Contrôle du transfert de l’information par la
dynamique calcique présynaptique aux synapses
formées par les fibres moussues de l’hippocampe
Thèse
Simon Chamberland
Doctorat en neurobiologie
Philosophiae doctor (Ph. D.)
Québec, Canada
© Simon Chamberland, 2017
Contrôle du transfert de l’information par la
dynamique calcique présynaptique aux synapses
formées par les fibres moussues de l’hippocampe
Thèse
Simon Chamberland
Sous la direction de :
iii
Résumé
Les neurones encodent l’information dans le nombre et la fréquence des potentiels d’action qu’ils déchargent. Les patrons de décharge de potentiels d’action enregistrés dans les animaux vivants varient fortement dans leur nombre et leur fréquence. Les variations dans la fréquence et le nombre de potentiel d’action déchargés affectent drastiquement la plasticité à court terme et le transfert de l’information vers la cellule postsynaptique. Comment les terminaux présynaptiques décodent la fréquence et le nombre de potentiel d’action par des dynamiques calciques spécifiques demeure inconnu.
Afin d’explorer cette question, nous avons combiné l’imagerie calcique par microscopie deux photons à accès aléatoire avec l’électrophysiologie dans les tranches aiguës d’hippocampe. Nous avons procédé à l’analyse de l’ultrastructure des terminaux synaptique par immunohistochimie et microscopie électronique.
Nous avons découvert que la propagation des potentiels d’action des cellules granulaires aux cellules principales du CA3 était dépendante du nombre de potentiel d’action dans une bouffée, mais était indépendante de la fréquence moyenne des potentiels d’action dans la bouffée. Le nombre de potentiel d’action dans une bouffée était encodé par le terminal présynaptique dans l’homogénéisation spatiale des microdomaines calciques. Cette globalisation des microdomaines calciques dans les terminaux présynaptiques supportait le recrutement de site de relâchements additionnels, suffisant pour augmenter grandement l’amplitude des courants postsynaptiques. De plus, les canaux calciques de type P/Q couplés faiblement aux senseurs calciques et localisés à une distance plus grande des zones actives étaient l’élément clé permettant l’homogénéisation des microdomaines calciques et le recrutement de sites de relâchement additionnels.
Ainsi, les fibres moussues de l’hippocampe propagent les potentiels d’action vers les cellules principales du CA3 en fonction du nombre de potentiel d’action dans la bouffée, indépendamment de leur fréquence. Cette transmission est possible grâce à la dynamique calcique présynaptique hautement spécialisée qui optimise l’utilisation d’un grand nombre de sites de relâchement.
iv
Abstract
Neurons encode information in the number and frequency of action potentials they discharge. Action potentials typically occur in bursts of varying number and frequency, with variations in these two parameters dramatically affecting short-term plasticity and the transfer of information to the postsynaptic neuron. How presynaptic terminals decode the frequency and the number of action potentials through calcium dynamics to gate neurotransmitter remains unknown.
To investigate this question, we combined random-access two-photon presynaptic calcium imaging in large mossy fiber terminals and electrophysiology in acute hippocampal slices. We further probed the ultrastructure of the mossy fiber terminals using immunohistochemistry and electron microscopy.
We found that action potential propagation from hippocampal granule cells to postsynaptic CA3 pyramidal cells was dependent on the number of action potentials (AP) in the granule cell burst, but was independent of the AP burst average frequency. Interestingly, the number of action potentials in a burst was encoded in presynaptic terminals by the spatial homogenization of calcium microdomains. This globalization of calcium microdomains within single presynaptic terminals supported the recruitment of additional release sites, sufficient to increase the EPSC amplitude several fold. Additionally, loosely-coupled P/Q-type VGCCs from calcium sensors provided the functional basis for the homogenization of calcium microdomains and proved essential for the recruitment of additional release sites. Therefore, hippocampal mossy fiber terminals propagate action potentials to CA3 pyramidal cells as a function of the number of action potentials in the burst, but not the frequency. This counting logic is made possible through specialized spatiotemporal calcium dynamics which optimize the use of a large number of release sites.
v
Table of contents
Résumé ... iii
Abstract ... iv
Table of contents ... v
List of figures ... xii
Abbreviations ... xv
Acknowledgements ... xvii
Foreword ... xviii
Chapter 1 - Introduction ... 1
A brief history of neuroscience leading to the study of synapses ... 1
The neuron doctrine ... 1
Propagation of electrical signals in nerves ... 3
Synapse: a specialized site of contact ... 4
The membrane potential ... 4
Neurotransmitter release and generation of postsynaptic currents ... 6
Probing the activity of individual neurons with the patch-clamp technique ... 8
The age of light... 9
Presynaptic terminals ... 10
Ultrastructure of presynaptic terminals ... 10
Molecular machinery of the presynaptic terminal ... 12
Voltage-gated sodium channels ... 15
Voltage-gated potassium channels ... 16
Voltage-gated calcium channels ... 17
Synaptic vesicles ... 19
Calcium sensors ... 21
Lifecycle of synaptic vesicles ... 23
Synaptic vesicle recycling through endocytosis ... 24
Synaptic vesicle docking and priming ... 26
Synaptic vesicle exocytosis ... 26
Synaptic vesicle pools ... 27
Readily-releasable pool ... 28
Recycling pool ... 29
vi
Modes of neurotransmitter release ... 30
Spontaneous release ... 31
Synchronous release ... 32
Asynchronous release ... 32
Multivesicular release ... 33
Main determinants of synaptic transmission ... 33
Organization of voltage-gated calcium channels ... 34
Presynaptic calcium dynamics ... 35
VGCC to vesicle coupling configurations ... 36
Postsynaptic domain of glutamatergic synapses ... 36
Short-term synaptic plasticity ... 37
Short-term facilitation ... 39
Calcium cooperativity of release ... 40
Calcium sensor specialized for short-term facilitation ... 40
Calcium-induced calcium release ... 41
Activation of presynaptic receptors ... 41
Multivesicular release ... 41
Amplification of presynaptic calcium current ... 42
Saturation of endogenous calcium buffers ... 42
The hippocampus ... 43
Anatomy of the hippocampus ... 44
CA3 pyramidal cells ... 47
Anatomical properties of the mossy fiber to CA3 pyramidal cell synapse ... 49
Functional properties of giant mossy fiber terminal to CA3 pyramidal cell synapses ... 51
Questions and Hypothesis ... 54
Chapter 2 – Presynaptic calcium dynamics translate bursts of action potential to count the number of action potentials ... 55
Foreword ... 55
Résumé ... 56
Abstract ... 58
Introduction ... 59
Material and Methods ... 61
vii
Whole-cell patch-clamp recording ... 61
Random-access two-photon calcium imaging ... 62
Analysis of electrophysiological and calcium imaging data ... 63
Results... 64
Action potential transmission to CA3 pyramidal cells by in vivo-like granule cell firing . 64 Synchronous glutamate release is encoded in the number of APs but not their frequency ... 65
Homogenization of calcium microdomains counts the number of action potentials ... 68
Discussion ... 71
Bibliography ... 74
Chapter 3 – Interplay between synchronization of multivesicular release and recruitment of additional release sites support short-term facilitation at hippocampal mossy fiber to CA3 pyramidal cells synapses ... 76
Foreword ... 76
Résumé ... 77
Abstract ... 79
Introduction ... 80
Material and methods ... 82
Slice preparation ... 82
Electrophysiological recordings ... 82
Random access two-photon microscopy ... 83
Two-photon glutamate uncaging and calcium imaging ... 84
Data analysis ... 85
Results... 87
Two mechanisms support short-term facilitation at MF-CA3 synapses ... 87
