Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Research Paper (National Research Council of Canada. Division of Building
Research); no. DBR-RP-407, 1969-07-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=c8c18b26-681a-46c8-ae69-3e0e1b6a7bab
https://publications-cnrc.canada.ca/fra/voir/objet/?id=c8c18b26-681a-46c8-ae69-3e0e1b6a7bab
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001537
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Simultaneous moisture and heat transfer in porous systems with
particular reference to drying
SIMULTANEOUS MOISTURE AND HEAT
TRANSFER
IN POROUS SYSTEMS WITH
,,
AL$E%ED
PARTICULAR REFERENCE T O DRYING
T . 2 . H A R M A T H Y
Division of Building Research, National Research Co~mcil of Canada, Ottawa, Canada
Based on an examination of the liquid-vapor equilibria and of the mass and energy transfer processes in porous systems, a theory has been developed for simultaneous mass and heat transfer during the pendular state of such systems. A set of differential equations is presented which, when solved with the appropriate initial and boundary conditions, yields the complete moisture content, temperature, and pressure history of the system. By theoretically simulating various drying processes it is shown that during the falling rate period the evaporation-condensation mechanism is the governing mechanism of drying. The application of the theory to transient heat conduction in the presence of moisture and to transient vapor transfer through porous media is also illustrated.
R O M among the several theories so far suggested to explain migration of moisture in porous media, three have won general recognition: the diffusion theory, the capillary flow theory, and the "evaporation-condensation" theory.
The movement of moisture by diffusion was explicitly proposed as the principal flow mechanism by Lewis (1921), Tuttle (1925), Sherwood (1929, 1930, 1932), Sherwood and Comings (1933), Newman (1931), Childs (1936), Kamei (1937), and many other research workers.
The fundamentals of the capillary flow theory were laid down by Buckingham (1907), who introduced the concept of "capillary potential" and postulated the mechanism of "un- saturated capillary flow." The latest version of this theory is presented by the basic assumption that the moisture flux is proportional to the gradient of the chemical potential of the moisture (Edlefsen and Anderson, 1943). With this and other assumptions the fundamental equation of moisture migration turns out to be identical with, or similar to, Fick's second law with concentration-dependent diffusion coefficient (Miller and Miller, 1955; Remson and Randolph, 1962).
The evaporation-condensation theory assumes that migra- tion of moisture takes place entirely in the gaseous phase (in the pores). Gurr et al. (1952), Hutcheon (1958), and Kuzmak and Sereda (1957) proved that when the system is subjected to a temperature gradient this assumption is essen- tially correct, even at relatively high pore saturation. The evaporation-condensation mechanism was utilized by Henry (1939), Cassie et al. (1940), Walker (1961), and others in de- scribing the movement of moisture in beds of textile ma- terials.
There are three comprehensive theories of simultaneous moisture and heat transfer processes not directly related to the above described three basic theories: those advanced by Philip (1957a, b, 1958a, b), Krischer (1942), Lykov and Mykhaylov (1961), and Lebedev (1961).
Characteristics of Drying Processes
Early work by Keen (1914), Fisher (1923, 1927), Sherwood (1929, 1930, 1932), and Sherwood and Comings (1933) in- dicated that under constant environmental conditions the process of drying can be divided into a "constant rate" and one or two "falling rate" periods. When the initial moisture
content is high enough, a considerable amount of moisture leaves the porous medium at a very high, approximately constant rate, which is roughly equal to the rate of evaporation from a continuous water surface under identical environmental conditions. During this initial period the temperature of the system also remains constant (Figure 1, a) and, as a rule, equal to the wet-bulb temperature of the environment.
At a point before the end of the constant rate period the temperature begins to rise slowly, and a few, apparently "dry" patches show up on the surface of the system (taken here as of the form of an infinite slab). This point, C , in Figure 1, a, referred to as the "upper critical point" of drying, indicates the beginning of the breakdown of the "funicular state" and the unfolding of the "pendular state" of the system. [In the "funicular state" there are continuous threads of moisture in the pores; in the "pendular state" there are not. For further discussion see the work of Haines (1927, 1930)].
U p to this point the moisture distribution is fairly uniform throughout the system (Figure 1, b), because of the high mobility of moisture in the funicular state. With the break- down of the continuous moisture threads, however, the mobility decreases sharply. The upper critical point thus also marks the inception of a gradual changeover to the migration of moisture by the evaporation-condensation mechanism.
The point at which the departure from the constant rate drying is first noticed in a plot of average moisture content us.
time is commonly referred to as "the critical point" of drying
(C*, Figure 1, a). That at which the last "wet" patches dis- appear from the surface (point
c,)
is called in this study the "lower critical point." Here the transition of the system from the funicular to the pendular state is essentially completed, and henceforth moisture movement takes place predominantly in the gaseous phase-i.e., by the evaporation-conclensationmechanism.
I n this study the interval I,,
<
t<
t , , is referred to as the "transient" period, and the region t>
t c l as the falling rate period of drying. Following point C l , the temperature of the system first rises very rapidly, then tends to level off at the so- called "pseudo-wet-bulb temperature" (Bell and Nissan, 1959; Nissan et al., 1959). Shortly before the end of the drying process it rises again at an increased rate to the level of final temperature.Reprinted from I&EC FUNDAMENTALS, Vol. 8, Page 92, February 1969
Copyright 1969 by the American Chemical Society and reprinted by permission of the copyright owner
t~ t~ t~ 0
L
T I M E S E C T I O N A C R O S S S L A B
Figure
1 .
Characteristic curves of a drying porous slaba. Average moisture content and temperature
b. Moisture distribution
T h e moisture distribution curves shown in Figure 1, 6 , are based on information published by Kamei (1937), Johnson (1961), and Corben and Newitt (1955).
Outline of Present Theory
T h e present theory is based on two fundamental assump- tions. T h e first is implied by the statement that it is an evaporation-condensation theory.
Assumption 1. All movement of moisture in a porous system takes place in the gaseous phase.
This assumption is not a n inherent feature of the theory, but because of the lack of knowledge concerning the mechanism of liquid-phase flow it is a t present a n unfortunate necessity. Even though moisture migration in the liquid phase is never completely extinct, there is ample evidence (Christensen, 1944; Osoba et a/., 1951 ; Philip, 1957a; Richards, 1931 ; Richardson, 1961; Richardson et al., 1952) that in the pendular state the mobility of moisture in the liquid phase is so small that in most practical problems it need not be considered.
Because of Assumption 1 the applicability of the present theory must be limited to the pendular state of porous systems in general, and to the falling rate period in connection with drying problems.
I n contrast with Assumption 1, Assumption 2 is concerned with the modeling of the texture of the porous system, and is therefore a n inherent feature of the theory. Before introducing Assumption 2, however, it is necessary to describe briefly the "constituents" of a "porous system." I n general, this system can be modeled as three phases, solid, "liquid" (adsorbed), and gaseous; and three components, the material of the solid,
water, and "air." (A component that appears in two phase is regarded as yielding two "constituents.")
I n this theory the solid phase is looked upon as a single- component material that exhibits isotropic characteristics in macroscopic dimensions. I t contains a network of inter- connected pores, the surfaces of which are the source of a force field. This field manifests itself in the depression of equilibrium vapor pressure of the liquid contained in the pores.
