HAL Id: hal-01228553
https://hal.archives-ouvertes.fr/hal-01228553
Submitted on 17 Nov 2015
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
To cite this version:
Safia Benkoula, Olivier Sublemontier, Minna Patanen, Christophe Nicolas, Fausto Sirotti, et al..
Water adsorption on TiO2 surfaces probed by soft X-ray spectroscopies: bulk materials vs. isolated
nanoparticles. Scientific Reports, Nature Publishing Group, 2015, 5, pp.15088. �10.1038/srep15088�.
�hal-01228553�
Water adsorption on TiO
2
surfaces probed by soft X-ray
spectroscopies: bulk materials vs.
isolated nanoparticles
Safia Benkoula1, Olivier Sublemontier2, Minna Patanen1, Christophe Nicolas1,
Fausto Sirotti1, Ahmed Naitabdi3, François Gaie-Levrel4, Egill Antonsson1, Damien Aureau5,
François-Xavier Ouf6, Shin-Ichi Wada7, Arnaud Etcheberry5, Kiyoshi Ueda8 & Catalin Miron1,9
We describe an experimental method to probe the adsorption of water at the surface of isolated, substrate-free TiO2 nanoparticles (NPs) based on soft X-ray spectroscopy in the gas phase using
synchrotron radiation. To understand the interfacial properties between water and TiO2 surface,
a water shell was adsorbed at the surface of TiO2 NPs. We used two different ways to control the
hydration level of the NPs: in the first scheme, initially solvated NPs were dried and in the second one, dry NPs generated thanks to a commercial aerosol generator were exposed to water vapor. XPS was used to identify the signature of the water layer shell on the surface of the free TiO2 NPs
and made it possible to follow the evolution of their hydration state. The results obtained allow the establishment of a qualitative determination of isolated NPs’ surface states, as well as to unravel water adsorption mechanisms. This method appears to be a unique approach to investigate the interface between an isolated nano-object and a solvent over-layer, paving the way towards new investigation methods in heterogeneous catalysis on nanomaterials.
Titanium dioxide (TiO2) is undoubtedly one of the most studied materials owing to its technological
relevance to various fields, such as photonics, electronic devices, self-cleaning materials and photocataly-sis1–4. Considerable research effort has been devoted to the understanding of the link between the surface
properties of TiO2 and water adsorption mechanisms on its surface5–8. These mechanisms are known to
be essential in photocatalytic processes, affecting for instance charge recombination rates9,10. The water
- TiO2 interface is thus of crucial importance, and the proper control of the surface properties of TiO2
appears to be even more crucial, as soon as we reach the nanometer scale where the surface-to-bulk ratio is considerably larger than in the infinite solid. Several studies, based on techniques such as high res-olution scanning tunneling microscopy (HRSTM)7,11,12 or X-ray Photoelectron Spectroscopy (XPS)13,14, 1Synchrotron SOLEIL, L’Orme des Merisiers, Saint-Aubin, BP 48, 91192 Gif-sur-Yvette Cedex, France. 2CEA/IRAMIS/
NIMBE/Laboratoire Edifices Nanométriques, CEA Saclay, 91191 Gif-sur-Yvette, France. 3Sorbonne Université
UPMC, Univ Paris 6, UMR 7614, Laboratoire de Chimie Physique Matière et Rayonnement, 11 rue Pierre et Marie Curie, 75005 Paris, France. 4Laboratoire national de métrologie et d’essais, département “métrologie des gaz et
des aérosols”, 1 rue Gaston Boissier, 75724 Paris Cedex 15. 5Institut Lavoisier de Versailles, Université Versailles-St
Quentin, UMR CNRS 8180, 78035 Versailles, France. 6Institut de Radioprotection et de Sûreté Nucléaire (IRSN),
PSN-RES, SCA, LPMA, 91192 Gif-Sur-Yvette, France. 7Graduate School of Science, Hiroshima University,
Higashi-Hiroshima 739-8526, Japan. 8IMRAM, Tohoku University, Sendai 980-8577, Japan. 9Extreme Light Infrastructure
- Nuclear Physics (ELI-NP), “Horia Hulubei” National Institute for Physics and Nuclear Engineering, 30 Reactorului Street, RO-077125 Măgurele, Jud. Ilfov, Romania. Correspondence and requests for materials should be addressed to O.S. (email: olivier.sublemontier@cea.fr) or C.M. (email: catalin.miron@synchrotron-soleil.fr)
Received: 30 January 2015 Accepted: 14 September 2015 Published: 14 October 2015
have been reported in the literature and aimed at understanding and controlling TiO2 nanoparticles
(NPs)’ surface properties. However, despite the wide interest devoted to this subject, the photocatalytic activity of TiO2 NPs and its correlation with water adsorption is still a matter of debate both
theoreti-cally and experimentally because of the complex interplay between the surface structure at the atomic scale and the nature of the adsorption mechanisms5,7,15–17. The degree of complexity is also enhanced by
the fact that the NPs are usually deposited on a substrate, resulting in sample modifications during the deposition process itself, interactions between the substrate and nanosystem under study, and sample charging effects. Identifying the key factors influencing the adsorption mechanisms and mastering the degree of hydration of TiO2 NPs are important challenges for photocatalysis. Here we address the water
adsorption problem on the surface of isolated TiO2 NPs using a novel experimental technique, which has
recently proved its efficiency in the characterization of isolated nano-objects18–20. Our approach consists
in using synchrotron radiation (SR) based soft X-ray electron spectroscopy to analyse the properties of a collimated beam of differently hydrated NPs generated and focused to the interaction region with the SR by an Aerodynamic Lens System (ADLS). This experimental approach offers the opportunity of avoiding any interaction between the sample and a substrate, thus giving access to the sole, intrinsic information about the NP surface. Several questions have been addressed to evaluate the feasibility of controlling the hydration state of freestanding TiO2 NPs and to achieve insight into the factors which can influence the
water adsorption mechanisms on isolated TiO2 NPs in the gas phase.
