Publisher’s version / Version de l'éditeur:
Cement and Concrete Research, 3, January 1, pp. 41-54, 1973-01-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/0008-8846(73)90060-4
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Action of triethanolamine on the hydration of tricalcium aluminate
Ramachandran, V. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=e9df9822-cedb-4fc0-a6fb-843c162b4d3a https://publications-cnrc.canada.ca/fra/voir/objet/?id=e9df9822-cedb-4fc0-a6fb-843c162b4d3a
CEMENT and CONCRETE RESEARCH. Vol
.
3 , pp. 41 -54, 1973. Pergamon P r e s s , I n c . P r i n t e d i n t h e U n i t e d S t a t e s .ACTION O F TRIETHANOLAMINE ON THE HYDRATION O F TRICALCIUM ALUMINATE
V. S. Ramachandran
Division of Building R e s e a r c h , National R e s e a r c h Council of Canada Ottawa KIA 0R6, Ontario, Canada
(Communicated by P. J
.
Sereda)ABSTRACT
Hydration c h a r a c t e r i s t i c s of t r i c a l c i u m aluminate and t r i c a l c i u m
aluminate -k gypsum w e r e studied following addition of 0. 5, 1. 0,
5. 0 o r 10. 0% triethanolamine (TEA) at a s o l u t i o n / C 3 ~ r a t i o of
1. 0 a f t e r hydration p e r i o d s of 1 t o 60 min. TEA a c c e l e r a t e d the
hydration of C3A t o t h e hexagonal aluminate h y d r a t e and i t s con- v e r s i o n t o the cubic aluminate h y d r a t e . T h e r a t e of hydration i n c r e a s e d with i n c r e a s e d amounts of TEA, which a l s o a c c e l e r a t e d the f o r m a t i o n of ettringite in t h e C3A - g y p s u m - H 2 0 s y s t e m .
S OMMAIRE
L e s c a r a c t C r i s t i q u e s d'hydratation d e l ' a l u m i n a t e d e t r i c a l c i u m
et de l'aluminate d e t r i c a l c i u m -k gypse ont CtC CtudiCes apr'es
l'addition de 0. 5, 1. 0, 5. 0 ou 1070 de trigthanolamine (TEA) dans
un r a p p o r t s o l u t i o n / C 3 ~ de 1. 0 apr'es de pCriodes d'hydratation
allant de 1 2. 60 min. La TEA accCl'ere l'hydratation du C3A en
h y d r a t e d'aluminate hexagonal et s a conversion en h y d r a t e d'aluminate cubique. La vites s e d'hydratation augmente en proportion d i r e c t e de l a quantitC de TEA, qui accCl'ere Cgalement l a f o r m a t i o n de l ' e t t r i n g i t e dans le syst'eme C A-gypse-H 0.
42
ADMIXTURE, TRICALCIUM ALUMINATE, HYDRATION, RATE V o l . 3, No.
1
I n t r o d u c t i o n T r i e t h a n o l a m i n e ( T E A ) i s u s e d i n c e r t a i n a d m i x t u r e f o r m u l a t i o n s i n c o n c r e t e . In t h e w a t e r r e d u c i n g
-
a c c e l e r a t i n g t y p e of a d m i x t u r e T E A h a s b e e n r e p o r t e d t o c o u n t e r a c t t h e e x c e s s i v e r e t a r d a t i o n effect of t h e w a t e r - r e d u c i n g agent. T h e m a i n a d v a n t a g e i n u s i n g T E A i n s t e a d of C a C l i s t h a t it 2 d o e s not p r o m o t e c o r r o s i o n of embedded m e t a l s u s e d i n r e i n f o r c i n g c o n c r e t e . T h e m e c h a n i s m of t h e a c t i o n of T E A i n t h e h y d r a t i o n of p o r t l a n d c e m e n t is not c l e a r . It i s g e n e r a l l y t e r m e d a n a c c e l e r a t o r , but i t h a s notb e e n c o m p l e t e l y a c c e p t e d a s one (1-4). It i s p o s s i b l e t h a t T E A m a y a c t a s e i t h e r a r e t a r d e r o r a c c e l e r a t o r of h y d r a t i o n of p o r t l a n d c e m e n t , depending on t h e c h e m i c a l and m i n e r a l o g i c a l c o m p o s i t i o n of t h e c e m e n t and t h e a m o u n t of T E A added t o i t . F r o m a p r a c t i c a l standpoint, a d i r e c t s t u d y of t h e influence of T E A on t h e h y d r a t i o n of c e m e n t would b e useful. T h e a d m i x t u r e m a y a c t i n a c o m p l e x w a y t o affect t h e h y d r a t i o n of individual p h a s e s and t h e i r h y d r a t i o n p r o d u c t s , h o w e v e r , and i t s e e m s t o be m o r e m e a n i n g f u l t o i n v e s t i g a t e t h e r o l e of T E A i n t h e h y d r a t i o n of t h e individual, b i n a r y and t e r n a r y s y s t e m s b e f o r e extending t h e s t u d y t o t h e c e m e n t i t s e l f .
