and a low (20kHz, 100W/cm2) frequency
ultrasonictransducerswhoirradiatethecathodicarea;constant current electrolyses werecarried out intetrahydrofuran, under ultrasonification,and10nmsizedZVI-nPareproducedwhenthe lowandthehighfrequencytransducersareusedsimultaneously. Chenetal.[9],claimthatZVI-nPrangingbetween1-20nmwere synthetized using aqueous 1M FeCl3 in the presence of
cetylpyridiniumchlorideas a dispersingagent; atwo platinum electrodes‘classicalelectrochemicalcell’,‘entirely’immersedinto anultrasonicbath(20kHz),wasusedandgalvanostatic electrol-yses were carried out under ultrasonification. Other metallic nanoparticles(silver,palladium,platinum,zinc,nickel,gold)have beensynthesizedbythesonoelectrochemicalmethod,see[17]for areview.Previousstudiesweregenerallydevotedtodetermining thebestoperatingparameters(pulsetimes,current,temperature, ultrasoundsintensity,stabilizers,etc.)foragivenset-upallowing thesynthesisofdesirednanoparticles.
Effectsofpowerultrasoundonvariouselectrochemicalsystems have also been extensively studied. Ultrasounds are generally appliedusingatitaniumultrasonichornactingastheultrasound generator placed in front of the working electrode (‘face-on’ configuration); the working electrode can also be directly integratedontheultrasonichorn([18]).Anotherpossibilityisto immersetheelectrochemicalcellinanultrasonicbath[19].Among other effects and regardless of the experimental configuration, ultrasounds induce cavitation bubbles and acoustic streaming whichenhancemass-transferleadingtoanincreaseofthecurrent [20–25,19].Currentfluctuationsarealsoobservedduetoviolent bubblecollapses[21,26,27].
Theabilityofthefluidmotionandalsotheshocksinducedby ultrasoundto‘clean’theelectrodesurfacehasbeeninvestigatedin severalworks.Cavitationbubblecollapsesinducedbyultrasound werefoundtobeabletoactivateelectrodesavoidingpassivationby ‘eroding/roughening’ the electrode material [28,19]. Coupling mercury electrodeposition and ultrasonification simultaneously on a vitreous carbonelectrode has led to a ‘steadystate’regime where the quantity of electrodeposited Hg on the electrode remains constant(electrodepositionflux=ablationflux)[29].Under ultra-sonification,theelectrodepositionofmetalssuchasZn,Co,Pb,and Hg, was investigated on a vitreous carbon substrate by cyclic voltammetryin[30](seealso[31]).Theauthorsanalyzedtheratioof anodictocathodicchargesasafunctionoftheultrasoundintensity, for theirparticular experimentalconditions (potential scanrate, sonoelectrochemicalsystem,electrodematerial...),andshowed thatitdependsonboththeultrasoundintensityandthemetalused. Thissuggests theimportance of metal/substrate affinityon the electrodepositsdispersion.Consideringthecaseofaparticlelyingon asubstrateandsubmittedtoultrasonificationin[32],theauthors discussedthecompetitionbetweenthehydrodynamicforcesand theadhesionforceasafunctionoftheparticlesize;theyshowedthat ultrasoundsshouldbeabletoremovesubmicrometerparticlesbut not smaller particles such as molecular adsorbates (adhesion outweighshydrodynamicsforces).
Thesepreviousstudiesshowthattheefficiencyofultrasoundto remove/disperseelectrodepositedmetalsshoulddependon:
-theultrasound intensity (and theexperimental configuration used)[30,31]
-thesize/morphologyandthespatialdistributionofdeposited metallicparticles[32]
-theadhesionenergyofelectrodepositedmetalontheelectrode material
Note that, todate, theeffect of the adhesion energy of the electrodepositedmetalontheelectrode substrate,hasnotbeen directlyinvestigated.
In the present work, the dispersion by ultrasound of iron electrodepositsisstudiedforvariouselectrodematerials(goldand vitreouscarbons)whichallowstovarytheadhesionenergy.The effect of the electrode material on the morphology of the electrodepositedironisalsostudiedbySEMimaging.Theinfluence of the electrode surface roughness is investigated using VC electrodeshavingdifferentlevelsofpolishing.
Nomenclature
A geometricsurfacearea(m2)
Aelect Hamakerconstantof electrode material,
elect=AuorVC(J)
Aelect=L=Fe Hamakerconstantforelectrodematerial (elect = Au or VC) and iron interacting acrosstheliquid(J)
Ar Argon
AFe;AL Hamaker constants of the iron and the
liquid(J)
CHþ Protonsconcentration(mol/m3) d Separationdistance(m) D Diffusioncoefficient(m2/s)
e Thicknessofthedeposit(m)
Eadh AdhesionenergyofirononVCelectrode= WFe=VCSFe=VC (J)
E Electrodepotential(V)
F Faradayconstant(96,500C/mol) h Localsurfaceheight(m) jlim Limitingcurrentdensity(A/m2)
I,Iapplied,Ilim Current, applied current and limiting
current(A)
MFe Molecularweightofiron(kg/mol)
n Electronsnumber
Qc,Qa,andQaref Respectivelythecathodic,theanodicand
theanodic referenceamountof charges (C)
r Potentialscanrate(mV/s) ra Arithmeticroughness(m)
rw Wenzel's roughness = ratio between
actualsurfaceandgeometricalsurface s Dispersionrateconstant(s#1)
Sdisk Surfaceareaofthediskelectrode(m2)
t Time(s)
tUS Ultrasonificationduration(s)
WFe=elect Workofadhesionofironontheelectrode,
elect=AuorVC(J/m2)
x,y Cartesiancoordinates(m) Greekletters
g
a Surfacetensionofmediuma(J/m2)g
a/b Interfacialtensionbetweenbothmediaaandb(J/m2)
g
elect Surfaceenergyoftheelectrode,elect=AuorVC1orVC2(J/m2)
g
dandg
p Respectively thedispersiveand thepolarcom-ponentsofthesurfaceenergy(J/m2)
h
Overpotential(V)r
Fe Volumetricmassofiron(kg/m3)n
Kinematicviscosity(m2/s)F
a/b Adjustmentparameterwhichdependsoninter-actionsbetweenbothinteractingmediaaandb
v
Angularvelocity(rpm)Thestudyisbasedontheanalysisofelectrochemical measure-ments:voltamperometriesandgalvanostaticelectrolysescoupled simultaneously or sequentially with ultrasonification. Experi-mentswererealizedinaclassicalelectrochemicalcellimmersed in an ultrasonic bath. Two different salts (FeCl2 and (NH4)2Fe
(SO4)2)wereusedasironelectrodepositprecursors.
Experimentalresultsonironelectrodepositsmorphologyand adhesion are discussed on the basis of a theoretical analysis, enablingtheestimationoftheworkofadhesionandtheinterfacial tensionbetweenironandthesubstrate,whichispresentedinthe Appendix.
2. Materialandmethods
2.1.Chemicalsandexperimentalset-up
Allsolutions,preparedusingultrapurewater(18.2M
V
.cm),were deaeratedbeforeexperiments(Argon,1bar),foraduringofatleast 15min; argon sparging duringthe experiments whichwereachieved atroom temperature(18<T($C)<22),exceptfor experimentsunderultrasonicirradiation,forwhichthetemperatureslightlyincreases (
D
T<5$C).Solutionscontaining0.01Mofiron (II)werepreparedbydissolvingNormapursolid FeCl2or(NH4)2Fe(SO4)2 (Mohr’ssalt)
supplied by Sigma-Aldrich. Solutions also contain respectively 0.1MKClor0.05MK2SO4assupportingelectrolytes,andtheirpH
wasadjustedto4.0,usingHClorH2SO4respectively.
The iron(II)was preferredtoiron (III)inordertoavoidthe oxidationofelectrodepositedironbythelatter.Moreover,using two different counter-ions (chlorideor sulfate)allows tostudy theireffectonthemorphologyoftheelectrodepositediron.
FeCl2wasselectedbecauseitisasimple,solubleandcheapiron
(II) salt;it has already been used to study iron nucleationon vitreouscarbonin[33].Furthermore,anotherwork[9]hasshown thatironnanoparticlescouldbesynthetizedby sonoelectrochem-istryusingFeCl3.
(NH4)2Fe(SO4)2complex,preferredtothesimplesaltFe(SO4)2,
was alsostudiedthankstoits‘chemical stability’againsttothe oxidationbyoxygen.
Electrochemical experiments were performed in a classical threeelectrodescell(50mL),eitherbycyclicvoltammetricscansor bygalvanostaticelectrolyses;arotatingdiskwasusedasaworking electrode (Radiometer,EDI101T),a platinumfoilconstitutesthe counter electrode, and a saturated calomel electrode (SCE) immersedwithinaLuggincapillaryisthereferenceelectrode.
Solutionwasalwaysstirredbytherotationofthediskelectrode (angular velocity
v
=1000rpm). For cyclic voltammetry, the potential scan rate r is equal to 20mV/s except for particular cases where lower scan rates were used. For galvanostatic electrolyses, theappliedcurrent isequal to90%of thelimiting current correspondingtoFe(II)reduction which ismeasured oncyclicvoltammetryscans(withoutultrasound).
