HAL Id: hal-01291479
https://hal.archives-ouvertes.fr/hal-01291479
Submitted on 21 Mar 2016
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Continuous-Flow Photochemistry: a need for chemical
engineering
Karine Loubiere, Michael Oelgemoeller, Tristan Aillet, Odile Dechy-Cabaret,
Laurent E. Prat
To cite this version:
Karine Loubiere, Michael Oelgemoeller, Tristan Aillet, Odile Dechy-Cabaret, Laurent E. Prat.
Continuous-Flow Photochemistry: a need for chemical engineering. Chemical Engineering and
Pro-cessing: Process Intensification, Elsevier, 2016, 104, pp.120-132. �10.1016/j.cep.2016.02.008�.
�hal-01291479�
O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 15641
To link to this article : DOI : 10.1016/j.cep.2016.02.008
URL :
http://dx.doi.org/10.1016/j.cep.2016.02.008
To cite this version :
Loubiere, Karine and Oelgemoeller, Michael
and Aillet, Tristan and Dechy-Cabaret, Odile and Prat, Laurent E.
Continuous-Flow Photochemistry: a need for chemical engineering.
(2016) Chemical Engineering and Processing: Process
Intensification, vol. 104. pp. 120-132. ISSN 0255-2701
Any correspondence concerning this service should be sent to the repository
administrator:
staff-oatao@listes-diff.inp-toulouse.fr
Continuous-flow
photochemistry:
A
need
for
chemical
engineering
Karine
Loubière
a,b,*
,
Michael
Oelgemöller
c,
Tristan
Aillet
a,b,
Odile
Dechy-Cabaret
a,d,
Laurent
Prat
a,baCNRS,LaboratoiredeGénieChimique(LGCUMR5503),4alléeEmileMonso,BP84234,31432Toulouse,France bUniversitédeToulouse,INPT,ENSIACET,F-31432Toulouse,France
cJamesCookUniversity,CollegeofScience,TechnologyandEngineering,Townsville,Queensland4811,Australia
dCNRS,LaboratoiredeChimiedeCoordination(LCCUPR8241),205routedeNarbonne,BP44099,F-31077Toulouse,France
Keywords: Flowphotochemistry Chemicalengineering ProcessIntensification Microstructuredreactors Modelling Dataacquisition ABSTRACT
Thepresentpaperaimstoillustratethatchemicalengineeringenablestoaddresssomeofthecurrent challenges and issues in continuous-flow photochemistry. For that, some common limitations encountered in industrial photochemistry are firstly highlighted and a general overviewon flow photochemistryequipmentispresented.Themainchallengeslinkedtophotochemical(micro)reactor engineeringaresubsequentlystated.Byconsideringonlythecaseofapurelydirectphotochemical reactions A!hyB in homogenous medium, the key factors to consider when implementing such photochemicalreactionsinmicrostructuredtechnologiesareoutlined.Theirinfluenceontheoutputs (conversion,productivity,photonicefficiency)ofthissimpletypeofphotochemicalreactionisthen discussed.Thesignificanceofchemicalengineeringframeworksisfinallydemonstratedusingseveral examplesconcerningtheunderstandingofthecouplingbetweenthedifferentphenomenainvolved,the predictions of the performances obtained,the acquisition of kinetics data and the elaborationof strategiesforphotochemicalprocessintensificationandsmartscale-up.Inthefuture,thechallengewill betointegratethecomplexityofphotochemistry(e.g.heterogeneousphasereactions)intothepresent modelling tools so as to enlarge the spectrum of strategies devoted to photochemical process intensification.
1.Introduction
Organicphotochemistryhasthepotentialtoemergeasakey synthesis pathway in sustainable chemistry. In recent years, photochemicalreactionshavesignificantlyenrichedthe method-ologyoforganicsynthesis[1–4].Incontrasttothermalreactions, photochemicalreactionsareinducedviatheelectronicallyexcited state possessing a different electron configuration than their corresponding thermal ground states [5–8]. Consequently, the chemicalreactivityofexcitedmoleculesisconsiderablydifferent from that of ground state molecules. The following points are particularlyinterestinginthecontextofsustainability:(i) multi-stepsynthesesofcomplexmoleculesareshortenedandsimplified; often,ahighmolecularcomplexityisgeneratedinonestepfrom simpleprecursors,(ii)aportfolioofnovelcompoundfamilies(e.g. strainedrings)isbecomingaccessible ormoreeasilyaccessible,
and(iii)inmanyreactions,thephotonactsasa“tracelessreagent”, and nochemical catalysts (acid, base, metal,etc.)or activating groups are needed [9–11]. The 12 guiding principles of Green Chemistry [12,13] are thus addressed by photochemistry. In addition, photochemical reactions are currently becoming an indispensable tool in the search of new biologically active compounds for applications in medicine, fine chemical and pharmaceuticalindustries, as wellasin many otherfields (e.g. materialandenvironmentalsciences)[14–23].
Atthesametime,continuous-flowtechnologies,inparticular microstructuredreactors,haveemergedasalternativestobatch processing and their implementation in process intensification strategies is likewise crucial for sustainable chemistry [24]. Recently,variousworkshaveshownthat thesetechnologiesare also suitable and beneficial for preparative photochemistry
[25–31],boostingtheinterestincontinuous-flowphotochemistry. Thepresentpaperaimstoillustratethatsomeofthecurrent challengesandissuesincontinuous-flowphotochemistrycanbe addressed using a chemical engineering framework. Such a frameworkisindeedessentialtoelaborateaprocessintensification strategy which enables adaptation of the microstructured
*Correspondingauthorat:CNRS,LaboratoiredeGénieChimique(LGCUMR
5503),4alléeEmileMonso,BP84234,31432Toulouse,France.
E-mailaddress:Karine.Loubiere@ensiacet.fr(K.Loubière).
photoreactor design (channel design, dimensions, light source, etc.)tophotochemicalreactionspecificities,andmoregenerallya transferfrombatchtocontinuousmodeoperations.
Firstly, the common limitations encountered in industrial photochemistrywillbeidentifiedandageneraloverviewonflow photochemistry equipment presented (Section 2). The main challenges linked to photochemical (micro)reactor engineering willthenbeexposed(Section3).Byconsideringonlythecaseof purelydirectphotochemicalreactionsA!hyBoccurringin homoge-nousmedium, thekey factors toconsider when implementing sucha photochemical reaction in microstructured technologies willbeoutlinedbasingonmodelingconsiderations(Section4).In thelastsection(Section5),someexampleswillbepresentedto illustrate,forthisparticularcaseofaphotochemicalreaction,how a chemical engineering framework enables to understand and formalizethepositiveeffectofmicrostructuredtechnologiesfor photochemistry.
2.Industrialphotochemistry:a«renaissance»?
Since1975,8000photochemicalreactionsfororganicsynthesis havebeen referenced [32]. Despite this huge portfolio, organic photochemistry hasnot found widespread implementations in chemicalindustry[33,34].Itisdifficulttoobtainaglobaloverview on currently existing photochemical activities as industrial processesareoften keptconfidential. Nevertheless,it is known
that many industrial photoreactions have been established decadesagoand havesincebeenoperationallargelyunchanged
[35].BasedontheinformationavailablebyBraunetal.[34],the worldwideelectricalpowerinstalledfortheradiationsourcesused in preparative photochemical equipment represents almost 30MW, thus demonstrating its significant importance. Photo-chemicalsynthesisismostlyappliedbychemicalcompaniesthat produceintermediateand/orfinechemicals(e.g.pharmaceutical, agrochemical, foodprocessing andfragrance industries)and by companiesproducingbasicorfinalproducts(e.g.,food,electronic, automotive, furniture, building and packaging industries). It shouldbenotedthattheproductionofhighlypricedfinechemicals (e.g. fragrance, pharmaceutically active compounds)represents the minor fraction of the installed electrical power previously mentioned [34].Amongthewell-known examples ofindustrial photochemistry,onecanmentionthesynthesisofvitaminD3and
vitamin A (BASF, Hoffmann-LaRoche), the photooximation of cyclohexane (Toray), the photochlorination of toluene, the synthesis of rose oxide (Symrise) [36] and more recently the synthesisofartemisinin[37].