E a r l i e r w o r k on t h e effect of d i f f e r e n t a m o u n t s of T E A on t h e h y d r a t i o n of t r i c a l c i u m s i l i c a t e h a s r e v e a l e d a n u m b e r of p o i n t s (5). R e g a r d l e s s of t h e method of a n a l y s i s , DTA, TGA, X - r a y o r c h e m i c a l a n a l y s i s , t h e amount of l i m e found a t a n y t i m e i n a s a m p l e t r e a t e d w i t h T E A i s l e s s t h a n t h a t found i n a n u n t r e a t e d s a m p l e . Addition of T E A t o t r i c a l c i u m s i l i c a t e a l s o i n c r e a s e s t h e induction o r d o r m a n t p e r i o d , p r o m o t e s t h e f o r m a t i o n of a c a l c i u m s i l i c a t e h y d r a t e with a h i g h e r C ~ O / S ~ O r a t i o , and e n h a n c e s t h e f o r m a t i o n of n o n - 2 c r y s t a l l i n e C a ( 0 H ) 2' T h e e a r l y s e t t i n g c h a r a c t e r i s t i c s of p o r t l a n d c e m e n t a r e influenced by t h e t r i c a l c i u m a l u m i n a t e (C A) p h a s e . A l i t e r a t u r e s u r v e y h a s i n d i c a t e d t h a t 3 p r a c t i c a l l y no w o r k h a s b e e n r e p o r t e d on t h e influence of T E A on t h e h y d r a t i o n of C A. C i a c h and Swenson s t u d i e d t h e m o r p h o l o g i c a l c h a n g e s o c c u r r i n g i n 3 C A i n t h e p r e s e n c e of 0. 5% T E A a t 5 m i n , 1 h r , 4 h r and t h e r e a f t e r
(6).
3 T h e y concluded t h a t i n t h e f i r s t few d a y s t h e h y d r a t i o n p r o c e s s of C A p a s t e 3V o l . 3, N o .
1
4 3 ADMIXTURE, T R I C A L C I U M A L U M I N A T E , H Y D R A T I O N , R A T Ewith T E A i s s i m i l a r t o t h a t of a p a s t e without i t . T r i c a l c i u m a l u m i n a t e r e a c t s r a p i d l y with w a t e r and i s t h e r e f o r e u s e f u l i n studying t h e h y d r a t i o n p r o c e s s a t v e r y e a r l y t i m e s . A s D i f f e r e n t i a l T h e r m a l A n a l y s i s i s known t o b e e m i n e n t l y s u i t e d t o following h y d r a t i o n c h a r a c t e r i s t i c s of C A (7), i t w a s 3 u s e d i n t h e w o r k now r e p o r t e d of t h e h y d r a t i o n of C A and 3 C 3 A - g y p s u m m i x - t u r e s f o r p e r i o d s of 1 t o 60 m i n u s i n g different a m o u n t s of T E A . E x p e r i m e n t a l M a t e r i a l s T r i c a l c i u m a l u m i n a t e of high p u r i t y w a s p r e p a r e d by c a l c i n a t i o n of
CaCO and A1 0 T h e s a m p l e w a s ground t o p a s s a 200 - m e s h s i e v e and h a d
3 2 3' a Blaine s u r f a c e a r e a of 4350 s q ~ m / ~ . F r e e l i m e w a s s c a r c e l y d e t e c t a b l e by X - r a y . Only v e r y f a i n t l i n e s of C A could b e d i s c e r n e d . T h e c h a r a c t e r i s - 12 7 t i c s of C A have a l r e a d y b e e n r e p o r t e d (8). T r i e t h a n o l a m i n e of c e r t i f i e d 3
g r a d e (supplied by F i s h e r Scientific G O . ) and a r e a g e n t g r a d e gypsum w e r e u s e d .