Depending onthe experiment, the electrochemical cell was immersedinanultrasonicbath(FisherScientificFB15057,37kHz, 750W,6.9L).ApotentiostatVoltalabPGZ100from Radiometer-Analytical isusedtoachievetheelectrochemicalmeasurements. Voltamaster4istheelectrochemicalsoftwareusedtocontrolthe potentiostat. Working electrode material is either gold (2mm diameter,purity>99.99%)orvitreouscarbon1(VC1,3mmdiameter, purity=99.96%)orvitreouscarbon2(VC2,3mmdiameter,purity= 99.99%). VC1 was provided by Carbone Lorraine Company and VC2wasprovidedbyOrigalysCompany.Chemicalcompositionof these VCelectrodesareslightlydifferent:0.04%ash contentand 50ppmsulfurandboretracesforVC1, 0.0042%ashcontentand 13.5ppmmetaltracesforVC2(dataprovidedbysuppliers).Themain differencebetweenVC1andVC2isthatVC1hasfabricationdefects (smallvoidinclusionsinitsvolume)andthusitssurfaceexhibits
holeswith diametersof about1-10
m
m(even afterpolishing at 0.3m
m), while the VC2 has no defects. VC1 and VC2 surfaces characterizationisgiveninsection3.3wheretheeffectofelectrode surfaceroughnessonelectrodepositadhesionisdiscussed.Electrodeswerepolishedusingaluminaaqueoussuspensionof 9,5,1and0.3
m
monarotatingpad.Theelectrodesweresonicated for 5minutes in a 50:50 ethanol/water mixture between each polishing and 5minutes more in ultrapure water to remove remainingaluminaparticles.2.2.Electrodesurfacecharacterization
Thesurfacesof theelectrodes werevisualized byanoptical microscope(ZeissAxiolab)andsurfaceprofilesweremeasuredby aninterferometricsurfaceprofiler(Zygo3D).Usingdataprovided byZygo3Dmeasurements,Wenzel’sroughnessrw(definedasthe
ratio between actual surface and geometrical surface) can be estimatedusingthefollowingequation:
rw¼1A Z A ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi 1þrhðx;yÞ2 q dxdy ð1Þ
whereAisthegeometricsurfacearea,histhelocalsurfaceheight andx;yaretheCartesiancoordinates.rwwascomputedusinga
home-madeMatlabprogram.Arithmeticsurfaceroughnessrawas
computedbytheZygosoftware.
2.3.Measurementofsurfaceenergiesofelectrodes
Electrodesurfaceenergyorsurfacetension
g
elect(elect=AuorVC1orVC2)isanimportantparameterallowingtheestimationof both the work of adhesion of metallic iron on the electrode materialinelectrolyticsolutionWFe=elect and alsotheinterfacial
tensionbetweentheelectrodeandtheiron
g
Fe=elect.ThesurfacetensionsofthegoldandVCelectrodes,
g
Au,g
VC1andg
VC2,wereobtainedbymeasuringthecontactanglesofaseriesofliquidsof known surface tensions directly on the polished electrodes surfaces(Owens-Wendt‘oneliquidmethod’[34]).Contactangles ofultrapurewater, glycerol,dimethylsulfoxideanddecane were measuredwith a GBXDigidrop goniometer DGD fast/60 using !2
m
Lliquiddropletinambientconditions.Themeasurementof contact angles was reproducible in the range )5$. Absoluteuncertaintyon
g
electmeasurementislowerthan4mJ/m2.2.4.Electrodepositmorphologyanalysis
MorphologyofironelectrodepositswasobservedbySEMwith aMEBFEGJEOLJSM7100FTTLSoraMEBFEGJEOLJSM7800F Prime-EDS. Before observation, the sample was rinsed with ultrapure water (to avoid electrolyte crystallization) and dried underambientconditions.Toavoidelectronsbeamdeviation(due to the presence of insulating material surrounding the disk electrode),thewholesamplesurfacewascoatedbyananometric layer of gold by vapor deposition, if required. An electric connectionbetweenelectrodesandtheSEMsupportwasinsured byabrassstudadaptedtotheelectrode.
3. Resultsanddiscussion
3.1.Ironelectrodepositionbycyclicscanvoltammetryundersilent conditions
3.1.1.Fe(II)electrochemicalbehaviourongoldandvitreouscarbon
electrodes
The electrochemical behavior of Fe(II) salts used (FeCl 2 and
electrolytesand onthreedifferentelectrodespolishedwiththe finestaluminasuspension(0.3
m
m):VC1,VC2andgold. Voltam-perometriccurvesobtained(understirring)withoutFe(II)(residualcurrents,seeinsetsinFig.1aandb)revealadiffusion-limitedwave attributedtothereductionofthefreeprotonsatpH=4:
Fig. 1.CyclicvoltammetryscansandthecorrespondingSEMpictures.a)andb):curves(forwardandbackward,seearrowsina))obtainedonarotatingdiskelectrodeforthree differentmaterials:VC1,VC2andgold.a):Mohr'ssaltsolution,b):FeCl2solution.Insets:Residualcurrents(forwardcurves).SEMpictures(takenattheendofthecathodic
cycle,seethearrowona)andb)):1)to3)ironelectrodepositsusingMohr’ssaltsolutiononrespectivelyVC1,VC2andgoldelectrodes;4)to6)ironelectrodepositsusingFeCl2
H++e#
!1/2H2. (2)
Curves obtained with the electrolyte K2SO4 alone (inset in
Fig.1a)exhibit a resolute diffusion-limited wave,of which the limitingcurrent,evenlowmagnitude(0.65mA/cm2),appearstobe
constantforthethreeexaminedmaterials(samehydrodynamics). ConcerningthecurveobtainedinpresenceofKCl(insetinFig.1b), it doesnot showa clearsignalfor theH+ reductionbeforethe
reductionofwater(2H2O+2e#!H2+2OH#),exceptforthegold
cathode.IndeedtheH+reductionontheVCisnotvisible(seeinset
in Fig.1b)becauseitis shiftedtomorenegativepotentialsand screenedbywaterreduction.Thisphenomenonhasalreadybeen observedbyGrujicicandPesic([33])whostudiediron electrode-positionfromsulfateandchloridesolutionsonVC.Moreover,for the gold cathode, thecorresponding limiting current (0.34mA/ cm2)ishalfofthemagnitudeofsulfatesystemones(0.68mA/cm2).
Thisratioof2betweentheselimitingcurrentvaluesisattributed tothedi-acidnatureofH2SO4comparedtothemono-acidnature
of HCl. Indeed, a solution of sulfuric acid and K2SO4 at
pH=4 contains thefollowing species: H+, HSO
4#, SO42#and K+,
soafter theconsumptionof thefreeH+,HSO
4#dissociates and
supplyadditionalH+whichcouldbereduced.Thisleads,forthe
sulfatesystem,toanapparentH+concentrationtwotimeshigher
thantheoneforthechloridesystem,andexplainswhyalimiting current(whichisproportionaltoconcentration)thatistwotimes higherhasbeenmeasuredforthesulfatesystem.
Valuesforlimitingcurrentdensity,jlim,canbeestimatedusing
Levich'sequation([35]): jlim¼0:62:n:F:D2=3:v#1=6:C
Hþ:
v
1=2; ð3Þwheren,F,D,v,CHþand
v
arerespectivelytheelectronsnumber, the Faraday constant, the diffusion coefficient, the kinematic viscosity,theprotonsconcentrationandtheangularvelocity.TakingthevaluesforD,CHþ,and
n
ofrespectively9.3+10#9m2/ s([36]),10#4Mand10#6m2/sleadstojlim=0.33mA/cm2whichis
in accordance withthe measuredvalueof jlim for the chloride
system(0.34mA/cm2,C
Hþ=10#4M).
Inthepresenceofironsalts(Fig.1aandb),allcurves(1000rpm, 20mV/s) exhibit an additional signal attributed to the Fe(II)
reduction:
Fe(II)+2e#!Fe(0). (4-a)
Forallexaminedelectrodematerials(gold,VC1andVC2),the firstscan(startingat+0.2V/SCEtowardcathodicpotentials)clearly indicatesaslowredoxsystem,reductionsofFe(II)toFestartingat
around-1.2to-1.3V/SCEontheinitiallybareelectrodes.Duringthe firstscan,vitreouscarbonsandgoldarepartiallycoveredby zero-valentiron,sooncetheswitchingpotentialisreached,substrates
tendtoactasanironelectrode.Indeedatthepotentialscanrate used, cathodiccurves obtainedduring the return scan (-1.4 to -0.4V/SCE),exhibitaresolutediffusion-limitedwave, correspond-ingtotheFe(II)reductionsonthenativeiron surface.It canbe
noticedthatforMohr'ssaltsolutions(Fig. 1a),thediffusion-limited wave(pseudo-plateau)isnotwelldefinedduetotheco-reduction of free protons on electrodeposited iron followed by water reduction.Thisdistinctionbetweenchlorideandsulfatesystems forironelectrodepositionhasalreadybeenobservedbyGrujicic andPesic([33]).
Forthebackwardscans,thecathodiccurrentwascanceledat theequilibriumpotentialswhichlieintherange[!-0.9to!-0.8V/ SCE]fortheKClmediumand[!-1to!-0.9V/SCE]fortheK2SO4
medium.ThesepotentialsremainrelativelyfarfromtheFe2+/Fe
Nernst'spotentialE=-0.44-0.25+0.03x(log(10#2/1))=-0.74V/
SCE.Thiscouldbeexplainedbythefollowingreasons:
-theFe(II)ionscomplexedwithchlorideorbyammonium/sulfate ions(Mohr'ssalt),sotheredoxsysteminvolvedisdifferentfrom thesimpleFe2+/Fesystem
-co-reduction of free H+ (even at the equilibrium potential)
disturbstheredoxpotential oftheFe2+/Fesystem duetothe
increaseofthepHatthecathode.
The anodic curves obtained during the backward scan for potentialshigherthan-0.8V/SCE,exhibitpeaksattributedtothe ironelectrodepositoxidation(forbothKClandK2SO4mediaand
forthethreesubstrates,Fig.1aand1b): Fe(0)
!Fe(II)+2e#. (4-b)
The sharp decrease in current, clearly indicates complete oxidationoftheelectrodepositandtheregenerationoftheinitially baresurfaceoftheworkingelectrode(gold,VC1andVC2).Thisis theendofonecompletecycleoftheoperatedexperiments. 3.1.2.Influenceofelectrodematerialonironelectrodeposit morphology
Ironelectrodeposits,obtainedonthethreesubstratesandfor bothchlorideandsulfatemedia,wereobservedbySEMattheend ofthecathodiccycle.TheresultingpicturesareshowninFig.1, pictures1–6.
