Hépatite virale chronique C
Texte intégral
Figure

Outline
Documents relatifs
data series... Fichtner et al. Title Page Abstract Introduction Conclusions References Tables Figures J I J I Back Close Full Screen / Esc.. Printer-friendly Version
By investigating a large cohort of 1112 HDV-infected patients followed in France but coming from differents areas of the world we were able to determine that HDV genotype, the place
To explore the relationship between starting ribavirin doses, expressed as mg/kg body weight and both rapid viral response at treatment week 4 [RVR] and sustained virological response
Au quatrième trimestre 2018, en France métropolitaine, 68,1 % des personnes inscrites à Pôle emploi en catégories A, B, C, D, E ou dispensées de recherche d'emploi sont indemnisables
20 we decided to assess the ability of high doses of pegylated IFN- α with standard or high doses of ribavirin to induce a significant antiviral response in genotype 1
Patients were random- ly assigned to one of four treatment groups: the T12PR24 group, which received telaprevir (VX-950, Vertex Pharmaceuticals), peginterferon alfa-2a
In hepatitis B “e” antigen (HBeAg) positive patients with hepatitis B virus (HBV) mono-infection, intensification of nucleos(t)ide analogue treatment with pegylated-interferon
Conclusion The sofosbuvir+daclatasvir combination is associated with a high rate of SVR12 in patients infected by genotype 1, with an optimal duration of 12 weeks in non-cirrhotic and
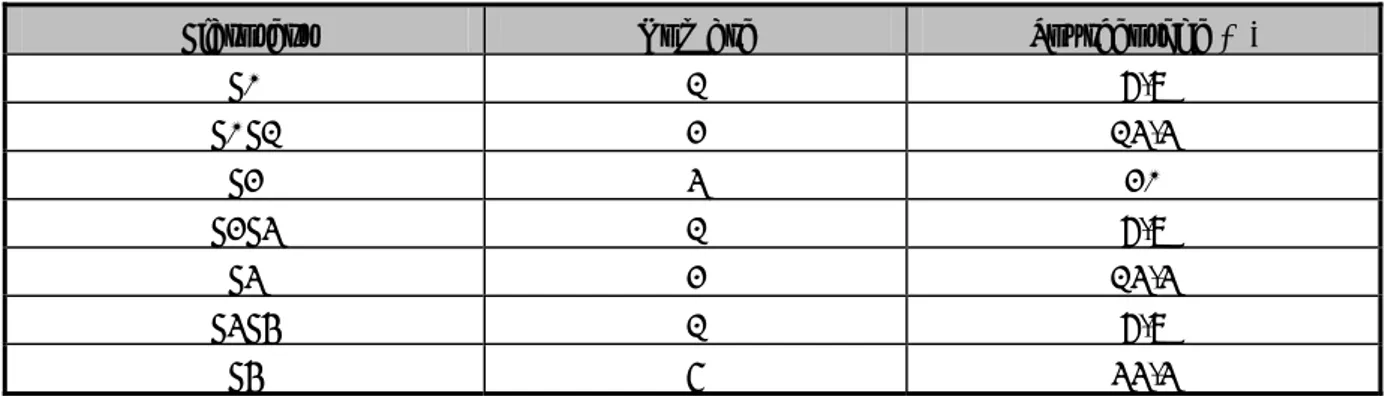

![Figure 7 : Le génome et les protéines du VHC [16].](https://thumb-eu.123doks.com/thumbv2/123doknet/1965879.385/58.892.120.797.546.1005/figure-genome-proteines-vhc.webp)
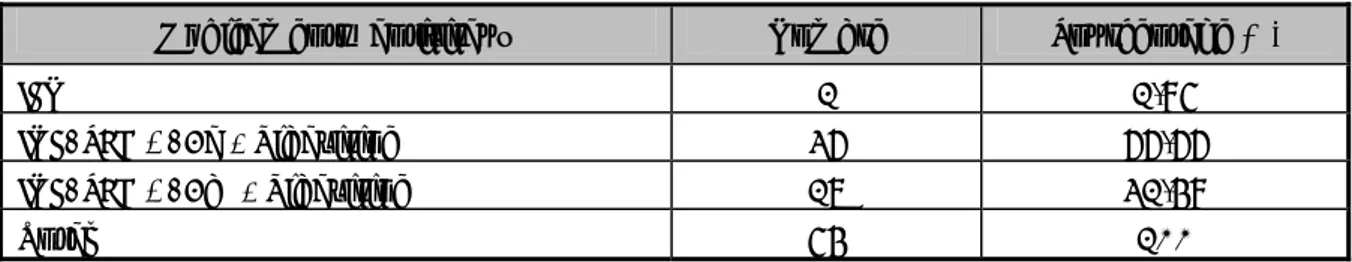
![Figure 8 : La microscopie électronique de virions VHC purifiés dans une culture de cellule [21]](https://thumb-eu.123doks.com/thumbv2/123doknet/1965879.385/59.892.260.663.120.791/figure-microscopie-electronique-virions-vhc-purifies-culture-cellule.webp)