Thereluctancetotransferpreparativephotochemistryto large-scaleis mainlydue tothelimitations of thecurrentlyavailable technology, which requires outdated immersion-type reactors, oftenoperatingin semi-batchmode(circulationofthereaction medium between a large central reservoir and the reactor), equippedwithexpensiveandenergy-demandingmercurylamps. Intheseinstallations,processlimitationsarenumerousduetothe uncontrolled coupling between hydrodynamics, light, mass transferandphotochemicalkinetics.Asaresult,lowerselectivity andyieldsthanonlab-scalearecommonlyobtained.Manyofthese systems furthermore need optical filters to cut off undesired radiation,largedilutionstoovercomeunfavorablelightabsorption andintensivecoolingtocountertheheatgenerationbythelamps. Bycombiningthebenefitsofmicro-scalewithcontinuous-flow mode, microstructured reactors enable, when compared to conventionalphotochemicalequipment, higherconversionsand selectivitieswhilereducingirradiationtime[25–31].Someoftheir specificadvantagesare:(i)extensivepenetrationoflight,evenfor concentrated chromophore solutions, (ii) minimization of side reactionsordecompositionsbyflow-operation,(iii)easycontrolof theirradiationtimeand(iv)saferconditions(forexamplewhen involvingheat-sensitiveoxygenatedintermediates).The combina-tionof microstructuredtechnologywithnewlight sources(e.g. Light-EmittingDiodes(LED)orexcimerlamps)additionallyoffers promising perspectivesin termsof energy-savings [38]. Conse-quently,thereisatpresentanincreasinginterestin continuous-flow photochemistry, leading to a “renaissance” of preparative photochemistry.Moststudiesarededicatedtotheproductionof small quantities in often improvised ‘in house’-made reactors (Fig.1a).Theresultsobtainedhaveneverthelessstrengthenedthis technologyandhavesparkedthedevelopmentofdedicatedand more advanced equipment. Currently, commercialtechnologies (e.g.fromthecompaniesYMC[39],Mikroglas[40],Ehrfeld[41], FutureChemistry[42])(Fig.1b)andinternallydevelopedreactors
[43–45]mainlyenablecontinuous-flowphotochemistryon lab-scales,althoughisolatedexamplesofmeso-scalephotoreactionsin flow have been reported as well. However, a scale-up to industriallyrelevantamounts,i.e.aboveafewhundredkilograms per year, has not been realized yet. Thus far, very few flow photoreactorsareavailableforseveralgramsperday(Vapourtec UV-150[46])orkilogramperdayoperations(Corning1G1Photo
Reactor [47], Heraeus Noblelight [48])(Fig.1b).A flow-photo-chemical production facility for the synthesis of low-volume anticancer compounds has recently been erected by Heraeus Noblelight[48],thusdemonstratingthepotentialofthisemerging newtechnology.
Nomenclature
A0e Referenceabsorbance(")
airrad Specificirradiatedarea(m"1) C Concentration(molm"3)
C0 Initialconcentration(molm"3)
DaI DamköhleronenumberdefinedinEq.(13)(") DaII DamköhlertwonumberdefinedinEq.(14)(") dpen Lightpenetrationdistance(m)
Dm Diffusioncoefficient(m2s"1)
e Characteristic dimension of the microphotoreactor withrespecttothelightpenetrationdirection(m)
ea Local volumetric rate of photon absorption (mol
photonm"3s"1)
E Sphericalirradiance(molphotonm"2s"1)
F0 Photonfluxdensityreceived atthe
microphotoreac-tor’swalls(molphotons"1m"2)
Fo Fouriernumber(")
L Lengthofthemicrophotoreactor(m)
rA RateofconsumptionofthespeciesA(molm"3s"1) RX ProductivitytoreachaconversionX(mols"1)
qp Incidentphotonflux(molphotons"1)
U Meanvelocityinthemicroreactor(ms"1)
Vr Volumeofthemicrophotoreactor(m3) X Conversion(")
Greeksymbols
a
Napierianlinearabsorptioncoefficient(m"1)b
A Competitive absorbance factor with respect to the speciesA(")k
Napierianmolarabsorptioncoefficient(m2mol"1)l
Wavelength(m)h
X Photonicefficiency(")f
Quantumyieldofthereaction(molmolphoton"1)t
Residencetime(s)Microstructuredtechnologiesthusprovidenewscientificand technologicalsolutions(i)forovercomingproblemsofmassand photonictransfersencounteredinclassicalphotochemicalunits, (ii)foroptimizingphotochemicalreactionprotocolsand(iii) for theirsubsequentimplementationinmeso-scalecontinuous-flow reactorsundergreener, saferandresource-efficientand energy-efficient conditions. Despite this huge potential, there are at presentfewattempts(i)tounderstandthepositiveeffectsofthe small-scale onthephotochemicalreactionperformances,(ii)to predictthereactionoutputsattheoutletofthemicrophotoreactor and/or (iii) to compare the performances obtained in
microstructured technologies with the ones in conventional equipment [27–31]. This research gap is yet essential for implementing photochemical reactions in intensified continu-ous-flowprocessescompatiblewithanindustrialproduction,and foraddressingissuesrelatedtobatch-to-continuoustransferand smartscale-up.
Motivatedbythisperspective,ourpreviousworkproposed,for a purely direct photochemical reactions A!hyB occurring in homogenous medium, different modeling approaches (one- or two-dimensional, taking into account, if necessary, the
Fig. 1.Microstructured technologies for preparative photochemistry: (a) examples of improvised ‘in house’-made microreactors, (b) examples of commercial
polychromatic character of the light source) to predict the conversionatthemicroreactoroutlet[47,53],toestablishsome guidelinestoavoidmass-transferlimitationsin microphotoreac-tors [54] or to acquire some kinetics data on photochemical reactions[56].Thismodellingbackgroundwillconstitutethebasis ofthechemicalengineeringapproachpresentedinthefollowing sectionstoaddresscurrentchallengesandissuesin continuous-flow-photochemistry.
3.Challengeslinkedtophotochemical(micro)reactor engineering
As forthermal reactions,thestarting point forphoto(micro) reactor engineering is the law describing the kinetics of the photochemicalreaction.Thislawisnotalwayseasytoexpress,in particular when heterogeneous photoreaction systems are in-volvedorwhenphotochemicalandthermalstepsarecombined. Forillustrationpurposes,apurelyphotochemicaltransformation
A!hyBisconsideredhere.Thekineticrateisexpressed,atagiven locationinthemicroreactorandatagivenwavelength
l
,as:rA;l¼
f
l$eaA;l ð1Þwhere
f
listhequantumyieldofthereaction(molmolphoton"1)and ea
A;l thelocal volumetric rate of photon absorption of the speciesA(molphotonm"3s"1).Thesetwoparametersarespectral
physicalquantities.
Several variants for thedefinition of quantumyield can be encountered, depending whether it is related to the primary photochemicalprocessortotheoverallprocess[33].However,it canbereasonablydefinedastheratiobetweenthemolarfluxof moleculesreactingduringthephotochemicalreactionandtheflux ofphotonsabsorbedbythemolecule.Thiskeyparameterprovides informationonthereactionmechanism:
f
l>1meansthatitisa chain reaction (the photochemical step solely initiates the reaction), andf
l<1 that it is a quasi-stoechiometricreaction inwhichsomedeactivationprocessesoccur(asdescribedbythe Jablonski’sdiagram)orsomeotherreactions(includingquenching mechanisms)areincompetitionwiththephotochemicalstep.For example, when considering sensitized photooxygenations, the expressionofthisquantumyieldbecomesmorecomplicateddue tothecontributionsofthequantumyieldfortheformationofthe tripletstateofthesensitizer,oftheefficiencyoftheenergytransfer fromthesensitizertoformsingletoxygenandoftheefficiencyof theformationoftheproductfromsingletoxygen[33].It is important todifferentiate thequantum yield fromthe chemicalyield.Forexample,ahighchemicalyieldcanbeobtained
withalowquantumyield,butthesereactionswouldrequirelong irradiationtimes.
ThesecondparameterinvolvedinEq.(1)isthelocalvolumetric rateofphotonabsorptionofthespeciesA,ea
A;l.Thislocalquantity representstheamountofphotonsabsorbedbythespeciesAper unitoftimeandperunitofreactorvolume.Itisexpressedas:
ea
A;l¼
a
A;lEl¼k
A;lCAEl ð2Þwhere
a
A;l is theNapierian linearabsorptioncoefficientof the speciesA(m"1),k
A;ltheNapierianmolarabsorptioncoefficientof thespeciesA(m2mol"1),C
A theconcentrationofthespeciesA
(molm"3)andE
lthesphericalirradiance(molphotonm"2s"1). ThecombinationofEqs.(1)and(2)showsthat,todetermine the mean reaction rate (i.e. averaged over the whole reactor volume),it isnecessarytoknow theconcentrationfieldsofthe different species (which depend on the hydrodynamics condi-tions),butalsotheabsorptionpropertiesofthemediumandthe irradiance field. When compared to thermal chemical reactor engineering,anewcouplingisthusintroduced:thecouplingofthe radiativetransferequationwithotherconservationequations,via thephotochemicalkineticterm.
The first consequence is that, even if the photoreactor is assumed“ideal”fromahydrodynamicpointofview(i.e.perfectly mixedor plug flow),a heterogeneous field of thereactionrate exists,duetheexponentialattenuationoflightinsidethereactor (Fig.2).Thewell-knownconceptof“idealreactors”shouldbethen thoughtagaininphotochemicalreactorengineering.
Theotherdirectconsequenceisthattheoccurrenceofsome gradientsof concentrations,duetolight attenuationbutalsoto hydrodynamics conditions(mixing), caninducephysical limita-tions,whichwillslowdownthephotochemicalreactionrateand decreasetheperformances(productivity,photonicefficiency).To identify these limitations, and thus to elaborate a strategy to overcome or limit them, modelling is an essential tool as it formalizes the coupling between the different phenomena involved.
