UNIVERSITE TOULOUSE III – PAUL SABATIER Ecole doctorale Biologie, Santé, Biotechnologies
INSERM U563, Centre de Physiopathologie de Toulouse – Purpan Dana-Farber Cancer Institute, Boston, MA, USA
THESE
Pour obtenir le grade de
DOCTEUR DE L’UNIVERSITE TOULOUSE III Discipline : Immunologie
Présentée et soutenue par
Fay MAGNUSSON
le
25 mars 2008
Titre :Etude de la tolérance périphérique aux antigènes du soi dans
un modèle de souris transgéniques
---Directeur de thèse : Pr. Roland LIBLAU Co-directeur de these: Dr. Khashayarsha KHAZAIE
---JURY
Prof J. van Meerwijk President Dr. D. Kaiserlian Rapporteur Dr. N. Cerf-Bensussan Rapporteur Dr. N. Vergnolle Examinateur
A
CKNOWLEDGEMENTS
I would like to thank Khash Khazaie for giving me the opportunity to pursue Ph.D. studies in his lab and for always being available and open for scientific discussions and advice. He has been a great mentor and I appreciated his support and kindness throughout the years.
I would like to thank Roland Liblau for giving me the opportunity to pursue a Ph.D. under his joined mentorship. He has always given me prompt and useful advice, and I am very grateful for his valuable help and support.
I would like to thank Harald von Boehmer for welcoming me in his lab and for the financial support he has given me.
I would like to thank all the members of the lab throughout the years that have kindly given help to me: Karsten Kretschmer, Mikael Pittet, Elias Gounaris, Dina Laznick, Kristy Melton, Olivier Herbreteau, Cliff Restaino.
I am grateful to Cecile Cassan for having gone through enrollment in the graduate school for me at the beginning of each school year.
I would like to thank my close friends, Luyen Nghiem and Clemence Racimora, and my brother and sister, Finn and Liv Magnusson, for their moral support.
I would like to give special thanks to my partner Ali Ergut and my mother Gwen Magnusson. They have given me unconditional love and support throughout the years, have always been encouraging during down times and have always believed in me. I owe my perseverance and confidence to them.
Last but not least, I would like to dedicate this work to my beloved father, Finn Aaron Magnusson, who recently passed away. He has been a great father and has always been there for me when I needed help.
T
ABLE OF CONTENTS
ACKNOWLEDGEMENTS... 3 TABLE OF CONTENTS... 4 RÉSUMÉ... 6 ABSTRACT... 7 ABBREVIATIONS... 8 INTRODUCTION...10 I. THE IMMUNE SYSTEM...11 A. HEMATOPOEISIS...11 B. LYMPHOID ORGANS...151. Primary lymphoid organs...15
Thymus...15
Bone marrow ...16
2. Secondary lymphoid organs ...16
Lymph nodes...16
Spleen ...16
Mucosal-associated lymphoid tissue...17
C. INNATE IMMUNITY...17
1. Barriers of the innate immune system ...18
Anatomic barriers ...18
Physiologic barriers ...18
Endocytic and phagocytic barriers ...19
Barriers created by the inflammatory response ...19
2. Cells of the innate immune system ... 20
Mast cells ...20
Macrophages...21
Neutrophils ...22
Dendritic cells...22
Basophils and eosinophils...22
Natural killer cells ...23
D. ADAPTIVE IMMUNITY... 23 1. B cells... 24 2. T cells ... 25 Cytotoxic T cells...25 Helper T cells...26 Memory T cells...33
Molecular and functional characterization of CD4+ CD25+ Tregs: role of Foxp3 in function
and maintenance of Tregs...41
Generation of Tregs in the thymus...42
Generation of Tregs in the periphery ...43
Mechanism of Treg suppression ...44
2. Immunologic ignorance... 47
3. Anergy... 47
4. Deletion ... 48
III. INFLAMMATORY BOWEL DISEASE... 53
A. GENERALITIES... 53
B. ANIMAL MODELS OF IBD ... 54
1. Genetics ... 56
2. Innate immune response... 57
3. Adaptive immune response... 58