Hydration
C A w a s mixed with double-distilled w a t e r a t a
water/^
A r a t i o of3 3
1. 0, p l a c e d i n tightly c o v e r e d polyethylene c o n t a i n e r s and r o t a t e d continu-
o u s l y o v e r r o l l e r s , At s p e c i f i e d i n t e r v a l s , which v a r i e d f r o m a few m i n u t e s
t o 1 h r , e a c h s a m p l e w a s p l a c e d i n a n e x c e s s of cold a c e t o n e , f i l t e r e d by
washing with cold acetone, and s u b s e q u e n t l y evacuated f o r 2 4 h r u s i n g liquid
a i r i n t h e t r a p . C a r e w a s t a k e n t o p r e v e n t contamination with CO A s i m i l a r 2' method w a s followed f o r h y d r a t i o n e x p e r i m e n t s i n t h e p r e s e n c e of 0. 5, 1. 0 and 5. 0% T E A a t a solution/C A r a t i o of 1. 0. 3 T h e influence of T E A on t h e h y d r a t i o n c h a r a c t e r i s t i c s of C A-gypsum 3 m i x t u r e s w a s studied a s d e s c r i b e d above. T h e C ~ / ~ y p s u m r a t i o w a s e i t h e r 3
20 o r 4, and thorough mixing w a s achieved with a Wig-1 -bug v i b r a t o r . T e c h n i a u e
A l l s a m p l e s w e r e s u b j e c t e d t o D i f f e r e n t i a l T h e r m a l A n a l y s i s , and s o m e t o conduction c a l o r i m e t r i c , X - r a y d i f f r a c t i o n and scanning e l e c t r o n m i c r o s c o p e investigations.
44
Vol.
3,
No. 1
ADMIXTURE, TRICALCIUM ALUMINATE, HYDRATION, RATE
DTA w a s c a r r i e d out i n a i r and i n a flow of N u s i n g a n a p p a r a t u s 2
supplied by R. L. S t o n e and Co. T h e h e a t i n g r a t e w a s m a i n t a i n e d a t 1 0 * l ° C .
In e a c h r u n 100 m g of t h e s a m p l e w a s u s e d t o f a c i l i t a t e a r e a s o n a b l e c o m p a r i - s o n of t h e r e l a t i v e i n t e n s i t i e s of t h e r m a l e f f e c t s developed a t d i f f e r e n t s t a g e s of h y d r a t i o n . D i f f e r e n t i a l t e m p e r a t u r e i s plotted on t h e y - a x i s of t h e t h e r m o - g r a m s . X - r a y p h o t o g r a p h s w e r e obtained by a N o r e l c o unit u s i n g a Debye- S c h e r r e r c a m e r a of d i a m e t e r 114. 6 m m
A conduction c a l o r i m e t e r containing s i x c h a m b e r s , supplied by t h e I n s t i t u t e of Applied P h y s i c s , Delft, w a s u s e d t o d e t e r m i n e t h e r a t e of h e a t
development of C A and C A
+
gypsum h y d r a t e d a l o n e and i n t h e p r e s e n c e of3 3
T E A . Although t h e c h a m b e r s w e r e c o a t e d with teflon t h e h y d r a t e d p r o d u c t s
tended t o s t i c k t o t h e s u r f a c e s o t h a t polyethylene l i n e r s w e r e u s e d . T h e s e n s i t i v i t y of t h e c a l o r i m e t e r w a s 20 ~ v / w ; t e m p e r a t u r e of t h e bath, 2 5 ° C . E x a m i n a t i o n of t h e h y d r a t e d s a m p l e s w a s a l s o c a r r i e d out by e l e c t r o n m i c r o - s c o p e u s i n g a C a m b r i d g e S t e r e o s c a n M a r k 2A unit. R e s u l t s and D i s c u s s i o n H y d r a t i o n of C A i n t h e P r e s e n c e of T E A 3 F i g u r e 1 r e f e r s t o t h e t h e r m a l c u r v e s of C A h y d r a t e d f o r 1. 5, 10, 3 30 and 60 m i n a t a w a t e r / C A r a t i o of 1. 0. C u r v e s of T E A w e r e obtained a t 3
0. 5, 1. 0 o r 5. 0% by m i x i n g with r e q u i r e d a m o u n t s of A1203. Unhydrated
C A d o e s not i n d i c a t e any t h e r m a l inflection and i s not shown. At 1 m i n t h e 3 C3A s a m p l e e x h i b i t s e n d o t h e r m a l e f f e c t s a t about 150 a n d 250°C (8, 9 ) . t y p i c a l of a m i x t u r e of hexagonal d i c a l c i u m a l u m i n a t e h y d r a t e , 2 C a 0 . A 1 2 0 3 . 8 H 2 0 (C2AH ) 8 and t e t r a c a l c i u m a l u m i n a t e h y d r a t e , 4CaO. A1 0
.