Results
A variety of studies was preliminary conducted in view of the structural characterization of the com-mercial TiO2 nanopowder. The TEM images obtained for the TiO2 nanoparticles reveal an important
size dispersion of the nanometer grains (Fig. 1), ranging from 20 nm to 120 nm. The sample from Sigma Aldrich, made of a mixture of the two prevalent crystalline phases of TiO2, namely rutile and
ana-tase (Fig. 1a) shows clearly the presence of two distinguishable morphotypes on the grain structure – a faceted and a cluster-like structure – which can be attributed to the two different crystalline phases present in the sample. This assumption is supported by the absence of such an inhomogeneity on the micrograph performed on a commercial sample of pure anatase TiO2 nanopowder (MK Impex Corp.)
(Fig. 1b). A systematic TEM study of different samples additionally shows that the amount of anatase and rutile phases present in the mixture is not equivalent. The X-ray diffraction (XRD) patterns of all samples (not shown here) evidence a predominant anatase phase in the mixture with large anatase (101) and (200) peaks, along with a weak rutile (110) peak. Using Spurr and Myers formula21, the fraction
of anatase in the commercial nanopowder was thus evaluated to be 0.8. Some DFT calculations and experimental studies have shown that the water sorption mechanisms are dependent on the crystalline structure22, as well as on the orientation of nanocrystals5,23,24. However, as our gas-phase experiment
results to an averaging of the contributions from all crystal orientations, the difference between anatase and rutile becomes meaningless. The mixture sample of 100 nm was thus chosen to be close to a “real-istic” sample commonly used in applications. Even if the anatase-rutile NPs mixture of 25 nm (P25) is the most used in commercial photocatalysis systems, it is poorly focused with our ADLS. Consequently, the choice of the average size of nanopowder sample (100 nm) was mainly guided by our aerodynamic focusing efficiency requirements.
Soft x-ray photoelectron spectroscopy was initially performed on dry TiO2 NPs sprayed out by the
nanopowder aerosoliser and then hydrated thanks to the setup described in the Methods section. In order to ensure the dryness of the particles prior to aerosolisation, an annealing process has been performed, similarly to the protocols used in bulk surface science. For annealing, the nanopowder has been kept
Figure 1. TEM images of commercial TiO2 nanopowder mixture of rutile and anatase from Sigma Aldrich
in a vacuum oven at 150 °C under N2 atmosphere during 24 h. The temperature has been chosen
delib-erately low in order to avoid any crystalline phase transition, according to previous observations25,26.
The effect of annealing on the O 1s XPS spectra can be seen in Supplementary Figure S1 online. The annealed nanopowder was then transferred in the aerosoliser chamber before being sprayed through the ADLS. In these conditions, the exposure of the nanopowder to the ambient moisture is strictly limited; however a brief exposure during the transfer to the aerosoliser chamber remains possible. Figure 2 dis-plays O 1s XPS core-level spectra obtained for annealed nanopowder before hydration (a) and during hydration (b) by water evaporation as described in the Methods section. The incident photon energy used to record the O 1s spectra was 630 eV, leading to an inelastic mean free path of about 0.6 nm in TiO2, as determined by using Seah’s equation for inorganic compounds27. The experimental resolution
(originating from the convolution of the monochromator bandwidth and of the electron spectrometer resolution) was about 960 meV, resulting in a total FWHM in the range 1.2–1.5 eV for each compo-nent (see Supplementary Table S2 online). It is worth stressing that NPs samples present a non-cleaned, non-oriented surfaces which are all measured at the same time, and each of the spectral lines represents an ensemble of atomic arrangements in the NPs with slightly different chemical environments (leading to slightly different binding energies). Also, on top of the instrumental and lifetime broadenings, linew-idths are affected by phonon and final state vibrational broadenings (FSVB). In case of absorbed species the FSVB can lead to broader photolines than their gas phase counterparts due to the formation of new vibrational modes28,29. For example several OH-vibrational modes having frequencies up to 0.5 eV have
been reported on hydroxylated TiO2 surfaces30.
A systematic energy calibration has been performed, by recording the Ar 2p photoemission lines in the gas phase. A Voigt profile was used to fit the data, and the background was assumed to have a Shirley-type shape31,32. In order to achieve a reasonable fit of the broad structure originating from O 1s
photoemission, four symmetric peaks with their experimental linewidths as fixed parameter were used. For information, the error bars representing the standard deviation have been reported for the individual fitting components. The relative energy positions of the components have also been kept constant (within the error bars), except for the higher binding energy (BE) peak whose position is shifted as discussed below.
Hence, after Shirley background subtraction and deconvolution, the spectrum gives rise to four com-ponents as shown in Fig. 2. The positions of each component used to fit the spectra can be found in Supplementary Table S3 online. The main peak at 533.8 ± 0.2 eV BE is interpreted as originating from bulk oxygen atoms in the TiO2 NPs lattice, and the component at 535.6 ± 0.2 eV BE is linked to the
adsorption of water on the TiO2 surface, mainly as hydroxylated chemisorbed species, which is in
agree-ment with several previous bulk studies14,25,31,33,34 and confirmed by the fact that before hydration, this
component is substantially reduced (Fig. 2a) and increases as a function of the water temperature during hydration (Fig. 2b). Let us point out that even without hydration, a small residual component attributed to the position of OH species still remains in the spectrum (a). This can be linked to the spectroscopic signature of the two-fold coordinated O-bridging, which has been already evidenced by Bullock et al. and other groups32,35. However, a small hydration due to ambient moisture is not totally excluded to explain
the presence of this peak.