Short-term facilitation is of presynaptic origin ... 91
Gradual increase in Q during trains in conditions of low release probability ... 93
Increased cleft glutamate concentration during facilitation in conditions of low release probability ... 96
Calcium stores contribute to synchronization of multivesicular release ... 99
Intraterminal calcium hotspots support recruitment of additional release site ... 101
Compartmentalization of calcium microdomains is restricted to large MF terminals in granule cell axons ... 104
viii
Calcium dynamics gates MF-CA3 short-term facilitation ... 108
Compartmentalized calcium elevations in MF boutons ... 109
Bibliography ... 111
Chapter 4 – Distinct contribution of presynaptic CaV2.1 and CaV2.2 to glutamate release from hippocampal mossy fiber terminals ... 119
Foreword ... 119
Résumé ... 120
Abstract ... 122
Introduction ... 123
Results... 125
Distinct roles of P/Q- and N-type VGCCs in synchronous glutamate release ... 125
Spatiotemporal dynamics of calcium elevations in MF boutons ... 129
Ca2+ elevations generated by P/Q- VGCCs are spatially homogeneous ... 130
P/Q-type VGCCs support short-term facilitation by recruiting additional release sites 132 Ca2+ influx through P/Q- have access to more release sites than N-type VGCCs ... 136
Ca2+ influx through P/Q-type have access to release sites controlled by N-type VGCCs 139 Homogenization of calcium microdomains during trains of action potentials ... 143
Ca2+ homogenization through loosely-coupled P/Q-type VGCCs ... 147
Discussion ... 152
Spatiotemporal calcium dynamics in giant MF terminals ... 152
Calcium handling in giant MF terminals ... 153
Short-term facilitation and vesicle release ... 154
Experimental Procedures ... 157
Hippocampal slices preparation ... 157
Electrophysiology ... 157
Random-access two-photon microscopy ... 158
Presynaptic calcium imaging ... 159
Pharmacology ... 160
Electrophysiological data analysis ... 161
Coefficient of variation analysis ... 161
Covariance analysis ... 162
Non-stationary variance-mean analysis ... 163
ix
References ... 166
Chapter 5 – Fast two-photon imaging of subcellular voltage dynamics in neuronal tissue with genetically encoded voltage indicators ... 172
Foreword ... 172
Résumé ... 174
Introduction ... 176
Results... 178
Improving GEVI characteristics for two-photon imaging of action potentials ... 178
Characterization of GEVIs in excitatory cells ... 181
Fast random-access two-photon imaging of GEVI responses in hippocampal slices ... 185
Tracking spike propagation in slice with ASAP2 and random-access two-photon imaging ... 189
Two-photon microscopy of subcellular voltage responses to physiological stimuli in vivo ... 191
Discussion ... 195
Materials and methods ... 199
Plasmid Construction ... 199
HEK293A Cell Culture, Patch Clamping, and Voltage Imaging ... 199
Quantifying Voltage Sensor Brightness and Photostability in HEK293A Cells ... 200
Generation, Cell Culture, and Voltage Imaging of Stem Cell-Derived Cardiomyocytes 202 Neuronal Cell Culture and Transfection ... 203
Confocal Imaging of Rat Cortical Neurons ... 204
Patch Clamping and Voltage imaging of Dissociated Neurons ... 204
Preparation and Electroporation of Organotypic Hippocampal Slices... 205
Slice Electrophysiology ... 205
Random-Access Two-Photon Voltage Imaging and Analysis ... 206
Transgenic Flies ... 207
In Vivo Two-Photon Imaging ... 207
Analysis of In Vivo Imaging Data ... 208
Statistical Analyses ... 209
Acknowledgements ... 210
References ... 211
Chapter 6 – Summary, Discussion and Perspectives ... 216
x
Discussion ... 216
Information transfer in neuronal networks ... 218
How do neurons transfer information? ... 220
Counting logic at the mossy fiber to CA3 pyramidal cell synapse ... 223
Properties of the counting logic at mossy fiber to CA3 pyramidal cell synapse ... 224
What are the advantages of the counting logic at the MF to CA3 pyramidal cell synapse? ... 227
Behavioral correlates of the counting logic in another system ... 229
Triggering the detonator ... 231
Interplay between neurotransmitter release and postsynaptic driving force ... 231
How do giant MF terminals release large quantities of glutamate? ... 236
Synchronization of multivesicular release and recruitment of additional release sites support AP counting ... 241
Calcium spatiotemporal dynamics in giant mossy fiber terminals ... 245
Mechanisms for calcium entry in giant mossy fiber terminals ... 246
Temporal properties of calcium elevations in giant mossy fiber terminals ... 246
What are the spatial properties of calcium elevations in giant mossy fiber boutons? . 248 How do P/Q- and N-type VGCCs control calcium elevations in giant MF terminals? 249 Repetitive activity homogenize presynaptic calcium microdomains ... 249
Limitations of presynaptic Ca2+ imaging measurements and technical considerations ... 250
How do specialized calcium spatiotemporal dynamics trigger glutamate release and shape short-term facilitation? ... 254
Dual coupling configuration in giant mossy fiber terminals ... 254
How do P/Q- and N-type VGCCs support glutamate release and short-term facilitation through their specific calcium dynamics? ... 256
What are the advantages of a dual coupling system for a presynaptic terminal with multiple release sites? ... 259
Overview of action potential counting through short-term facilitation at the mossy fiber synapse ... 261
Optical nanophysiology: probing electrical activity in submicrometer compartments ... 263
Perspectives ... 270
Conclusion ... 271
Bibliography ... 272
xi Foreword ... 294
xii
List of figures
Figure 1-1. Drawings of neurons by Santiago Ramon y Cajal. ... 2
Figure 1-2: Luigi Galvani’s discovery of bioelectricity. ... 3
Figure 1-3 : Hodgkin and Huxley’s experiment. ... 5
Figure 1-4 : Exploring the nature of neurotransmitter release. ... 7
Figure 1-5 : Ultrastructural features of the synapse. ... 11
Figure 1-6 : Molecular composition of a presynaptic terminal. ... 13
Figure 1-7 : Molecular machinery gating the interactions between the synaptic vesicle and the presynaptic membrane controlling neurotransmitter release. ... 14
Figure 1-8 : The different states of voltage-gated sodium channels. ... 15
Figure 1-9 : Voltage-gated potassium channels can be in a closed or open state and do not have an inactivated state. ... 16
Figure 1-10: Structure and function of voltage-gated calcium channels. ... 17
Figure 1-11 : Molecular anatomy of a synaptic vesicle. ... 20
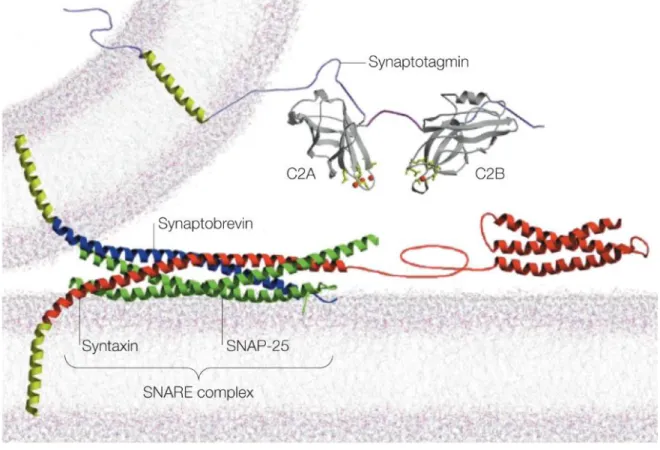
Figure 1-12 : Synaptotagmins sense ambient calcium concentration to trigger vesicle exocytosis. ... 22
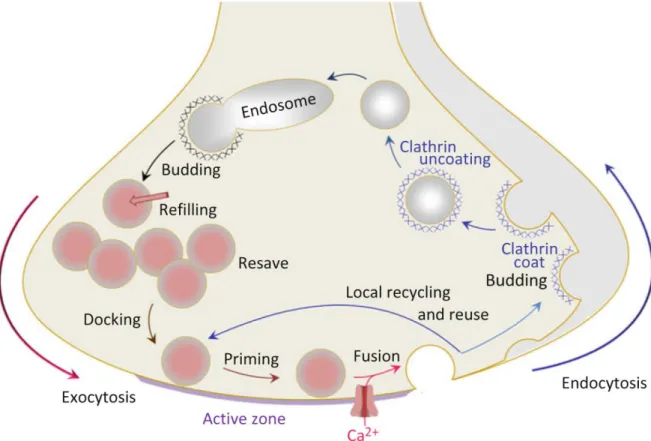
Figure 1-13 : Endocytosis, priming and exocytosis of vesicles support the function of neurotransmitter release from nerve terminals. ... 23
Figure 1-14 : The three pool model and the gradual release of vesicles in these pools during stimulation of the presynaptic terminal. ... 28
Figure 1-15 : Different patterns of activity may selectively recruit vesicles from distinct pools. ... 30
Figure 1-16 : Three modes of release govern synaptic transmission. ... 31
Figure 1-17 : Different profiles of short-term plasticity can be mediated at both sides of the synapse. ... 39
Figure 1-18 : The hippocampus ... 44
Figure 1-19 : Picture of a horizontal slice of the hippocampal formation showing the different regions. ... 45
Figure 1-20 : Properties of hippocampal granule cells firing in vivo... 46
Figure 1-21 : Anatomical properties of CA3 pyramidal neurons ... 48
Figure 1-22 : Ultrastructural anatomy of the MF to CA3 pyramidal cell synapse. ... 49
Figure 1-23 : Short-term dynamics of neurotransmitter release at two hippocampal mossy fiber targets. ... 52
Figure 2-1 : Transmission of AP to CA3 pyramidal cells depends on the number of APs in a burst ... 64
Figure 2-2 : MF terminals count the number of APs to release glutamate ... 66
Figure 2-3 : Homogenization of presynaptic calcium microdomains support AP counting ... 69