T h e liquid phase is also considered to be a single-component phase. I n the presence of the above-mentioned force field the physical and thermodynamic properties of this phase are more or less dependent on the amount in the pores-i.e., on the relative pore saturation.
T h e gaseous phase is regarded as an ideal binary mixture of water vapor and of fictitious air molecules.
Assumption
2.
T h e phases of a porous system are so finely distributed that from the macroscopic standpoint the system is a quasi-one-phase system. (It is not implied that the phases are distributed into macroscopically small units. I n fact, each phase may form a macroscopically large, continuous network.)An important limitation of the analogy between porous and truly one-phase systems must not be overlooked. I n a truly one-phase system the components are dispersed on a molecular scale, so that the mass and energy fluxes are associated with molecular transport phenomena (diffusion and conduction, respectively). I n a quasi-one-phase system, on the other hand, there are separate phases in any macroscopically small volume element. As the relative movement of the phases takes place by convection, the convective mass and energy transfers are primary transport phenomena. I n addition,
Table I. Expressions for Local Volume and Mass Concentrations of Phases and Constituents of the Porous System
Pliase Constituent Vol. Concn., Cc./Cc. Mass Concn., G./Cc.
Gaseous Vapor Air O (1
-
d'
~ ) c f ,Liquid Water e - c f , e - O
Solid Solid I - e 1 - 6
Total 1 1
conduction of heat occurs in all phases and mass transfer by diffusion in those that contain more than one component.
Assumption 2 implies three additional assumptions, the first two of which simply represent the extension to quasi-one-phase systems of two basic assumptions of nonequilibrium thermo- dynamics (Fitts, 1962). These "subassumptions" are as follows:
ASSUMPTION 2A. I t is possible t o assign local values to the mass concentrations of the constituents a n d to the specific internal energy-in other words, to specify 6,,(r, t), 6,,(r, t), 6L(r, t), 6,(r, t), and E ( r , t). These quantities are averages over microscopically large but macroscopically small periods of time and cells in space. Some expressions for the mass (and also volume) concentrations of the various phases and constituents of the porous system are summarized in Table I .
As the sllght dependence of the density of liquid phase on the relative pore saturation can be disregarded, as far as the mass concentrations are concerned the porous system is an ideal system.
ASSUMPTION 2B. Even if the system as a whole is a non- equilibrium system, there is a local equilibrium within each macroscopically small cell of it. For a truly one-phase system this assumption takes care of the extensibility of all thermo- dynamic relations to nonequilibrium systems. For a qwsi- one-phase system it has another implication:
ASSUMPTION 2C. I n a quasi-one-phase system the local values of the chemical potential for each cornponest are the same in all phases. F Q ~ o r d i n a d por~s,system> the ~ a c t i c a l meaning of this assumption is that the partial pressure of water vapor in the gaseous phase is equal to the equilibrium vapor pressure of the liquid (adsorbed)-phase.
.
The validity of Assumption
2
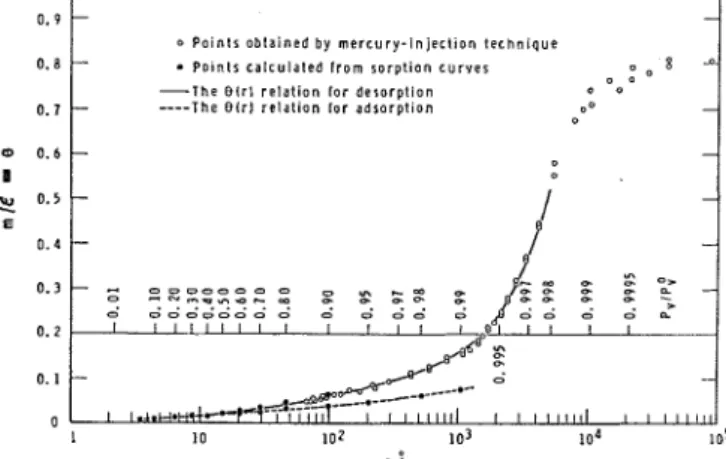
and.all its implications depends primarily on how closely the actual texture of the porous system approximates a quasi-one-phase texture. As for Assumption 2C, calculations indicate that in drying processes the equilibrium between the liquid and gaseous phases can be taken for granted in most practical problems if the adsorption of moisture is essentially a surface phenomenon.Liquid-Vapor Equilibrium
T h e relation expressing the liquid-vapor equilibrium, the so-called sorption relation, is characteristic of the particular porous system. I t is of the form
The most common representation of this relation is by the sorption isotherms (Brunauer et al., 1938; Kiselev, 1958)- i.e., by the family of curves m = m(P,) or m = m(P,/P,O) for
T
= const.A logical way of including all three variables, m, P,, a n d
T,
in a single relation was shown by Vassiliou and White (1948), who presented the sorption relation as a relation be- tween the moisture content and the mean normal curvature of the liquid surfaces in the pores-i.e., as m = m(r). An equiva- lent relation isB(= m/e) = B (r) (2)
If r is expressed from the Kelvin equation
and Equation 3 is substituted in the O(r) relation, an expression is obtained that correctly represents the dependence of m on
Po and T.
I n a strict sense the Kelvin equation is applicable only to the capillary condensation region (P,/P/
>
0.4), where sorption is brought about by the development of curved liquid surfaces in the pores. I n the region of true adsorption (P,/P,O<
0.3) the retention of water molecules is caused by inter- molecuiar forces, and the surface of the liquid (adsorbed) phase follows more or less the geometry of the pore surfaces. However, an m us. r relation, with r from Equation 3, correctly describes the dependence of m on P, and T i n almost the cntire 0<
P,/P,O<
1 sorption domain. Thus thc validity of Equa- tion 2 can be extended to a major portion of the region of true sorption, provided that the original physical mcaning of r is overlooked and it is regarded simply as a convenient param- eter.T h e generalized liquid-vapor equilibrium relation (as presented by Equation 2) for a particular porous system can be .calculated from its sorption isotherms with the aid of Equation 3. T h e main difficulty is the hysteresis phenomenon; be- cause of it m is not a single-valued function of P,/P,O in the region of capillary condensation. An "effective" sorption isotherm has to be selected, the shape of which depends on the nature of the process to be examined. For example, for most technical drying problems the effective sorption isotherm consists of a desorption scanning curve (Rao, 1941) and a section of thy; main desorption isotherm. I t seems, however, that drying processes are not overly sensitive to minor changes in the effective equilibrium isotherm, so that it is usually per- missible to use the main desorption branch as representing the effectivg m us. Po/#/ relation.