Two different OH groups have been distinguished in the literature of hydrated TiO2 surfaces32,35,36:
OH-groups bond to 5-coordinated Ti4+ cations forming Ti-OH basic groups, and acidic OH-groups
linked to the bridging oxygens, here called ObrH. Sham and Lazarus reported already in 1979 a study
of hydrated rutile (001) surface, where they observed these chemisorbed acidic and basic groups as well as physisorbed components in the O 1s XPS36. Evaluating from the spectra they present, the BE shifts
from the bulk O 1s component are approximately + 1.6 eV and + 2.6 eV for ObrH and Ti-OH
compo-nents, respectively. Perron et al. reported a binding energy shift of + 1.3 eV for O-bridging component, and + 2.5 eV for Ti-OH. Unfortunately, we cannot resolve these peaks, and only one broad component
Figure 2. Experimental fit performed on O 1s XPS spectra obtained for annealed TiO2 nanopowder before
(FWHM = 1.5 eV) is fitted with a binding energy shift of + 1.8 eV compared to the bulk O, representing non-hydrated O-bridging, ObrH, and Ti-OH.
To fit the experimental data, two more components have to be added: an intermediate peak at 537.4 ± 0.2 eV BE which results from the adsorption of molecular water in the upper layer, as previ-ously shown by Sham and Lazarus36, H. Perron et al.32 and other groups37, and a higher BE component
whose position relative to the bulk component seems to fluctuate. This peak can be related to oxygen from organic contaminants at the surface of TiO2 NPs resulting either from the annealing process38 or
from air exposure. The energy location of the carbon contamination signature is known to depend on the nature of the adsorbate and to vary for different organic contaminants as a function of the chemical partner of the carbon (e.g. oxygen or hydrogen)39. This chemical shift can thus be assigned to the
differ-ent neighboring of the organic adsorbdiffer-ent species before (a) and during (b) hydration. It is important to stress that the energy scale of all spectra is referenced to the vacuum level, due to the gas phase configu-ration. Consequently, a systematic shift varying between 3.5 eV and 4 eV of the whole spectrum is to be considered to compare with the data from the literature dealing with deposited NPs, which corresponds to the TiO2 work function.
Evaluations of the peak areas relative to the bulk component for different hydration levels are shown in Fig. 3. The relative humidity (RH) measured for each step of water-heating temperature is also reported. The curves reveal that the peak weight at 535.6 eV related to the OH species adsorbed is strongly depend-ent on the RH, whereas the H2O peak seems to stay constant whatever the hydration level is. It has been
shown that molecular physisorbed water is easily desorbed under ultrahigh vacuum conditions32,37, and
hence cannot be directly linked to the hydration level. The higher BE peak is also independent of the state of hydration which supports the assignment to oxygen atoms from organic contaminants, whose signa-ture is also confirmed by the slightly grey color observed on our nanopowder samples after annealing.
Figure 3. Area ratio of each component of the O 1s spectra relative to the bulk component after peak fitting. The first point corresponds to the spectrum recorded before hydration. Error bars are within the size
of markers.
Figure 4. O 1s XPS spectra of dry (red) and hydrated (blue) deposited TiO2 NPs, obtained with an Al
To validate this interpretation of the spectra, additional XPS measurements have been performed for chemical specification of our sample with a monochromatized Al Kα source on TiO2 NPs deposited on
a substrate, as described in the Methods section.
Figure 4 displays the O 1s XPS spectra obtained with the latter setup. Charge effects are compensated thanks to a flood gun correction and a systematic calibration by recording C1s position. Fitting has been performed with the commercial Thermo scientific Avantage software, using a Voigt line shape. The bulk oxygen peak arises at 530.2 eV BE in perfect agreement with the position observed by Hugenschmidt et
al.40. The BE shift of the overall spectrum with regards to Fig. 2 - corresponding to gas phase
configura-tion - enables to extract a work funcconfigura-tion equal to 3.6 eV.
As was the case for the isolated nanoparticles, a second component arises at 1.6 eV higher BE upon hydration (blue spectrum) which is fully consistent with the peak position attributed to OH chemisorbed species in Fig. 2, observed at + 1.8 eV from the bulk O peak. This peak was verified to be independ-ent from any C-contamination, by recording C 1s XPS spectra before and after hydration. It has to be stressed that due to a lower surface sensitivity in the latter conditions, the NPs were saturated with liquid water in order to get a signal from surface-adsorbed water, resulting in a higher coverage in this case. It might also be noted that no other component is observed at higher energy, contrary to the spectra shown in Fig. 2. This confirms the assignment of the peak at 537.4 eV BE in Fig. 2 to physisorbed molecular water in the upper layer, which tends to be more easily desorbed under the present higher vacuum con-ditions. It explains why this peak has been evidenced only at low temperature41 or by tilting the sample32.