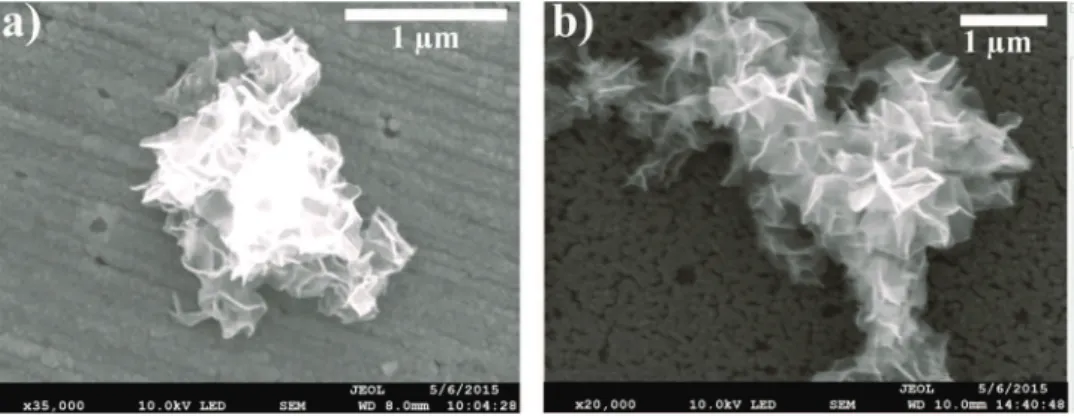
Inchloridemedium,a clearmorphologicaldifferencecanbe noticedbetween iron electrodeposits obtained on goldand on vitreouscarbonelectrodes(Fig. 1,pictures4–6).Indeed,onthegold electrode (Fig. 1, picture 6), the SEM image reveals iron electrodepositasacompactlayerthatcoverswelltheelectrode surface.Thislayerconsistsofcubicstructuressizedintherangeof afewhundrednanometers.Conversely,ontheVCsubstrates(Fig. 1,
Fig.2.SEMpicturesofironelectrodepositsformedafteracathodiccyclebyvoltamperometry(startingat0.2V/SCE,reversedat-1.3V/SCE,stoppedat-0.8V/SCE)using Mohr’ssaltsolutionforthegoldelectrodea)andfortheVC2electrodeb)respectively.
pictures4and5)ironelectrodepositsappearassmallmicrometric dendriticstructureswithheightsnotexceeding1
m
m.InMohr’ssaltmedium,ironelectrodepositedongold(Fig.1, picture3)andonVCsubstrates(Fig.1,pictures1–2)presentquite similarmorphologywithathinlayerofironcoveringthesurface (verified by Energy Dispersive X-Ray Spectroscopy) with iron micrometricstructuresonit.However,amoredetailedanalysisof thisthinlayerofiron(Fig.2)showsthatitcoversmoreonthegold electrode(Fig.2a)thanontheVC2electrode(Fig.2b).Indeed,on theVC2electrode,thethinlayerofironisnotcontinuous,some holes(darkspots)arevisiblerevealingtheVCsubstratethrough them.On contrary,onthegoldsubstrate,thislayer isfarmore continuous.
IronelectrodepositmorphologydependsontheFe(II)saltand
thesolutioncompositionaswellastheelectrodematerial.Ithas beenshownthatspecificcounter-ionsadsorptioncouldinfluence electrodepositmorphology([37]).Here,wefocusontheeffectof theelectrodematerialontheironelectrodepositmorphology,and ourobservationsshowthatelectrodepositedironhasmoreaffinity withgoldthanwithvitreouscarbonelectrodes.Ironspreadsbetter ongoldthanonVCduringitselectrodepositionforbothmedia used.Ithastobenoticedthatnodifferenceisobservedbetween electrodepositsonVC1andthoseonVC2.
Fromtheelectrodepositiontheory([38]),itisshownthatthe growthmode (from2D to3D)ofa crystalstronglydepends on theinterfacialenergyofinterfacebetweentheelectrodepositand the electrode
g
Fe=elect (with elect corresponding either to AuelectrodeortoVCelectrodes).Crystalsobtainedforlowvaluesof
g
Fe=electarealmostflat(2Dgrowth)andconverselycrystalsgrowpreferentiallyperpendicularlytotheelectrodesurface(leadingto poorlycoveredelectrode surface)forhighvaluesof
g
Fe=elect (3Dgrowth).
Contactanglemeasurementswereachievedfor goldandVC substrates, see Table 1. Using a theoretical development, the differencebetween
g
Fe=VC andg
Fe=Aucanbeestimatedfromthemeasurementsofsurfaceenergies(seetheAppendix).Apositive andnon-negligiblevaluelyingintherange[205.4mJ/m2;58.9mJ/
m2]wasdeducedfor
g
Fe=VC#g
Fe=Au whichisinaccordancewiththemorphologicalobservationsofironelectrodeposits.
Voltamperometriccurvesindicatedin Fig.1a)and 1b),show thattheoverpotentials
h
requiredforironreductionontheinitially baregoldelectrode(thefirstforwardscan)h
Auaresystematicallylowerthan
h
VC1!h
VC2 forboth mediaused.Therefore,alowerenergy(lowercellvoltage)isrequiredtocreate/increasetheFe/Au interfacethantheFe/VCinterface.Thisisinaccordancewiththe previous analysis showing that a higher energy is required to increasethesurfaceareaoftheFe/VCinterfacethantoincreasethe one of the Fe/Au interface during iron electrodeposition ð
g
Fe=VC>g
Fe=AuÞ.To conclude,during a cathodiccycle onVC electrodes, iron electrodepositsbegintogrowfollowinga3Dmode,leadingrapidly toadendriticgrowth.Onthegoldelectrode,ironelectrodeposits begintogrowfollowinga2Dmode(thinfilmgrowth)whichdelays theappearanceofthedendriticgrowthmodeasitisclearlyvisible inthecaseofchloridemedium(Fig.1,pictures4–6).
3.1.3.Influenceofironelectrodepositmorphologyonlimitingcurrent ofFe(II)reduction
The differences in iron electrodeposit morphologies, as discussed above, can be revealedby a detailed analysis of the voltamperogramsforbothmedia(chlorideandsulfate).
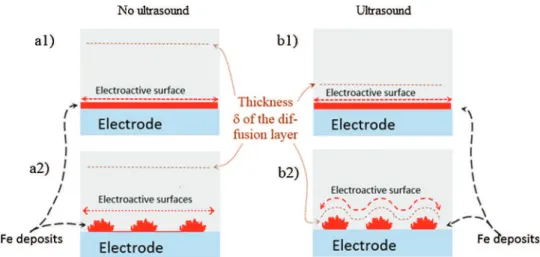
Inchloridemedium,thegreatdifferenceinironmorphology observed for gold and VC electrodes (see Fig.1, pictures 4-6) explainstheslight,butsystematicdifferenceoflimitingcurrent values, measured for both substrates (cathodic backward scan, Fig.1b).Aspreviouslymentioned,onthegoldelectrode,theSEM imagerevealsquasitotalcoverageoftheelectrodesurfacebyathin ironlayer.Therefore,theelectroactivesurfaceareaforthegolddisk canbeconsideredasthegeometricalsurfacearea(seeaschematic representationoftheelectrodepositsinFig.3aforgoldand3bfor VC).Conversely,ontheVCelectrodes(Fig.1,pictures4and5)iron electrodeposits consist of relatively small dendrites of which heights (<1
m
m) are lower than the thickness of the diffusion boundary layer thickness (estimated at !14m
m). For the VC electrodescase,electroactivesurfaceareaislimitedtothesumof surface areas of the growing dendrites tops(projected surface area) which is lower than the geometrical surface areaof the electrode(seeFig.3b).ThefactthatthelimitingcurrentsofFe(II)reductioninchloridemedium,usingVCelectrodes,arelowerthan thoseusinggoldcanbesimplyexplainedbya reduced electro-activesurfaceareaassociatedwithirondendriticgrowthontheVC electrodes,butnotonthegoldelectrode.
In Mohr’s salt medium, similar magnitudes of the pseudo-limiting current were measured for gold and VC electrodes (cathodicbackwardscansinFig. 1a,correspondingtothereduction ofFe(II)onthesubstratecoveredbytheironelectrodeposit).This
differentbehaviouris explainedbytheveryslightdifferencein iron electrodeposit morphologies (comparatively to chloride medium)obtainedongoldandonVCelectrodes(Fig.1,pictures 1–3).
3.1.4.Fe(II)/Fe(0)kineticsonthegoldelectrode
Inadditiontothedifferencesobservedinthelimitingcurrent forthethreeelectrodematerials,differencesalsoappearbetween goldandVCelectrodesforlowcurrents,onthebackwardcathodic curves (Fe(II) reduction) on electrodeposited iron; while these
curvesdonotoverlapinchloridemedium(Fig.1b),theyoverlapin sulfatemedium(Fig.1a).Moreover,inchloridemedium(Fig.1b), thebackwardcurvesforVC1andVC2overlapandexhibitlower overpotential(activationareastartingat!-0.9to!-1.05V/SCE)in comparison to the backward curve obtained using the gold electrode (higher overpotential, activation area starting at !-0.95 to !-1.15V/SCE). The iron electrodeposits morphology (Fig.1, SEM pictures 4–6) could explain the surprising lower overvoltageobservedonVCelectrodes;indeed,theiron electro-depositonthegoldelectrode(picture6inFig.1)ismoreuniform but not really ‘massive’, while iron electrodeposited on VC electrodes (pictures 4 and 5 in Fig. 1) presents micrometric dendritesinametalliciron‘massive’orbulkform.