1 3 H 2 0 (C AHl3). 2 3 4A t about 5 m i n a l l s a m p l e s show a l a r g e e n d o t h e r m a l effect a t about 300°C, followed by a s m a l l e r e n d o t h e r m b e t w e e n 400 and 500°C. T h e s e i n f l e c t i o n s a r e t y p i c a l of t r i c a l c i u m a l u m i n a t e h e x a h y d r a t e
3 C a 0 . A1203. 6 H 2 0 (C3AH ) 6
V o l . 3 , N o .
1
4 5 ADMIXTURE, T R I C A L C I U M A L U M I N A T E , HYDRATION, RATEu
u
I l l 1 l l 1 1 i : u0 - T t A 0 57 T E A 1 0‘ T E A 5 - T E A
FIG. 1
Hydration of C A with Different Amounts of Triethanolamine
3
and r e p r e s e n t a stepwise dehydration of
6
m o l e s of H 0.2
The intensity of t h e peaks i n c r e a s e s a s hydration p r o g r e s s e s . Data
r e v e a l that C A h y d r a t e s t o the hexagonal aluminate hydrate p h a s e immediately
3
on contact with water and that i t t r a n s f o r m s t o t h e cubic f o r m within 5 min. The r a t e of formation of the hexagonal aluminate hydrate and i t s t r a n s f o r m a - tion t o the cubic f o r m depend on t e m p e r a t u r e and water/C A ratio. At a low
3
water/^
A r a t i o of 0. 12 i t m a y be 2 h r before t h e hexagonal f o r m t r a n s f o r m s 3t o the cubic f o r m
(9).
C3A s a m p l e s containing 0. 5, 1. 0 o r 5.070 TEA show t h e r m a l c h a r a c -
t e r i s t i c s different f r o m those of untreated C A. At 1 min, 0. 5 and 1. 070 TEA
3
p r o m o t e t h e formation of l a r g e r amounts of hexagonal aluminate hydrates, a s evidenced by t h e g r e a t e r intensity of the peak a t about 150°C (revealing
thereby t h a t a c c e l e r a t i o n of hydration of C A h a s taken place). The endo- 3
t h e r m a l effect a t about 250°C does not i n c r e a s e i n proportion t o that a t about 150°C because of t h e masking effect of t h e exothermal effect of TEA o c c u r r i n g between 200 and 300°C. At 5.070 TEA t h e r e i s a l a r g e amount of C AH even
3
6
a t 1 min, indicating that a c c e l e r a t i o n of hydration of C A i s enhanced by 3
46
Vol.
3 ,
No. 1
A D M I X T U R E , T R I C A L C I U M A L U M I N A T E , H Y D R A T I O N , R A T E
i n c r e a s e d amount of TEA. It w a s difficult, b e c a u s e of t h e e x p e r i m e n t a l l i m i t a t i o n s of stopping h y d r a t i o n efficiently within a few s e c o n d s , t o confirm
whether C A
+
57'0 T E A f o r m e d a hexagonal a l u m i n a t e h y d r a t e a s a p r e l u d e t o3
f o r m a t i o n of t h e cubic f o r m .
C A
+
57'0 T E A shows a n e x o t h e r m a l effect between 400 and 5 0 0 ° C a t3
60 m i n of hydration, a n effect not r e a d i l y a p p a r e n t i n o t h e r s a m p l e s . T h i s p e a k cannot be a t t r i b u t e d t o t h e oxidation effect of f r e e T E A b e c a u s e f r e e T E A
exhibits e x o t h e r m a l p e a k s between 200 and 300" C. Leaching of t h e s a m p l e
with ethyl alcohol does not r e m o v e t h e e x o t h e r m i c peak. F r e e T E A i s soluble i n alcohol and i t m a y be i n f e r r e d t h a t T E A r e a c t s with t h e a l u m i n a t e h y d r a t e t o exhibit t h e e x o t h e r m i c effect a t about 450°C. C a l c i u m lignosulfonate i s
shown t o be i r r e v e r s i b l y a d s o r b e d by t h e C AH p h a s e (10). 3 6 T h e a b s e n c e of e x o t h e r m i c p e a k s i n a l l o t h e r s a m p l e s m a y be due t o one o r m o r e of t h e following c a u s e s : ( 1 ) T h e amount of C AH f o r m e d i n o t h e r s a m p l e s i s l e s s and h e n c e l e s s T E A 3 6 i s bound. ( 2 ) A l a r g e r contact t i m e m a y b e r e q u i r e d f o r r e a c t i o n of t h e T E A admixture. ( 3 ) T E A m a y e x i s t i n t h e f r e e f o r m .