Ti 2p XPS spectra have also been recorded on freestanding TiO2 NPs, before and after hydration of
previously annealed TiO2 NPs. The Ti 2p core-level spectra (Fig. 5) do not reveal any obvious signature
of water adsorption. However, some studies report the presence of a shoulder at the lower binding energy side of the Ti 2p doublet31,33,42,43 attributed to reduced species (Ti3+ and Ti2+) in non-stoichiometric
defective TiO2 films. This asymmetry is observed to be quenched after subsequent water exposure33,43,
resulting in a “healing” of surface defects as shown by Wang et al.33. The absence of such an asymmetry
in our situation and the high similarity between the two spectra in Fig. 5 reveals that these Ti 3d states are absent or below the detection threshold in our NPs and suggests that they do not take part in the adsorption path. Thus, we can conclude that the Ti-OH are not most likely dominating species on the
Figure 5. Ti 2p XPS spectra obtained on annealed NPs without hydration (red spectrum) and during hydration with water evaporation at 68 °C (blue spectrum) using synchrotron radiation. The incident
photon energy was 560 eV.
Figure 6. (a) Valence spectra obtained with a suspension of solvated NPs sprayed out with an atomizer
(blue) and on “as-received” dry NPs sprayed out with an aerosoliser (green) at 100 eV photon energy. (b) Comparison of the edge of the valence band (4–12 eV) for “as-received” dry NPs (green) and hydrated NPs (blue). The spectra have been normalized relative to the top of the valence band.
surface of NPs and in the O 1s spectra the strong increase of OH-group contribution is mostly due to the ObrH-groups.
However, a clear evidence of water adsorption can be seen in the valence spectra. Indeed, Fig. 6a depicts the valence spectra obtained on solvated TiO2 NPs sprayed out by atomization (blue spectrum)
and on an “as-received” nanopowder sprayed out with the commercial nanoaerosoliser (green spectrum). The peak at 15.9 eV BE corresponds to Ar 3p valence states, and has been deliberately kept for calibration purposes. The spectrum corresponding to solvated TiO2 NPs gives rise to three molecular states of water
in the valence band region, labelled as 1b1, 3a1 and 1b2 – as previously described by M.A. Henderson44
– which are absent or strongly attenuated in the spectrum of dry NPs. Water peak assignment was based on Kimura’s et al.45 valence states study. The presence of molecular water states in the valence band
region (blue spectrum) is assigned to the atomization procedure of NPs in water suspension. However, it is difficult to distinguish the signal of adsorbed surface water from gas phase water also present in the interaction region, because the binding energy shift between them is very small in the valence band region. To better illustrate water adsorption in the valence band region, the spectra have been normalized relative to the top of the valence band (Fig. 6b) and energy calibration was achieved using the Ar 3p photoline. A difference between dry and solvated NPs cases is evidenced: the band bending of the valence edge accompanying the hydrated state and the shift towards higher BE is in full agreement with the observation made by Kurtz et al.46 on bulk hydrated TiO
2 surfaces. The same behaviour was highlighted
with DFT calculations47 and was attributed to a solvation of TiO
2 surface states due to water adsorption.
Discussion
Considerable effort has been dedicated to unravel the reactivity of water on TiO2 surfaces. However, the
literature suffers from insufficient information regarding the nanoparticles’ case. Even on the well-known bulk situation, several factors have been shown to influence the water sorption scheme. Some stud-ies have argued for a coverage-dependency on the adsorption mechanism, with a two-step process in which a low coverage dissociative adsorption is followed by a molecular adsorption at higher cover-age16,43. Another point of view defends influence of the sample temperature on the adsorption scheme46.
However, a large consensus seems to be achieved for a molecular adsorption on defect-free surfaces, whereas dissociation occurs only on defective surfaces24 especially at O-vacancies (O
vac)8,11,25. Walle
et al.41 have recently shown that dissociation can also take place on vacancy-free surfaces, resulting in a
mixed dissociative and molecular water adsorption qualified as a “pseudodissociated state” at monolayer coverage, where the temperature of the substrate plays a crucial role in the experimental observation of such a mixed state.
SR-based XPS of a rutile (110) single crystal was recorded to follow the hydration signature of a bulk solid in ultra-high vacuum conditions for comparison with the isolated NPs experiment using the same photon energy (Fig. 7a).
In good agreement with the experiments of Walle et al.41 these results show that (1) no trace of molec-ular water can be visible at room temperature (RT) whereas it is clearly evidenced at −193 °C through the structure around 534 eV, (2) a small shoulder appears at 532.2 eV at both temperatures, and seems to be related to OH species chemisorbed at Ovac. Contrary to Walle’s experiment, the present study is made on
a defective surface with Ovac – as observed with the STM characterization performed on the rutile (110)
monocrystal (not shown here) – supporting the view that in this case, OH species are preferentially adsorbed at Ovac sites.
For comparison, the data obtained with our isolated nanoparticles (mixture of rutile/anatase) are shown in Fig. 7b on the annealed nanopowder progressively hydrated. As previously discussed, the spec-trum corresponding to dry-annealed NPs, as well as the spectra associated to a controlled-hydration, display four components with a clear signature of OH and H2O – as supported by the fit – the whole
hydration procedure being made at RT. Moreover, still in contradiction with the bulk situation, the hydration is accompanied by a strong increase of the OH component - with a weak effect on the H2O
Figure 7. O 1s XPS spectra recorded on bulk rutile (110) monocrystal at the TEMPO beamline (a) and on
peak - giving rise to the second peak at 535.6 eV, which corresponds to the small shoulder observed on the (110) surface (Fig. 7a).