Becabse of some experimental advantages the 0.9
<
P,/I',O<
1 (or r>
100 A.) section of the equilibrium rcblation in desorption can be more conveniently studied with the aicl of the so-called mercury-injection technique (Purccll, 1949; Richardson, 1961). Some experimental data conccrnihg the liquid-vapor equilibrium, in both desorption and adsorption, for a certain, brown clay brick are plotted in Figure 2. T h e curves, referred to hereafter simply as the O(r) functions, have been tabulated and will be used later as the cffective liquid- vapor equilibrium relations for this kind of brick.As the gaseous ~ h a s e is regarded here as an ideal binary mixture
With the aid of this and the following two empirical equations
1 MWX
P' = - exp
(
-
-)
a n d
D P a i n t r o b l a i n e d b y m e r c u r y - i n j e c l i o n t e c h n i q u e P D i n l r c a l c u i a l e d l r o m s o r p t i o n c u r v e s
- The B l r l r e l a l i o n l o r d e s o r p t i o n 0 (I
9 0 0.1 ----The e l r l r e l a t i o n l o r a d l a r p t i o n o 0
Figure 2. Generalized liquid-vapor equilibrium relation for a certain clay brick
Equation 3 becomes
2Mw. U O - B T r = - -
T l n a9P
+
b whereor may be regarded as an empirical constant. With Equation
7 the basic effective equilibrium relation now can be presented in the following more explicit form:
The derivatives of this relation, with respect to p, P, and T,
will also be used later as part of a series of background informa- tion. The (bO/br)(r) function is obtainable by numerical differentiation of the appropriate O(T) function and is, in general, also presented in tabulated form.
Molecular Transport Processes
Accortlin~ to lllc model of tllc porous system prcscnlctl, s i ~ r l ~ ~ l t ; u ~ c . o ~ ~ s rr~olccl~liir mass r l ~ l t l lleiit triillsfi'r can take placc
o111y in t l ~ c Kaseous pl~ase. I t is known from Onsayer's work (1931) that in suctl simultaneous processes coupling occurs bctween the flux-force pairs, giving rise to various secondary phenomena. [The words "flux" and "force" (or "driving force") are used according to the terminology of nonequilib- rium thermodynamics.] A numerical study of these phenom- ena (Harmathy, 1967) revealed that in drying processes the mass fluxes induced by driving forces other than concentration gradients are negligible. Admittedly, the conduction heat flux and the Dufour energy flux may assume values of the same order of magnitude; yet because of the insignificant magnitude of the total molecular energy flux in the gaseous phase, neglecting the Dufour energy flux is justifiable.
By virtue of these arguments the molecular fluxes occurring in the gaseous phase of a porous system may be satisfactorily described by the following two equations:
Energy flux (excluding the fluxes associated with mass fluxes) :
q o = - + k 0 v T Mass fluxes (Bird et al., 1960) :
Here, because of the isotropy of the porous medium, the effec- tive porosity,
a,
could be used for the fractional free area. Because of the tortuosity of the pores D is some fraction of the coefficient of diffusion of water vapor in air outside the pores.Equations 13 and 14 can be recognized as Fourier's law of heat conduction and Fick's law of diffusion, respectively.
Convective Mass Transport
The mass transport described by Equation 14 takes place on a molecular level in relation to the local center of mass of the gaseous phase. I n general, however, the local center of mass is also in motion (convection) in relation to the stationary phases of the system.
The equation of motion (in a macroscopic sense) of the gaseous phase is obtained by the application of Darcy's law:
Here v, is the (local) mass average velocity of the gaseous phase:
Darcy's "law" is an experimentally obtained expression. I t can also be derived from the fundamental Navier-Stokes equations of motion under certain simplifying assumptions (Harmathy, 1967). Because of these assumptions the applica- bility of Darcy's law must be limited to the Poiseuille flow of liquids occurring a t very small pressure gradients, but it is permissible to extend it formally to gases if the permeability, K ~ , is not regarded as a property of the porous medium alone but as a factor that depends also on some characteristics of the gas (Creutz and Zumwalt, 1962).
When the porous medium is partially saturated with mois- ture, K~ in Equation 15 must be interpreted as the "effective"
permeability, which is related to the "limiting" permeability- i.e., to the permeability in the absence of moisture-K,O, as
K O = {KO0 (17)
where
[,
thcr rc!lativc ~)ermcability, is a function of t t ~ c rclative pore satumtiorl, 0 . I:,xl)c:rinirnt;il t l i t t ; ~ c:onc:crrlin~ this ft~nc-tion for thr brown clay brick ~nc:ntionrtl ;~lx)vc (wit11 Ircliurn as the gaseous phase) are
l lotted
in 1"igur-c 3. In tile prac:- tically important rangc of 0<
O<
0.4, where tlic moisture (or the "wetting phase," in general) is practically immobile (Osoba et al., 1951; Richardson, 1961; Richardson et al., 1952), the experimental finding may be satisfactorily described by the following empirical equation (shown as a continuous line in the figure) :Such a function is expected to be practically independent of the properties of the gas, and probably also of the pore struc- ture of the medium (Osoba et al., 1951 ; Richardson, 1961).
Table 11. Coefficients in Equation 43 A I = ( M . / M ) D e ( l
-
8 ) Az = - ( M w / M ) D e ( l-
8 ) Aa = 0 BI = (~nO/vo)S-v Bz = (~n"/vn)S-(l-
( P ) Ba = 0 C1 = 0 Cz = O C3 = k Dl = - ( M a / M ) D O v Dz = ( M w / M ) D O v Da = O El = ( K ~ O / ? ~ ) ( P ~ P Ez = (l(nO/ln)(l-
P ) ~ P Ea = (~n"/17n)( FI =o
FZ= o
FS = ( a k / a ) ( a e / a T ) GI =( ~ ~ ~ / ~ ~ ) [ ( ~ ( a ~ - i ~ ) ( a e / a ~ )
+
S-I
+
GZ = ( ~ ~ ~ / ~ , ) [ ( 1-
~ ) ( a S - l b B ) ( b B / a ~ )-
Ga = 0 ( M , / M ) D O prl
- ( M W / M ) D O p Hi = - ( M . / M ) D O T HZ =( M ~ / M ) D G
H3 = (ak/bB)(bB/dp)-
c ( P / R T ) X ( M w M o / M ) D ( l - B)[(cp)n- ( c P ) , ] J1 = - ( ~ p ~ / v p ) v W ~ J 2 =-
( ~ ~ O / 1 n ) ( l-
' P ) ~ T J3 = (dkL&)(bB/ap)+
( ~ n O / 1 1 n ) r 2
K~ = E [ - ~ ( b ~ i a ~ )+
(1 -ell
KZ = € [ - ( I - v ) ( M / ~ v )-
(1-
Ka = - ~ Q ( b e / a ( ~ ) LI =€ 1 -
Y ( & / ~ P )+
(1-
O ) ( ~ / P ) I L2 = (1-
~ ) O P -
La = e [ - & ( b B / a P )-
(1-
8)1 Mi = e l - p ( b B / a ~ ) - (1-
B ) ( v / T ) ] Ma = - ( I-
~ ) O T M s = -e&(bB/dT)+
e(1-
8)Z+
2'
where2'
= pwe8(cp)w+
p.(l-
~ ) ( C P ) .0
= pW[Q+
NaQ/&)l M = M,V+
~ ~-
V ) ( 1wp
= ( u p )+
( a ~ - / & ) ( w a p )0,
=-
M . ) I M I ( I-
8 )+
€ ( M i a v ) , JF-T = ( s - / T )-
( a r / a e ) ( b B / a T ) OP = e [ ( l-
8 ) l P - (bBlaP)l Y = v-
( p w / M w ) ( R T / P ) OT = e [ ( l-
e ) / T+
(bB/dT)l Z = ( P / R T ) [ M W ( c p ) v v+
Mo(cp)o(l -v)l
0 0.2 0.4 0.6 0.8 1.0 R E L A T I V E S A T U R A T I O N m l &-
0Figure 3. Relative permeability of a certain clay brick to gases in presence of moisture
Equations of Continuity of Matter
By examining the expressions listed in Table I and taking into account the following relation between the relative pore saturation and effective porosity
T o determine the cp(r, t), P ( r , t), and T ( r , t) functions, three independent equations are needed. Two equations can be obtained by applying the equation of continuity to the two mobile constituents of the system, water vapor and air (Bird et al., 1960) :
where
no, = .~,,v,, = j,,
+
6,,v, (22)noa = 60avoa = joa ~oavo (23)
and as the j's are to be referred to the mass average velocity, v,, their sum adds up to zero.