In s u c h s a m p l e s t h e l a r g e e n d o t h e r m of t h e C3AH effect, commencing a t
6
about 200°C, m a y m a s k t h e e x o t h e r m of t h e f r e e TEA. In t h e C3A
+
070 T E As a m p l e s i t m a y be o b s e r v e d t h a t e n d o t h e r m a l deviation of t h e p e a k o c c u r s a t
about 200°C. In s a m p l e s containing T E A t h e deviation o c c u r s beyond a
t e m p e r a t u r e of 200" C.
T h e a c c e l e r a t i n g effect of T E A on t h e h y d r a t i o n of C A was f u r t h e r 3
confirmed by r a t e of h e a t development i l l u s t r a t e d by t h e conduction c a l o r i
-
Vol.
3,
No.
1
4
7
A D M I X T U R E , T R I C A L C I U M A L U M I N A T E , H Y D R A T I O N , R A T E
a f t e r 10 to 12 min for C A hydrated without 3
TEA, and a s the amount of TEA i s increased, s o i s the r a t e of heat liberation enhanced. The inflection corresponding to the maximum r a t e of heat development o c c u r s e a r l i e r a s the
amount of TEA i s increased from 1. 0 t o 10. 070;
1
the maximum r a t e o c c u r s at only 3, min f o r the sample with 1070 TEA.
The total amount of heat developed in the f i r s t 30 min i s n e a r l y twice a s much for the
4 "
sample with 1070 TEA and about one and one half
2 3
a s mach for t h e sample with 470 TEA a s for corresponsing s a m p l e s containing no TEA. It
IS
should be noted that the r a t e of hydration d e t e r - mined by conduction c a l o r i m e t r y i s not directly
comparable to data obtained through DTA t h e r - r1:1c ? ~ I N U T E C
m o g r a m s because, in the c a l o r i m e t e r , the
sample-solution mixture i s not continuously FIG. 2
agitated during hydration. The method c l e a r l y Conduction C a l o r i m e t r i c
demonstrates the accelerating effect of TEA Curves of 3 C a 0 A1203
With Different Amounts on the hydration of C A.
3 of Added TEA
The exact mechanism of the accelerating effect of TEA on the h y d r a -
tion of C A i s difficult to prove conclusively. On exposure to water C A
3 3
f o r m s hexagonal aluminate hydrates on the s u r f a c e , and the r a t e of reaction a t the s u r f a c e and the p a s s a g e of water through the layer of hexagonal
aluminate hydrates control the o v e r - a l l r a t e of reaction. Triethanolamine is an aminoalcohol and cation active, and it i s possible that it i n t e r f e r e s with the formation of t h e usual protective layer of t h e hexagonal aluminate hydrate on the s u r f a c e of C3A. The formation of a complex of TEA with the hexagonal
phase was not detected in the t h e r m o g r a m s . It i s possible that, i f formed, this complex i s soluble in an aqueous medium.
48
ADMIXTURE, T R I C A L C I U M ALUMINATE, HYDRATION, RATE
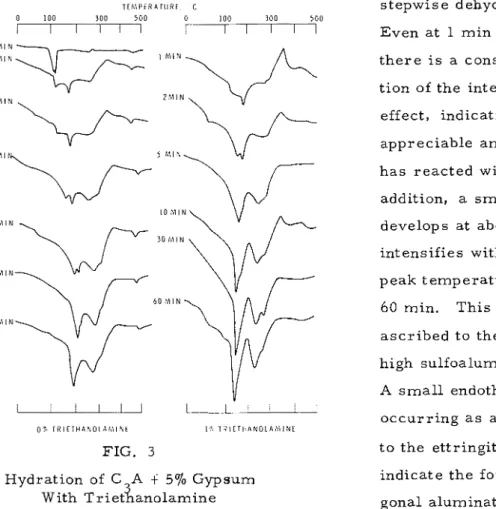
Hydration of C,A t Gypsum in t h e P r e s e n c e of TEA
V o l . 3, N o . 1
F i g u r e 3 shows t h e t h e r m a l behaviour of C 3 A hydrated in t h e p r e s e n c e
of 57'0 gypsum with and without 170 TEA. The unhydrated C A t gypsum m i x - 3
t u r e exhibits a typical endothermal doublet between 100 and 150" C due t o t h e
TEhlPiRATURL c stepwise dehydration of gypsum.
0 100
d
o
o
-
6
"
Even a t 1 m i n of hydrationO M l N
t h e r e i s a c o n s i d e r a b l e diminu- tion of the intensity of t h i s
2 M I N ,
effect, indicating that an appreciable amount of gypsum
h a s r e a c t e d with C A. In
3
addition, a s m a l l endotherm
! O ? < l l N develops at about 170" C that
intensifies with a shift in t h e
I
I peak t e m p e r a t u r e at 30 and
60 min. T h i s peak m a y be a s c r i b e d t o t h e p r e s e n c e of high sulfoaluminate (ettringit e).