Figure 3-1 : Two mechanisms are involved in short-term facilitation at MF-CA3 synapses .... 88
Figure 3-2 : Short-term facilitation involves two components during prolonged activity and requires increased intracellular calcium ... 90
Figure 3-3 : Mechanisms involved in short-term facilitation are of presynaptic origin... 92
xiii Figure 3-5 : Increase in cleft glutamate concentration accompanies EPSC facilitation in
condition of low release probability ... 97
Figure 3-6 : Intracellular calcium stores contribute to short-term facilitation in low extracellular Ca2+ ... 100
Figure 3-7 : Calcium compartmentalization in single mossy fiber terminals revealed by simultaneous multi-site recordings of calcium elevations ... 103
Figure 3-8 : Compartmentalized calcium elevations in large MF boutons but not in en passant terminals ... 106
Figure 4-1 : Ca2+ influx through P/Q- and N-type VGCCs differently control glutamate release ... 126
Figure 4-2 : Effect of blocking P/Q- or N-type VGCCs on EPSC amplitude ... 127
Figure 4-3 : Blocking both P/Q- and N-type VGCCs abolish EPSCs ... 128
Figure 4-4 : Spatiotemporal properties of Ca2+ elevations in MF boutons ... 130
Figure 4-5 : Ca2+ elevations through P/Q-type VGCCs are more spatially homogeneous ... 131
Figure 4-6 : P/Q-type VGCCs contribute to short-term facilitation through recruitment of additional release sites and N-type VGCCs control multivesicular release ... 134
Figure 4-7 : Ca2+ influx from P/Q-type VGCCs have access to more release sites than N-type VGCCs ... 137
Figure 4-8 : Varying the contribution of intra- and intersite variability in variance-mean analysis ... 139
Figure 4-9 : P/Q-type VGCCs contribute to release at active zones initially controlled by N-type VGCCs ... 141
Figure 4-10 : Blocking P/Q-type VGCCs limits maximal EPSC amplitudes ... 142
Figure 4-11 : Homogenization of presynaptic Ca2+ microdomains during trains of APs ... 144
Figure 4-12 : Amplitudes of Ca2+ elevations are unchanged during trains of 20 APs ... 146
Figure 4-13 : P/Q-type VGCCs homogenize Ca2+ microdomains to recruit additional release sites ... 148
Figure 4-14 : Effect of EGTA-AM on EPSC amplitude and short-term facilitation ... 150
Figure 5-1 : Improving GEVI characteristics for two-photon imaging of action potentials. .. 179
Figure 5-2 : Development of ASAP2. ... 180
Figure 5-3 : Characterization of GEVIs in excitatory cells. ... 182
Figure 5-4 : Voltage imaging of cardiomyocytes derived from induced pluripotent stem cells. ... 184
Figure 5-5 : Fast random-access two-photon imaging of GEVI responses in hippocampal slices. ... 185
Figure 5-6 : Kinetics and signal-to-noise ratios of GEVI responses... 187
Figure 5-7 : Tracking spike propagation in slices with ASAP2 and random-access two-photon imaging. ... 189
Figure 5-8 : Pooled data from optical tracking of spike propagation in multiple neurons. .... 190
Figure 5-9 : Two-photon microscopy of subcellular voltage responses to physiological stimuli in vivo. ... 191
Figure 5-10 : Photostability of ASAP2 and non-responsiveness of a FRET-opsin sensor in two-photon in vivo imaging. ... 194
xiv Figure 6-2 : A network of neurons is a data transmission system. ... 221 Figure 6-3 : Action potentials encode the intensity of a physical stimulus. ... 222 Figure 6-4 : How do neurons encode information? ... 223 Figure 6-5 : The counting logic generates postsynaptic firing following a fixed number of presynaptic APs, independent of their frequency. ... 224 Figure 6-6 : Errors in action potential timing introduce noise in temporal precision and this noise is compounded to the interspike interval. ... 229 Figure 6-7 : The counting logic allows action potential generation in the postsynaptic CA3 pyramidal cell independent of the previous activity frequency. ... 233 Figure 6-8 : Proposed ideal granule cell burst to trigger CA3 pyramidal cell firing in an energy-efficient manner. ... 235 Figure 6-9 : Univesicular and multivesicular release from a single active zone. ... 237 Figure 6-10 : Fast two-photon glutamate uncaging to study the summation of proximal
synaptic inputs on CA3 pyramidal neurons... 265 Figure 6-11 : Subcellular voltage imaging indicates that APs propagate to dendritic spine without attenuation ... 267
xv
Abbreviations
γ-DGG γ-D-Glutamylglycine ACSF Artificial cerebrospinal fluid
AP Action potential
AMPA α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate acid ASAP Accelerated sensor of action potential
ATP Adenosine triphosphate
AZ Active zone
BAPTA 1,2-bis(o-aminophenoxy)ethane-N,N,Nʹ,Nʹ-tetraacetic acid
Ca2+ Calcium
[Ca2+]
e Extracellular calcium concentration
CA1/CA3 Cornu ammonis region 1/3 CNS Central nervous system
CV Coefficient of variation
DCG-IV (2S,2'R,3'R)-2-(2',3'-Dicarboxycyclopropyl)glycine
EGTA Ethylene glycol-bis(β-aminoethyl ether)-N,N,Nʹ,Nʹ-tetraacetic acid EPSC Excitatory postsynaptic current
EPSP Excitatory postsynaptic potential
GABA γ-aminobutyric acid
GEVI Genetically-encoded voltage indicator
GTP Guanosine triphosphate
IPSC Inhibitory postsynaptic current IPSP Inhibitory postsynaptic potential
K+ Potassium
Kd Dissociation constant
MF Mossy fiber
N Number of release sites
Na+ Sodium
NMDA N-methyl-D-aspartic acid
OGB-1 Oregon green bapta-1 Pocc Probability of occupancy
Prel Release probability
PC Pyramidal cell
Q Quantal size
RAMP Random-access multiphoton microscopy RIM Rab3-interacting molecule
RRP Readily-releasable pool
SNARE Soluble NSF attachment protein receptor VAMP Vesicle associated membrane protein VGCC Voltage-gated calcium channel
xvi
VGKC Voltage-gated potassium channel VGSC Voltage-gated sodium channel
VM Variance-mean
xvii
Acknowledgements
First and foremost, I would like to thank my thesis supervisor, Katalin Tóth. Her curiosity for biological questions is highly contagious. Her interest spans multiple fields; she is probably the only person suggesting her students to read a paper about how woodpeckers do not suffer from concussion, how dolphins sleep with one brain hemisphere and how Venus flytrap plants catch flies. Seriously, I hope I can still go to your office in the future to discuss how sunflowers track the sun. We need to finish that discussion. I hope to be able to pursue question-driven science wherever I go next, which is, fundamentally, what makes science so fun; sheer curiosity. Katalin, you taught me how to think instead of what to think, and that’s the greatest thing ever.
Thanks to the fridge repair crew. I’ve had a chance to meet these people approximately 8 years ago. You guys were crucial to my success in the program. Not only did you contribute to the scientific projects as colleagues, you also supported me through all those years and became close friends. For this, I am extremely grateful to Charleen Salesse, Alesya Evstratova, Modesto ‘’Dino’’ Peralta III, Yanina Mircheva and Philippe Lemieux. Hopefully, we will never get over fixing the freon leak. Special thanks to Alesya for working on so many projects with me. Those nights when we finished the papers were awesome. Thanks to Charleen for being a great scientific colleague for so long and always helping me out with complicated things. Working with you was awesome. Big thanks to Dino for working on projects with me that will work (soon!) and correcting my written English. At $50 a mistake, a man learns quickly. Thanks to all the people who supported me during my studies. Notably, the members of my committee, Dr Paul De Koninck and Dr Martin Parent for providing guidance during my studies. I would also like to thank Profs. Armen Saghatelyan, Martin Deschênes and Mohamed Chahine for giving me a big thumbs up at a much needed time. Sometimes, a few good words lead to big changes. I also had the pleasure of meeting, discussing and working with fantastic people at the CRIUSMQ over the years. Thanks to everyone for creating such an environment. I would also like to thank the Centre
thématique de recherche en neurosciences (CTRN) for providing me with a PhD scholarship
that allowed me to complete my PhD studies.