As the gaseous phase is regarded as an ideal mixture
po = MwP/RT (24)
pa = MaP/RT (25)
and
P, = P/RT [Mwcp f Ma(l - cp)l (26) By substituting j,, and j,, from Equation 14 and v, from Equation 15 (neglecting the p,g term which, for gaseous flow, is always very small) in Equations 22 and 23 and making use of Equation 17 and some expressions in Table I one obtains
MwMa
l-K
Onow = -@poD --
vcp
-
pocp 4 VPM= 'lo (27)
MwMa
l-K
OnOa = ap0D Vcp
-
pa(l-
P) -P- VPM Z 'lo
After substituting from Equations 24 to 28 and from 'Tablr I into Equations 20 and 21, two srcontl-ordcr, nonlinc;~~, partial differential equations arc obtainccl:
A1V2v
+
BlV2P+
D i ( ~ c p ) ~+
E I ( v P ) ~+
Gtvcp.
VI'+
bcp b P b T
it may be seen that a porous system is unambiguously defined
z-z1vcp
.
VT+ j,vp.
VT = K~ -+
L~ - -+
M, - - (29)by the O(r, t), ~ ( r , t), P(r, t), and T ( r , t) functions. Because bt bt bt
of the assumed local equilibrium 0 can be expressed in terms of
Q, P, and T (see Equation 9), so that the model system actually
A2V2cp
-b
B2V2P+
D z ( v Q ) ~+
E~(vP)'+
GZVP.
V P+
dcp b P b T
possesses three degrees of freedom. For practical reasons Q, H~~~
.
v~
+ J ~ V P.
V~ = K~-
+ ,r2 - + M~ - (30)P, and T a r e used here as the three independent state variables. bt bt dt
The coefficients are, in general, functions of (o, P, and T. Their expressions are listed in Table 11.
Energy Transpod Equation
T o obtain the third equation one can use the law of conserva- tion of energy. Here the following form of this law seems to be the most convenient starting point (Bird et al., 1960):
The applicability of this equation rests on Assumption 2. I t has also been assumed that only gravitational forces are effective.
The mass average velocity for the entire porous system is defined as
According to Assumption 1 the liquid and solid phases are stationary phases-i.e., n, = n, = v, = v, = 0. By com- bining Equations 16 and 32 and using plausible values for the 6's it is easy to show that, in general, Ivl
<
0.0005 Ivgl. I t will be assumed that v=
0; thus the term v VP disappears from Equation 31.The enthalpy change can be written as
For the gaseous constituents and for the material of the solid (bH/bO)p,T = 0. For the liquid phase (bHl/b8)P,T =
-
(dQ/be),,, because of the relationFurthermore, p, = const., p l s p,
--
const., and (c,)~ =(c,), = const. (Berezin et al., 1963). Thus, with the aid of well known thermodynamic relations, the following expressions are obtained for the four constituents of the system:
'I'lic (bQ/bO),,,, term in Equation 38 is generally negligible ol~tuitlc thc [lomain of truc ntlsorption.
q iri I<cluatiot~ 31 ia tlic cor~ductivc cnergy flux in a macro- xopically srrr;rll but r~licroscopically largc volurnc clemcnt. (Since v
--
0, it is approximately equal to the heat flux in a stationary coordinate system.) By making use of Assumption 2 one can extend the validity of Fourier's law (see Equation 13 for the gaseous phase) to macroscopically small cells of the system by writingq = -kVT (39)
Because of its simplicity, the following empirical equation has been selected to describe k for a porous system (Kingery, 1960) :
k = [k14(1
-
8)+
k I n 8+
kan(l-
e)]'ln (40)where -1
5
n<
1 and is to be determined experimentally.A in Equation 31 can be expressed, on a macroscopic scale, as -@vo
-
VP; consequently, with the aid of Equations 15 and 17 asAfter expanding the first two terms in Equation 31 and com- bining them with the equations of continuity (Equations 20 and 21), substituting from Equations 34 to 39 and 41 and from Table I in Equation 31, and taking into account that v
=
0, one obtains:The coefficients C3.
. .
M3 are also listed in Table 11.All three equations of the simultaneous moisture and heat transfer in porous systems can be written in the following general form:
where k = 1,2,3. Some of the coefficients Ak
. .
.
M k are equalto zero; a number of others are negligible in many practical applications.
Initial Conditions
The initial conditions are presented by the following three expressions:
PO = ~ ( r , O), PO = P(r, 0), T O = T(r, 0); r in [V] (44) Because of Assumption 1 the present theory is applicable only if the porous system is in a pendular state. This seems to be the case whenever 0," is less than its value a t r 2000 A. according to the effective equilibrium e(r) relation. This statement implies that the finer the pore structure of the porous medium, the wider the applicability of the theory.
The fact that in studying drying processes the theory is limited to the falling rate period is of little practical significance, since the history of average moisture content during the con- stant rate period is amenable to simple calculations (Bagnoli, 1963).
I n general, m,, a t the lower critical point-i.e., at t = t c r is the only available information for the theoretical simulation of the falling rate period of drying. As the time t = tcl be- comes thc t = 0 lcvel for thc simulation, onc may write (mo)., for m,, at t =
'rIi(.
clistribution of moisturc at thr lower critical point is, unforturlately, far frorn 11nifor111 (1:igure 1, 1)).'1'0 elilninatc the use of estiniated moisture clistribution it is aclvisable to start t11r tlieorrtical simulation a t somc highcr uniform moisture content-i.e., a t some 711-0
>
(NJO)., tliatoccurs a t some (undefined) t-0
<
0 time-and then disregard the results obtained for the period t-0<
t<
0.As (D has been selected as one of the three state variables
of the system, actually Po(r) [or p(t-~)], not mo(r) [or m(t-o)], should be specified in the initial conditions. The conversion from m to P is done with the aid of the following equation,
which is obtained by combining Equation 7 with the expres- sion of liquid vapor equilibrium, Equation 2 [r(B) is the inverse of the B(r) relation].
The selection of To(r) (within reasonable limits) seems to have very little effect on the moisture history of the system. The errors associated with its inaccurate selection fade away in relatively short time.
Boundary Conditions
T h e boundary conditions are, in general, presented by the following equations :
When the first set of conditions does not apply, expressions for ~ ( p r , t) and v T ( r , t) can be obtained by examining no,
and q a t the boundary of the porous system.