In a classical paper by Sham and Lazarus36, chemisorbed and physisorbed water on a freshly
intro-duced ambient sample of TiO2 (001) surface were observed. When the sample was allowed to stay
in UHV vacuum conditions for a week, the high energy side of the O 1s spectrum was substantially decreased, indicating that these features corresponded to physisorbed water. A similar effect can be observed in our spectrum of the (110) surface (Fig. 7a) evidencing the presence of physisorbed water: when the temperature is decreased, the residual moisture of the experimental chamber condenses on the TiO2 surface and the physisorbed molecular water peak becomes evident. Additionally, in their study
Sham and Lazarus mechanically scraped the surface before exposing it to water, thus creating a lot of surface defects. To record the O 1s XPS they used Mg Kα radiation, resulting to higher kinetic energies and thus longer escape depth of the photoelectrons, and in order to be more sensitive to the surface, the sample was tilted which clearly enhanced the intensity of the hydroxylated peaks. The effect is very similar to what can be seen in the evolving hydration process of NPs (Fig. 7b). However, compared to our results obtained on a rutile (110) monocrystal and to the (001) surface studied by Sham and Lazarus, the NPs data show stronger increase of the OH-species.
The first explanation for this difference in intensity of the OH component can be attributed to the cov-erage, which is higher in the case of isolated TiO2 NPs than with a well-controlled surface, the hydration
process being hardly quantifiable in our gas phase environment. This coverage dependency is moreover confirmed by the presence of a molecular H2O peak in Fig. 7b, which can be observed only at low
temperature on the experimental data of the (110) surface (Fig. 7a) or on Walle’s study41. Decreasing
the temperature might result in adsorption of residual water on the surface of TiO2 and prevent from
complete desorption of adsorbed species as shown in other studies24,37,41. This supports the idea that at
higher coverage the adsorption can also occur molecularly on the OH sublayer acting as “anchor sites” for H2O molecules, as reported by Yamamoto et al.43 for the TiO2 (110) surface. Indeed, the presence
of the molecular H2O component was also experimentally evidenced by H. Perron and co-workers32 on
a (110) rutile surface at RT where the hydration process was performed during 24 h, resulting in a pre-sumed higher hydration amount than in Fig. 7a, and more comparable with our isolated NPs hydration conditions.
Let us point out that the relative coverage is moreover maximized at the nanometer scale, where the surface-to-bulk ratio is larger, resulting in an exaltation of the water-peaks, which become almost com-parable to the bulk component as the water layer thickness increases.
A clear indication for the occurrence of a dissociative adsorption mechanism is hence visible through our NP spectra via the appearance of a RH-dependent OH component, and this is consistent with the fact that our commercial nanopowder sample might contain Ovac at the NPs’ surface, especially after
the low-temperature annealing process carried out to dehydrate the nanopowder. The presence of induced-surface defects is confirmed by the appearance of a shoulder in the valence gap region for the annealed nanopowder samples (Fig. 8), which was absent from the non-annealed sample.
These valence gap states are usually attributed either to Ovac or Ti interstitials37. However Yim et al.48
have shown that these states are mainly resulting from Ovac rather than Ti interstitials, which is
consist-ent with the fact that no Ti3+ component was observed in our Ti 2p core-level spectrum. A DFT study
has also attributed this peak at + 1 eV above the VB of TiO2 to a “poorly solvated” configuration of OH
species49.
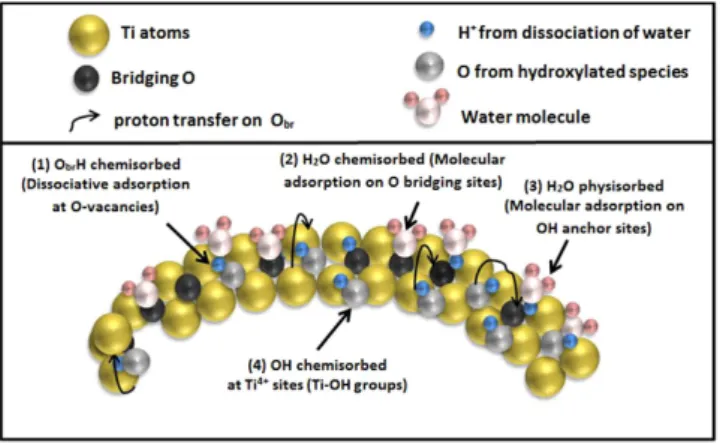
Based on this XPS study, Fig. 9 summarizes the main adsorption schemes which can occur at the surface of a TiO2 NPs and evidenced through XPS spectra measurements. The dissociative mechanism
(1) at O-vacancies (grey O atoms) have been shown to be dominating, resulting in a highly hydroxylated surface. A proton transfer on a neighbouring O-site can subsequently occur (black O atoms), resulting in a OH weight which is twice the density of defects, as already shown11,46. Related to this “chemisorption
path” a molecular H2O adsorption which could contribute to the peak arising under hydration (Fig. 7b)
is not totally excluded (2). Increasing the coverage, the H2O physisorption takes place in the upper layers
(3), which is enhanced by the presence of underlying OH sites. Another dissociative mechanism (4) via 5-coordinated Ti atoms adds also the amount of ObrH species when the proton is transferred from Ti
to the neighboring O-bridging atom (schematically represented by black arrows). This pathway is con-cluded to be a smaller contribution compared to the mechanism (1), based on the fact that the Ti 2p XPS spectra remained unchanged during the hydration process. Also the fitted OH-related peak in the hydrated nanoparticle XPS shows a BE shift closer to the ObrH value from literature, but one has to be
careful when comparing the importance of these methods based on O 1s XPS since both mechanisms (1) and (4) contribute to the ObrH species.