V
v
A s m a l l endothermal effectu
u
o c c u r r i n g a s a doublet adjacent
OL T I I I E T H A N O L d ? l l N t I ' T H l t T H A N O L A h . l l \ t
FIG. 3 t o t h e ettringite peak m a y
Hydration of C A
f
57'0 GypsumWith ~ r i e t i a n o l a m i n e
indicate t h e formation of h e x a - gonal aluminate h y d r a t e . The endothermal humps between 250 and 300°C (containing two endothermal effects) m a y r e p r e s e n t t h e e x i s - t e n c e of the hexagonal aluminate h y d r a t e and i t s solid solution with t h e low
sulfoaluminat e.
E t t r i n g i t e i s t h e f i r s t sulfate-containing product that f o r m s in t h e h y - dration of C A with gypsum and c o n v e r t s , subsequently, t o t h e low sulfoalu-
3
m i n a t e f o r m , depending on t h e r a t i o of C A t o gypsum. In C A containing
3 3
57'0 gypsum t h e formation of ettringite, the hexagonal. aluminate hydrate, and conversion of ettringite t o t h e low sulfoaluminate o r solid solution s e e m t o occur together in the f i r s t hour of hydration.
V o l . 3, N o . 1 4 9 ADMIXTURE, T R I C A L C I U M A L U M I N A T E , H Y D R A T I O N , RATE
The C A t gypsum m i x t u r e containing 170 TEA a l s o shows endother-
3
ma1 e f f e c t s s i m i l a r t o those of the m i x t u r e containing no TEA. The r a t e of formation of various h y d r a t e s i s different : gypsum s e e m s t o be
consumed m o r e rapidly; ettringite f o r m s in l a r g e r quantities, even a t 2 min, and continues t o i n c r e a s e up t o 60 min; and the amount of low sulfo- aluminate i s enhanced. T h e s e r e s u l t s a r e evidence that TEA a c t s a s an
a c c e l e r a t o r in the hydration of C A t gypsum m i x t u r e .
3
In o r d e r t o accentuate some of t h e s e effects, a f u r t h e r set of e x -
p e r i m e n t s was c a r r i e d out by hydrating a C A t gypsum m i x t u r e containing
3
25% gypsum ( s e e t h e r m o g r a m s , T E I I P L R A T U R C c
0 1 0 0 3 0 0 5 0 0
Fig. 4). T h e r m a l c u r v e s of C A
m
0+Fr?14u
3 h l l N 0
t gypsum hydrated in the p r e s e n c e
of TEA a r e different from those
without TEA. Hydration s e e m s t o 2,,,
s t a r t in the f i r s t minute of contact
of H 0 with the C A t gypsum
2 3
mixture. At 5 m i n a l a r g e r amount of gypsum h a s disappeared from the sample containing TEA; a t
10 and 30 min it i s evident that the ettringite peak o c c u r r i n g between 150 and 200°C i s m o r e intense. Beyond 30 min t h e two s a m p l e s (with and without TEA)
exhibit almost s i m i l a r intensities.
1
It a p p e a r s that formation of U J
-
0 % T R I E T H A N O L A h l l N E 1% T R I E T H A N O L A h I I N E
ettringite i s a c c e l e r a t e d between 5
FIG. 4
and 10 min in the TEA-treated
Hydration of C A 3 t 25Y0 Gypsum
sample, w h e r e a s in the untreated With Triethanolamine
s a m p l e t h i s o c c u r s between 10 and 30 min. S i m i l a r t r e n d s m a y be observed
in C A t 5% gypsum m i x t u r e s (Fig. 3 ) . Additional peaks corresponding t o
3
those f o r hexagonal aluminate h y d r a t e s a r e not indicated in the C A
+
25703
gypsum m i x t u r e s . Where t h e r e i s an e x c e s s of gypsum, C A p r e f e r e n t i a l l y 3
5 0 V o l . 3, N o . 1 ADMIXTURE
,
T R I C A L C I U M A L U M I N A T E , HYDRATION, RATEf o r m s t h e sulfoaluminate products. L a r g e amounts of low sulfoaluminate h y d r a t e a r e formed between 10 and 30 min. A s m a l l endothermal effect between 100 and 150°C p e r s i s t i n g t o 60 m i n m a y r e p r e s e n t r e s i d u a l gypsum; it m a y a l s o be contributed t o s o m e extent by the ettringite phase.
T h e a c c e l e r a t i n g influence of TEA in the hydration of C A t gypsum
3
should be reflected in t h e amount of heat developed during the r e a c t i o n and m a y be followed through the conduction c a l o r i m e t r i c c u r v e s ( F i g . 5).