Only one set of boundary conditions is discussed in detail here: that applicable to a system completely surrounded by air of controlled (not necessarily constant) water vapor content, pressure, and temperature. The expression for P a t the bound- ary is immediately obvious
P ( r , t) = Pm(t), r in [S], t
>
0 (47) According to the film concept of mass transfer, the diffusive flux of water vapor a t the pore openings through a laminar boundary layer of air (referred to unit area of the system) canbe expressed as
where v takes care of the possible extension of the actual transfer area due to convective mass transport. T h e heat flux through the same boundary layer may be expressed analogously as
q = -h[T(r, 1 )
-
Tm(t)]; r in [S], t2
0 (49) T h e terms in the square brackets in Equations 48 and 49 are vector quantities oriented (for positive values) perpendicularly toward the boundary surface. [In a strict sense the transfer co- efficients defined by these two equations are applicable only if n,,-
0 and n, +- 0 (Bird et al., 1960).]By combining Equations 48 and 49 with Equations 14 and 39 the following t y o boundary conditions result:
R T
v9
= v a-
[ ~ ( r , 1)-
cp,(t)] ; 7 in [S], t2
0 (50)PD
.
h T = - T ,t)-
( t ) ] r in ($1, t>_
0 (51) kFor transfer processes taking place between a vertical slab (with x = 0 and x = L boundary planes) and air a t atmospheric
pressure and a temperature T, (within, say, O0 and 50' C.)
contained in a n enclosure of a temperature T,, the transfer coefficients can be expressed as follows:
where a* and c are empirical constants and can be determined from simple drying experiments (Harmathy, 1967). I n general T,
=
T,.Table Ill. Expressions Used in Computer Calculations To Describe Expressions Used
Liquid-vapor equilibrium Tabulated 0 = 0(r) functions (Figure 2) with r from Equation 7
Relative permeability us. Equation 18 relative saturation rela-
tion
Heat of sorption Q = A, bQ/bO = 0 Thermal conductivity Equation 40
Simultaneous heat and moisture transfer
F o r O < x < L Equations 54, coefficients listed in t
>
t-0 or t>
0 Table I1F o r O < x < L m - ~ or mo = const. (uniform)
1 = or t = 0 T-O or TO = const. (uniform)
P - 0 or PO = const. (uniform)
cp-0 or cpo to be obtained by sub- stituting values of 0-0, T-0, and P-o or O0, To, and Po in Equation 45
For x = 0 or x = L P = Pnt,,
t
2
I-o or t>
0 Equation 55 with a from Equation 52 Equation 56 with h from Equation 53and k from Equation 40
Table 1V. Values of Constants Appearing i n the Expressions Listed in Table Ill
Symbol Value Appearing in Source Material Properties (cP), 1.0063 X 107 Table I1 a ( C P ~ 0.815 X 107 Table 11 Exptl. (cp), 1 ,8646 X 107 Table I1 a (cP).. 4.1793 X 107 Table I1 a
b-
' - 0.128 Table I1 Est. e 0 . 8 Ea. 53 Est. ko 0:02613 X 106~ 4 .
40 oka 2.207 X
lo6
Eq. 40 Exptl. kw 0.616 X 106 Eq. 40 a Ma 28.952 Table I1 a M W 18.016 Table I1 a e 0.26 Table I1 Exptl. 1 . 8 3 X 10-4 Table I1 'lo 0xoo 2.5 X 10-10 Table I1 Exptl. X 2 4418 X 1 0 ' 0 Table 111 a Table II ~ x p t l . Table I1 a Physical Constants 1.01325 X 108 Table 111 8.3149 X 107 Table I1 5.67 X 10-6 Eq. 53 Geometric Constants Eq. 52 Table 111 Empirical Constants 1.209658 X 10-l2 Eqs. 7,45 5080 Eqs. 7, 45 0.25 Eq. 40 b Eq. 52 0.167 Eqs. 7, 45 1 .o Eq. 55 121.2 Eqs. 7, 45 a a a Exptl. Exptl. Exptl. Exptl. Est. Exptl. Est. Est. Est.
a Values listed in several well known handbooks. b Given in captiom
0j"figures.
Experimental Work and Computer Calculations
A number of drying experiments and computer calculations have been performed. I n the experiments the variation of the weight and the temperature (at the center) of clay brick slabs (of the material mentioned) of 1-cm. thickness, 18.39 cm. square, were recorded under controlled environmental conditions. Some characteristic curves concerning three runs (cases 1, 2, and 3) are plotted in Figures 4, 5, and 6, together with the corresponding curves of theoretical simulation.