Theintroductionof thisnewcouplingsignificantlyincreases thedegreeofcomplexityofthemodellingapproach,inparticular duetotheintrinsiccomplexityoftheradiativetransferequation (integro-differentialequation,dependenceonspatialandangular coordinates, light emission model, scattering, etc.). As the analytical solutions available in some simplified configurations (intermsofgeometryandlightemission)cannotbealwaysused, advancedmethods,oftentime-consuming,mustbeimplemented instead,for examplethe Monte-Carlo methodor flux methods (discreteordinate, two-fluxmethod, etc.)[57–64]. Forthat, the
literatureonthetheoryofphotoreactorengineeringcanbeusedto thoroughly derive reactionengineering principles and radiative energytransportfundamentals[59–71].Thechallengeswillthus be(i)tobridgethegapbetweentwoscientificfields,namelyto integratefundamentalprinciplesofradiativetransferand photo-chemistryintoengineeringmodellingmethods,and(ii)tofindthe mostsimpleandcomprehensivemodelsallowingtorepresentina sufficiently accurate way all the phenomena involved in a microphotoreactor, and their couplings. This willbe illustrated inthefollowingsections.
4.Flowphotochemistry:whicharethekeyinfluencing parameters?
Inthefollowingsub-sections,thekeyparametersinfluencing the photochemical reaction outputs when carried out in a microstructured technology willbe highlighted,based onbasic modelling.Toillustratethemethod,ithasbeenchosentoconsider:
-a monochromatic, mono-directional and collimated light source, uniformly distributed along the reactor walls and perpendicularly to the flow direction; the subscript“
l
” will bethenvoluntarilyomittedtosimplifynotationsrelatedtoall wavelength-dependentphysicalquantities.-a straightmicroreactor which characteristicdimensionalong thelightpenetrationdirectionisnotedeandthematerialofthe opticalsurfacesisnon-reflective.
-a purely photochemicaltransformation A!hy
Bwhereboth the speciesAandBareabsorbingtheincidentphotonsatagiven wavelength
l
;theperformanceswillbethenevaluatedonlyin termsofconversion.Formorecomplexreactionalschemes,one shouldalsoconsiderselectivity.4.1.Incidentphotonfluxdensityandspecificirradiatedarea
Letusconsiderthatthemicrophotoreactorbehavesasa plug-flowreactor.Inthiscase,Ailletetal.[54]showedthat,underthe assumptions previously reported, a simple equation can be established to describe the variation of the concentration in speciesA,CA,withrespecttotheresidencetime,
t
.Forthat,alocalmassbalanceis written(ananalytical solutionfor theradiative transferequationisthenconsidered),followedbyanintegration overthewholereactorvolume.Itleadsto:
"dCA d
t
¼F
qp Vr f¼F
qp Vrk
ACAk
ACAþk
BCB 1"exp½"ðk
ACAþk
BCBÞe) ð Þ ð3Þwhere qp is the incident photon flux (molphotons"1), V
r the
volumeofthemicrophotoreactor,ethecharacteristicdimensionof the microphotoreactor with respect to the light penetration
direction(pathlength),
k
Aandk
BtheNapierianmolarabsorptioncoefficientsrelatedtothespeciesAandB,respectively.
InEq.(3),thefactor
F
qpVrcanbeseenasakineticconstantofa zero-orderreaction andf is calledthephotokinetic factor. It is interestingtonotethatthis equationis stillvalidina perfectly mixed batch reactor by replacing the residence time by the irradiationtime[53,54].Furthermore,theparameterqpVrcanbealso expressedas: qp Vr ¼F0$Sirrad Vr ¼F0$airrad ð4Þwhere F0 is the photon flux density received at the
micro-photoreactor’swalls(molphotons"1m"2)and a
irrad thespecific
irradiatedarea(m"1)
definedbytheratiobetweentheirradiated surface,Sirrad,andthereactorvolume,Vr.
FromEqs.(1),(3)and (4),onecandeducethatthemaximal averagereactionrate(molm"3s"1)that canbeachievedin the
microphotoreactor(i.e.ifalltheincidentphotonsareusedbythe speciesA), rA E max D ,isequalto: rA E max¼
F
qp Vr¼F
$F0$airrad # ð5ÞEq.(5)isfundamentalasithighlightsthatthephotochemical reactionrateis directlyproportional tothephotonfluxdensity receivedatwallsandtothespecificirradiatedarea.F0andairrad
arethusthetwoleverstointensifyaphotochemicalreaction;they aredependentonbothcharacteristicsofthemicroreactorandof thelightsource,andalsoofthewaythemicroreactorisexposedto the light source. Eq. (5) also gives implicitly the main reason explainingwhymicrostructuredtechnologies,combinedwiththe newlightsources(likeLED),enableenhancedreaction perform-ances when compared toconventional technologies:they offer significantlyhigherspecificirradiatedarea(few1000m"1)andthe
photon flux densities received at walls are higher and can be adjusted.
AnotherconsequenceofEq.(5)isthattheincidentphotonflux,
qp(i.e.thephotonfluxreallyreceivedin thesystem)shouldbe imperatively known when the objective is to compare results obtainedindifferent(micro)photoreactorsortodesigna micro-reactorforagivenphotochemicalreaction.Indeed,consideringthe photonfluxemittedbythelightsourceisnotsufficientbecause onlyapartoflightemittedisreallyreceivedinthesystem,mainly duetothenon-collimatednatureofthelightsourceand/ortothe reflectanceandtransmittanceofthemicroreactormaterial.With respecttothesmalldimensionsinvolvedinmicrophotoreactors, direct measurements using a radiometer are not possible; the moreefficientalternativeis,asproposedbyAilletetal.[55],to implement an actinometry method, which involves a simple photochemicalreactionwithaknownquantumyield.
Table1
Plug-flow microphotoreactors(irradiatedwithamonochromatic, mono-directionalandcollimatedlight source,uniformlydistributedalong thereactorwallsand
perpendicularlytotheflowdirection):definitionsofthecharacteristicdimension,e, and of the parameterqp
Vr depending on the geometry.
Parallelplatemicroreactorirradiatedfromtheoutside Tubularmicroreactorirradiatedfromtheoutside Annularmicroreactorirradiatedfromtheinside
qp Vr¼ F0 Wand e¼W qp Vr¼2 F0 R and e¼2R qp Vr¼2 F0Ri ðR2 e"R2iÞ and e¼Re"Ri
Finally,itisinterestingtonotethatEq.(3)canbegeneralizedto three types of geometries of plug-flow microphotoreactors (irradiated with a monochromatic, mono-directional and colli-matedlightsource,uniformlydistributedalongthereactorwalls and perpendicularly to the flow direction). These geometries consistinparallelplatemicroreactorsirradiatedfromtheoutside, tubular microreactors irradiated from the outside and annular microreactors irradiated from the inside. For that, an ad hoc definitionforthecharacteristicdimension,e,andforqpVr(thesetwo parameters being sufficient to fully characterize ideal micro-photoreactors),asshowninTable1.
4.2.Mediumabsorbanceandcompetitiveabsorptionfactor
LetusdefinetheconversionXas:
X¼1"CA
CA0 ð6Þ
Eq.(3)canthenbewrittenagainas:
"dX d
t
¼F
qp Vrb
A CA0 1"X 1"X ð Þb
Aþð1"b
AÞX 1"exph"A0eðð1"XÞb
Aþð1"b
AÞXÞi & ' ð7Þwhere CA0 is theinitial concentration of the species A,
b
A the competitiveabsorbancefactorwithrespecttothespeciesAandA0 eareferenceabsorbancedefinedas[54]:
b
A¼k
Ak
Aþk
B ð8ÞA0e¼ð
k
Aþk
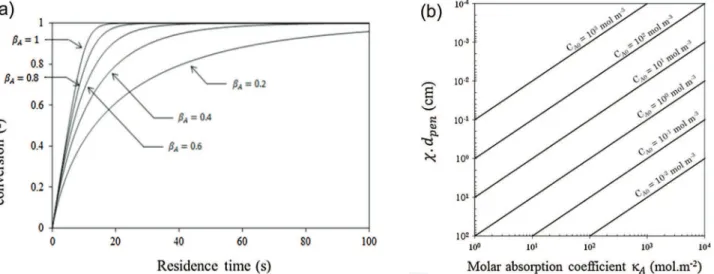
BÞCA0$e ð9ÞConsequently, Eq.(7)highlights twootherimportant param-eters for consideration when carrying out a photochemical reaction in continuous (micro) photoreactors: the competitive absorbancefactorandthemediumabsorbance.