Theeffectofthepotential scanrate onthecurrent-potential curvesshape,aswellasonthedepositstructure,forthereduction of Fe(II) on gold substrate, was examined in Fig. 4. Decreasing
potential scan rates(20!8!2mV/s)ledtoan increasein the amount of iron produced during the cathodic scan (5.7!14.7! 62.7 mC). Consequently, SEM pictures in Fig. 4b and4cofirondeposit,producedonthegoldsubstrateduringa cathodicscan,showthat increasingtheamountof irondeposit leadstoachangeinitsstructure.
Aspreviouslymentioned,irondepositsproducedongoldduring thecathodicscan,spreadontheelectrodesurfaceasathinlayer.The SEM picture (Fig. 4c) reveals that for a scan rate of 20mV/s, thethicknessofthedepositisabout90nmwhichisclosetothe
Table1
SurfaceenergiesofVC1andVC2electrodes.gd and gp are respectively the
dispersive and the polar components of the total surface energy
gelect¼gdþgp.
gd(mJ/m2) gp(mJ/m2) gelect(mJ/m2)
VC1 19)2 10)2 29)2 VC2 21)1 12)2 34)4
calculated thickness: equivalent compact layer thickness e¼ðMFeR I:dtÞ=ð2F
r
FeSdiskÞ=67nm. This confirms the spreadingofironanda2Dgrowthofthedepositonthegoldelectrode.A3D growthofthedepositisobtainedonlywhenalargerquantityof ironisdeposited(Fig.4b)forlowscanrates8mV/sand2mV/s.Asa firststep,ironspreadsonthegoldsurfaceandoncethesurfaceis totallycovered,deposit startstogrowthfollowinga 3Dgrowth modeanddendriticmorphologyappears.Indeed,decreasingthe scan rate from 20mV/s to 8mV/s leads to a change from a homogeneousandultrathindeposit(Fig.4c)toamicrometricand dendriticdepositthatcanactasabulkironelectrode(Fig.4b).
TheI=f(E)curvesobtainedforthethreescanrates(Fig.4a)are similartothoseindicatedinFig.1b;theforwardcathodicscans exhibit a diffusion-limited plateau at -1.2V/SCE for which the magnitudeofthelimitingcurrentisnotaffectedbythepotential scanrate.Thismeansthatforthethreecurvesthesystemoperates inasteadystate;thelimitationofthecurrentisduetotheconstant agitation applied. Concerning the backward cathodic scan, the curvesshowthat,decreasingthepotentialscanrate(20!8!2 mV/s)causes thehalf-wavepotential of theFe(II)reduction on
native iron, to shift to the anodic values (-1.014!-0.992! -0.938V/SCE).Thisfactsuggestsasystemwhichtendstobecome morereversible(toreachthebehaviourofa‘bulkiron’electrode)at low scan rates, because for longer electrolysis durations the
amountofirondepositedishigherand adendriticstructure(of micrometricsize)isobtained.
Thereforetheshapeofthecathodiccurvesisdictatedbythe structureofirondepositedandthereforebythequantityofiron produced.
Potentialscanratealsoaffectsthepotentialoftheoxidationof theiron-depositsignal;themorethescanratedecreases(20!8 !2mV/s),themoretheoxidationoftheirondepositedonthegold electrode becomes easier (-0.550, -0.566 and -0.598V/SCE), suggestinga‘bulkironbehaviour’.
Itcanbeconcludedthattheelectrodepositobtainedat20mV/s issothin(67nm)thatitselectrocatalyticpropertiesareaffectedby the electronic collector (gold) and the Fe(II) reduction (and oxidation)appearsasaslowersystem.Thiseffectisnotobserved onVCelectrodesbecause,evenat20mV/s,thecorrespondingiron electrodepositsconsistsofmicrometricirondendrites,whichare inabulkironform(Fig.1,SEMpictures4and5).
3.2.Ironelectrodepositionbycyclicscanvoltammetryunder ultrasonification
TheelectrochemicalbehaviourofFe(II)during
ultrasonification wasinvestigatedinchlorideandsulfatemediaandforthethree electrodespolishedwiththefinestaluminasuspension(0.3
m
m): VC1,VC2andgold.TheelectrochemicalcellwasimmersedintheFig.3. Schematicrepresentationoftheironelectrodepositmorphologiesobtainedinchloridemedium.a):onthegoldelectrode(relatedtotheSEMpictures6inFig. 1),b):on theVC1andVC2electrodes(relatedtotheSEMpictures4–5inFig.1).
Fig.4. a)Potentialscanratedependenceofthevoltamperometrycurves(forwardandbackward,seearrowsina))obtainedonarotatinggolddiskelectrode,immersedinthe FeCl2solution.SEMpicturesofironelectrodepositobtainedongoldafteracathodiccycle(startingat0.2V/SCE,reversedat-1.25V/SCE,stoppedat-0.8V/SCE)atb)8mV/sand
ultrasonicbathandspecialcarewastakentoalwaysputthecellin thesameplaceinthebathsoastogeneratethesameultrasound intensity into the cell. Cyclic voltamperometry was performed underultrasonificationorundersilentconditionsandthesolution was continuously stirred(
v
=1000rpm, potential scan rate=20 mV/s).ResultsaregiveninFig.5.Aspreviously,forwardscanstarts fromtheopencircuitpotential(OCP!+0.2V/SCE)andgoestothe cathodicdirection(to-1250mV/SCEforthegoldelectrodeandto -1350mV/SCEforVCelectrodes);thenthescanwas reversedto potentialsofaround0V/SCEinordertogetthebackward‘cathodic andanodicparts’oftheI=f(E)curve.3.2.1.Effectofultrasoundonmasstransportduringironelectrodeposit growth
Current-potential curves obtained under ultrasonification (Fig.5)exhibithigherFe(II)reductionlimitingcurrents(obtained
duringbackwardscan)thanthoseobtainedundersilentconditions (withtheexceptionofcurrent-potentialcurvesinFig.5cwhich willbediscussedbelow).Thisisduetoultrasoundsthatinduce cavitation bubbles and acoustic streaming, causing additional convection near the electrode and then increasing the mass-transport flux of the electroactive species [20–25,19].Current fluctuations,that arehighly visible,especially onthe diffusion-limited plateau of Fe(II) reduction (see Fig. 5a, b, eand f),are attributedtosuccessiveeventsofthecollapseofcavitationbubbles
Fig.5.Cyclicvoltammetryscans(forwardandbackward,seearrowsina))obtainedonarotatingdiskelectrodeforthreedifferentmaterials:gold(a)andd)),VC1(b)ande)) andVC2(c)andf)).Curvesina),b)andc)obtainedwithMohr'ssaltsolution;curvesind),e)andf)obtainedwithFeCl2solution.Dashedlines:silentconditions,solidlines:
closetotheelectrodesurfaceinducinglocalandtransientvigorous stirring([21,26,27]).Itisinterestingtonotethatforcurrentlower thanthelimitingcurrent,intheactivationarea(duringbackward scans, reduction of Fe(II) on Fe(0)), current
fluctuations are not observed(Fig.5a,b,c,eandf)andthecurves,withandwithout ultrasound,tendtooverlap(especiallyinFig.5bande).Thisisin agreementwithpreviousstudiesshowingthatultrasoundsaffect electrochemical processes mainly via mass-transport enhance-mentandonlyslightlytheelectrodekinetics[39,20,30].
TheincreaseinlimitingcurrentofFe(II)reductiononthegold
electrode in chloride medium, when ultrasounds are activated (Fig.5d),islow(relativeincreaseof7%), comparedtotheother onescorrespondingtocurvesinFig.5a,b,eandf(relativeincrease from15to60%).Onthebasisofelectrodepositedironmorphology obtainedbySEMimaging(Fig.1,pictures1–6),itappearsthatthe electrodepositthat hasgrownonthegoldelectrode inchloride mediumandundersilentconditions(Fig.1,pictures6)istheonly onetogrowasathinfilm.ForallotherFe(II)saltsandelectrode material combinations, electrodeposited iron presents micro-metric dendrites reaching about 1
m
m height (Fig. 1, pictures 1–5).Submittingthesolutiontoultrasoundscausesanincreasein thelimitingcurrentassociatedwiththedecreaseindiffusionlayer thickness [21,22]. Furthermore, the fast growth of the iron electrodeposits probablyleadstohigherdendrites withheights greaterthan1m
m,similartothethicknessofthediffusionlayer. Consequently,theelectroactivesurfaceareaincreasesleadingtoa currentmagnitudeincrease(seetheschematicrepresentationin Fig.6).Forelectrodepositgrowthasafilm(thedendriticgrowthis delayed and such a couplingis notexpected), theelectroactive surfacearearemainsequaltothegeometricalsurfaceareaofthe electrodeandthecurrentincreasesonlyduetotheenhancement ofmasstransportbyultrasounds.Therefore,thisanalysisallowsto giveanexplanationfortheobserveddifferencesin thelimiting currentincreaseswithultrasoundsbetweenthethinfilmgrowth modeandthedendriticgrowthmode.3.2.2.Effectofultrasoundonironelectrodepositsdispersion Theintegrationofbothcathodic(forwardandbackward)waves andanodicpeak(Fig.5)allowtogettheamountofchargeforFe(II)
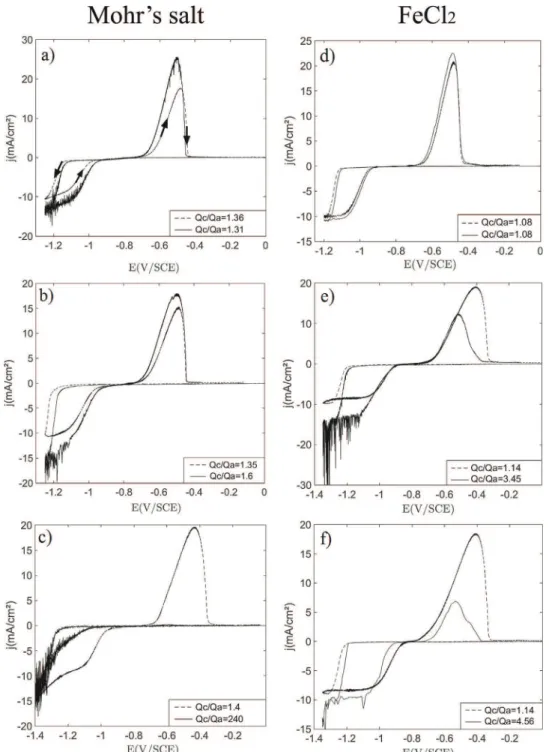
reduction,Qc,andforFe(0)oxidationQa.Thecomputedvaluesof
theratioQc=QaaregiveninlegendsofFig.5.Qc=Qavalues,higher than1inallcases,areusedbelowascriteriontodiscusstheeffects of adhesion and ultrasound on iron electrodeposits dispersion fromtheelectrode.