T I M E , hr.
Figure
4.
Drying experiment 1 a n d its theoretical simulation Initial Conditions m a = 0.1 0 5 cc./cc. (uniform) T 4 = 306.2' K. (uniform) P-0 = Pat, (uniform) Boundary Conditions q, = q , ~ = ,pm2 = 0 . 0 1 0 5 3 9 mole/mole T, = T,I = To12 = 3 1 8.9' K. P, = P,1 = Pm2 = Patm a* = U I * = u2* = 2.478 X mole/ sq. cm. sec.Equilibrium represented b y O ( r ) relation for desorption (Figure 2)
0 1 2 3 4 5 6 1 8 9 10 11 I2 13 Id
TIME, h r .
Figure
5.
Drying e x p e r i m e n t2
a n d its theoretical simulationInitial Conditions Boundary Conditions
m-a = 0.1 0 5 CC/CC (uniform) q, = q-1 = q d = 0 . 0 0 9 0 0 9 mole/ mole
T-a
-
302.7' K. (uniform) T, = T,I = T d = 308.8' K.P - a = Pat, (uniform) P, = P,I = P d = Pat,
a* = a l * = u2* = 2.478 X 1 0-8 mole/ sq. cm. sec
Equilibrium represented b y H r ) relation for desorption (Figure 2)
T o one-dimensional transfer problems the following forms bp
-
= (-l)'"+l "a RTof Equation 43 are applicable bx
b p bT bP bT The various equations and conditions used in the computer
bx bx bx bx calculations are summarized in Table 111. The values of some
ap
bP bT constants appearing in these equations and in the expressionsKk-+ L,-
+
Mk-
br br br (k = 1, 2,3) (54) of coefficients in Table
I1
are given in Table IV. [Anymaterial property could be regarded as a function of T, P,
The boundary conditions are now and p (or 0) without unduly complicating the numerical
1 . 0 ( 0 . , a f i n q * r a t e p e r i D d I - E x p e r l m e n la1 - - - E s t i m a t e d
--
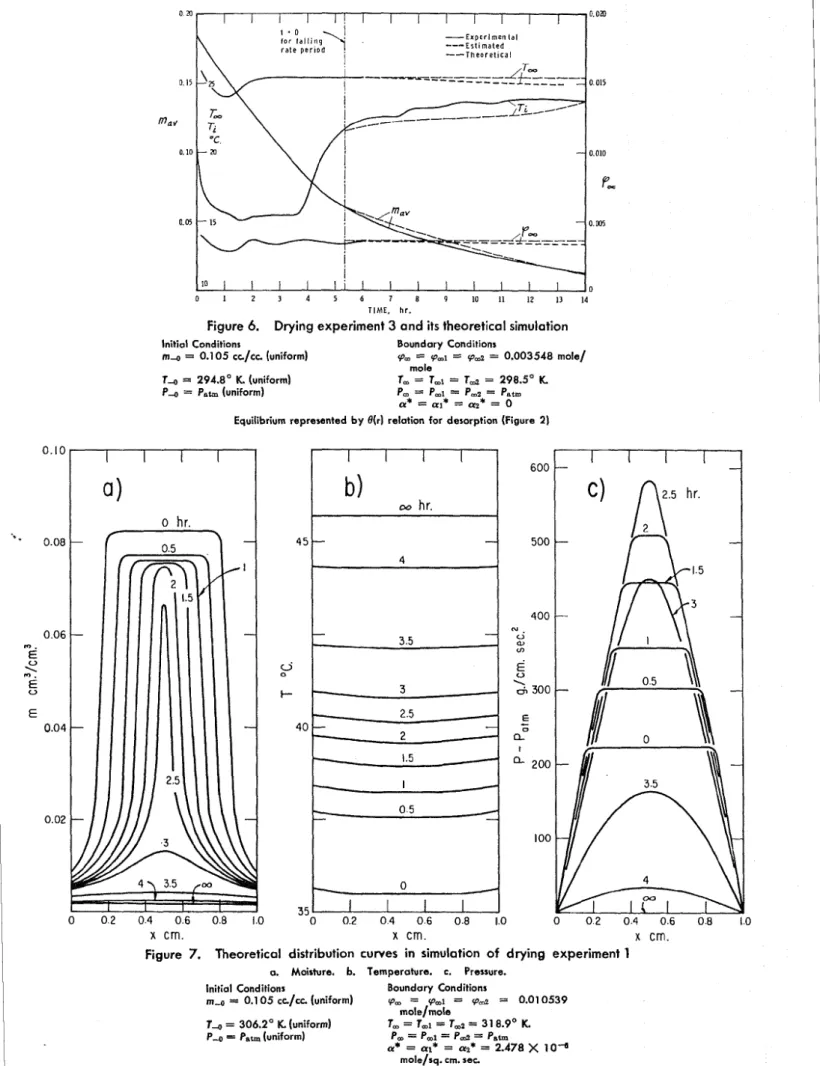
T h e o r e t i c a l 0 I 1 1 1 1 I I I I I 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 T I M E , h r .Figure 6. Drying experiment 3 and its theoretical simulation Initial Conditions m a = 0.1 05 c c / c c (uniform) T 4 = 294.8' K. (uniform) Pa = Pat, (uniform) Boundary Conditions (P, = (P-1 = (P& = 0.003548 mole/ mole T, = T,I = T d = 298.5' K. P, = P,1 = P,z = Pat,
,+
= al' = a2+ = 0 Equilibrium represented b y O(r) relation for desorption (Figure 2)x cm. x cm. x cm. 0.10
'.
0.08 45 0.06 m .5
\ m"
i
u! - .
E 0.04 40Figure 7. Theoretical distribution curves in simulation of drying experiment 1
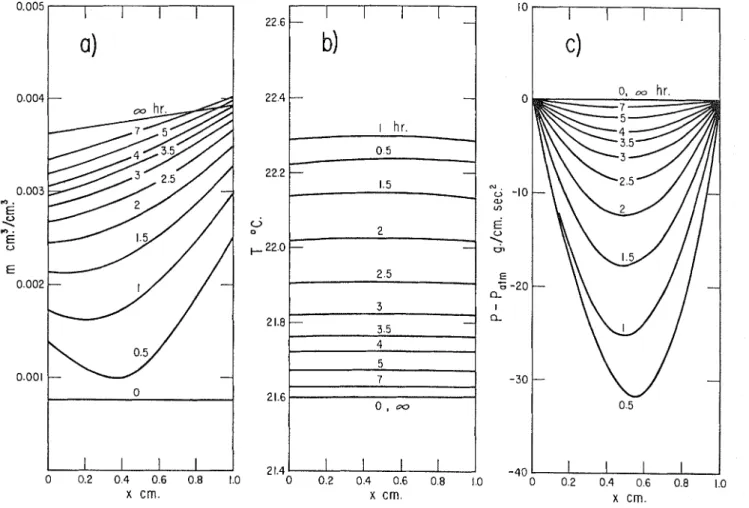
a . Moisture. b. Temperature. c. Pressure. Initial Conditions Boundary Conditions
m-0 = 0.1 05 c c / c c (uniform) (P, = 9-1 = v a = 0.01 0539 mole/mole T4 = 306.2' K. (uniform) T, = T,l = T-2 = 31 8.9' K. P4 = P u b (uniform) Pm = P,1 = P d = P u b a* = a,* = az* = 2.478 X 10- mole/sq. em. s e c
I
I
I
b
oo hr.-
-
4-
3 . 5-
3-
2.5-
-
-
2Equilibrium represented b y B(r) relation for desorption (Figure 2) 600 500 400 N u' a, U) € 0 \ oi300 E
-
a 100 I & E C F U N D A M E N T A L S I a 200 100 1.0 0 0.2 0.4 0.6 0.8 1.0 1.5-
0.02 35 'Z I / i 0.5-
/-
-
0 .---I
0 0.2 0.4 0.6 0.8 1.0 0 0.2 0.4 0.6 0.8x
cm.
xcm.
xcm
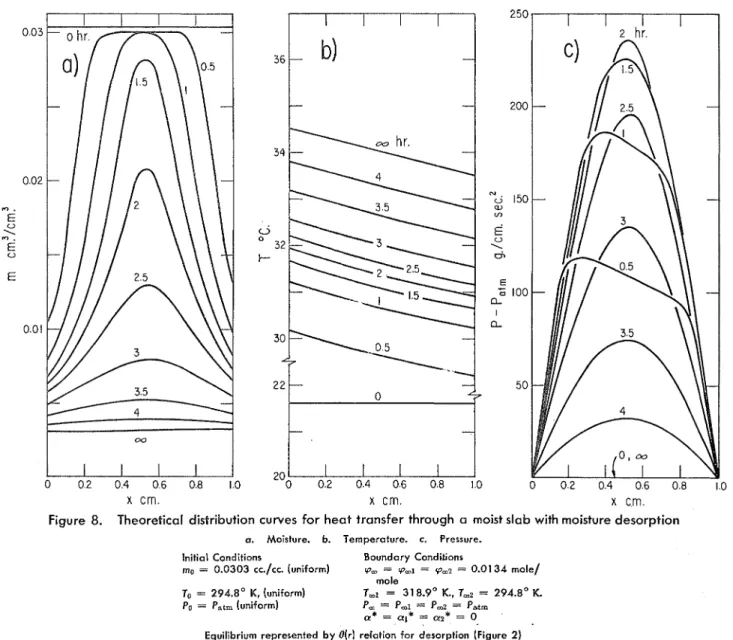
Figure 8. Theoretical distribution curves for h e a t transfer through a moist slab with moisture desorptiona. Moisture. b. Temperature. c. Pressure.
Initial Conditions Boundary CondiL'ons
mo = 0.0303 cc./cc. [uniform) rp, = rp,~ = q,? = 0.0134 mole/ mole
To = 294.8' #, (uniform) T,I = 31 8.9' K., T,? = 294.8' K
Po = Pat, (uniform) P, = P-1 = Pmz = P,,tm
a* = = 0. Equilibrium represented b y O(r1 relation for desorption (Figure 2)
procedure.] T h e values of the constants related to the initial and boundary conditions have been selected as representative of the experimental processes (Figures 4 to 6).