Bydefinition,
b
Agivesinformationonthelevelofcompetition between the reactant A and the product B for absorbing the incidentphotons.Toillustrateitsinfluence,anexample(deduced fromtheresolutionofEq.(7))ofthevariationoftheconversionasa functionofresidencetime for differentcompetitiveabsorbance factorsispresentedinFig.3a.Thisfigureshowsthatthemoretheproduct B is absorbing
b
A!0Þ, the more the reaction rate is sloweddown,thepartofphotonsabsorbedbythereagentAbeing decreasingasfarastheconversionincreases.Thisphenomenon, intrinsictothereactioncharacteristics,willbemorepronouncedin thecaseofnon-idealmicrophotoreactorsforwhichmasstransfer limitationsexist(seeSection5.1).Concerning the medium absorbance, Ae, this parameter changes asfaras thereaction progresses,asdepending onthe conversionXaccordingto:
Ae¼ð
k
ACAþk
BCBÞe¼A0e$½b
Að1"XÞþXð1"b
AÞ)¼A0e$b
A$x
with
x
¼ð1"XÞ þXð1"b
AÞb
A ð10Þwhere
x
is a function of X and ofb
A (x
¼1 when X¼0, andx
¼1"bAbA whenX¼1)andA 0
edefinedinEq.(9).Itisinterestingto
observe that the medium absorbance can be also seen as a dimensionlessnumber:
Ae¼ e
dpen ð11Þ
wheredpenisthecharacteristiclightpenetrationdistance(also
calledopticalthickness),definedaccordingto
dpen¼ 1
k
ACA0x
ð12Þ
AsshownbyEq.(11)andFig.3b,thelightpenetrationdistance decreaseswhenincreasingtheinitialconcentrationofthespecies Aandthemolarabsorptioncoefficient
k
A.IfthespeciesBdoesnotabsorb ð
b
A¼1Þ, dpen will increase as far as the conversionincreases(decreaseofthefunction
x
):themediumbecomesthen more and more transparent,whereas, whenb
A*0:5,dpen willdecreaseand themediumwillbecomemoreand moreopaque. Classically,oneconsidersthatAe<1correspondstogreatoptical thickness (i.e.e<dpen) and Ae>1 to small optical thickness
(i.e.e>dpen). In the second case, the fraction between the
irradiatedandthereactorvolumeswillbesmallerthanone,thus implyingtheappearanceofdarkzones.Intheextremecase,when thisfractiontendstowardszero,theirradiatedvolumeislocatedin a narrow zone close totheoptical walls, leadingto a “surface reaction”or “filmreaction”. Inthis case, theroleofthemixing (masstransfer)willbecrucialtoefficientlyrenewthiszone.
Fig.3.(a)Variationoftheconversionasafunctionofresidencetimefordifferentcompetitiveabsorbancefactorinthecaseofaplug-flowmicrophotoreactor(Fqp
Vr¼10
"4
molL"1s"1,k
A¼500 Lmol"1cm"1, CA0¼0:01molL"1, e¼1cm).(b)Light penetrationdistance,weighted bythefunction x(Eq.10)asafunction ofthe molar
As the molarabsorption coefficients of species are intrinsic propertiesofthereaction,thetwoleversavailabletochangethe mediumabsorbancearetheinitialconcentrationofthespeciesA,
CA0, and/or thecharacteristic dimensionof themicroreactor, e.
Thus,anotheradvantageofmicrostructuredtechnologiesbecomes apparent:thankstotheirsmalldimensions,itispossibletowork withconcentratedmediawhileconservinganacceptablelevelof absorbance. For the “surface reactions” previously mentioned, fallingfilmmicroreactorsprovetobeparticularlyadapted[51,52].
4.3.Masstransferlimitations
In the previous sub-sections, a plug-flow behavior of the microphotoreactorwasassumedtohighlight,inasimpleway,the key parameters (
f
, F0, airrad, Ae,b
A) influencing the reactionoutputs. Unfortunately, such kind of approach is often not sufficient todescribe thecoupling betweenallthe phenomena insidea microphotoreactor,andinparticulartheeffectof mass-transfer limitations (mixing). To fill this gap, more advanced modellingtoolsarerequired.Inthisperspective,Ailletetal.[54]
have proposed a bi-dimensional model enabling to predictthe spatial distributions of concentrations and irradiance inside a straightmicrophotoreactorinvolvingalaminarflow(theradiative transferequationissolvedbythetwo-fluxmethod[73]).Usinga dimensionless set of equations, they showed that the reaction outputs are always controlled by the competitive absorbance factor
b
AandthereferencemediumabsorbanceA0e,butalsobytwo otherdimensionlessnumbers:theDamköhlerIandIInumbers,DaIandDaII.Thelatterdimensionlessnumbersareexpressedas
theratiobetweenresidencetime,
t
,andphotochemicalreaction time,t
r,andbetweentransversemixingtime,t
d,andphotochem-ical reaction time, respectively. Considering a photochemical reactionA!hyB, a monochromatic collimated light source and a laminarflowinsideastraightmicroreactorirradiated perpendicu-larlytoitswidthfromoneorbothsides(
y
=0or1)(parallelplate geometry,seeTable1),theycanbeexpressedas[54]:DaI¼
t
t
r¼ L U CA0eF
ð1þy
Þb
AF0 ð13Þ DaII¼t
dt
r¼ e2 Dm CA0eF
ð1þy
Þb
AF0 ð14ÞwhereUisthemeanvelocityinthemicroreactor,Landethelength (alongtheaxialdirection)andthetransversedimension(alongthe lightpenetrationdirection)ofthemicrophotoreactorandDmthe
diffusioncoefficient.
These two dimensionlessnumbers arecommon inchemical engineering,buttheirtranspositiontophotochemicalreactionsis not direct as it implies to correctly define the characteristic reaction time. Contrary to thermal reactions, for which some intrinsickineticslawsareformulated,thecharacteristictimeofthe photochemical reaction is process-dependent (i.e. no more intrinsic to the reaction system) because the reaction rate, r,
dependsonthevolumetricrateofphotonabsorption,ea(Eq.(1))
andthusontheirradiance,E(Eq.(2)).Itsdefinitionshouldthenbe adapted,foreach reaction,butalsoforeachlight source/micro-reactortechnology.Thecharacteristictime reportedinEqs.(13)
and (14) was deduced, for strongly absorbingmedia, fromthe average of the local reaction times over a conversion varying between0and1,andnotfromthereactionrateatthebeginningof
thereactionwhentheconversionisnull,asitisclassicallydonefor thermalreactions;suchmethodenablestotakeintoaccountthe effectof
b
Aonthereactiontime[54].TheDamköhlerInumbercanberegardedasameasureofthe conversionthatcanbeachieved:highvaluesofDaImeancomplete
conversionsatthemicroreactoroutlet.Itisinterestingtonotethat
DaI isalsodirectlylinkedtothedose, thatis,totheamountof
photonsreceived duringtheresidence time perunit of reactor volume(molphotonm"3),definedas:
dose¼qp Vr
t
ð15Þ thusimplying: DaI¼doseCF
A0b
A ð16ÞTheDamköhlerIInumbercanberegardedasameasureofthe efficiencyofthemixing(ormasstransfer)alongtheopticallight path,which is induced,inEq. (14),by moleculardiffusion.The latterrepresentsoneofthetwomainphenomenaresponsiblefor the occurrence of concentration gradients in the transverse direction,namelythelightattenuation(theotheronebeingthe heterogeneousvelocityfieldalongthetransversedirectiondueto thelaminarflow).AvalueofDaIIsmallerthanonemeansthatthe
transverse masstransfer time is shorterthan thecharacteristic time of the reaction. Another advantage of microstructured technologiescanbepointedouthere:duetotheirsmallscales, thetransversemasstransfertimesaresignificantlyreduced,which enablestoovercomethistypeoflimitationscommonly encoun-tered in conventional photochemical equipment and thus to improvereactionoutputsand/ortocarryoutreactionsundersafer conditions.
Naturally,bothDamköhlernumbersarelinkedviatheFourier number,Fo,as:
DaII¼
1
FoDaI ð17Þ
FromEq. (17), a diagram canbe established toidentify the different zones in which a microphotoreactor can operate, as shownbyAilletetal.[54].The“optimal”domainavoidingmass transferlimitations(noconcentrationgradients alongthe trans-verse direction, plug-flow behavior) corresponds to the cases whereDaII<1and1=Fo<1.
It is important to notethat, even if some strategies can be elaboratedtoavoidmass-transferlimitations(seeSection5.1),this presupposesindirectlythatthelifetimeofelectronicallyexcited speciesislongenoughtoenablethereactiontooccur.Ifthisisnot thecase,mixingwillbenolongerthelimitingparameterandother strategies (a change of solvent for example) will have to be implementedtoincreasethelifetimeoftheexcitedspecies.
When more complex geometries of microphotoreactors (meandering channels for example) are involved or when heterogeneousreactionsare carriedout, itwillbenecessary to adapt or complete this analysis based on the dimensionless numbers. Mass transfer coefficients should be in particular considered.
4.4.Productivityandphotonicefficiency
Fromachemicalengineeringpointofview,theperformances obtainedinagivenmicrophotoreactorcanbegenerallyevaluated throughbothproductivityandphotonicefficiency.
Theproductivity,RX,isdefinedforagivenconversionX,as:
RX¼ n
t
Xwherenis thenumberof molesof productformedand
t
XtheresidencetimenecessarytoreachagivenconversionX.According toEq. (3),this residencetime isinverselyproportionalton=qp.