Inabsenceofultrasonification,Qc=QaobtainedinFeCl2(!1.10)
islowerthanthoseobtainedinMohr'ssalt(!1.35),becauseofthe co-reduction of bothH+and partially ofwater during theFe(II)
reduction(asalreadysaidinsection3.1.1).Notethatthecurrent consumptionbyH+andwaterreductionconstitutesa drawback
(lossinfaradicyieldforFe(II)reduction),butalsotwoadvantages:i)
consumption of H+ in the diffusion layer, so limitation of the
corrosionofzero-valentirondeposit,andii)productionofalittle partofH2whichallowslimitationoftheoxidationbytheresidual
oxygen.
Concerningtheadhesion/dispersionoftheironelectrodeposit onthegoldelectrode(Fig.5aandd),theeffectoftheultrasounds appears minor in both FeCl2 and Mohr’s salt media; indeed
comparisonoftheratioQc=Qa(seelegendsinFig.5aandd)with ultrasonification(1.31inMohr'ssalt;1.08in FeCl2)andwithout
(1.36 in Mohr’s salt; 1.08 in FeCl2) shows very similar values,
demonstratingthatallironelectrodepositedduringFe(II)reduction
remains on the gold surface and oxidizes totally during the backwardanodicscan.Conversely,forVCelectrodes(seeFig.5b,c, eandf),ironelectrodepositsdispersionisobservedineverycase, Qc=Qaobtainedwithultrasoundisatleastequalto1.6anditis alwayshigherthanQc=Qavaluesobtainedundersilentconditions (Fig.5b,c,eandf).
NotethatfortheelectrolysesusingtheMohr'ssaltsolutionand theVC1electrode(Fig.5b),eveniftheremovalofthedepositis observedQc=Qa=1.6),theanodicpeak(andalsotheamountof charge),whenultrasoundsareactivated,ishigherthantheone obtainedundersilentconditions.Asdiscussedin[30]and[31],this isduetotheenhancementofmass-transferbyultrasoundleading tolargerdepositionrateandthenalargerquantityofironisformed ontheelectrodecomparedtothesilentconditioncaseevenifsome partofthedepositisdispersed.
Ironelectrodepositappearstoadheremuchmoreonthegold electrodesurfacethanontheVCelectrodessurfaces,becauseof thehighadhesionenergybetweenbothmetals;thesoliddeposit requires higher ultrasonification power to be removed and dispersed in the liquid. These results are in agreement with estimationofworksofadhesionpresentedintheAppendix:work of adhesion of iron on gold WFe=Au=148.7mJ/m2 and work of
adhesionofirononVCWFe=VC=17.3mJ/m2.
ConcerningVC1electrode(Fig.5bande),similarcurvesand Qc=QaratioareobtainedinMohr’ssaltandinFeCl2.ForMohr’s
salt,Qc=Qareaches1.6withultrasound,andthemainpartofthe irondeposit(62.5%) remainsontheVC1electrode surfaceeven underultrasonification.ForFeCl2,ultrasonificationappearstobe
Fig.6.Schematicrepresentationsofpossiblediffusionlayerconfigurationsforthetwomaintypesofironelectrodepositmorphologyencountered,thinfilm(a1andb1)and dendritic(a2andb2),undersilentconditions(a1anda2)andwithultrasonification(b1andb2).
moreefficient,Qc=Qareaches3.45withultrasounds.Nevertheless, ultrasonification effect on iron deposit dispersion appears less efficient onVC1 than on VC2 (see Fig 5b, c, e and f). Indeed, VC2electrodeledtothemostinterestingresults(Fig.5candf) particularlywithMohr'ssaltsolutions(Fig.5c).Theanodicpeak correspondingtotheremainingiron,electrodepositedduringthe cathodicscan,ismissing,indicatingthatalltheironelectrodeposit wasremovedfromtheelectrodesurfaceandQc=Qareaches240 (instead 1.4 without ultrasound)! In addition, the backward cathodicscan(Fig.5c)tendstooverlaptheforwardcathodicscan, meaningthatFe(II)reductiontakesplaceonanalmostclean/bare
surface,whereironelectrodepositsarerapidlyandcontinuously removedthankstoultrasounds.Ironelectrodepositsadhesionon VC2electrodesurfaceisveryweakandtheyaredispersedeasily duringtheirformation.Thesameeffect,evenlessimportant(in comparisonwiththeMohr’ssalt),isobservedwithFeCl2onVC2
(Fig.5f),Qc=Qareaches4.56underultrasonification.
A similar analysis of the evolution of the ratio Qc=Qa as a functionoftheultrasoundintensityhasbeenachievedin[30]for the electrodeposition of metals (Zn, Co, Pb and Hg) on a VC electrodeusingaface-onconfiguration.Theoverlappingofboth thecathodicbackwardandtheforwardscans(asobservedinthe presentstudy,Fig.5c)wasnotobservedinthislaststudy,evenfor thehigherultrasoundintensity.Thissuggeststhat electrodepos-itedmetalsparticleswerealwayspresentonVCelectrodesurface duringthecathodicpartofthescan.Asdiscussedin[30](seealso [31]),thedispersionratewasnotsufficientlyhightoovercomethe deposition rate during the cathodic part of the scan. The experimental configuration, used in the present study, clearly shows that iron particles can be very rapidly removed by ultrasound allowing to operate continuously (with an almost ‘bare’substrate).
Thislastobservationisimportantfromapracticalpointofview becauseitshowsthatitispossibletodrivetheelectrodepositionof ironsimultaneouslywithitsalmosttotaldispersionthatshould leadtothe continuoussynthesis of fineiron particles(limiting theirgrowth).Onanotherside,asitcouldbeseeninFig.5c,the cathodicchargeislowerthanintheothercases,leadingtoalower synthesisrate.Asdiscussed,in[30]and[31],thecompetitiveeffect ofmetaldepositionand itsdispersioncouldbeadjusted bythe
control of both the Fe(II) concentration and the ultrasound
intensity.
Tosumup,maindifferencesinthedispersionofiron electro-depositedongoldandVCelectrodes,areduetoa differencein workofadhesionbetweenironandthesesubstrates.
However,differencescanalsobeobservedbetweenapparently similarVCelectrodes:VC1andVC2.Dispersionbyultrasonification ismoreefficientonVC2thanonVC1,suggestingthatironadheres lessonVC2thanonVC1.Todemonstratethisassumption,contact anglemeasurementwereachievedforVC1andVC2,andresults wereindicatedinTable1,giving
g
VC1andg
VC2.ThevaluesofthesurfacetensionsofVC1andVC2electrodesare very similar (
g
VC1!g
VC2, averaged value=30mJ/m2).
g
VC2 isfoundtobeslightlyhigherthan
g
VC1probablybecauseofaslightdifferenceintheirchemicalcompositions(seesection2.1)andthis couldinduceabetteradhesiononVC2thanonVC1(seeAppendix). Nevertheless,experimentshaveclearlyshownabetteradhesion onVC1thanonVC2,implyinganotherparameterasresponsibleof thisobserveddifference.TheeffectoftheVCelectrodessurface roughnessisanalyzedinthefollowingsections.
3.3.InfluenceoftheroughnessoftheVCelectrodeonthedispersionof electrodepositediron
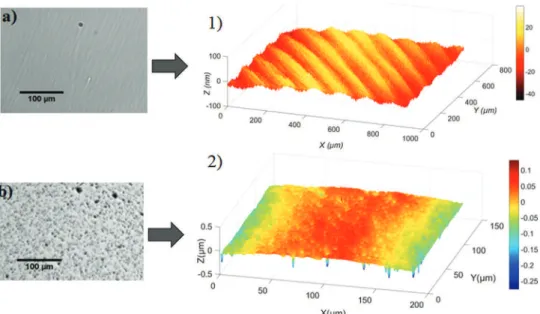
Theroughnessofthesurfaceof theelectrodeis aparameter whichcouldinfluencetheadhesionofiron.BothVCelectrodes’ surface were characterized by optical microscopy and by an interferometricsurfaceprofiler(Zygo3D),Fig.7.Thesmallvoid inclusions withinthe VC1 (visible asholes onits surface with diametersintherange1to10
m
m,Fig.7band7-2)correspondto manufacturingdefects.Conversely,VC2doesnotexhibitholeson itssurface;remainingpolishingstripesarevisibleonthesurface profileandtheirheightsremainlessthan30nm(Fig.7-1).Decreasing the roughness of the electrode surface enables easier electrodeposit dispersion in the liquid. In order to specifically study the effect of the electrode roughness on electrodeposit dispersion, independently ofiron growth, in the nextsectionwefocusonthedispersionkineticsofiron electro-depositedundersilentconditions.