I
I I I I I I I I Generally, the r a t e of h e a t develop-ment in T E A - t r e a t e d s a m p l e s i s higher. T h e t o t a l heat developed in
t h e f i r s t 30 min in the C3A t 570
0 - -
gypsum s a m p l e with and without TEA, i s in the r a t i o 1:O. 77; the c o r r e s p o n d -
ing r a t i o for the C A t 2570 gypsum
3
i s 1:O. 9. As h a s a l r e a d y been stated,
t h e r a t e of heat development d e t e r - mined by conduction c a l o r i m e t r y i s not d i r e c t l y c o m p a r a b l e with DTA c u r v e s . T h e c u r v e s , however, demon- s t r a t e t h e r e l a t i v e r a t e s of reaction of
C A t gypsum s a m p l e s with and with-
3 out TEA.
0
0 I 0 20 30 4 0 5 0 No definite conclusions could
TlhlE M I N U T E S
FIG. 5
be drawn f r o m the X - r a y data. 3A l i n e s become somewhat weaker a s
Conduction C a l o r i m e t r i c hydration p r o g r e s s e s . T h e C3A t
C u r v e s of 3 C a 0 A1203
Hydrated in t h e P r e s e n c e gypsum
+
H 2 0+
570 TEA s a m p l e showsof C a S 0 4 2H 0 and TEA
2 a broad band in the r a n g e 9 . 8 t o 10A,
not indicated in the untreated s a m p l e s possibly owing t o t h e formation of m o r e e t t r i n g i t e in the TEA-treated sample.
E l e c t r o n m i c r o s c o p i c examination provided observations of morpho
-
logical changes o c c u r r i n g in the s y s t e m a t different p e r i o d s of hydration. In general, the ettringite needles predominated in a l l s a m p l e s during the e a r l y
Vol.
3, No.
1
51
A D M I X T U R E , T R I C A L C I U M A L U M I N A T E , H Y D R A T I O N , R A T E
FIG. 6A FIG. 6B C A+
Gypsum+
H 0 a t 5 m i n 2 C A t Gypsum+
H20
+
TEA (170) 3 3 ( x 1400) 5 m i n ( x 1400) FIG. 6C FIG. 6D C A t Gypsum+
H
0 a t 5 m i n 3 2 C A t Gypsum t H 3 2 0+
T E A (170) ( x 3650) 5 m i n ( x 3650)p e r i o d s of h y d r a t i o n . At 5 m i n t h e C A t 570 gypsum m i x t u r e with TEA
3
indicated a m o r e d e n s e f o r m a t i o n of e t t r i n g i t e n e e d l e s on t h e C A s u r f a c e t h a n 3
did t h e u n t r e a t e d s a m p l e ( F i g . 6A t o D ) . T h e r e w a s a l s o s o m e indication t h a t
m o r e unhydrated p a r t i c l e s e x i s t in t h e u n t r e a t e d s a m p l e . At 60 m i n of h y d r a - t i o n a p l a t y s t r u c t u r e f o r m e d in a l l s a m p l e s , c o r r e s p o n d i n g t o t h e hexagonal a l u m i n a t e h y d r a t e , low s u l f o a l u m i n a t e o r i t s solid solution (Fig. 6 ~ t o G).
52 V o l . 3, N o . 1 ADMIXTURE, T R I C A L C I U M A L U M I N A T E , HYDRATION, R A T E FIG. 6 E FIG. 6 F C A 3
+
G y p s u m+
H 0 a t 60 m i n 2 C A t G y p s u m t 3 H 2 O + T E A (1%) ( x 7500) a t 60 m i n ( x 7500) FIG. 6G C A+
G y p s u m t H 0+
T E A (5%) 3 2 a t 60 m i n ( x 7500) In t h e h y d r a t i o n of C A t gypsum m i x t u r e s i t i s g e n e r a l l y believed 3 t h a t a l a y e r of h i g h s u l f o a l u m i n a t e f o r m s on t h e a n h y d r o u s C A s u r f a c e and 3 r e t a r d s t h e r e a c t i o n by d e c r e a s i n g diffusion of s u l f a t e i o n s t h r o u g h i t . With t i m e , m o r e s u l f a t e c o m p l e x f o r m s u n t i l t h e l a y e r b r e a k s . It a p p e a r s t h a t t h e addition of T E A i n t e r f e r e s with t h e f o r m a t i o n of a n i m p e r m e a b l e l a y e r of s u l f o a l u m i n a t e b y c o m p e t i n g f o r a d s o r p t i o n on t h e C A s u r f a c e . T r i e t h a n o l a - 3 + t t++
m i n e is c a t i o n a c t i v e and i s c a p a b l e of r e p l a c i n g A 1 and C a i o n s . T h e p o s s i b i l i t y of t h e f o r m a t i o n of a l e s s c r y s t a l l i n e e t t r i n g i t e i n t h e p r e s e n c e of T E A i s difficult t o p r o v e , but t h e r e i s i n d i c a t i o n of a s t r o n g e x o t h e r m i n t h eVol.