Consequently, the productivity is proportional to the incident photonflux,qp,andusingEq.(4),onefinds:
RX/qp leadingtoRX/F0$airrad$Vr ð18Þ
In a process intensification strategy, Eq. (18) has important consequences as it implies that an increase in productivity necessarily requiresan increase in qp. For that, several choices
areavailable: increasingthephoton flux densityat the reactor wallsðF0Þ and/or theirradiated specific areaðairradÞ and/orthe
reactorvolumeðVrÞ.Inthisperspective,photochemicalequipment
basedon theconcept of plate heat-exchangers are particularly interesting,astheyallowtomaintain,foreachfluidicmodulein series,identicalF0andairradthankstoLEDarraysplacedonboth
sidesofeachfluidicmodule.Consequently,theproductivitycanbe simply increased by raising the reactor volume, namely by multiplyingthenumberoffluidicmoduleinseries(seeRef.[47]). Nevertheless,oneshouldkeepinmindthattheproductivityis notonlycontrolledbytheincidentphotonflux,butalsobythe absorbancepropertiesofthemedium(iftheabsorbanceislow,few photonswillbeabsorbedandtheproductivitywillbelow).Forthis reason, one should introduce another criteria: the photonic
efficiency,
h
X.It isdefined,for a givenconversionX,astheratiobetweenthenumberofmolesofreactantconverted(orproduct formed)andthenumberofphotonsreceivedinthemicroreactor, correctedbythequantumyield[53]:
h
X¼ nf
$qp$t
Xð19Þ
It evaluatestheoptimaluse ofphotonsin the microreactor. Indeed,asquantifiedbythequantumyield(seeSection3),notall ofthephotonsabsorbednecessarilyleadtotheconversionofthe compoundA.However,otherphenomenacanincreasethenumber ofphotonsrequiredtoformtheproductB:
-thehydrodynamicsinsidethemicroreactor.Forexample,when theproductBorotherspeciesabsorbtheincidentphotons,poor mixing conditions can generate an overexposure of these moleculestothedetrimentofthereactantA,andthusreduce thepartofphotonsavailableforA(seeSection5.1),
-themedium absorbance.If themediumabsorbance islow,a significantpartofthephotons aretransmitted overtheback opticalwallsifthislatteristransparent(noreflector).
Thephotonicefficiencyenablesthetwolatterphenomenatobe takenintoaccount.Itiscorrectedbythequantumyieldtofreeit
Fig.4.Effectofmixinglimitations:(a)ConversionattheoutletofthemicroreactorversusDamköhlerIInumberfordifferentcompetitiveabsorbancefactors(A0e=10).
(b)Concentration fieldsinsidethe microphotoreactorfor twolimitcases (DaII!0 andDaII!1),dependingon thecompetitiveabsorption factorbA (A0e=20).
Fig.5.Predictionsoftheperformancesobtained:(a)synthesisofapentacycliccagecompound[47,53],(b)conversionversusirradiationtimeinacapillarytower
from the effect of deactivation processes intrinsic to the photochemicalreactionmechanisms.Ideally,itshouldtendto1 (onemoleofphotonsusedtoformonemoleofproduct).
From Eqs. (17) and (19), the productivity and the photonic efficiencyarecloselylinked[54]accordingto:
RX¼
f
$qp$h
X¼f
$F0$airrad$Vr$
h
X ð20ÞEq. (20) confirms that, to maintain a constant productivity betweentwoplug-flow(micro)reactors,oneshouldconserveboth incidentphotonflux&qp'andphotonicefficiency(
h
X).Eq.(20)
also shows that, ifthe intrinsic parameters of thereaction are known (quantumyield, molarabsorptioncoefficients), one can determine the requirements in terms of incident photon flux density(designofthelightsource),ofirradiatedspecificareaand reactorvolume(designofthemicroreactorandintegrationofthe light source around it), and of photonic efficiency (medium absorbance)in ordertoreacha givenproductivityinplug-flow (micro)reactors.
5.Illustrativeexamples
Thispartwillpresentsomeillustrativeexamplesextractedfrom our previous studies. In all these examples, purely direct photochemicalreactionsA!hyBoccurringinhomogenousmedium areconsidered.Theobjectiveistodemonstratehowachemical engineering framework, such as presented in the previous sections,enablestounderstandthecouplingbetweenthedifferent phenomena involved(Section 5.1), to predicttheperformances obtained(Section5.2),toacquirekineticsdataonaphotochemical reaction(Section5.3)ortoelaborateastrategyforphotochemical processintensification(Section5.4).
5.1.Understandingthecouplingbetweenthedifferentphenomena
involved
Asmentionedintheprevioussection,themixingalongthelight penetrationdepthcanhaveaninfluenceofthereactionoutputs. Thisphenomenonhasbeenhighlighted,numericallyand experi-mentally,byAilletetal.[54]andAilletetal.[74]respectively,inthe case ofa photochemicalreactionA!hyB.For illustrativepurpose,
Fig. 4a reports, for a strong absorbing medium &A0e¼10', numericalresults describing theconversionatthemicroreactor
outlet,X,asafunctionoftheDamköhlerIInumber,DaII(Eq.(14))
andofthecompetitiveabsorbancefactors,
b
A.Onecanobserve that a significant decrease in conversion exists when increasingDaII, namely when the transverse mixing becomesslowerandslower.Thesmallestthecompetitiveabsorptionfactor is
b
A (i.e. the highest the molar absorption coefficient of the productB,k
B),themorepronouncedtheeffectofDaIIis.To physically understand such trends, the corresponding concentrationfieldsinthemicrophotoreactorshouldbeanalyzed (Fig.4b).WhentheproductBabsorbsatthesamewavelengththan the reactant A ð
b
A<1Þ and when the medium is strongly absorbing &A0e¼10', strong concentration gradients appear as farasDaIIincreases.Thisisdirectlyduetotheformation,fromtheinitialmomentsofthereaction,ofalayerofproductBclosetothe microreactorwallwherethelightisthemostintense.Thislayer playstheroleofascreenorafilter,whichpreventsthephotonto penetratefurtherinside themicroreactorandtoreactwiththe reactantA.Itpersiststhroughoutthemicroreactorlengthasthe mixing(masstransferbydiffusion)doesnotenablethefluidatthe wall to be efficiently renewed. Another way to evaluate this phenomenonis tonumericallycalculatetheaveragevolumetric rates of photons absorbed by the compounds A and B in the microreactor, ea A E D and ea B E D
[54].Onecanthenobservethatthe amountofphotonsabsorbedbythecompoundBincreaseswhen increasingDaII.Inpractise,specialattentionshouldbepaidtothis
fact because, in the case of light-sensitive products, some photodecomposition may occur and may thus impact on the reactionselectivity.
Ailletetal.[54]haveidentifiedtwoparticularcasesforwhich the occurrence of mass transfer limitations ðDaII>1Þ has a
negligibleinfluenceontheconversion:
- WhenthespeciesAisthesingleabsorbingspeciesð
b
A¼1Þ.In this case, the absorbing layer shifts to the center of the microreactorasfarastheconversionprogresses(seeFig.4b), thus meaning that the medium becomes more and more transparent.- When the medium absorbance is low &A0e<5'. The micro-reactoristhenfullyilluminated.
In both cases, the transverse mixing slightly impacts the conversionbecausethemolecules ofreactantA donot needto
travel efficiently along the light penetration direction as the photonscanpenetrateinsidethemediumtoreachthenon-excited molecules.Formicroreactormodelling,thisisanoptimalsituation asitimpliesthatthemicroreactorcanbeconsideredasaplug-flow reactor.
5.2.Predictingtheperformancesobtained
The synthesis of a pentacyclic cagecompound (Fig. 5a) was carriedoutbyAilletetal.[53]inaclassicalimmersionwellreactor (Vr=225mL, e=0.62cm) and a capillary-tower microreactor
(Vr=0.81mL,e=0.0508cm),bothirradiatedbyamediumpressure
mercurylamp.Itwasobservedthatfullconversionswereachieved within a few minutes of residence times in the microreactor whereasirradiationtimeslongerthan20minwererequiredinthe batchreactor(Fig.5b).Asthecagecompounddoesnotabsorbthe incident photons at 365nm ð
b
A¼1Þ, the following analytical solutionfortirrad couldbeobtainedfromEq.(7):tirrad¼ Vr qp CA0
F
Xþ 1 A0 e ln1"exp&"A0e' 1"exp "A0eð1"XÞ & ' 2 4 3 5 0 @ 1 A ð20Þ
which can be also written, using Eq. (13) and considering
t
¼tirrad,as DaI¼dose:F
CA0 ¼ Xþ1 A0 e ln1"exp&"A0e' 1"exp "A0eð1"XÞ & ' 2 4 3 5 0 @ 1 A ð21Þ
As shown in Fig. 5b, the experimental variations of the conversion, X, as a function of the irradiation time, tirrad, are successfully predicted by Eq. (20) in both reactors. Such good agreementvalidatesthat,when
b
A¼1,thebatchreactorcanbe describedasaperfectlymixedreactorandthemicroreactorasa plug-flowreactor(seeSection5.1).ItisalsointerestingtouseEq.(20)asameantounderstandwhy theirradiationtimesaresodifferentinbothreactors.Indeed,the irradiationtime ratio,
x
Xt,requiredtoreacha conversion X,for
exampleequalto90%,atagivenabsorbanceA0e,canbecalculated as:
x
Xt ¼ðtirradÞbatch tirrad ð Þmicro ¼ qp=Vr & ' micro qp=Vr & ' batch $ðCAOÞbatch CAO ð Þmicro ¼ðairrad$F0Þmicro airrad$F0 ð Þbatch$ CAO ð Þbatch CAO ð Þmicro ð22ÞUsingthedatareportedinRef.[53]andtheresultsobtainedby actinometry[55],anirradiationtimeratiocloseto17isobtained, whichisinperfectagreementwiththeexperimentalratio(Fig.5b). Anin-depthanalysisrevealsthatsucharesultisduetoadifference intermsofinitialconcentrations(A0eisconstantinbothreactors), but also in terms of irradiated specific area (2530m"1 in
microreactor against 133m"1in batch reactor) and of incident
photon flux density (2.55$ 10"3molphotonm"2s"1 in
micro-reactoragainst0.23$10"3molphotonm"2s"1inbatchreactor).