Details of the numerical procedure have been given (Har- mathy, 1967). A backward difference or implicit scheme (Douglas, 1961) was selected that is known to yield stable solutions for any selection of the space and time grid spacings. T h e moisture content history-i.e., the m(x, t) function, which is undoubtedly the principal information sought-is not available explicitly from the solution of Equation 54. I t has to be obtained by additional calculations, using the p(x, t), P(x, t), and T ( s , t) solution functions and the liquid-vapor equilibrium relation Equations 2 and 7.
The agreement between the experimental and theoretical curves is, in general, satisfactory (Figures 4 to 6). Some discrepancies are attributable to the insufficient constancy of
T , and p, during the experiments (due to certain experimental
difficulties), the not strictly one-dimensional character of the actual transfer processes, and certain compromises made in the selection of some material properties (especially the Q =
X
=const. assumption).
I n Figure 7 the computed moisture content, temperature, and pressure histories of the brick slab (for the falling rate period) are plotted for case 1. T h e moisture distribution curves are similar to those plotted on the basis of experimental data in Figure 1, b .
T h e drying processes so far discussed come to a n end with the development of equilibrium between the system and the surroundings. T o show the general validity of the present theory, two entirely different processes (cases 4 and 5) were also simulated on the computer. T h e information in Tables I11 and I V is applicable to these computations also.
I n case 4 a desorption process is examined that eventually reaches a steady state characterized by the absence of mass fluxes and by a constant energy flux. The steady-state mois- ture content distribution will be governed by the desorption liquid-vapor equilibrium corresponding to a constant water vapor pressure and a n approximately linear temperature dis- tribution (Figure 8), in agreement with the observations of Vassiliou and White (1948) and Paxton and Hutcheon (1952). Case 5 represents an adsorption process. T h e steady state is now characterized by the absence of conductive heat flux and by a constant water vapor flux. T h e steady-state mois- ture content distribution is determined by the (local) adsorp- tion equilibrium, corresponding to a constant temperature and a linear vapor pressure distribution.
Discussion
A large number of other computer calculations indicate that the effective liquid-vapor equilibrium is one of the major factors that determine the shape of the m, us. t relation in drying. Also, for any realistic selection of the initial moisture
22.4
-
-
I hr.-
0.5 22.2 --
1.5-
m Ee
nv
2c
,-
22.0 - * E 2.5 3 21.8 - - 3.5 4 5 7 0 2 1.6 0 ,I
1
2 1.4I
I
0 0.2 0.4 0.6 0.8 1.0 0 0.2 0.4 0.6 0.8 1.0 x cm.x
cm.Figure
9.
Theoretical distribution curves for vapor transmission through aa. Moisture. b. Temperature. c. Pressure.
-40
-
0 0.2 0.4 0.6 0.8 1.0
x
cm.moist slab with moisture adsorption
Initial Conditions Boundary Conditions
mo = 0.00077 cc./cc. (uniform) qml = 0.00364 mole/mole To = 294.8' K. (uniform) qmz = 0.01339 mole/mole Po = Pat, (uniform) T, = Tml = T,a = 294.8" K.
P, = P,1 = P d = Pat, a* = al* = az* = 0
Equilibrium represented b y the B(r) relation for adsorption (Figure 2 )
distribution a t the lower critical point, double S-shaped moisture distributions will arise for some time (Figure 7, a). Bagnoli (1963) believed that such distributions are brought about by capillary flow mechanism only.
A plot of log m, us. t is approximately a straight line, a t least for some time after the onset of the pendular state condi- tions. A t a given slab thickness the slope of this line appears to be proportional to some power of the rate of drying during the constant rate period.
If the "apparent thermal conductivity," k.,,, of moist ma- terials is defined by replacing the conductive heat flux by the total energy flux in Equation 39, the following equation can be obtained:
I t has long been believed that the presence of moisture always increases the value of k,,,. Cammerer (1939) ex- pressed the percentage increase in the value of k,,, as a unique function of the moisture content. Krischer and Rohnalter (1940) developed a more elaborate expression, which is applicable to the steady state of systems at very high relative saturation.
Figures 7 to 9 and Equation 57 reveal that very often, and in drying processes inevitably, k,,
<
k. I n processes charac-terized by the n,, -+ 0 condition in steady state, k can differ from k.,, only under transient conditions, if the system is in its pendular state.
Acknowledgment
I n selecting this area for study the author was influenced by
N. B. Hutcheon. The author thanks R . F. Legget for per- mission to use this work as a doctoral dissertation and A. F. Orlicek and Kurt Peters, University of Technology, Vienna, Austria, for consenting to its publication. I n the preparation of the computer program S. D. Baxter, J. D. Taylor, and T. A. Hills yielded invaluable help.
Nomenclature
Some symbols used as the coefficients of Equation 43 or
as expressions in these coefficients (see Table 11) are not listed. a = empirical constant, cm. s e ~ . ~ / g r a m
b = empirical constant o M,X/R, O K . B = height of vertical surface, cm.
c p = specific heat at constant pressure, sq. c m . / ~ e c . ~ O K.
D
= effective coefficient of diffusion of water vapor in air inside a porous medium, sq. cm./sec.e = empirical constant, dimensionless
E = specific internal energy, sq. cm./sec2
g = gravitational acceleration, c m . / ~ e c . ~ h = heat transfer coefficient, g r a m / s e ~ . ~ O K. H = specific enthalpy, sq. cm./sec.2
j
= mass flux relative to mass average velocity, gram/sq. cm. sec.k = thermal conductivity, gram c m . / ~ e c . ~ O K.
L = thickness, cm.
m = volumetric fraction of liquid-phase moisture, "mois- ture content," cc./cc.
M = molecular weight; without subscript: molar mean weight of gaseous phase, gram/mole
n = empirical constant, dimensionless
n = mass flux with respect to stationary coordinates, gram/sq. cm. sec.
P
= partial pressure; without subscript: total (macro- scopic) pressure, gram/cm. ~ e c . ~P,o = equilibrium vapor pressure of free water, gram/cm. S ~ C . ~
q = conductive energy flux relative to mass average veloc-
ity, g r a m / ~ e c . ~
Q
= latent heat of sorption of water, sq. cm./sec.Z7 = parameter; radius or mean normal curvature of liquid surface, cm.
r = position vector, cm.
R = gas constant, gram sq. c m . / ~ e c . ~ O K. mole
[S]
= surface of porous systemt = time, sec.
T = temperature, O K. (unless otherwise stated)
v = velocity; without subscript o r with subscript g: mass average velocity, cm./sec.
[V] = volume bounded by surface
[S]
x = coordinate, cm.
a = coefficient of mass transfer, moles/sq. cm. sec.
a* = empirical constant, moles/sq. cm. sec.
fl = empirical constant, g r a m / s e ~ . ~ O K.
6 = mass concentration, gram/cc.
A = viscous dissipation per unit volume, gramcm./ ~ e c . ~
E = true porosity, cc./cc.
{ = relative permeability, dimensionless
7 = coefficient of shear viscosity, gram/cm. sec. 0 = relative saturation = m/c, dimensionless
K~ = effective permeability of condensed phases of porous
system to gases, sq. cm.
K,O = limiting permeability of porous medium to gases in absence of liquid phase, sq. cm.