On the personal side, I would also like to thank my fiancée Caroline Lachance. Thank you so much for all the support during these years. Part of this success goes to you.
xviii
Foreword
The present thesis is based on four manuscripts that are either published or in the submission process for publication on which I am first or co-first author. The structure of the thesis is as follows. First, I will briefly introduce the main findings from past work leading to the study of synaptic physiology. Important discoveries that shaped today’s neuroscience will be reviewed. As I progress towards more recent concepts, a general introduction will explore in more details key ideas that represent the intellectual basis for the original work that will be presented in the results section. The results section of the thesis is divided in four chapters, each dedicated to an individual manuscript. The first three manuscripts represent our work on short-term facilitation and calcium dynamics at hippocampal mossy fiber terminals. The fourth research paper explores the advantages of using a novel voltage-sensitive indicator to study neuronal physiology and how this technique will improve our ability to probe electrical activity in small compartments. Next, I will summarize and discuss these findings given the scientific literature and highlight how these results advance our knowledge on presynaptic neurotransmitter release and synaptic transmission. This section will be followed by perspectives on the possible new research directions generated by our results. Finally, I will state general conclusions that stem from the work presented in this thesis.
Individual chapters in the results section will be introduced by a foreword describing the rationale and the specific questions we addressed. Furthermore, it will describe the work that was done by each author of the study as required by Université Laval’s guidelines.
1
Chapter 1 - Introduction
A brief history of neuroscience leading to the study of synapses
The brain has fascinated philosophers and scientists for centuries. Historical records indicate that it was first correctly identified as the organ where the mind, the intelligence and memory are located by the Greek Alcmaeon of Croton in the 5th century BC (Debernardi et al., 2010). Anatomical studies further advanced this knowledge and provided description of the structures, including the distinction of the cerebrum and the cerebellum by Herophilus of Chalcedon in the 2nd century BC (Acar et al., 2005). Therefore, initial philosophers interested in the location of intelligence and the mind correctly identified the brain as a central computing unit that allows us to interact with our environment. On the other hand, the brain is located relatively far from the sensory organs. This raised an important question. How does the nervous system propagate signals to and from the brain?
Initial beliefs on the nervous system functionality held that long, continuous fibers spanned the whole animal body. Acting much like electrical cables, these long wires provided the means to transmit and receive crucial information allowing animals to interact with their environment. This theory embraced the idea that information from our environment can be transmitted across the body to reach the brain, where it can be computed. Although very much imperfect, this theory implicated the brain as the key structure governing our actions and emphasized the role of communication between the brain and the sensory organs (Melzack, 1996). Furthermore, this theory had the advantage of providing a fast, simple and efficient way of information to be transferred. Indeed, there was no need for relays between neurons.
The neuron doctrine
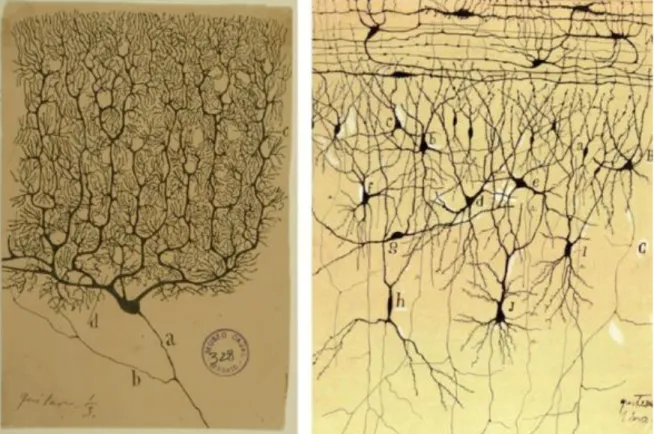
Rather than long continuous fibers, the first neuroanatomical studies by Ramon y Cajal demonstrated that neurons were discrete structures (Figure 1-1) (Ramon y Cajal, 1888, 1890; Garcia-Lopez et al., 2010). Using a staining technique known as Golgi staining and a microscope, Ramon y Cajal skillfully drew by hand hundreds of neurons from several brain
2
structures of different species. The careful drawings of Ramon y Cajal showed that neurons were restricted to a much smaller area than previously imagined. Furthermore, these highly accurate and detailed drawings showed that neurones are composed of a cell body, a dendritic arbour and an axon (Figure 1-1). The finding of these fine structures revolutionized the ideas held at the time, and led to the creation of a whole new scientific field, in which neurons were seen as discrete and individual elements.
Figure 1-1. Drawings of neurons by Santiago Ramon y Cajal.
These drawings illustrate that neurons are discrete elements presenting a complex anatomy. Illustrations of a Purkinje cell and cortical neurons highlight the anatomical diversity between different types of neurons.
Ramon y Cajal’s findings were crucial to the elaboration of the neurone doctrine. His discoveries correctly identified neurons as the simplest units involved in neurotransmission. He showed that neurons are not continuous elements, but rather contiguous parts of an ensemble. Furthermore, Ramon y Cajal demonstrated that neurons are composed of dendrites, a soma and an axon. Last, he rightfully hypothesized that neurons are polarized elements; information is integrated in dendrites, travels through the cell body and then
3
transmitted by the axons. Based on these results, it was postulated that neurons must communicate between each other to relay information.
Propagation of electrical signals in nerves
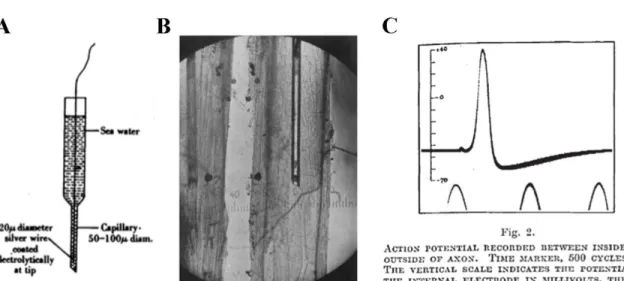
How can neurons relay information between each other? As neurons are small, discrete and interconnected elements, information must travel across networks of neurons. Furthermore, this propagation of activity must be exquisitely rapid. Late in the 18th century, Luigi Galvani found that nerves and muscles could be controlled by electricity (Fulton J., 1936). This finding was coined bioelectricity. Historical records say that by accidentally touching the nerve of a dead frog with a charged scalpel, Galvani moved the frog’s leg (Figure 1-2). Although correctly identifying electricity as the effector of muscle movement, Galvani incorrectly believed that it was due to the movement of an electrically charged liquid. Further research on the subject by Alessandro Volta indicated that cells must have a membrane potential. Attempting to prove his point, Volta invented the electrical battery. Therefore, neurons were identified as electrically-active cells.
Figure 1-2: Luigi Galvani’s discovery of bioelectricity.
Pictured on the left is the important experiment demonstrating that by touching a dead frog’s nerve with a charged scalpel, Galvani could generate fast muscle twitches.
4
Synapse: a specialized site of contact
Nerves transmit electrical signals. Work by Ramon y Cajal had demonstrated that neurons were not continuous, but rather contiguous elements. As such, connections between individual neurons would be required for information transmission to occur. It was postulated that communication between neurons occurs at specialized contact sites, with these sites possessing key anatomical features. At the neuromuscular junction, the connection between the nerve and the muscle was termed synapse by Sir Charles Sherrington (Sherrington, 1906). This term originates from the contraction of two Greek words, syn and haptein, meaning to
clasp together. This specialized site of contact between a presynaptic and a postsynaptic
element was also used to describe connections between neurons. Presynaptic components were rightfully identified as small burgeoning sites from axons. On the other side, the postsynaptic domains were identified as dendritic spines. The size of synapses between neurons is much smaller than the neuromuscular junction. Therefore, the size of these structures precluded further detailed analysis; microscopy techniques at the time were limited by the diffraction limit of light. Advances in microscopy techniques led to the development of the electron microscope in the decade between 1930 – 1940, which took advantage of the shorter wavelength of electrons compared to photons (Franklin, 1978). Ultrastructural analysis through electron microscopy of synapses later showed that indeed, synapses between neurons form the structural basis of neurotransmission. Anatomical studies subsequently showed that synapses release neurotransmitter from neurotransmitter-filled vesicle.
The membrane potential
Major work on the electrophysiology of neurons next came from Hodgkin and Huxley at the Marine Laboratory (Hodgkin and Huxley, 1952). Trying to understand the basics of the mechanisms generating the membrane potential, Hodgkin and Huxley showed that neurons possess a semipermeable membrane which is dynamically regulated to become more or less permeable to different ions. The membrane potential is generated by the flow of potassium and sodium ions through the membrane. Cleverly, they demonstrated that sodium and potassium had reversal potentials, at which the flow of these ions could be reversed (Hodgkin
5
and Huxley, 1952). It was later shown that neurons spend energy to maintain their intracellular ionic concentrations at a constant level. Indeed, neurons spend energy to maintain a high concentration of potassium ions inside their membrane (relative to the outside), but a relatively low concentration of sodium ions. This process is accomplished by the sodium-potassium exchanger, which requires energy, as it moves ions against their concentration gradient (Skou, 1957). The membrane potential is generated by the movement of positively charged potassium ions through voltage-gated potassium channels from the inside of the neuronal membrane to the extracellular milieu. Therefore, this process generates a membrane potential that is more negative on the inside of the cell membrane. Measurements of the membrane potential across several cell types differ, but is always hyperpolarized relative to the extracellular space. Disturbance of this ionic balance generates electrical signals that are propagated in the neuron. For example, after reaching a certain threshold of membrane potential, action potentials are generated in the initial segment of the axon (Figure 1-3).