Iron deposition was achieved by galvanostatic electrolysis undersilentconditionsusingtherotatingdiskelectrode(
v
=1000Fig.7. Opticalimagesandsurfaceprofilesofthepolished(aluminasuspensionat0.3mm)VCelectrodesusedinthepresentstudy;forVC2a)opticalimageand1)surface profile;forVC1b)opticalimage,2)surfaceprofile.
rpm) and the sameelectrolytes composition. In order toavoid significant hydrogen production, the applied current, Iapplied,
correspondsto90%ofthelimitingcurrent,Ilim,obtainedduring
thebackwardcathodicscanundersilentconditions.Theduration of electrolysis
D
t was chosen in order to obtain an iron electrodeposit withan equivalentiron-compact-layer of 85nm. Afterelectrolysis, therotationofthedepolarizeddiskelectrode (cathode disconnected from the anode), was maintained and ultrasonificationwasimmediatelyactivated(ornot)foraspecific durationtUS.Inordertoevaluatetheeffectofultrasonificationonthe dispersionof the deposit,an anodic scan was immediately carried out after the ultrasonification phase (from the OCP !-0.730V/SCE to +0.2V/SCE at 20mV/s, maintaining electrode rotationandwithoutultrasonification).
Theamountofcharge(Qa)correspondingtotheoxidationof theremainingdepositontheelectrodesurfacewasthenevaluated. NotethatQawascomparedwithanamountofcharge,thuscalled Qaref,whichwasmeasuredduringa‘referenceanodicscanwithout
ultrasounds’;Qarefcorrespondstotheoxidationoftheirondeposit
not submittedtoultrasonification(tUS=0)afterelectrolysis.The
results obtained for the operating parameters investigated are presented in Fig. 8, where the ratio (Qa/Qaref) is plotted as a
functionoftUS.Points oneachcurvecorrespondtotheaverage
valuesQa/Qarefmeasuredforatleastthreeexperiments;errorbars
correspondtostandard deviations.Asindicated above,theiron depositonthegoldelectrodeisnotaffected(orveryslightly)by ultrasounds; thereisnodispersion.Forthis reason,thissection focusesonthevitreouscarbonelectrodesVC1andVC2only,andit presentstheeffectofbothprecursors(FeCl2andMohr’ssalt)onthe
dispersionofthesolidirondeposit.ThecurvesQa/QarefversustUS
(Fig. 8) are analyzed with the help of SEM pictures of the
electrodepositedironnotsubmittedtoultrasonification(insetsin Fig.8).
3.3.1.Ironelectrodepositsmorphology
The Morphologyof iron electrodeposits formedby galvano-staticelectrolysis(insetsinFig.8)isdifferentfromthoseformedby voltammetry(Fig.1).ForFeCl2andbothVC1and VC2(insetsin
Fig. 8a and b), electrodeposited iron morphologies consist of micrometric‘rounded particles’and submicrometric iron struc-tures covering the remaining VC surfaces, while dendritic structures were obtained with voltammetry. It can be noticed thattheroundedparticleshavecoalescedinsomecases,forming biggerflakes.ThisisclearlyobservedontheVC2electrode(insetin Fig.8b).
Themorphologiesobtainedbygalvanostaticelectrolysisappear more‘homogeneous’ (lessdendritic)than theonesobtainedby voltammetry.Amongothereffects,thiscouldbeattributedtothe electrodepositionmodeandthecathodiccurrentemployed;with voltammetry, electrodeposition is conducted under continuous evolutionoftheappliedvoltageandtheresultingcurrent(which reacheditslimitingvalue),whilegalvanostaticelectrolysescarried outwitha currentclose(90%) tothelimiting current.Asoften observedingalvanostaticelectrodeposition,electrodeposittends to be dendritic/powdery when applied current is equal to, or higherthanthelimitingcurrent([40–42]).Evenwhentheapplied currentisjustbelowthelimitingcurrent,a‘lessrough’and‘less dendritic’electrodepositmorphologycouldbeobtained[41,42].
ForMohr'ssaltandbothVC1andVC2(insetsinFig.8candd), metallicirontakestheformofmicrometricstructuresasforthe depositionobtainedbyvoltammetry;buthere,thesemicrometric structures seem to have grown under the form of a ramified
Fig.8.DimensionlessironquantityQa/Qarefremainingontheelectrodeasafunctionoftheultrasonificationduration(tUS).Experimentsa)(VC1)andb)(VC2)wereachieved
intheFeCl2solution.Experimentsc)(VC1)andd)(VC2)wereachievedintheMohr’ssaltsolution.PointscorrespondtotheaveragevaluesQa/Qarefmeasuredforatleastthree
structurecoveringallthesubstratesurface.FortheVC2electrode (insetinFig.8d),aproportionoftheirondepositpartiallypeeled off the electrode surface, probably solely due to the effect of electroderotation.Comparatively,theiron depositfromMohr’s salt on VC1 (inset in Fig. 8c) consists of the same deposit morphologybutitremainsstuckontheelectrodesurface.
IntheFeCl2media,itseemsthattheseironparticles(especially
theflattenedones,insetin Fig.8b)alsopartiallypeeledoffthe electrodesurfaceinthecaseofVC2electrode.Therefore,theseSEM images, taken just before theultrasound phase, showthat the adhesion of iron electrodeposits to the electrode seems to be weakerfortheVC2thanforVC1.Indeed,inthefollowingsection, thisobservation isconfirmedbyelectrochemicalmeasurements indicatedontheplotofQa=Qaref asafunctionoftUS
3.3.2.Dispersionbyultrasonificationofiron‘electrodepositedunder silentconditions’
3.3.2.1. Ultrasound action for two VC substrates and two salt precursors.
Theeffectofthesubstrate(VC1andVC2)ontheirondispersionfor both FeCl2 and Mohr’s salt precursors were examined. All the
curves(exceptFig.8b)exhibitadecreaseoftheratioQa=Qaref asa
functionoftUS,meaningthatthequantityofthedispersediron
increasesprogressivelywithtimeduringultrasonification. Forallexperiments,anultrasonificationdurationof60sallows theremovalofmost(!90%)oftheirondeposit,anddispersesit intotheliquid.StandarddeviationoftheratioQa=Qaref hasbeen
determinedin both mediaand with both substrates. For short ultrasonificationtime,resultsarenothighlyreproducible,butthey becomemorereproducibleforlongerdurations.Furthermore,the quantityoftheirondepositremainingonthesubstratedecreases progressively with time; this is particularly visible for the VC1electrode(Fig.8a andc). FortheVC2electrode, dispersion istoofasttoobservethisbehavior,especiallyinthecaseofFeCl2
medium(Fig.8b).
The progressive removal of iron from the VC1 electrode is explainedbytheoperatedmodeofultrasonicdispersion.Indeed, constant-power-ultrasound produces cavitation bubbles in the liquidata constantrateperunit ofvolume.However,onlythe collapsesofcavitationbubblesinthevicinityoftheirondeposit surfacecancauseitslocaldispersion.Thefluxofremovedparticles shouldthenbeproportionaltothequantityofirondepositpresent ontheelectrodesurface.Thus,decreasingtheironquantityatthe surface,reducestheremovedparticlesflux,asobserved experi-mentally. A simple first order kinetics model can be built to
quantifythisbehavior: d dt Qa Qaref % & ¼#sQaQa ref ; ð5Þ
withsbeingthedispersionrateconstant(s#1)thatshoulddepend
on the ultrasound power, on the adhesion energy Eadh¼WFe=VCSFe=VC (SFe=VC beingthesurface areaof theiron/VC
interface) and on the size and distribution of particles on the electrode. Equation (5) leads to the exponential decrease: Qa/ Qaref(t)=exp(#st).Theplotofln(Qa/Qaref)versustUSusingdata
fromFig.8aandcareshowninFig.9.Dispersionaroundthelinear bestfitcurvesisobserved(duetotheresultsthatarenottotally reproducible).Nevertheless,itshouldbepointedoutthatthisvery simplemodelcapturestheoveralldynamicofthephenomenon.
This resultis in agreementwithresults fromother studies, mentioning a progressive ‘erosion/roughing’ of Pt electrode submitted to ultrasonification [28], or a progressive ablation associated with cracks formation in glass surrounding a disk electrodesubmittedtoultrasound[32].
Inbothmedia,resultsindicatethattheirondepositwasmore easilyremovedfromtheVC2thanfromtheVC1electrode,after 15s of ultrasonification,100%of theiron deposit is dispersed, confirmingwhathasbeenobservedpreviouslyonthebasisofSEM imagesininsetsofFig.8.Incomparison,onVC1,!60%(inFeCl2)
and!90%(inMohr’ssalt)isremovedafter15sofultrasonification (Fig.8).
TheaboveobserveddifferencesinironadhesiononVC1and VC2cannotbeexplainedbydifferencesintheworkofadhesion betweenironandVCsubstrates(WFe/VCinJ/m2).Asalsoindicated
above,anotherdifferencebetweenVC1andVC2electrodesistheir surfaceroughness’.MicrometricholesonVC1,thatinduceahigher roughness(Fig.7),actas‘anchors’fortheelectrodepositediron. Roughness induces higher iron/VC interfacesurface areaSFe=VC
leading to higher adhesion energy, Eadh¼WFe=VCSFe=VC, as said
before. ‘More cavitation bubbles’ (which provide mechanical energy)arethen requiredtodetach theironparticles fromthe electrode, which takes more time (using always the same ultrasound power) and leads to a slower iron removal as experimentallyobserved.