3,
No. 1
53
A D M I X T U R E , T R I C A L C I U M A L U M I N A T E , H Y D R A T I O N , R A T E
r a n g e 300 t o 400°C, even a t 1 m i n , i n T E A - t r e a t e d s a m p l e s . T h i s effect i s a b s e n t a t s u b s e q u e n t p e r i o d s of h y d r a t i o n . It m a y r e p r e s e n t a c r y s t a l l i z a t i o n effect of t h e d e h y d r a t e d e t t r i n g i t e . Analogous e x o t h e r m a l effect i n t h e
t h e r m a l c u r v e s of C A. C a C l . 6 H 0 h a s b e e n r e p o r t e d ( 1 1 ) . 3 2 2 C o n c l u s i o n s T r i e t h a n o l a m i n e a c t s a s a r e t a r d e r i n t h e h y d r a t i o n of t r i c a l c i u m s i l i c a t e by extending t h e induction p e r i o d . It a c t s , h o w e v e r , a s a n a c c e l e r a - t o r i n t h e h y d r a t i o n of t r i c a l c i u m a l u m i n a t e , by both i n c r e a s i n g t h e f o r m a t i o n of t h e h e x a g o n a l a l u m i n a t e h y d r a t e s and p r o m o t i n g c o n v e r s i o n t o t h e cubic a l u m i n a t e h y d r a t e . T E A a l s o a c c e l e r a t e s t h e f o r m a t i o n of e t t r i n g i t e i n t h e C A - g y p s u m - H 0 s y s t e m . 3 2 Acknowledgment
T h e a u t h o r i s g r a t e f u l t o P. E. Grattan-Bellew for useful d i s c u s s i o n and acknowledges with t h a n k s t h e v a l u a b l e e x p e r i m e n t a l c o n t r i b u t i o n of
G. M. P o l o m a r k and t h e a s s i s t a n c e of E. G. Quinn and P. J. L e f e b v r e .
T h i s p a p e r i s a c o n t r i b u t i o n f r o m t h e Division of Building R e s e a r c h , National R e s e a r c h C o u n c i l of Canada, and i s published with t h e a p p r o v a l of t h e D i r e c t o r of t h e Division.
R e f e r e n c e s
1. S. M. Royak, V. S. Klemet'eva, and G. M. T a r n a r u t s k i i . Z h u r n a l
P r i k l . Khimii, 43, 8 2 (1970).
-
2 . ACI C o m m i t t e e 212, A d m i x t u r e s i n C o n c r e t e , J. A m e r . C o n c r e t e I n s t . , 1 6 , 113 (1954).-
3. K. E. F l e t c h e r , and M.H. R o b e r t s . C o n c r e t e , 5, 142 (1971).-
4. V. S, R a m a c h a n d r a n . (unpublished r e s u l t s ) . 5. V. S , R a m a c h a n d r a n . "fluence of t r i e t h a n o l a m i n e on t h e h y d r a t i o n c h a r a c t e r i s t i c s of t r i c a l c i u m s i l i c a t e P . T o b e published. 6. T . D . C i a c h , a n d E . G , Swenson. C e m e n t C o n c r e t e R e s , , 1, 1 4 3 ( 1 9 7 1 ) .-
7. V. S. R a m a c h a n d r a n . Applications of D i f f e r e n t i a l T h e r m a l A n a l y s i s i n54 V o l . 3, N o . 1 ADMIXTURE, T R I C A L C I U M A L U M I N A T E , HYDRATION, RATE
Cement C h e m i s t r y , C h e m i c a l Publishing Co.
,
New York, pp. 308,(1969).
8. V. S. R a m a c h a n d r a n and R. F. -Feldman. Cement Technology,
-
2, 121(1971).
9. R. F. F e l d m a n and V. S. Ramachandran. J. A m e r . C e r a m . S o c . , 49,
-
268 (1966).
10. V. S. R a m a c h a n d r a n and R. F. Feldman. M a t e r i a u x et Constructions,
5, 67 (1971).
-
11. S. J. Ahmed, L. S. Dent G l a s s e r and H. F. W. T a y l o r . P r o c . Fifth