Figure 1-3 : Hodgkin and Huxley’s experiment.
A, Drawing of the equipment used by Hodgkin and Huxley to record the membrane potential in squid giant axons. B, Schematic of the recording configuration, with an electrode impaling the squid giant axon. C, Electrophysiological traces showing action potentials.
The action potential is due to the opening of voltage-gated sodium channels, and subsequent invasion of sodium ions in the neuron (Hodgkin and Huxley, 1952). As the resting concentration of sodium ions is kept low in the neuron but relatively high on the outside, the
6
opening of voltage-gated sodium channels cause a massive entry of sodium ions through their concentration gradient. The large influx of positively charged ions causes a large depolarization of the neuron. These revolutionary findings obtained by Hodgkin and Huxley were obtained from the giant squid axon (Figure 1-3). This experimental preparation had several advantages, most notably its size. At more than 1 mm in diameter, it was easily penetrated by an electrode, and changes in membrane potential could be measured across the membrane. Using this simple preparation and gradients of ion concentration, they described in mathematical detail the generation of the membrane potential and the action potential.
Neurotransmitter release and generation of postsynaptic currents
The large amplitude electrical signal generated by action potentials is rapidly propagated throughout the whole neuron, with a passible propagation into the dendrites but active propagation in the axon. In the dendrites, the action potential is gradually attenuated due to cable filtering. In the axon, voltage-gated sodium channels contribute to the active propagation of the action potential. Upon reaching the presynaptic terminals, the action potential induces a large depolarization of the membrane. The action potential is dependent on sodium ions and is actively propagated in the axon. How do action potentials support communication between neurons? This key question was explored by the group of Bernard Katz at the frog neuromuscular junction. Release of neurotransmitter quanta from the neuromuscular junction was correctly identified by Fatt and Katz (Fatt and Katz, 1952b) and explored in further details by del Castillo and Katz (Del Castillo and Katz, 1954). The group of Katz had two ground-breaking findings. First, they identified that neurotransmitter release was dependent on calcium ions. Second, they found that neurotransmitter release was quantal in nature. These two findings will be explored below.
First, their results quickly identified an important conundrum for neurotransmitter release: while the action potential is dependent on sodium ions, neurotransmitter release appeared to be dependent on calcium ions (Fatt and Katz, 1952a). By lowering the extracellular concentration of calcium to values near 0, they found that they could abolish the spontaneous fluctuations in postsynaptic membrane potentials at the neuromuscular junction (Del Castillo and Katz, 1954). Conversely, when the extracellular calcium
7
concentration was increased, the frequency and the amplitude of spontaneous changes in membrane potentials caused by the release of acetylcholine were amplified. Next, they found that neurotransmitter release could still occur in the absence of nerve impulses (Katz and Miledi, 1967d), when these nerve impulses were blocked with the highly-selective voltage-gated sodium channel antagonist tetrodotoxin (Katz and Miledi, 1967a). This phenomenon was termed spontaneous miniature release (Katz and Miledi, 1967c). Katz and colleagues found that calcium ions are a key messenger in the steps leading to the release of neurotransmitters (Katz and Miledi, 1967c). Why are calcium ions important in neurotransmitter release, but not in action potential propagation? Katz and Miledi later demonstrated that calcium ions enter the presynaptic terminals through calcium channels, which are enriched in the presynaptic terminals (Katz and Miledi, 1967b, d, c). Therefore, Katz and colleagues demonstrated the importance of calcium ions for neurotransmitter release. Later studies confirmed that the influx of calcium is required for release and that vesicle fusion depends on this intermediary step, rather than the voltage change by itself (Zucker and Lando, 1986).
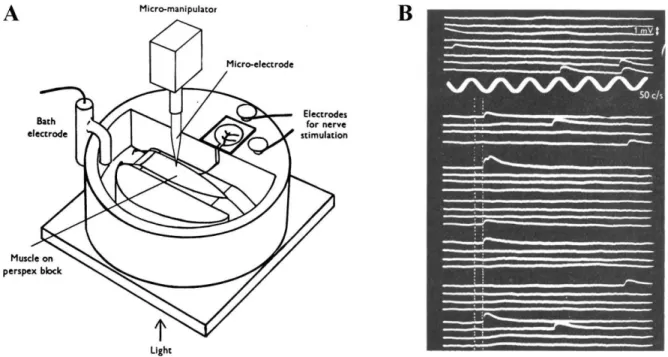
Figure 1-4 : Exploring the nature of neurotransmitter release.
A, Experimental design used by the group of Bernard Katz leading to the discovery of the calcium-dependency of neurotransmitter release and the quantal nature of neurotransmitter release. Figure from (Fatt and Katz, 1951). B, Spontaneous (above) and evoked (below) end-plate potentials recorded from the muscle. The vertical dotted lines show the electrical
8
stimulus timing. The low proportion of successes is explained by the low calcium concentration in this particular experiment. Figure from (Del Castillo and Katz, 1954).
The second major finding of Bernard Katz and colleagues was that end-plate potentials could also be observed in the absence of presynaptic nerve stimulation (Fatt and Katz, 1952a). These release events were termed miniature end-plate potentials. Extensive quantitative and statistical examination of the evoked postsynaptic currents and the miniature end-plate potentials revealed that neurotransmitters were released in discrete packets (Del Castillo and Katz, 1954). They obtained this conclusion because their analysis revealed that the larger end-plate potentials were multiplicative of the miniature end-plate potentials.
The depolarization generated by the action potential propagates through the axon and causes the entry of calcium ions in the presynaptic terminal. This signal than triggers neurotransmitter release. The findings by Katz and colleagues provided crucial evidence that the propagation of electrical signals from a neuron to another requires a central step, which is the release of the neurotransmitter. Therefore, when an action potential invades the presynaptic terminal, it causes the release of neurotransmitter quanta in the synaptic cleft. These finding indicated a central role for presynaptic terminals in the transmission of information.
Probing the activity of individual neurons with the patch-clamp technique
These key electrophysiological studies proved to be essential to our understanding of synaptic transmission. They established the general principles governing the electrical excitability of neurons, the rules regulating the membrane potential and revealed fundamental principles of neurotransmission. However, such electrophysiological studies could not yet be performed in the central nervous system of higher-order animals, mainly due to the small size of neurons and the inability to prepare living preparations allowing such recordings to be performed. Pioneered in the late 1970s by Erwin Neher and Bert Sakmann, the patch-clamp technique opened the possibility to study the electrical activity of neurons and synapses in more details (Neher and Sakmann, 1976; Hamill et al., 1981). Using live brain preparations under a microscope, the activity of single neurons and the current generated by single ionic channels could be recorded. To achieve recording from single neurons, a very
9
fine glass micropipette connected to an electrode is lowered on brain slices observed under a microscope. The desired recording configuration, such as the whole-cell configuration, is then obtained by applying gentle negative pressure after positioning the glass micropipette on the structure of interest. This technical development opened up the possibility to probe synaptic transmission between mammalian neurons in exquisite details.
The age of light
Direct electrophysiological recordings from neurons revealed much about their properties and the key roles of synapses in neuronal communication. Many pioneering results started to identify small neuronal compartments such as dendritic spines and presynaptic terminals as key elements for the computational power of neurons. Therefore, investigators started to question the properties of these small structures. However, these submicrometer compartments were less amenable for direct electrophysiological recordings because of their small size. Investigators needed to access these structures, but how?