3.3.2.2.Effectofdifferentpolishinglevelondepositdispersion. Inordertoconfirmtheeffectoftheelectroderoughnessoniron depositadhesion,threedifferentpolishingswereappliedtothe VC2electrodesurface:thesubstratewaspolishedwithpapergrid P800(electrodeVC2P800),P1200(electrodeVC2P1200)andwith 0.3
m
maluminasuspension(electrodeVC20.3m
m).Aspreviouslystated,theelectrodessurfaceswerecharacterizedusinganoptical surfaceprofiler(Zygo3D)andresultsareshowninFig.10.Dueto mechanical polishing stripesare clearly visible forall levels of polishing.Theonedimensionalprofiles,presentedinFig.10,show a peak-to-peakamplitudeof approximately2
m
mand 1m
mfor VC2P800andVC2P1200respectively.OnVC2,polishedwiththe 0.3m
maluminasuspension,thesurfaceappearstobesmoother, presenting apeak-to-peakamplitudeofapproximately0.03m
m. The values of the Wenzel's roughness rw and the arithmeticroughnessraaregiveninTable2.
Ironelectrodepositsobtainedaftergalvanostaticelectrolysison VC2,polishedwithP800andP1200paper,arepresentedinFig.11a (insets). The effect of the ultrasonification duration on iron dispersionwasexaminedbyplottingoftheratio(Qa/Qaref)asa
functionoftUS(Fig.11a),forthethreedifferentpolishinglevels.As
forVC1(Fig.8aandc,andFig.9),apracticallyexponentialdecrease was observed, that validates the fact that iron electrodeposit dispersion by ultrasonification follows a first order kinetics (Equation 5). The lowest dispersion efficiencies are indeed obtainedfor electrodesthat present thehighestroughness. For example, after 5 s of ultrasonic duration, the quantity of iron depositremainingonthesurfaceforVC2,decreasesfrom!45%to <5%when polishingwas achievedrespectivelywithpapergrid P800,P1200and0.3
m
maluminasuspension.Thisconfirmsthe effectoftheroughnessonthedispersionkinetics.For VC2P800and VC2P1200, thesame quantity of ironwas depositedduringtheelectrolyses,butasrw(ratiobetweenactual
surfaceand geometricalsurface)ishigherforVC2P800thanfor VC2P1200 (Table 2), SFe=VC is larger for VC2P800 than for
VC2P1200. This induces a larger Eadh¼WFe=VCSFe=VC for
VC2P800and then a slower dispersionkinetics, asobserved in Fig.11a.
Depositsobtainedontheseroughsurfacescanbecompared withdepositsobtainedonasmoothVC2electrode(polishedwith 0.3
m
m alumina suspension, inset of Fig. 8b). On the smooth surface,ironisdepositedrandomly,formingroundedparticlesand micrometric plates/flakes (Fig. 8). On the rough surfaces (VC2P800andVC2P1200),micrometricplatesarealsoobserved, buttheygrowalongthestripes,followingthetopographyofthe substrates.Furthermore,SEMpictures,takenwith40$ tilt,showthatironpreferentiallygrowsatthebottomofthestripes(Fig. 11b). Thisbehaviourdoesnotcorrelatewiththeclassical phenome-nology of galvanostatic electrodeposition on rough substrates. Whenthediffusionlayerthickness(!14
m
mhere)isgreaterthan theheightofthestripes(2m
mhere,macroprofile),due tolocal variations of the diffusion layer thickness, metal should be preferentiallydepositedatthetopofthestripes[41].Wecannot explainthisobservationatthistime.However,linearvoltammetry conducted on these three surfaces reveals that the greater theroughness,thelessertheoverpotentialofinitialFe(II)reduction (not shown). By analogy with the analysis undertaken in section3.1.2,thisfactsuggeststhatpolishingstripesofferanareaFig.10.SurfaceprofilesofVC2electrodesobtainedbyanopticalsurfaceprofiler(Zygo3D)andtheironedimensionalprofiles(perpendiculartothestripes),a)VC2P800,b) VC2P1200,c)VC20.3mm.
Table2
VC2surfaceroughness’fordifferentlevelsofpolishing:with0.3mmalumina suspension,withpapergridP800andP1200.
Amplitudemax(mm) rw ra(nm)
VC20.3mm 0.03 1.0000 8
VC2P1200 1 1.0156 162
where
g
Fe=VCislowered,facilitatingirondeposition.g
Fe=VCshouldthereforebeloweratthebottomofthestripesthanatthetop, leading to an improved adhesion of these ‘anchored’ deposits (WFe/VC=
g
Fe/L+g
VC/L#g
Fe/VC,seetheAppendix).Anotherpoint is thatiron depositedontothestripescanbe protectedfromthefluidstreamandultrasonicperturbationthat cancausethedetachmentofthedeposit.Indeed,thesamequantity of iron is deposited on the rough substrates (VC2P800 and VC2P1200)asthatonthesmoothone.Butirondepositedinsidethe stripesof theroughsubstrates hasan iron/liquidinterface less exposedtocavitationbubbles.
These results clearly confirm the major importance of VC electrodesurfaceroughnessontheadhesionofirondepositand demonstratethat dispersion kineticseffectively slows down as electrodesurfaceroughnessincreases.
4. Conclusion
Theobjectiveofthisworkwastostudyphenomenainvolvedin thesynthesis of zero-valent iron nanoparticles by sonoelectro-chemistry.Theroleoftheelectrodesubstrateonthemorphologyof theelectrodepositediron and its dispersionby ultrasound was moreparticularlyinvestigated,consideringthefollowingthreekey parameters: 1) the interfacial energy of the iron/electrode interface
g
Fe=elect,2)theworkofadhesionoftheelectrodepositedironontheelectrodeand3)theelectrodesurfaceroughness. ChoosinggoldorVCaselectrodematerials,exhibitingdifferent interfacialenergies
g
Fe=VC>g
Fe=Au, allowstoobservesignificantdifferencesin both themorphologyof iron electrodeposits and theirdispersionbyultrasound.Nodispersionwasobservedusing ultrasound when iron was reduced on the gold; the electro-deposited iron adheres strongly and it spreads easily along electrodesurface(2Dgrowthmode)beforetheeventualstarting ofadendriticgrowth(dependingontheprecursorsaltused).On VCelectrodes,electrodepositedironcoverspartiallytheelectrode surface (3D growth mode); its weak adhesion facilitates its dispersionbyultrasound.
A particular regime was observed, using the smoother VC electrodeduringcyclicvoltammetryscanunderultrasonification: electrodepositdispersioniscomplete,allowingcontinuous regen-eration of the bare substrate; fine iron particles should be synthetizedduringthisprocess.Theseresultsallowtohighlight theimportanceoftheelectrodematerialchoicefortheelaboration of sonoelectrochemical devices dedicated to the synthesis of metallicnanoparticles.Thelowsurfaceenergyandthelowaffinity
of VC substrates against the deposited metal offer the double benefit:
-tofavorthelowspreadingofthemetalontheelectrode (3D growth),promotingtheformationofisolatedfineironparticles insteadofthinfilms
-tofacilitatethe‘ultrasound-assisted’dispersionofthe electro-deposit.
Theironelectrodepositsdispersionbyultrasoundappearstobe describedbyafirstorderkineticsbecauseoftheconstantremoval action of the cavitation bubbles. Moreover, dispersion kinetics slowdownwhentheelectrodesurfaceroughnessincreases,the latterinducingenhancedadhesionoftheironelectrodeposits.
Evenifthesynthetizedquantityofdispersedironparticlesis lowwiththedeviceused(micro-electrolyses),somepreliminary electrolyses were carried out (without surfactant) by applying ultrasound(VC2polishedat0.3
m
m,Mohr'ssalt).The characteri-zation by dynamic light scattering revealed the presence of !200nmsizedparticles.Acknowledgments
This study was supported by the MSR Graduate Research FellowshipandtheauthorswouldliketothankthePaulSabatier Universityforfundingtheresearch.ThanksarealsoduetoSophie Chambersforcheckingthemanuscript.
Appendix.Estimationofworksofadhesionandinterfacial tensions
The objective of this Appendix is toestimate the works of adhesionofiron(Fe)ontheelectrodematerialsusedinthisstudy, WFe=elect, withelect correspondingeither to Au (gold) or toVC
electrodes.Furthermore,theinterfacialtensionsoftheinterface betweenironandelectrodematerials,
g
Fe=elect,arealsoestimated.WFe=electand
g
Fe=electareestimatedusingthefollowingtheoreticaldevelopment and the values of electrode surface energies measuredbythecontactanglemeasurementmethod.
Theworkofadhesionofironontheelectrodecanbeestimated consideringonlythevanderWaalsinteractionsandfollowingthe theory developed in [43]. Considering the van der Waals interaction potential profile for iron interacting with electrode across the liquid phase, work of adhesion corresponds to the extremumvalue of this profile. It hasbeen shown, for several
Fig.11. Dimensionlessironquantity(Qa=Qaref)remainingontheelectrodeasafunctionoftheultrasonificationduration(tUS)forVC2inFeCl2solutionforvarious
polishinglevels.Points correspondtotheaveragevaluesQa=Qaref measuredforatleastthreeexperiments;errorbarscorrespondtostandarddeviations;insets
showSEMimages oftheelectrodepositsjustafter theelectrolysis(notexposedtoultrasonification)onVC2 polishedwithP800andP1200 papergrids.b)SEM imagewith 40$ tilt, of the iron electrodeposit onVC2P800; iron deposits located at the stripes bottom are visible inthe red ellipse.
material combinations, that this extremum is found for a separation distance between both interacting media (iron and electrode) d=0.165nm [43]. Work of adhesion can then be estimatedusingthefollowingequation([43]):
WFe=elect¼Aelect=L=Fe=ð12
p
d2Þ; ðA1Þwhere Aelect=L=Fe istheHamaker constantfor iron and electrode interactingacrosstheliquid(L).Aelect=L=Feiscomputedthankstothe
combiningrelation: Aelect=L=Fe¼ð ffiffiffiffiffiffiffiffiffiffiffi Aelect p # ffiffiffiffiffiAL p Þð ffiffiffiffiffiffiffiAFe p # ffiffiffiffiffiAL p Þ; ðA2Þ
whereAelect,ALandAFearethecomputedHamakerconstantsfrom
theLifshitztheoryofrespectivelyelectrode material,waterand iron; the following Hamaker constants values are used: AAu=45.3+10#20J([44]),AL=3.7+10#20J(Hamakerconstantof
water,[43])andAFe=26.0+10#20J([45]).ThevalueofAVCcouldbe
estimatedusingthemeasuredsurface energy oftheVCelectrodes,
g
VC= 30mJ/m2 (averaged value of surface energies measured for
VC1 and VC2,seeTable 1), and theequation: AVC¼24
p
d2g
VC([43]).