For the first time, we have experimentally demonstrated the presence of water at the surface of freestanding TiO2 NPs. To achieve this, we used aerodynamic focusing and SR-based X-ray
photoelec-tron spectroscopy. More importantly, we have shown that it is possible to control the amount of water adsorbed at the NPs’ surface and to unravel the adsorption mechanism. A comparison with the bulk case showed that the water signature is exalted in the O 1s XPS spectra through the OH component, attributed to a higher coverage and the higher surface-to-bulk ratio, enhancing the weight of the water components. This result is relevant and of high importance to the photocatalysis research, taking into account that the dissociation of water is favored at the surface of TiO2 NPs. The high surface sensitivity obtained in our
experimental conditions have proved to be crucial to disentangle the role of the surface state into the adsorption mechanism. Finally, consistent with previous studies on bulk TiO2 surfaces, we have observed
a clear evidence for a mixed dissociative and molecular adsorption mechanisms, explained by the high coverage obtained in our experimental hydration conditions for nanomaterials.
Methods
SR based soft X-ray electron spectroscopy measurements. The experiments were carried out at the French national SR laboratory SOLEIL (Saint-Aubin, France) at the ultrahigh resolution soft X-ray PLEIADES beamline (9–1000 eV), where soft X-rays with any kind of polarization can be generated using an Apple II – type permanent magnet HU80 (80 mm period) undulator starting from 60 eV. PLEIADES is dedicated to the spectroscopic studies of isolated species ranging from atoms50, ions51 and molecules52–55
to proteins56,57, clusters58 and nanoparticles20,59. The photoelectron spectra were recorded with a
com-mercial VG-Scienta R4000 spectrometer based on a hemispherical electron analyzer whose detection axis is perpendicular to the propagation direction of the SR. The pass energy and entrance slit are selected according to the experimental resolution targeted for each measurement and the polarization vector of the linearly polarized SR was chosen to be parallel to the electron detection axis.
The basic idea of the experiment is to create a collimated beam of isolated TiO2 NPs in interaction
with SR under high vacuum conditions. Briefly, a flow of nanoparticles is sprayed out in the aerosol phase using a carrier gas (usually Ar or N2) and the resulting solid aerosol is focused thanks to an ADLS and
injected through a 2 mm skimmer into the high vacuum chamber of the photoelectron spectrometer setup where the pressure is kept around 2 × 10−6 mbar during the experiment. The principle of the ADLS
was widely described by Zhang et al.60 or McMurry et al.61–63 and its relevance to the study of isolated
nanospecies has already been demonstrated at the PLEIADES beamline20,59,64. Complementary
meas-urements of single crystal TiO2 have been performed at the TEMPO beamline at the French national
SR facility SOLEIL (A. Naitabdi et al.). TEMPO is dedicated to the spectroscopic studies of condensed matter in the soft X-ray region (50–1500 eV), supplying a good complementarity to the gas-phase meas-urements performed at PLEIADES. The comparison is discussed in section “Discussion”.
Figure 9. Schematic diagram of the proposed adsorption mechanisms at the surface of a TiO2
nanoparticle. The four main mechanisms which can occur on a TiO2 nanoparticle surface are displayed on
injection in the ADLS. The moisture level was controlled by varying water temperature and the flow rate of N2. Water temperature was measured thanks to a type-K thermocouple and the humidity level was
measured in-situ with a commercial humidity sensor (Sensirion, kit EK-H5).
Classical XPS measurements. A better characterization of our nanopowder has been achieved by additional XPS measurements carried out at the French laboratory “Institut Lavoisier de Versailles”, on deposited TiO2 NPs, using the same sample which served for the SR studies. The spectra were collected
using an XPS apparatus (Thermo scientific) with a monochromatized Al Kα source, and the pressure in the analysis chamber was kept around 2 × 10−8 mbar.
References
1. Simon, P. et al. N-Doped Titanium Monoxide Nanoparticles with TiO Rock-Salt Structure, Low Energy Band Gap, and Visible Light Activity. Chem. Mater. 22, 3704–3711 (2010).
2. Scanlon, D. O. et al. Band alignment of rutile and anatase TiO2. Nature Mater. 12, 798–801 (2013).
3. Yola, M. L., Eren, T. & Atar, N. A novel efficient photocatalyst based on TiO2 nanoparticles involved boron enrichment waste for
photocatalytic degradation of atrazine. Chem. Eng. J. 250, 288–294 (2014).
4. Reddy, K. M., Manorama, S. V. & Reddy, A. R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys.
78, 239–245 (2003).
5. Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 48, 53–229 (2003).
6. Borodin, A. & Reichling, M. Characterizing TiO2(110) surface states by their work function. Phys. Chem. Chem. Phys. 13,
15442–15447 (2011).
7. Matthiesen, J. et al. Formation and Diffusion of Water Dimers on Rutile TiO2(110). Phys. Rev. Lett. 102, 226101 (2009).
8. Aschauer, U. et al. Influence of subsurface defects on the surface reactivity of TiO2: Water on anatase (101). J. Phys. Chem. C 114,
1278–1284 (2010).
9. Dimitrijevic, N. M. et al. Role of Water and Carbonates in Photocatalytic Transformation of CO2 to CH4 on Titania. J. Am. Chem.
Soc. 133, 3964–3971 (2011).
10. Tang, J., Durrant, J. R. & Klug, D. R. Mechanism of Photocatalytic Water Splitting in TiO2. Reaction of Water with Photoholes,
Importance of Charge Carrier Dynamics, and Evidence for Four-Hole Chemistry. J. Am. Chem. Soc. 130, 13885–13891 (2008). 11. Wendt, S. et al. Oxygen vacancies on TiO2(110) and their interaction with H2O and O2: A combined high-resolution STM and
DFT study. Surf. Sci. 598, 226–245 (2005).