In recent years, major advances in the development of microscopy and protein engineering revolutionized our ability to directly probe these structures. Two main advances allowed recordings of activity within fine subcellular compartments. First, the development of microscopy techniques, such as two-photon microscopy, allowed investigators to obtain a direct read-out of the biological activity in small structures (Denk et al., 1990). Using fluorescent molecules sensitive to biological activity, highly localized fluctuations in calcium fluxes or changes in membrane potentials could be recorded (Sabatini et al., 2002; Peterka et al., 2011). Second, protein engineering created molecules that could provide a read-out of biological activity, and actuators that were controlled by light (Scanziani and Hausser, 2009). Originally from the jellyfish Aequorea victoria, the green fluorescent protein was engineered to be expressed in mammalian cells (Shimomura et al., 1962; Tsien, 1998). Furthermore, a widely used family of genetically-encoded calcium sensors were developed using this fluorophore (Chen et al., 2013). Additionally, light-sensitive proteins gating ion channels were also engineered to be expressed in mammalian neurons (Boyden et al., 2005). Upon light exposure, these actuators cause the depolarization or hyperpolarization of the target neurons. This enabled researchers to study network dynamics with unprecedented spatial and temporal resolution to decipher the connectivity rules of the brain. By using light to probe
10
and control the activity of small neuronal compartments, investigators can now directly look into these structures, without a need for physical access. These tools, combined with electrophysiological approaches are therefore ideal to study synaptic transmission with a level of details previously not achievable.
Presynaptic terminals
As briefly described above, chemical neurotransmission occurs at specialized contact sites called synapses. Presynaptic terminals translate bursts of action potentials to calcium elevations for release of neurotransmitter (Schneggenburger and Neher, 2000; Sakaba and Neher, 2001). Therefore, the building blocks found in presynaptic terminals shape neurotransmitter release. Because presynaptic terminals are constructed with specific building blocks, neurotransmitter release can be synapse-specific (Wu et al., 1999; Toth et al., 2000; Waldeck et al., 2000; Bagnall et al., 2008). This allows presynaptic terminals to extensively modulate neurotransmitter release to perform their unique functions. First, I will describe the architecture of presynaptic terminals. Their morphological properties and their composition will be dissected, including the proteins making up the machinery required for neurotransmitter release.
Ultrastructure of presynaptic terminals
Presynaptic terminals are structures burgeoning from axons with highly variable anatomy. Most presynaptic terminals found in the central nervous system span only a few hundred nanometers, but some presynaptic terminals can reach several microns. While presynaptic terminals can be very different from each other, they share some common features. They are equipped with active zones, synaptic vesicles, vesicle trafficking elements and release machinery (Gray, 1963; Wilhelm et al., 2014). Neurotransmitter release occurs at the active zone. Active zones are specialized structures, where the molecular machinery required for release is found (Sudhof, 2012). Anatomically, active zones are identified based on the higher contrast from the adjacent presynaptic membrane (Gray, 1963). Furthermore, the postsynaptic specialization site is also denser than its surrounding. Electron microscopic images show that synaptic vesicles can be found in the presynaptic terminals, at varying
11
distance from the active zone. Synaptic vesicles can notably be found close to and docked at the presynaptic active zone.
Figure 1-5 : Ultrastructural features of the synapse.
A, Cartoon depicting the presynaptic terminal and the adjacent postsynaptic structure. The synaptic cleft separates both structures. The active zone is the physical location where neurotransmitter is released from the presynaptic terminal. B, Phosphotungstic acid staining reveals the pre- and postsynaptic partners under electron microscopy examination. C, Synaptic vesicles as observed under an electron microscope. Modified from Sudhof, 2012 and Kaeser et al., 2011.
First, the size and shape of presynaptic terminals in the central nervous system are highly variable. Presynaptic terminals can be small and simple structures burgeoning from the axon. For example, presynaptic terminals formed by hippocampal CA3 pyramidal cells on CA1 pyramidal cells show anatomical features of simple presynaptic terminals (Harris and Sultan, 1995; Schikorski and Stevens, 1997). These structures rarely exceed 1 µm in diameter. Indeed, most presynaptic terminals are small and relatively simple structure. On the other hand, giant presynaptic terminals also populate the nervous system. For example, the calyx of Held demonstrates a highly complex structure and can reach a diameter of 15 – 30 µm. These complex structures are also equipped with filopodial extensions. In the hippocampus, giant mossy fiber terminals possess a diameter of up to 10 µm and several filopodial extensions (Amaral and Dent, 1981; Chicurel and Harris, 1992). Therefore, the
12
size of presynaptic terminals range from less than 1 µm for small terminals up to 30 µm for giant terminals.
Second, the number of active zones vary between different presynaptic terminals. Most small-sized terminals, such as those from hippocampal CA3 to CA1 pyramidal cell possess only a single active zone (Harris and Sultan, 1995; Schikorski and Stevens, 1997). At the other end of the spectrum, hippocampal mossy fiber terminals possess up to 45 release sites (Rollenhagen et al., 2007), while the calyx of Held presynaptic terminal is equipped with up to 1106 release sites (mean value 678 ± 174) (Taschenberger et al., 2002). In the case of the giant mossy fiber terminals, release sites are separated by an average of 450 nm (Rollenhagen et al., 2007). These findings highlight that presynaptic terminals are highly heterogeneous, although they are composed of similar basic elements.
Molecular machinery of the presynaptic terminal
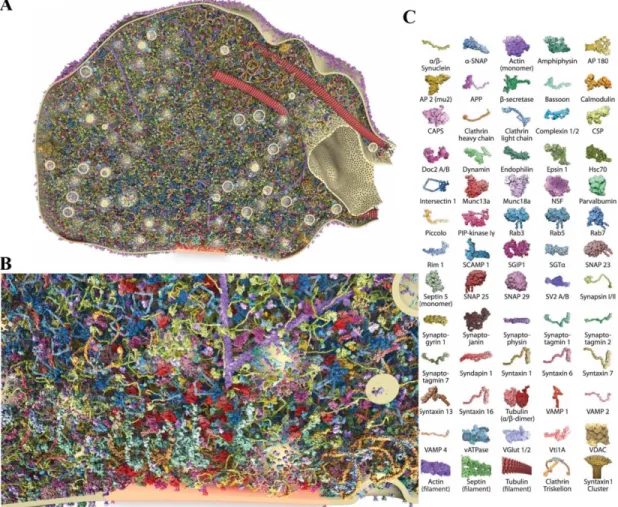
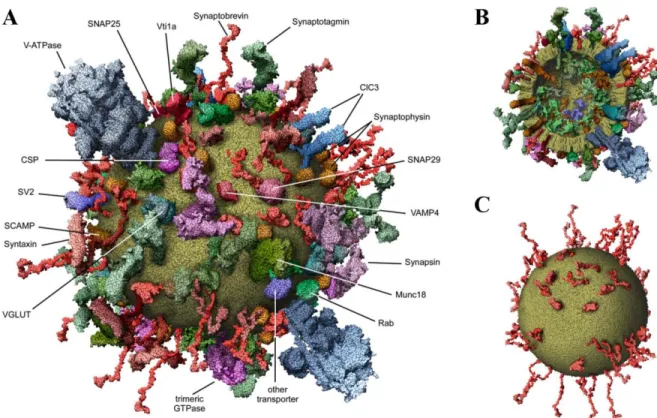
A surprisingly complex architecture of proteins is found in presynaptic terminals (Kaeser, 2011; Sudhof, 2012). Presynaptic terminals are filled with a large array of proteins, and are far from being empty structures as typically pictured (Wilhelm et al., 2014). This is shown in Figure 1-6 below (Wilhelm et al., 2014). Indeed, there are over 300 000 proteins in presynaptic terminals (Wilhelm et al., 2014). These proteins are involved in all the processes assumed by presynaptic terminals, such as neurotransmitter release, vesicle trafficking, vesicle endocytosis and long-term plastic changes. All these functions are controlled by specific molecular machines (Wilhelm et al., 2014). Interestingly, proteins that are part of the exocytotic process are present in high copy numbers in presynaptic terminals (Wilhelm et al., 2014).
Neurotransmitter release occurs through the fusion of neurotransmitter-filled vesicles to the presynaptic membrane. A vast repertoire of presynaptic proteins controls all the steps leading to this process. Presynaptic proteins controlling neurotransmitter release can be broadly classified between SNAREs and exocytotic cofactors such as the Munc and RIM families of proteins (Wilhelm et al., 2014). SNARE proteins are the main effectors of vesicle fusion. Their name originates from the contraction of Soluble NSF Attachment Protein Receptor. There are several isoforms of snare proteins. SNARE proteins are found on the
13
vesicles (v-SNAREs) and on the presynaptic terminals (t-SNAREs). For vesicle fusion to occur, the v-SNAREs and the t-SNAREs must bind together. This mechanism is known as the zippering of the trans-SNARE complex. This reaction yields a more stable protein complex, and is key to the speed of neurotransmitter release (Zhou et al., 2015).
Figure 1-6 : Molecular composition of a presynaptic terminal.
Figure modified from (Wilhelm et al., 2014). A, Inside view of a presynaptic terminal showing the expression of different proteins and their arrangement in the presynaptic terminal. This figure was generated through modelling approaches from experimental data. B, Close-up of the active zone from the image shown in A. C, Representation of the vast repertoire of proteins expressed in presynaptic terminals. The different proteins are represented with specific cartoons that can be found in A and B to show their spatial organization.