Therelevancyof thismodellingapproach (Eq.(20))hasalso been demonstrated when this reaction was carried out in the meso-scale continuous reactor commercialized by Corning (Corning1Advanced-FlowTM
G1photoreactor),eithercomposed byoneorfivefluidicmodules,asrecentlyillustratedbyElgueetal.
[47].
5.3.Acquiringkineticdata
Recently,microreactorswereused,forthefirsttime,asatool foracquiringkineticdataona photochemicalreaction[56].For illustration purpose, a thermal photochromic system (1,3,3-trimethylindolino-60-nitrobenzopyrylospiran, named TMINBPS)
was chosen (Fig.6a).It involves a reversible systemwhere the initialcolorlessspecies(closedform)reactsphotochemicallyviaa stepcharacterizedbythequantumyield
F
AB.Thespeciesformed(open form with a pink color) has a short lifetime and is transformedintoAbyathermalreactioncharacterizedbyarate
kt. The two kinetics parameters of the reaction (
F
AB,kt) weresuccessfully determinedbycombiningmodelling tools(such as describedinSections4.1and 4.2)andspecificexperimentsina spiral-shapedmicroreactorirradiatedbyaUV-LEDarray(Fig.6b). For that, the incident photon flux, qp, should be imperatively known; actinometry measurements were thus carried out, accordingtotheprotocoldefinedby[55].
Thiswork[56]offerspromisingperspectivesforanewusageof microreactors for photochemistry. Indeed, the ability to use microreactors for acquiring kinetic data of a photochemical reactionis anundeniableadvantage,andall themore thatthis can be done rapidly, with low volumes handled and in an experimentalwindowenlargedtooperatingconditions inaccessi-bleforbatchreactors(shortresidencetime,highconcentration). Thisisparticularlyinterestinginaprocessintensificationstrategy where an in-depth knowledge of reaction kinetic will ensure reliabilityinextrapolationandinprocessmodeling.
Fig.7. Elaborationofstrategiesforphotochemicalprocessintensification:(a)diagramfordeterminingthemaximalphotonfluxdensityandtheminimalresidencetimeto
avoidmasstransferlimitationsinmicroreactor.(b)nomasstransferlimitations:Iso-curvesforphotonicefficiency(foraconversionof95%)asafunctionofthedimensionless
numbersA0
5.4.Elaboratingstrategiesforphotochemicalprocessintensification
Using the modelling background presented in Section 4, strategies can be built todetermine the optimalconditions in whichamicroreactorshouldoperateortoaddressscale-upissues. Inthefollowing,someexampleswillbepresented,alwaysinthe caseofaphotochemicaltransformationA!hyB.
Theoccurrenceofmasstransferlimitations(slowmixingalong thelightpenetrationdirection)caninduceasignificantdecreaseof theconversionattheoutletofthemicroreactor(seeFig.4a),but alsoontheproductivityviathedecreaseofthephotonicefficiency (see[54]).Startingfromthesefindings,severalstrategiescanbe devisedformaximizingtheproductivityinmicroreactors.Oneof them is to identify the conditions under which the micro-photoreactor behaves as a plug-flow reactor (homogeneous concentration profiles in the transverse direction). For that, accordingtoSection4.3,theDamköhlerIInumber,DaII,should
bekeptbelow1whileconservingalsotheinverseoftheFourier number,1=Fo,below1.Eq. (14)showsthatonecanactontwo leverstofilltheseconditions:thecharacteristicdimensionofthe microphotoreactorinthetransversedirection,e,andtheincident photonfluxdensity,F0.Inthisperspective,adiagram(builtfrom
numericalresults)hasbeenproposedbyAilletetal.[54],enabling to determine, for a given characteristic dimension, e, and depending on the medium absorbance, A0e and competitive absorptionfactor,
b
A (associatedwitha photochemicalreactionA!hyB),theconditionsinwhichthemicroreactorshouldoperateto achieveahighconversion(forexample95%)whileavoidingmass transferlimitations(Fig.7a).Moreparticularly,theseconditions correspondto:
-a maximal photon flux density,F0;max, toimpose. Indeed, as predicted by Eq. (14), the reaction characteristic time,
t
r is inverselyproportionaltoF0;consequently,definingamaximalvalueforF0enablestoensurethatthetransversemixingtime,
t
d,remainssmallerthant
r;inFig.7a,F0;maxisdeducedfromthe coefficientklim reportedontheabscissaaxis(seeRef.[54] for details),- aminimalresidencetime(
t
min) toimpose,whichislinkedto thepreviousmaximalphotonfluxdensity.Itisdeducedfromthe residencetimerequiredtoreachaconversionof95%inthecase where
b
A¼1(notedt
0:95jbA¼1)whichisreportedonthe
right-sideordinateaxisofFig.7b.Thevalueof
t
0:95jbA¼1readonthe
diagramshouldbethenmultipliedbythefunctionH0:95&
b
A;A0e'defined in Ref. [54] toaccount for theeffectof the medium absorbanceandcompetitiveabsorptionfactor.
Suchadiagramcanbealsousedasatoolforquantifyingthe effectoftheminiaturizationofthemicroreactoronthe productiv-ity,RX,inthecasewherenomasstransferlimitationsexist[54].For that, F0;max and
t
min associated with different microreactor dimensions, e, have to be determined and then, from the knowledge of&b
A;A0e' and of the photonicefficiency (Fig. 7b),RXcanbededucedforeach[54].
Once having determined the conditions &F0;max;
t
min' with respect toavoiding masstransfer limitations in a given micro-reactor,orwhentheselimitationshaveanegligibleinfluence(see Section5.1),anewdiagramcanbeconstructed(fromEqs.(7)and(13)) reporting the variation of the Damköhler I number, DaI,
required to reach a given conversion, X, as a function of the
referenceabsorbance,A0e,andofthecompetitiveabsorbancefactor,
b
A.Suchadiagram,detailedinRef.[54],revealsthat,forstrongly absorbingmedia&A0e>5',theDamköhlerInumber,DaI,requiredto reach a given conversion, X, becomes independent on the absorbance,anddependsonlyof
b
A,as:DaI¼dose$
F
CA0
¼"ð1"
b
AÞðXþlnð1"XÞÞþb
AX ð24ÞThelatterequationgivestwoimportantguidelines:
- whentheproductBabsorbstheincidentphotonsð
b
A<1Þ,the valueofDaI (andthusofthedose)requiredtoreachagivenconversion,X,shouldbeincreasedtocounterbalancethefact thatapartoftheincidentphotonsisabsorbedbytheproductB. -aneasymethodtocalculatethedoserequiredtoreachagiven
conversion.
Inasmartscaleperspective(e.g.fromlab-scalemicroreactors producingfew
m
gh"1ormgh"1tomeso-scalecontinuousreactorsproducing few kgh"1), the latter information means that, to
maintainthesame conversionatboth scaleswhen A0e>5,one shouldconserveasinglecriteria:thedose;thiscanbeachievedby adapting the residence time and/or the incident photon flux (Eq.(15)).Nevertheless,oneshouldkeepin mindthatthedose alonedoesnotallowtofullydesignacontinuous(micro)reactor; theproductivity,RX,alsohastobeconsidered,whichdependson thephotonicefficiency,
h
X(Eq.(20)).AsshowninFig.7b,whennomass transfer limitations exist (or when their influence are negligible),themore themedium isabsorbingandtheless the speciesBisabsorbing,thehigherthephotonicefficiency
h
Xis.Thisshowsthatworkingwithlowabsorbancesisnotapriorioptimal firstlybecausedilutedmediumimplieshighersolventneedsand thusheavierdownstreamprocesses,butalsointermsofenergetic efficiencyasapartofphotonsis wastedbytransmittanceifno reflector is used. Nevertheless,in practice, when mass transfer limitations cannot be overcome (for example, when the light sourceemittedbythelampcannotbemodified),theusageoflow absorbancescanbeameanstolimittheirimpactonthereaction outputs (conversion, productivity and photonic efficiency); an optimumfortheabsorbancehasthentobefound[54].
To conclude, the understanding and the modeling of the phenomena involved (and of their coupling) are absolutely requiredtodeterminetheoptimaloperationdomaininwhicha givenmicrophotoreactorshould operatetomaximizereactions outputsandalsotohelp thedesignprocess.Nevertheless,in a photochemical process intensification perspective, it is only a globalprocessanalysis(asproposedbyLoponovetal.[29])that will enable to decide if microstructured technologies operate moreprofitable than otherphotoreactortechnologies. Thecost functionultimatelywillbethedecidingfactorandits optimiza-tionwillbeusedtorevealthetrueinteractionsbetweendifferent competingfactorsin acomplexindustrialsystem(thefindings obtained will strongly depend on the photochemical reaction considered).Inaddition,itisimportanttorememberthat,evenif their use for production purpose are not always profitable, microstructuredtechnologiesremainapowerfultoolat labora-tory-scale for synthetizinga few milligrams of a product used afterwards in early research and development, and also for investigating photochemical reactions (operating condition screening,kineticdataacquisition,seeSection5.3).