12. Du, Y. et al. Water Interactions with Terminal Hydroxyls on TiO2 (110). J. Phys. Chem. C 114, 17080–17084 (2010).
13. Chen, X. & Burda, C. The electronic origin of the visible-light absorption properties of C-, N- and S-doped TiO2 nanomaterials.
J. Am. Chem. Soc. 130, 5018–5019 (2008).
14. Iwabuchi, A., Choo, C.-K. & Tanaka, K. Titania Nanoparticles Prepared with Pulsed Laser Ablation of Rutile Single Crystals in Water. J. Phys. B: At., Mol. Opt. Phys. 108, 10863–10871 (2004).
15. Wesolowski, D. J. et al. Comment on “structure and dynamics of liquid water on rutile TiO2(110)”. Phys. Rev. B 85, 167401 (2012).
16. Liu, L.-M., Zhang, C., Thornton, G. & Michaelides, A. Structure and dynamics of liquid water on rutile TiO2(110). Phys. Rev. B
82, 161415 (2010).
17. Bolis, V. et al. Hydrophilic/hydrophobic features of TiO2 nanoparticles as a function of crystal phase, surface area and coating,
in relation to their potential toxicity in peripheral nervous system. J. Colloid Interface Sci. 369, 28–39 (2012).
18. Mysak, E. R., Starr, D. E., Wilson, K. R. & Bluhm, H. Note: A Combined Aerodynamic lens/ambient Pressure x-ray Photoelectron Spectroscopy Experiment for the On-stream Investigation of Aerosol Surfaces. Rev. Sci. Instrum. 81, 016106 (2010).
19. Meinen, J. et al. Core level Photoionization on Free sub-10-nm Nanoparticles using Synchrotron Radiation. Rev. Sci. Instrum.
81, 085107 (2010).
20. Sublemontier, O. et al. X-ray Photoelectron Spectroscopy of Isolated Nanoparticles. J. Phys. Chem. Lett. 5, 3399–3403 (2014). 21. Spurr, R. A. & Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-Ray Diffractometer. Anal. Chem. 29,
760–762 (1957).
22. Kavathekar, R. S., English, N. J. & MacElroy, J. Spatial Distribution of Adsorbed Water Layers at the TiO2 Rutile and Anatase
Interfaces. Chem. Phys. Lett. 554, 102–106 (2012).
23. Vittadini, A., Selloni, A., Rotzinger, F. P. & Grätzel, M. Structure and Energetics of Water Adsorbed at TiO2 Anatase (101) and
(001) Surfaces. Phys. Rev. Lett. 81, 3–6 (1998).
24. Henderson, M. A. Structural Sensitivity in the Dissociation of Water on TiO2 Single-Crystal Surfaces. Langmuir 7463, 5093–5098
(1996).
25. Rath, C., Mohanty, P., Pandey, A. C. & Mishra, N. C. Oxygen Vacancy induced Structural Phase Transformation in TiO2
Nanoparticles. J. Phys. D: Appl. Phys. 42, 205101 (2009).
26. Yeh, S.-W. et al. Characteristics and Properties of a Novel in situ method of Synthesizing Mesoporous TiO2 Nanopowders by a
simple Coprecipitation Process without adding Surfactant. J. Alloys Compd. 613, 107–116 (2014).
27. Seah, M. & Dench, W. Quantitative Electron Spectroscopy of Surfaces: A standard Data Base for Electron Inelastic Mean Free Path in Solids. Surf. Interface Anal. 1, 2–11 (1979).
28. Mårtensson, N. & Nilsson, A. Core-Level line shapes of adsorbates: effects of electronic and vibrational excitations. J. Electron Spectrosc. Rel. Phen. 52, 1–46 (1990).
29. Bancroft, G. M. et al. Toward a comprehensive understanding of solid-state core-level XPS linewidths: Experimental and theoretical studies on the Si 2p and O 1s linewidths in silicates. Phys. Rev. B 80, 075405 (2009).
Phys. Lett. 68, 426–432 (1979).
37. Walle, L. E., Borg, A., Uvdal, P. & Sandell, A. Probing the influence from residual Ti interstitials on water adsorption on TiO2
(110). Phys. Rev. B 86, 205415 (2012).
38. Jouan, P., Peignon, C., Cardinaud, C. & Lempérière, G. Characterisation of TiN coatings and of the TiN/Si interface by X-ray photoelectron spectroscopy and Auger electron spectroscopy. Appl. Surf. Sci. 68, 595–603 (1993).
39. Benoit, R., Durand, Y., Narjoux, G. & Quintana, G. Spectra and data base for XPS, AES, UPS and ESCA, photoelectrons. Available at: http://www.lasurface.com/database/elementxps.php (Accessed: 8th December 2014).
40. Hugenschmidt, M. B., Gamble, L. & Campbell, C. T. The interaction of H2O with a TiO2(110) surface. Surf. Sci. 302, 329–340
(1994).
41. Walle, L. E., Borg, A., Uvdal, P. & Sandell, A. Experimental Evidence for Mixed Dissociative and Molecular Adsorption of Water on a Rutile TiO2(110) Surface Without Oxygen Vacancies. Phys. Rev. B 80, 235436 (2009).
42. Kollbek, K. et al. X-ray Spectroscopic Methods in the Studies of Nonstoichiometric TiO2-x Thin Films. Appl. Surf. Sci. 281,
100–104 (2013).
43. Yamamoto, S. et al. In-situ x-ray Photoelectron Spectroscopy Studies of Water on Metals and Oxides at Ambient Conditions. J. Phys.: Condens. Matter 20, 184025 (2007).