The Munc family of proteins are also involved in vesicle release, although indirectly. While Munc18-1 is important for the docking of the vesicle and stabilization of the snare complex, Munc13 speeds the transition to the SNARE complex (Ma et al., 2011). This brings
14
the calcium sensor synaptotagmin-1 close to the voltage-gated calcium channel and allows it to sense calcium influx. Therefore, these interactions between these molecular machines are key to mediate neurotransmitter release. The calcium sensors and the different v-SNAREs will be treated in separate sections. Interactions between the different proteins to bring the vesicle to the membrane are illustrated below.
Figure 1-7 : Molecular machinery gating the interactions between the synaptic vesicle and the presynaptic membrane controlling neurotransmitter release.
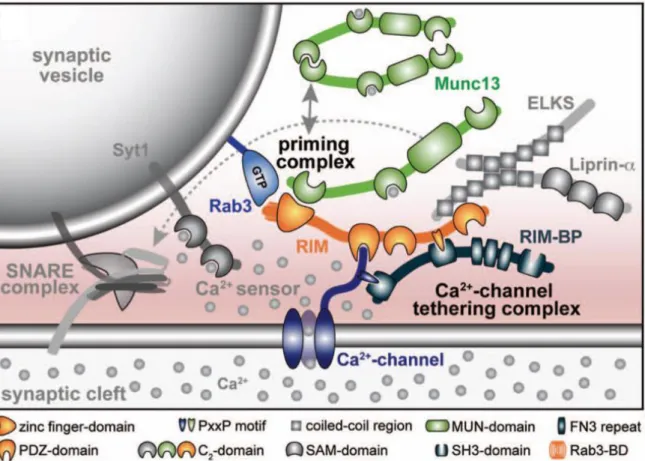
The SNARE complex is supported by a variety of proteins mediating the priming of the vesicle to the membrane. Figure modified from (Kaeser, 2011)
RIM proteins are important for synaptic vesicle priming and docking (Kaeser, 2011). They are implicated in tethering the synaptic vesicle to the voltage-gated calcium channel. In addition, RIM proteins were found to have important roles in basal neurotransmitter release and both short-term and long-term synaptic plasticity (Schoch et al., 2002; Kaeser, 2011). For example, RIM1α controls presynaptic long-term potentiation in hippocampal and cerebellar mossy fibers (Castillo et al., 2002).
15
Voltage-gated sodium channels
Voltage-gated sodium channels (VGSCs) are sodium permeant channels that open upon membrane depolarization and allow action potentials to be actively propagated along the axon (Hodgkin and Huxley, 1952; Hu and Jonas, 2014). VGSCs can be in a closed state, open state, or inactivated state. At resting membrane potential, VGSCs are closed and do not let sodium ions enter the cytosol. Upon membrane depolarization to a certain threshold (~50 mV, but varies from cell to cell), the VGSCs change to the open configuration and a transient influx of sodium ions occurs. This influx of sodium ions causes the rising phase of the action potential. This channel is self-inactivated through a ball and chain mechanism (Purves et al., 2001), which ensures that the sodium current is very brief.
Figure 1-8 : The different states of voltage-gated sodium channels.
Voltage-gated sodium channels fluctuate between closed, open and inactivated states as a function of membrane potential. Figure modified from (Purves et al., 2001)
Changes in membrane potential through the activation of VGSCs do not cause neurotransmitter release directly (Zucker and Lando, 1986; Zucker and Haydon, 1988; Mulkey and Zucker, 1991). Rather, voltage-gated sodium channels are enriched in synaptic terminals to amplify the depolarization, and ensure opening of voltage-gated calcium channels (Bischofberger et al., 2002; Engel and Jonas, 2005). VGSCs are quite heterogeneous, as there are 9 known subtypes of VGSCs (NaV1.x). These channels possess
specific properties, the assembly of which may confer specialized functions to presynaptic terminals. For example, NaV1.2 and NaV1.6 are enriched in hippocampal granule cells (Felts
16
et al., 1997; Schaller and Caldwell, 2000; Engel and Jonas, 2005). Therefore, specific assembly of VGSCs control the rising phase, the onset and the amplitude of the action potential in presynaptic terminals. In return, this controls indirectly the dynamics of neurotransmitter release.
Voltage-gated potassium channels
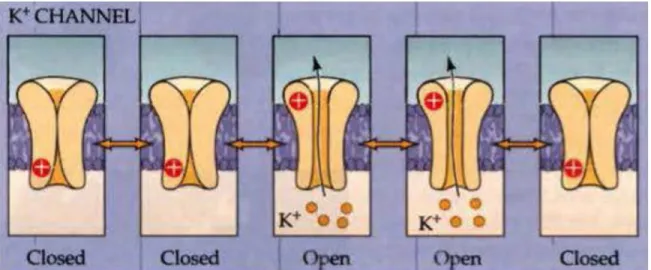
Voltage-gated potassium channels (VGKCs) are voltage-activated channels that gate the flux of potassium ions. Currently, there are approximatively 40 types of VGKCs known. In presynaptic terminals, some VGKCs are involved in the control of the neuronal membrane repolarization following the rising phase of the action potential. Close to the peak of the action potential (~0 mV), voltage-gated potassium channel are activated. At this membrane potential, opening of VGKCs cause an efflux of potassium ions, contributing to the repolarization of the neuronal membrane. Therefore, these channels ensure that the presynaptic terminal repolarizes following an action potential. Unlike voltage-gated sodium channels, VGKCs do not possess a self-regulation mechanism and do not have an inactivated state.
Figure 1-9 : Voltage-gated potassium channels can be in a closed or open state and do not have an inactivated state.
Similarly to voltage-gated sodium channels, the state of VGKCs fluctuates as a function of membrane potential Figure modified from (Purves et al., 2001).
17
In presynaptic terminals, voltage-gated potassium channels are known to mediate fast repolarization of the boutons following an action potential (Geiger and Jonas, 2000). Furthermore, the specific assembly of VGKCs confers dynamic properties to the presynaptic structure to control neurotransmitter release (Geiger and Jonas, 2000). At the mossy fiber synapse, repetitive firing causes a gradual broadening of action potentials, which generate a larger calcium current. In contrast, this phenomenon is not observed at the giant calyx of Held terminal (Taschenberger and von Gersdorff, 2000; Taschenberger et al., 2002). Therefore, voltage-gated potassium channels contribute to the control of neurotransmitter release. Much like voltage-gated sodium channels, specific incorporation of the different families of voltage-gated potassium channels in the presynaptic terminal will shape neurotransmitter release.
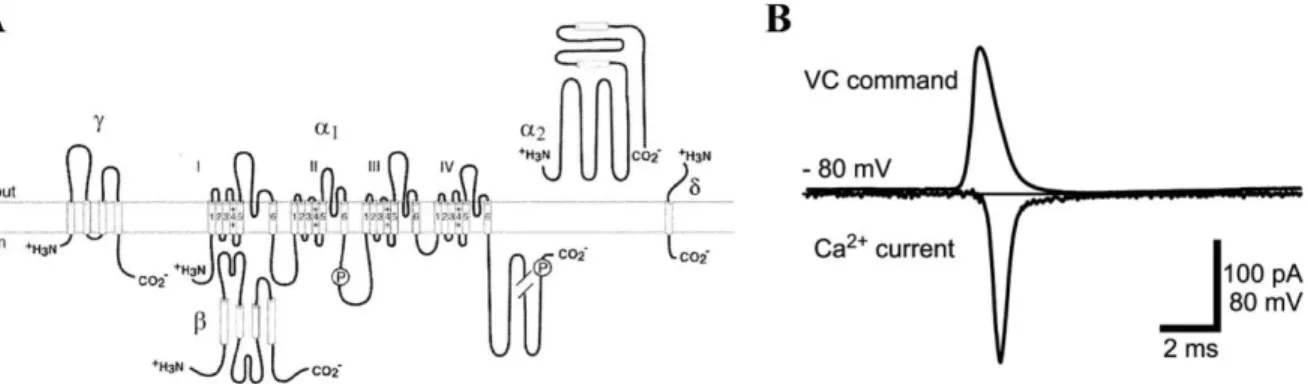
Voltage-gated calcium channels
The most important Ca2+ source in presynaptic terminals for neurotransmitter release comes from the opening of voltage-gated calcium channels (VGCCs), which are selective to Ca2+ ions. VGCCs are proteins inserted in the membrane composed of 5 subunits (α1, α2, β, γ, δ), with the α1 subunit dictating most biophysical properties of the channel (Hofmann et al., 1994; Catterall, 2000). VGCCs expressed in presynaptic terminals include P/Q-, N-, R-, and L-type VGCCs, with a relatively high-threshold of activation. These VGCCs share a common mechanism of activation and deactivation, but their activation doesn’t yield the same Ca2+
dynamics due to their distinct biophysical properties.