6.Conclusion
Continuous-flow photochemistryis thesubjectof a growing amountof research and industrial projects as microstructured reactors provide new scientific and technological solutions to optimize photochemical reaction protocols and to overcome problemsencounteredinconventionalphotochemicalequipment. Thispresentpaperillustratesthatsomeofthecurrentchallenges andissuesincontinuous-flowphotochemistrycanbeaddressed within a chemical engineering framework. Based on a basic modellingapproachand byconsideringonlythecase ofpurely direct photochemical reactions A!hyB occurring in homogenous medium, thekey factors toconsider when implementing such photochemical reactions in microstructured technologies were outlined,namelythephotonflux densityreceived atwalls,the irradiatedspecificarea,themediumabsorbance,thecompetitive absorbancefactorandtheinfluenceofthemixingalongthelight penetration direction. Their influence on the reaction outputs (conversion, productivity, photonic efficiency) was analyzed in detail.Theinterestofthisframeworkwasatlastdemonstrated,for thesetypesofphotochemicalreactions,usingseveralillustrative examplesextractedfromourpreviousstudies;theyconcernedthe understandingofthecouplingbetweenthedifferentphenomena involved, the predictions of the performances obtained, the acquisitionofkineticsdata,andtheelaborationofstrategiesfor photochemicalprocessintensification.
Inthefuture,thechallengewillbetointegratethecomplexity ofphotochemistry (e.g.heterogeneousphase reactions, indirect scheme, competitive or consecutive photoreactions) into the present modelling tools so as to enlarge the spectrum of photochemical process intensification strategies. To succeed, twoconditionswillberequired:
- using the current literature on the theory of photoreactor engineeringinordertorigorouslyderivereactionengineering principlesandradiativeenergytransportfundamentals. - closelyinterconnectingphotochemistryandchemical
engineer-ingfromthebeginningofastudyinordertoidentifyreaction andprocesslimitations assoonaspossible,and todevelop a strategytoovercomethese.
References
[1]C.-L.Ciana,C.G.Bochet,Cleanandeasyphotochemistry,Chimia61(2007) 650–654.
[2]N.Hoffmann,H.Bouas-Laurent,J.-C.Gramain,Synthèseparvoie photochimique,ActualitéChimique317(2008)6–11.
[3]N.Hoffmann,Photochemicalreactionsaskeystepsinorganicsynthesis,Chem. Rev.108(2008)1052–1103.
[4]T.Bach,J.P.Hehn,PhotochemischeReaktionenalsSchlüsselschritteinder Naturstoffsynthese,Angew.Chem.123(2011)1032–1077.
[5]H.E.Zimmerman,Mechanisticorganicphotochemistry,Angew.Chem.Int.Ed. 8(1)(1969)1–88.
[6]N.J.Turro,Geometricandtopologicalthinkinginorganicchemistry,Angew. Chem.Int.Ed.25(10)(1986)882–901.
[7]M.Olivucci,F.Santoro,Chemicalselectivitythroughcontrolofexcited-state dynamics,Angew.Chem.Int.Ed.47(2008)6322–6325.
[8]I.Schapiro,F.Melaccio,E.N.Laricheva,M.Olivucci,Usingthecomputerto understandthechemistryofconicalintersections,Photochem.Photobiol.Sci. 10(2011)867–886.
[9]N.Hoffmann,Photochemicalreactionsofaromaticcompoundsandthe conceptofthephotonasatracelessreactant,Photochem.Photobiol.Sci. 11(11) (2012)1613–1641.
[10]M.Oelgemöller,Greenphotochemicalprocessesandtechnologiesforresearch &development,scale-upandchemicalproduction,J.Chin.Chem.Soc.61 (2014)743–748.
[11]S.Protti,D.Dondi,M.Fagnoni,A.Albini,Photochemistryinsynthesis:where, when,andwhy,PureAppl.Chem.79(2007)1929–1938.
[12]P.T.Anastas,M.M.Kirchhoff,Origins,currentstatus,andfuturechallengesof greenchemistry,Acc.Chem.Res.35(9)(2002)686–694.
[13]P.Anastas,N.Eghbali,Greenchemistry:principlesandpractice,Chem.Soc. Rev.39(2010)301–312.
[14]H.Baumann,U.Ernst,M.Goez,A.Griesbeck,M.Oelgemöller,T.Oppenländer, M.Schlörholz,B.Strehmel,LichtalskleinstesReagenzundWerkzeug,Nachr. Chem.62(2014)507–512.
[15]A.Griesbeck,H.Ihmels,K.Licha,T.Mielke,M.Senge,B.Strehmel,D.Wöll,Licht fürMedizinundDiagnostik,Nachr.Chem.62(2014)612–616.
[16]J.Iriondo-Alberdi,M.F.Greaney,Photocycloadditioninnaturalproduct synthesis,J.Eur.J.Org.Chem.(2007)4801–4815.
[17]K.Matsuda,M.Irie,Diaryletheneasaphotoswitchingunit,J.Photochem. Photobiol.C:Photochem.Rev.5(2004)169–182.
[18]G.S.Kottas,L.I.Clarke,D.Horinek,J.Michl,Artificialmolecularrotors,Chem. Rev.105(2005)1281–1376.
[19]K.Szaciłowski,W.Macyk,A.Drzewiecka-Matuszek,M.Brindell,G.Stochel, Bioinorganicphotochemistry:frontiersandmechanisms,Chem.Rev.105 (2005)2647–2694.
[20]R.deRichter,S.Caillol,Fightingglobalwarming:thepotentialof photocatalysisagainstCO2,CH4,N2O,CFCs,troposphericO3,BCandother
majorcontributorstoclimatechange,J.Photochem.Photobiol.C:Photochem. Rev.12(2011)1–19.
[21]M.Tahir,N.S.Amin,Recyclingofcarbondioxidetorenewablefuelsby photocatalysis:prospectsandchallenges,Renew.Sustain.EnergyRev.25 (2013)560–579.
[22]M.R.Hoffmann,S.T.Martin,W.Choi,D.W.Bahnemann,Environmental applicationsofsemiconductorphotocatalysis,Chem.Rev.95(1995)69–96. [23]A.M.Bugaj,Targetedphotodynamictherapy—apromisingstrategyoftumor
treatment,Photochem.Photobiol.Sci.10(2011)1097–1109.
[24]K.F.Jensen,B.J.Reizman,S.G.Newman,Toolsforchemicalsynthesisin microsystems,LabChip14(2014)3206–3212.
[25]E.E.Coyle,M.Oelgemöller,Micro-photochemistry:photochemistryin microstructuredreactors.Thenewphotochemistryofthefuture?Photochem. Photobiol.Sci.7(2008)1313–1322.
[26]M.Oelgemöller,O.Shvydkiv,Recentadvancesinmicroflowphotochemistry, Molecules16(2011)7522–7550.
[27]J.P.Knowles,L.D.Elliot,K.I.Booker-Milburn,Flowphotochemistry:oldlight throughnewwindows,BeilsteinJ.Org.Chem.8(2012)2025–2052. [28]Y.Su,N.J.W.Straathof,V.Hessel,T.Noël,Photochemicaltransformations
acceleratedincontinuous-flowreactors:basicconceptsandapplications, Chem.Eur.J.20(34)(2014)10562–10589.
[29]K.N.Loponov,J.Lopez,M.Barlog,E.V.Astrova,A.V.Malkov,A.A.Lapkin, Optimizationofascalablephotochemicalreactorforreactionswithsingulet oxygen,Org.Proc.Res.Dev.18(11)(2014)1443–1454.
[30]D.Ziegenbalg,B.Wriedt,G.Kreisel,D.Kralisch,Investigationofphotonfluxes withinmicrostructuredphotoreactorsrevealinggreatoptimizationpotentials, Chem.Eng.Technol.39(1)(2016)1–13.
[31]Y.Su,K.Kuijpers,V.Hessel,T.Noel,Aconvenientnumbering-upstrategyfor thescale-upofgas–liquidphotoredoxcatalysisinflow,React.Chem.Eng. (2016),doi:http://dx.doi.org/10.1039/c5re00021a.
[32]K.-H.Pfoertner,T.Oppenlander,Photochemistry,Ullmann’sEncyclopediaof IndustrialChemistry,Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim, Germany,2000.
[33]A.M.Braun,M.-T.Maurette,E.Oliveros,TechnologiePhotochimique,Presses PolytechniquesRomandes,1986.
[34]A.M.Braun,G.H.Peschl,E.Oliveros,Industrialphotochemistry,in:A. Griesbeck,M.Oelgemöller,F.Ghetti(Eds.),CRCHandbookofOrganic PhotochemistryandPhotobiology,CRCPress,2012,pp.1–19.
[35]M.Fischer,Industrialapplicationsofphotochemicalsyntheses,Angew.Chem. Int.Ed.Engl.17(1978)16–26.
[36]M.C.DeRosa,R.J.Crutchley,Photosensitizedsingletoxygenandits applications,Coord.Chem.Rev.233–234(2002)351–371.
[37]T.J.Turconi,F.Griolet,R.Guevel,G.Oddon,R.Villa,A.Geatti,M.Hvala,K. Rossen,R.Göller,A.Burgard,Semisyntheticartemisinin,thechemicalpathto industrialproduction,Org.ProcessRes.Dev.18(2014)417–422.