Finally,combiningequations(A1)and(A2),wefindWFe=Au=
148.7mJ/m2andW
Fe=VC=17.3mJ/m2.Eveniftheobtainedvaluefor
WFe=Au is probably underestimated (because attractive forces
betweenmetals,suchaspossiblemetallicbonds,arenotconsidered bythevanderWaalsinteractions),weshowthatWFe=Au>>WFe=VC.
Ironshouldadheremuchmoreonthegoldelectrodethanonthe VCelectrodes.Indeed,thisiseffectivelydemonstrated experimen-tally,insection3.2.2.
Onthebasisofthevaluesoftheseworksofadhesion,interfacial tensions
g
Fe=electcanbeestimated.Indeed,theworkofadhesioncanbeexpressedasafunctionoftheinterfacialtensionsinvolved inthesystemusingtheDupré'sequation:
WFe=elect¼
g
Fe=Lþg
elect=L#g
Fe=elect; ðA3Þwhere
g
Fe/L,g
elect/L andg
Fe/elect are the interfacial tensionsrespectivelybetweenironand electrolyticsolution(L),between electrodeandelectrolyticsolutionandbetweenironandelectrode. WFe=elect corresponds to the energy per unit area required to separate the iron surface from the electrode surface in the electrolytic solution.Usingequation (A3),
g
Fe=VC#g
Fe=Au canbeexpressedas:
g
Fe=VC#g
Fe=Au¼g
VC=L#g
Au=LþWFe=Au#WFe=Vc ðA4ÞGirifalcoandGood[46]haveshownthatinterfacialtensionscan beapproximatelycomputedasafunctionthesurfacetensionsby:
g
a=b¼g
aþg
b#2F
a=bffiffiffiffiffiffiffiffiffiffiffi
g
ag
bp
ðA5Þ where
g
a=bistheinterfacialtensionbetweenphaseaandphaseb,g
aandg
barethesurfacetensionsofrespectivelyphaseaandb,F
a=bisanadjustmentparameterwhichdependsoninteractionsbetweenaandb.Therelation(A5)isappliedfortheVCelectrode andforthegoldelectrodeinterfaces,(respectively
g
VC=L¼g
VCþg
L#2F
VC=Lpffiffiffiffiffiffiffiffiffiffiffiffiffiffig
VCg
L andg
Au=L¼g
Auþg
L#2F
Au=Lpg
ffiffiffiffiffiffiffiffiffiffiffiffiffiAug
L).g
L istaken asthe purewater surfacetension, 73mJ/m2 at 20$C (i.e.
neglecting salts content). As previously, the value 30mJ/m2 is
chosen for
g
VC. Forgold surface, placed in ambientconditions,surfacecontaminationsoccur([47])andthevalueof
g
Au isthensignificantlylowerthanitsvaluemeasuredforpuregoldmaterial (!1500mJ/m2atthemeltingpoint,[48]).
g
Auhasbeenmeasuredby contact angle measurement,
g
Au= 27mJ/m2 (measured at)3mJ/m2).
Theadjustmentparameters
F
Au=LandF
VC=Laregenerallycloseto1.IndeedaccordingtotheexperimentsofGirifalcoandGood [46],carriedoutwitha lotof materialcombinations(including mercury asmetal), the adjustmentparameterliesin therange [0.31, 1.17]. Using extremes values (0.31 and 1.17), interfacial energies
g
VC=Landg
Au=Lareestimatedasbeingwithinthewiderange [74.0mJ/m2; 0mJ/m2] and [72.5mJ/m2; 0mJ/m2]
respec-tively(restrictingtopositivevalues).
Finally,applyingequation(A4),
g
Fe=VC#g
Fe=Auliesintherangeofpositivevalues[205.4mJ/m2;58.9mJ/m2]meaningthat
g
Fe=VC>g
Fe=Au.IronshouldthenspreadsmoreonthegoldelectrodesurfacethanontheVCelectrodesurface.Thisiseffectivelyexperimentally observedanddiscussedinsection3.2.1.
However,itshouldbekeptinmindthattheseestimatedvalues for
g
Fe=VC#g
Fe=Au andWFe=Auareprobablyunderestimatedsince,assaidabove,onlyvanderWaalsinteractionsareconsideredfor the computation of WFe=Au. A ‘upper limit’ for WFe=Au can be
estimatedusingthe‘apparent’Hamakerconstantscomputedfrom gold and iron surface tensions ðAFe=Au¼24
p
d2g
FeorAuÞ takingvaluescorrespondingtoa‘clean’surfacestate(
g
Au=1500mJ/m2
and
g
Fe=2475mJ/m2, [48]). Then, the value of 3138mJ/m2 is
obtainedforWFe=Au suggesting,asexpected, stronginteractions betweenironandthegoldelectrode.
References
[1]Y.J.Wang,S.M.Hussain,G.P.Krestin,Superparamagneticironoxidecontrast agents:PhysicochemicalcharacteristicsandapplicationsinMRimaging,Eur. Radiol.11(2001)2319–2331.
[2]A.Ito,M.Shinkai,H.Honda,T.Kobayashi,Medicalapplicationoffunctionalized magneticnanoparticles,J.Biosci.Bioeng.100(2005)1–11.
[3]A.Jordan,R.Scholz,P.Wust,H.Fähling,R.Felix,Magneticfluidhyperthermia (MFH):CancertreatmentwithACmagneticfieldinducedexcitationof biocompatiblesuperparamagneticnanoparticles,J.Magn.Magn.Mater.201 (1999)413–419.
[4]C.G.Hadjipanayis,M.J.Bonder,S.Balakrishnan,X.Wang,H.Mao,G.C. Hadjipanayis,MetallicIronNanoparticlesforMRIContrastEnhancementand LocalHyperthermia,Small4(2008)1925–1929.
[5]R.A.Crane,T.B.Scott,Nanoscalezero-valentiron:Futureprospectsforan emergingwatertreatmenttechnology,J.Hazard.Mater.211–212(2012) 112–125.
[6]C.Wang,W.Zhang,SynthesizingNanoscaleIronParticlesforRapidand CompleteDechlorinationofTCEandPCBs,Environ.Sci.Technol.31(1997) 2154–2156.
[7]D.P.Siantar,C.G.Schreier,C.Chou,M.Reinhard,Treatmentof 1,2-dibromo-3-chloropropaneandnitrate-contaminatedwaterwithzero-valentironor hydrogen/palladiumcatalysts,WaterRes.30(1996)2315–2322.
[8]S.Choe,Y.Chang,K.Hwang,J.Khim,Kineticsofreductivedenitrificationby nanoscalezero-valentiron,Chemosphere41(2000)1307–1311.
[9]S.Chen,H.Hsu,C.Li,Anewmethodtoproducenanoscaleironfornitrate removal,J.NanoparticleRes.6(2004)639–647.
[10]D.L.Huber,Synthesis,properties,andapplicationsofironnanoparticles,Small 1(2005)482–501.
[11]J.E.Muñoz,J.Cervantes,R.Esparza,G.Rosas,Ironnanoparticlesproducedby high-energyballmilling,J.NanoparticleRes.9(2007)945–950.
[12] L.B.Hoch,E.J.Mack,B.W.Hydutsky,J.M.Hershman,J.M.Skluzacek,T.E. Mallouk,Carbothermalsynthesisofcarbon-supportednanoscalezero-valent ironparticlesfortheremediationofhexavalentchromium,Environ.Sci. Technol.42(2008)2600–2605.
[13]M.Bystrzejewski,Synthesisofcarbon-encapsulatedironnanoparticlesvia solidstatereductionofironoxidenanoparticles,J.SolidStateChem. 184(2011) 1492–1498.
[14]F.He,D.Zhao,Manipulatingthesizeanddispersibilityofzerovalentiron nanoparticlesbyuseofcarboxymethylcellulosestabilizers,Environ.Sci. Technol.41(2007)6216–6221.
[15]J.Delplancke,J.Dille,J.Reisse,G.J.Long,A.Mohan,F.Grandjean,Magnetic nanopowders:Ultrasound-assistedelectrochemicalpreparationand properties,Chem.Mater.12(2000)946–955.
[16]A.Khachatryan,R.Sarkissyan,L.Hassratyan,V.Khachatryan,Influenceof ultrasoundonnanostructuralironformedbyelectrochemicalreduction, Ultrason.Sonochem.11(2004)405–408.
[17]V.Sáez,T.J.Mason,Sonoelectrochemicalsynthesisofnanoparticles,Molecules 14(2009)4284–4299.
[18]J.C.Eklund,F.Marken,D.N.Waller,R.G.Compton,Voltammetryinthepresence ofultrasound:anovelsono-electrodegeometry,Electrochim.Acta41(1996) 1541–1547.