X = latent heat of vaporization of free water, sq. c r n . / s e ~ . ~ v = empirical factor, dimensionless
p = density; true density, gram/cc.
o = surface tension of water, g r a m / s e ~ . ~ oo = empirical constant, g r a m / s e ~ . ~
o. = Stefan-Boltzmann constant, g r a m / ~ e c . ~ O K.*
= effective porosity: volume fraction occupied by gaseous phase, cc./cc.
p = mole fraction of water vapor in gaseous phase, moles/ mole
V
= nabla vectorv2
= Laplacian operator a = air a p p = apparent atm = atmospheric av = averagec = during constant rate period
C = at critical point e = enclosure g = gaseous phase
~a = air in gaseous phase of porous system
fiu = water vapor in gaseous phase of porous system
I = in irltcrior of slab
k = 1 , 2 , 3
1 = l i q u i d hase; lower
0 = ! = 0 rat lower critical point) -0 = beginning of theoretical simulation
s = solid phase
u = upper
u = w a t e r v a p o r w = water
m = in bulk of surrounding air 1 = on side x = 0
2 = on side x = L
Literolure Cited
Bagnoli, E., "Drying of Solids," "Chemical Engineers' Handbook," J. H. Perry, Ed., Sect. 15, p. 32, McGraw-Hill, New York, 1963.
~ e l l , i . . ~ . , Nissan, A. H., A.1.Ch.E. J . 5, 344 (1959).
Berezin, G. I., Kiselev, A. V., Sinitsyn, V. A,, RUSS. J . Phys. Chem:
37,167 (1963).
Bird, R. B., Stewart, W. E., Lightfoot, E. N., "Transport Phe- nomena," pp. 502, 555, 562, 637, Wiley, New York, 1960. Brunauer, S., Emmett, P. H., Teller, E., J . Am. Chem. Sod. 60,
309 (1938).
Buckingham, E., "Studies on the Movement of Soil Moisture," U. S. Dept. Agr., Bur. Soils, Bull. 38 (1907).
Cammerer, J . S., Warme- u. Kalfefech. 41, 126 (1939).
Cassie, A. B. D., King, G., Baxter, S., Trans. Faraday Sod. 36, 445, 453, 458 (1940).
Childs, E. C., J . Agr. Sci. 26, 114, 527 (1936). Christensen, H. It., Soil Sci. 57, 381 (1944).
Corben, R. W., Newitt, D. M., Trans. Znsf. Chem. Engrs. 33, 52 (1955)
, - - --,.
Creutz, E. S., Zumwalt, L. R., J . Appl. Phys. 33, 2883 (1962). Douglas, J., Jr., "Survey of Nu~ncrical Methods of Parabolic
Differential Equations," "Advances in Computers," by F. L: Alt, Ed., Vol. 2, p. 1, Academic Press, Ncw York, 1961. Edlefsen, N. E., Anderson, A. B. C., Htlgnrdza 15, 31 (1943). Fisher, E. A.,.J. Agr.Sci. 13, 121 (1923); 17, 407 (1927).
Fitts, D. D., "Nonequilibrium Thcrrnodynamics," McGraw-Hill, New York, 1962.
Gurr, C. G., Marshall, T. J., Hutton, J. T., Soil Sci. 74, 335 (1952).
,- - -
Haines, W. B., J . Agr. Sci. 16, 265 (1927); 20, 97 (1930). Harmathy, T . Z., "Simultaner Feuchtigkeits- und Warmetransport
in porigen Systemen mit besonderem Hinweis auf Trocknung," doctorial dissertation, Technische Hochschule, Wien, 1967. Henry, P.S. H., Proc. Roy.Soc. 171A, 215 (1939).
Hutcheon, W. L., "Moisture Flow Induced by Thermal Gradients within Unsaturated Soils," Highway Res. Board, Spec. Rept. 40,113 (1958).
Johnson, K. O., Tappi 44, 599 (1961 ).
Kamei, S., J . Soc. Chem. Znd. Japan, 40, 251, 257, 325, 366, 374 (1937).
\ - - - ,.
Keen, B. A., J . Agr. Sci. 6, 456 (1914).
Kingery, W. D,, "Introduction to Ceramics," p. 499, Wiley, ~lew'york, 1960.
Kiselev, A. V., "Structure and Properties of Porous Materials," D. H. Everett, F. S. Stone, Eds., Loxldon, Butterworths, p. 195, 1958.
Gischer, O., VDZ Forschungsh. 415, 1 (1942).
Krischer, 0.. Rohnalter, H., VDZ Forschunesh. 402, 11B, 1, (1940). Kuzmak, J. M., Sereda, P. J., Soil Sci. 84, 419 (1957).
Lebedev. P. D.. Intern. J . Heat Mass Transfer 1. 294 (1961 ).
Lewis, W. K., J. Znd. Eng. Chem. 13, 427 (i921 j.
Lykov, A. V., Mykhaylov, Y. A , , "Theory of Energy and Mass Transfer," Prentice-Hall, Englewood Cliffs, N. J., 1961. Miller, R. D., Miller, E. E., Proc. Soil Sci. Sod. Am. 19, 271 (1955). Newman, A. B., Trans. Am. Insf. Chem. Engrs. 27, 203, 310 (1931). Nissan, A. H., Kaye, W. G., Bell, J. I<., A.1.Ch.E. J . 5, 103 (1959). Onsager, L., Phys. Rev. 37, 405 (1931); 38, 2265 (1931).
Osoba, J. S., Richardson, J. G., Kerver, J. K., Hafford, J. A., Blair, P. M., Trans. AZME 192, 83 (1951).
Paxton, J . A., Hutcheon, N. B., Trans. A S H V E 5 8 , 301 (1952). Philip, J . R., "Physics of Water Movement in Porous Solids,"
Hiehwav Res. Board. S ~ e c . R e ~ t . 40.147 (1958a). ~hili;, J. R., Soil Sci. 83, g45, 435'(195ja).
Philip, J. R., Soil Sci. 84, 163,257, 329 (1957b). Philip, J. R., Soil Sci. 85, 278, 333 (1958b). Purcell. W. R.. Trans. AZME 186. 39 (1949). Rao, K.. S., J . Phys. Chem. 45,506-(1941).
Remson, I., Randolph, J . R., "Review of Some Elements of Soil- Moisture Theory," Geol. Survey Profes. Paper 411-D (1962). Richards, L. A., Physics 1, 318 (1931).
Richardson, J . G., "Flow through Porous Media," Section 16, "Handbook of Fluid Dynamics," V. L. Streeter, Ed., McGraw- Hill, New York, 1961.
Richardson, J. G., Kerver, J. K., Hafford, J. A., Osoba, J. S.
Trans. A I M E 195. 187 (1952).
herw wood,
T. K., ind. E;ig. Chcm. 21, 12, 976 (1929); 22, 132 (1930); 24, 307 (1932).Sherwood, T. K., Comings, E. W., Znd. En!. Chcm. 25, 311 (1933). Tuttle, F., .J. I*ianklin I n ~ f . 200, 609 (1925).
Vassiliou, U., White, J., Trans. Ceram. Soc. 47, 351 (1948). Walker, I. K., Ncw Zcaland J . Sci. 4,775,796 (1961).
RECEIVED for review March 4, 1968 ACCEPTED September 19, 1968 Contribution from the Division of Building Research, National Research Council of Canada, published with the approval of the Director of the Division.