44. Henderson, M. A. The interaction of water with solid surfaces: fundamental aspects revisited. Surf. Sci. Rep. 46, 1–308 (2002). 45. Kimura, K., Katsumata, S., Achiba, Y., Yamazaki, T. & Iwata, S. Handbook of HeI photoelectron spectra of fundamental organic
molecules (Japan Scientific Societies Press, 1981).
46. Kurtz, R. L., Stockbauer, R., Madey, T. E., Roman, E. & Segovia, J. L. D. E. Synchrotron Radiation Studies of H2O Adsorption on
TiO2(110). Surf. Sci. 218, 178–200 (1989).
47. Patel, M., Mallia, G., Liborio, L. & Harrison, N. M. Water adsorption on rutile TiO2(110) for applications in solar hydrogen
production: A systematic hybrid-exchange density functional study. Phys. Rev. B 86, 045302 (2012).
48. Yim, C. M., Pang, C. L. & Thornton, G. Oxygen Vacancy Origin of the Surface Band-Gap State of TiO2(110). Phys. Rev. Lett.
104, 036806 (2010).
49. Cheng, H. & Selloni, A. Hydroxide ions at the water/anatase TiO2(101) interface: structure and electronic states from first
principles molecular dynamics. Langmuir 26, 11518–11525 (2010).
50. Söderström, J. et al. Angle-resolved electron spectroscopy of the resonant Auger decay in xenon with meV energy resolution. New J. Phys. 13, 073014 (2011).
51. Gharaibeh, M. F. et al. K-shell photoionization of singly ionized atomic nitrogen: experiment and theory. J. Phys. B: At., Mol. Opt. Phys. 44, 175208 (2011).
52. Lindblad, A. et al. Vibrational scattering anisotropy in O2 dynamics beyond the Born-Oppenheimer approximation. New J. Phys.
14, 113018 (2012).
53. Kimberg, V. et al. Single-molecule x-ray interferometry: Controlling coupled electron-nuclear quantum dynamics and imaging molecular potentials by ultrahigh-resolution resonant photoemission and ab initio calculations. Phys. Rev. X 3, 011017 (2013). 54. Travnikova, O. et al. On Routes to Ultrafast Dissociation of Polyatomic Molecules. J. Phys. Chem. Lett. 4, 2361–2366 (2013). 55. Miron, C. et al. Site-selective Photoemission from Delocalized Valence Shells induced by Molecular Rotation. Nat. Commun. 5,
3816 (2014).
56. Milosavljevic, A. R. et al. Gas-phase Protein Inner-Shell Spectroscopy by coupling an Ion trap with a Soft x-ray Beamline. J. Phys. Chem. Lett. 3, 1191–1196 (2012).
57. Milosavljevic, A. R. et al. K-Shell Excitation and Ionization of a Gas-Phase Protein: Interplay between Electronic Structure and Protein Folding. J. Phys. Chem. Lett. 6, 3132–3138 (2015).
58. Patanen, M., Nicolas, C., Liu, X.-J., Travnikova, O. & Miron, C. Structural characterization of small Xe clusters using their 5s correlation satellite electron spectrum. Phys. Chem. Chem. Phys. 15, 10112–10117 (2013).
59. Miron, C. & Patanen, M. Synchrotron-Radiation-Based Soft X-Ray Electron Spectroscopy Applied to Structural and Chemical Characterization of Isolated Species, from Molecules to Nanoparticles. Adv. Mater. 26, 7911–7916 (2014).
60. Zhang, X. et al. A Numerical Characterization of Particle Beam Collimation by an Aerodynamic Lens-Nozzle System: Part I. An Individual Lens or Nozzle. Aerosol Sci. Technol. 36, 617–631 (2002).
61. Wang, X., Kruis, F. E. & McMurry, P. H. Aerodynamic Focusing of Nanoparticles: I. Guidelines for Designing Aerodynamic Lenses for Nanoparticles. Aerosol Sci. Technol. 39, 611–623 (2005).
62. Wang, X., Gidwani, A., Girshick, S. L. & McMurry, P. H. Aerodynamic Focusing of Nanoparticles: II. Numerical Simulation of Particle Motion Through Aerodynamic Lenses. Aerosol Sci. Technol. 39, 624–636 (2005).
63. Wang, X. & McMurry, P. H. An experimental study of nanoparticle focusing with aerodynamic lenses. Int. J. Mass Spectrom. 258, 30–36 (2006).
64. Lindblad, A., Söderström, J., Nicolas, C., Robert, E. & Miron, C. A multi purpose source chamber at the PLEIADES beamline at SOLEIL for spectroscopic studies of isolated species: Cold molecules, clusters, and nanoparticles. Rev. Sci. Instrum. 84, 113105 (2013).
Acknowledgments
The experiments have been performed at the PLEIADES beamline at the SOLEIL Synchrotron, France (Proposal No. 20130466). We thank Dr L. Chiodo for discussion and theoretical assistance, E. Robert for technical assistance, and the SOLEIL staff for stable operation of the equipment and storage ring during the experiments. The aerosol generation and focusing instrumentation used for this work has been funded by the Agence Nationale de la Recherche (ANR) under Grand No. ANR-07-NANO-0031
Additional Information
Supplementary information accompanies this paper at http://www.nature.com/srep Competing financial interests: The authors declare no competing financial interests.
How to cite this article: Benkoula, S. et al. Water adsorption on TiO2 surfaces probed by soft X-ray
spectroscopies: bulk materials vs. isolated nanoparticles. Sci. Rep. 5, 15088; doi: 10.1038/srep15088 (2015).
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Com-mons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/