Figure 1-10: Structure and function of voltage-gated calcium channels.
A, Cartoon depicting the structure of a voltage-gated calcium channel (Catterall et al., 2003). B, Mock action potential (top trace) evoked in a presynaptic terminal and associated calcium
18
current (bottom trace). The peak of the calcium current occurs during the repolarization phase of the action potential, when the calcium driving force is greatest (Bischofberger et al., 2002).
First, an action potential invades and depolarizes the presynaptic terminal. The abrupt positive change in voltage causes the opening of VGCCs, but not the direct influx of Ca2+ ions. Rather, the entrance of Ca2+ ions is delayed until the repolarizing phase of the action potential, where the electrochemical gradient of Ca2+ ions is greatest. The repolarizing phase of the action potential generated by the concerted action of voltage-gated potassium channels is faster than the deactivation time of VGCCs. This subtle difference provides a narrow time window for Ca2+ ions to cross the membrane. Presynaptic voltage-gated calcium channels vary in their ability to allow Ca2+ ions in terminals due to their specific biophysical properties,
determined by the identity of the α1 subunit (Tsien et al., 1995; Catterall et al., 2003; Li et al., 2007). P/Q-, N- and L-type VGCCs are known as high-voltage activated channels. On the other hand, R-type VGCCs are activated at intermediate levels of membrane depolarization. While the conductance of P/Q-, N- and R-type VGCCs is limited by the fast inactivation kinetics of the channels (50 to 80 ms) (McCleskey et al., 1987), L-type VGCCs demonstrate long inactivation kinetics, on the order of 500 ms (Catterall, 2000). Furthermore, the fraction of VGCCs opening after a single action potential varies in a terminal-specific manner. While several studies point to a low open probability of VGCCs in response to action potentials at most synapses (Luo et al., 2011; Sheng et al., 2012; Tarr et al., 2013), other synapses show high-open probability for VGCCs (Bischofberger et al., 2002; Li et al., 2007). This is possibly explained by the large variety of conductances cooperating in presynaptic terminals (Kimm et al., 2015). Additionally, VGCC biophysical properties even vary in a terminal-specific manner, due to presence or absence of specific subunits and interactions with other presynaptic machinery (Bezprozvanny et al., 1995; Felix et al., 1997; Hurley et al., 2004; Luvisetto et al., 2004). The presynaptic calcium currents will vary as a function of the VGCCs mediating calcium entry (Li et al., 2007). Therefore, the resulting Ca2+ influx is hard to predict based solely on the identity of the VGCC due to varying open probability and terminal-specific properties (Geiger and Jonas, 2000; Begum et al., 2016; Rowan et al., 2016).
Presynaptic voltage-gated calcium channels are distributed in a random and non-uniform manner in presynaptic terminals (Robitaille et al., 1990; Reid et al., 1997; Holderith
19
et al., 2012; Keller et al., 2015; Stanley, 2015b). The distribution of VGCCs can be described relative to the active zones. VGCCs are mainly found close to active zones (Sheng et al., 2012), but can also be found in more remote locations relative to the active zones (Wu et al., 1999). Furthermore, presynaptic VGCCs can be found in clusters and on their own (Jones et al., 1989; Nakamura et al., 2015). For example, at the calyx of Held, clusters of VGCCs are composed of 2 to 27 VGCCs and cover an area ranging from 0.002 µm2 to 0.0067 µm2 (Nakamura et al., 2015). Finally, it was demonstrated that VGCCs are also highly mobile in neurons and in the presynaptic membrane (Schneider et al., 2015). By using state-of-the-art imaging methods and tracking of single VGCCs, Schneider et al., (2015) found that approximately 60% of VGCCs are mobile in the presynaptic membrane. The diffusion coefficient of mobile VGCCs in the presynaptic membrane was approximately 0.02 µm2/s. This mobility was increased as a function of activity and decreased by buffering intracellular calcium ions (Schneider et al., 2015). Altogether, these studies support the notion that VGCCs are non-uniformly distributed in space. Furthermore, the different configurations can also be altered as a function of activity. Therefore, by itself, the identity or the arrangement of VGCC cannot predict neurotransmitter release.
Synaptic vesicles
Synaptic vesicles are sphere-shaped structures containing the neurotransmitter molecules. Their size is small and relatively uniform, with a diameter ranging from only 40 to 80 nm (Taschenberger et al., 2002; Takamori et al., 2006). Synaptic vesicles are composed of 60% protein and 40% lipid (Takamori et al., 2006). These values are comparable to the composition of the neuronal membrane (Stoeckenius, 1962). These fundamental properties and compositions do not appear to differ between the different populations of synaptic vesicle studied (Takamori et al., 2000; Takamori et al., 2006) or vesicles studied at different synapses (Taschenberger et al., 2002). The physical parameters of synaptic vesicles appear relatively uniform. They have a density of 1.1 g/ml and an outer diameter of 41.6 nm (Takamori et al., 2006). Therefore, these data indicate that synaptic vesicles are homogeneous in their basic constituents. However, the expression of a wide arsenal of proteins incorporated in their membrane makes synaptic vesicles extremely heterogeneous (Takamori et al., 2006).
20
Synaptic vesicles may express more than 100 different proteins on their membrane. The molecular composition of synaptic vesicles is a key parameter defining their identity and function (Neher, 2015; Chamberland and Toth, 2016).
Figure 1-11 : Molecular anatomy of a synaptic vesicle.
A, Model of a synaptic vesicle showing the expression of various components and the incorporation of key proteins. B, Vesicle cut in half to show the relative size of the membrane-bound proteins and the vesicle itself. C, Expression of synaptobrevin, the most abundant vesicle component. Synaptobrevin is part of the SNARE complex. Figure modified from (Takamori et al., 2006)
The surface of synaptic vesicles is heavily crowded with transmembrane domains of the proteins expressed. Indeed, synaptic vesicles possess on average 600 transmembrane domains, which cover approximately 20% of their surface. The molecular composition of synaptic vesicles likely define the type of synaptic vesicles and their functions (Neher, 2015). Proteins expressed on synaptic vesicles can be broadly categorized in three main classes: trafficking proteins, transporter and channel proteins, and cytoskeletal proteins (Takamori et al., 2006). Of these, the most numerous class of proteins are trafficking proteins. Trafficking proteins encompass SNAREs, endocytosis-related proteins, small GTPases and others. Of
21
these, the most diverse class are SNAREs (Takamori et al., 2006). Notably, v-SNAREs and t-SNAREs are responsible for all steps leading to vesicle fusion. SNARE proteins come in at least 20 different forms (Takamori et al., 2006). For example, VAMP1, 2, 4, SNAP-29, SNAP-47, and Syntaxin 6, 7, 13, 16b were all shown to reside on synaptic vesicles (Takamori et al., 2006). Given the large diversity of SNAREs expressed on synaptic vesicles, it is hypothesized that the assembly of different v-SNAREs contributes to the selective binding of the vesicle to their designated targets. It is fascinating that the most diverse class of synaptic vesicle proteins are trafficking proteins. This supports the notion that a rich repertoire of trafficking proteins are required to support the diverse functional roles of synaptic vesicles (Neher, 2015; Chamberland and Toth, 2016). Indeed, trafficking of synaptic vesicles to the appropriate physical location at a given moment is likely to be a major determinant of neurotransmitter release.
Calcium sensors
A wide repertoire of calcium sensors are expressed on synaptic vesicles. Calcium sensors are proteins such as the synaptotagmin (Syt) family. The proteins have the peculiarity that they can bind calcium ions. Binding of calcium ions will lead to activities such as synaptic vesicle docking and synaptic vesicle fusion. Furthermore, calcium sensors with different affinities provide a means for vesicles to be released by the correct activity patterns and calcium transients. Indeed, the calcium sensors found on vesicles possess different affinities (McCue et al., 2010). For example, the expression of particular calcium sensors can control the mode of neurotransmitter release.
For example, vesicles expressing Syt1 are more likely to be released than vesicles expressing Syt7 (Bacaj et al., 2013). Indeed, it was demonstrated that Syt1 functions as a calcium sensor important for spontaneous release of vesicles (Xu et al., 2009). Furthermore, short-term facilitation at the mossy fiber synapse was shown to be mediated in part by vesicles expressing the calcium sensor synaptotagmin-7 (Jackman et al., 2016). Therefore, according to this proposition, vesicles expressing specific synaptotagmin isoforms can be released with high temporal accuracy at the correct moment. This model of vesicle heterogeneity and their role in the different modes of release has been demonstrated in several