[38]W.-K.Jo,R.J.Tayade,Newgenerationenergy-efficientlightsourcefor photocatalysis:LEDsforenvironmentalapplications,Ind.Eng.Chem.Res.53 (2014)2073–2084.
[39]K.Terao,Y.Nishiyama,S.Aida,H.Tanimoto,T.Morimoto,K.Kakiuchi, Diastereodifferentiating[2+2]photocycloadditionofchiralcyclohexenone carboxylateswithcyclopentenebyamicroreactor,J.Photochem.Photobiol.A: Chem.242(2012)13–19.
[40]O.Shvydkiv,S.Gallagher,K.Nolan,M.Oelgemöller,Fromconventionalto micro-photochemistry:photodecarboxylationreactionsinvolving phthalimides,Org.Lett.12(2010)5170–5173.
[41]M.Nettekoven,B.Püllmann,R.E.Martin,D.Wechsler,Evaluationofa flow-photochemistryplatformforthesynthesisofcompactmodules,Tetrahedron Lett.53(2012)1363–1366.
[42]S.A.M.W.vandenBroek,R.Becker,K.Koch,P.J.Nieuwland,Microreactor technology:real-timeflowmeasurementsinorganicsynthesis, Micromachines3(2012)244–254.
[43]A.Vasudevan,C.Villamil,J.Trumball,J.Olson,D.Sutherland,J.Pan,S.Djuric, LOPHTOR:aconvenientflow-basedphotochemicalreactor,TetrahedronLett. 51(2010)4007–4009.
[44]B.G.Anderson,W.E.Bauta,W.R.Cantrell,Developmentofanimprovedprocess fordoxercalciferolviaacontinuousphotochemicalreaction,Org.ProcessRes. Dev.16(2012)967–975.
[45]T.Horie,M.Sumino,T.Tanaka,Y.Matsushita,T.Ichimura,J.-I.Yoshida, Photodimerizationofmaleicanhydrideinamicroreactorwithoutclogging, Org.ProcessRes.Dev.14(2010)405–410.
[46]S.Josland,S.Mumtaz,M.Oelgemöller,Photodecarboxylationsinanadvanced meso-scalecontinuousflowphotoreactor,Chem.Eng.Technol.39(1)(2016) 81–87.
[47]S.Elgue,T.Aillet,K.Loubière,A.-L.Conté,O.Dechy-Cabaret,L.Prat,R. Chemens,O.Horn,S.Vallon,Flowphotochemistry:ameso-scalereactorfor industrialapplications,Chem.Todays33(5)(2015)42–44.
[48]M.Oelgemöller,Highlightsofphotochemicalreactionsinmicroflowreactors, Chem.Eng.Technol.35(7)(2012)1144–1152.
[49]O.Shvydkiv,A.Yavorskyy,K.Nolan,A.Youssef,E.Riguet,N.Hoffmann,M. Oelgemöller,Photosensitizedadditionofisopropanoltofuranonesina365nm UV-LEDmicrochip,Photochem.Photobiol.Sci.9(2010)1601–1603. [50]A.Yavorskyy,O.Shvydkiv,N.Hoffmann,K.Nolan,M.Oelgemöller,Parallel
microflowphotochemistry:processoptimizationscale-upandlibrary synthesis,Org.Lett.14(2012)4342.
[51]O.Shvydkiv,C.Limburg,K.Nolan,M.Oelgemöller,Synthesisofjuglone (5-hydroxy-1,4-naphthoquinone)inafallingfilmmicroreactor,J.FlowChem.2 (2012)52–55.
[52]S.A.M.W.vandenBroek,R.Becker,K.Koch,P.J.Nieuwland,Microreactor technology:real-timeflowmeasurementsinorganicsynthesis, Micromachines3(2012)244–254.
[53]T.Aillet,K.Loubière,O.Dechy-Cabaret,L.Prat,Photochemicalsynthesisofa cagecompoundinamicroreactor.rigorouscomparisonwithabatch photoreactor,Chem.Eng.Process.ProcessIntensif.64(2013)38–47. [54]T.Aillet,K.Loubière,O.Dechy-Cabaret,L.Prat,Impactofthediffusion
limitationsinmicrophotoreactors,AIChEJ.61(4)(2015)1284–1299. [55]T.Aillet,K.Loubière,O.Dechy-Cabaret,L.Prat,Measurementofthephotonic
fluxreceivedinsideamicrophotoreactorbyactinometry,Int.J.Chem.React. Eng.12(1)(2014)1–13.
[56]T.Aillet,K.Loubière,O.Dechy-Cabaret,L.Prat,Microreactorsasatoolfor acquiringkineticsdataonphotochemicalreactions,Chem.Eng.Technol.39(1) (2016)115–122.
[57]W.A.Fiveland,Discrete-ordinatessolutionsoftheradiativetransportequation forrectangularenclosures,J.HeatTransfer.106(1984)699–706.
[58]A.Peraiah,AnIntroductiontoRadiativeTransfer:MethodsandApplicationsin Astrophysics,CambridgeUniversityPress,NewYork,2001.
[59]A.E.Cassano,C.A.Martin,R.J.Brandi,O.M.Alfano,Photoreactoranalysisand design:fundamentalsandapplications,Ind.Eng.Chem.Res.34(1995)2155– 2201.
[60]A.Brucato,A.Cassano,F.Grisafi,G.Montante,L.Rizzuti,G.Vella,Estimating radiantfieldsinflatheterogeneousphotoreactorsbythesix-fluxmodel,AIChE J.52(2006)3882–3890.
[61] G.LiPuma,A.Brucato,Dimensionlessanalysisofslurryphotocatalyticreactors usingatwo-fluxandsix-fluxradiationabsorption-scatteringmodels,Catal. Today122(2007)78–90.
[62]J.-F.Cornet,C.-G.Dussap,Asimpleandreliableformulaforassessmentof maximumvolumetricproductivitiesinphotobioreactors,Biotechnol.Prog.25 (2009)424–435.
[63]P.J.Valades-Pelayo,J.Moreira,B.Serrano,H.deLasa,Boundaryconditionsand phasefunctionsinaPhoto-CRECWater-IIreactorradiationfield,Chem.Eng. Sci.107(2014)123–136.
[64]Y.Boyjoo,M.An,V.Pareek,CFDsimulationofapilotscaleslurryphotocatalytic reactor anddesignofmultiple-lampreactors, Chem. Eng.Sci.111(2014)266–277. [65]A.E.Cassano,P.L.Silveston,J.M.Smith,Photochemicalreactionengineering,
Ind.Eng.Chem.59(1)(1967)18–38.
[66]J.C.Andre,M.L.Viriot,A.Saïd,IndustrialphotochemistryXI:comparison betweendifferenttypesofphotoreactorsandselectivefilteringfor monomolecularphotoreactions,J.Photochem.Photobiol.Chem.42(2–3) (1988)383–396.
[67]A.Bouchy,J.C.Andre,E.George,M.L.Viriot,IndustrialphotochemistryXIII: determinationofthemostsuitableirradiationconditionsformolecular photoreactions,J.Photochem.Photobiol.Chem.48(2–3)(1989)447–463. [68]N.Midoux,C.Roizard,J.C.Andre,IndustrialphotochemistryXVII:macroscopic
transporteffectsontheperformanceofphotochemicalreactors,J.Photochem. Photobiol.Chem.58(1)(1991)71–97.
[69]M.Mohajerani,M.Mehrvar,F.Ein-Mozaffari,PhotoreactordesignandCFD modellingofaUV/H2O2processfordistillerywastewatertreatment,Can.J.
Chem.Eng.90(3)(2012)719–729.
[70]M.Mohajerani,M.Mehrvar,F.Ein-Mozaffari,CFDModelingofmetronidazole degradationinwaterbytheUV/H2O2processinsingleandmultilamp
photoreactors,Ind.Eng.Chem.Res.49(11)(2010)5367–5382.
[71] S.Elyasi,F.Taghipour,PerformanceevaluationofUVreactorusingoptical diagnostictechniques,AIChEJ.57(1)(2011)208–217.
[73] J.-F.Cornet,Calculationofoptimaldesignandidealproductivitiesof volumetricallylightenedphotobioreactorsusingtheconstructalapproach, Chem.Eng.Sci.65(2)(2010)985–998.
[74]T.Aillet,K.Loubière,O.Dechy-Cabaret,L.Prat,Impactofphotonandmass transferonphotochemicalreactionoutputinsmall-scaleflowsystems,13th InternationalConferenceonMicroreactionTechnology(IMRET13),Budapest, June2014,2014.

![Fig. 5. Predictions of the performances obtained: (a) synthesis of a pentacyclic cage compound [47,53], (b) conversion versus irradiation time in a capillary tower microreactor and in an immersion well reactor (dotted lines: predicted values by Eq](https://thumb-eu.123doks.com/thumbv2/123doknet/13661866.429649/10.892.93.819.119.368/predictions-performances-synthesis-pentacyclic-conversion-irradiation-capillary-microreactor.webp)
![Fig. 6. Acquisition of kinetic data: (a) reversible reaction between the closed and the open forms of TMINBPS [56], (b) spiral-shaped microreactor irradiated by a LED array.](https://thumb-eu.123doks.com/thumbv2/123doknet/13661866.429649/11.892.117.763.836.1128/acquisition-kinetic-reversible-reaction-closed-tminbps-microreactor-irradiated.webp)