Effector T cell response...58
Regulatory T cell response ...60
Conclusion...63
RESULTS... 65
I. MECHANISMS OF PERIPHERAL CD8 TOLERANCE... 66
A. DIRECT PRESENTATION OF ANTIGEN BY LN RESIDING RADIO-RESISTANT CELLS AND CROSS-PRESENTATION... 66
1. Article 1 ... 66
Résumé des travaux...67
Protocoles experimentaux...70
Publication ...71
2. Article 2... 101
Résumé des travaux...102
Protocoles experimentaux...104
Publication ...105
B. CD4+CD25+F OXP3+ REGULATORY T CELLS... 141
1. Mechanism of suppression... 141
Résumé des travaux...141
Results ...143
Conclusion...152
2. Article in collaboration ...153
Résumé des travaux...154
Publication ...155
DISCUSSION AND PROSPECTS...162
Résumé...163
Mechanism of peripheral CD8 tolerance via direct presentation of self-antigen by LN stromal cells ...165
Thymic generation of CD4+ CD25+ Foxp3+ Tregs ...172
Mechanism of Treg suppression ...174
R
ESUME
Le système immunitaire joue un rôle important dans le maintien de l’intégrité du corps humain puisqu’il le protège contre diverses infections virales et bacteriennes, tout en lui attribuant tolérance envers les antigènes du soi. Plusieurs mécanismes de tolérance au soi nous protègent des maladies auto-immunes.
Dans le contexte de la tolérance périphérique et à l’aide de modèles murins transgéniques, nous avons étudié le rôle de la délétion via présentation directe d’antigènes par les cellules stromales de ganglions lymphatiques, dans les maladies inflammatoires de l’intestin causées par les lymphocytes T CD4 ou CD8. Nous avons également etudié la génération thymique de cellules T régulatrices (Tregs) spécifiques d’un néo-antigène du soi, et le mécanisme de la suppression des Tregs par comparaison de puces à ADN provenant de lymphocytes T CD8 régulés ou non par des Tregs.
A
BSTRACT
Several mechanisms of peripheral self-tolerance, including CD4+ CD25+ Foxp3+
regulatory T cells (Tregs), cross-presentation by dendritic cells (DCs) and the newly identified direct presentation of antigen by lymph node (LN) stromal cells, contribute to protecting our bodies and animals from self-damaging autoimmune diseases.
We first sought to determine the relative contribution of antigen-specific CD8 and CD4 T cells to intestinal autoimmunity. To this end, we studied autoimmune targeting of an ectopic antigen expressed by enteric glial cells, in transgenic mouse models. We found that direct presentation of antigen by LN stromal cells caused the activation induced cell death of specific CD8 T cells. In contrast, conventional CD4 T cells were not affected by this mechanism of tolerance and their targeting of enteric glial cells produced lethal intestinal autoimmunity. Thus, we conclude that in our mouse model, this novel mechanism of peripheral tolerance preferentially protects against CD8 T cell intestinal autoimmunity. Furthermore, we have established a new model of antigen-specific CD4 T cell-mediated small intestine autoimmunity.
To determine the relative contribution of Tregs, DCs and LN stromal cells for protection against autoimmunity, we sought conditions that make adoptively transferred naïve conventional CD8 T cells autoaggressive. Depletion of Treg cells, non-specific activation of the T cells through their lymphopenic expansion, and inhibition of TGF-β receptor signaling in T cells achieved autoimmunity. The CD8 T cells caused multi-organ autoimmunity, but the intestine was not affected. Since insensitivity to TGF-β rendered the T cells resistant to deletion through cross-presentation but not direct presentation of antigen, we conclude that the former was the critical mechanism determining the outcome of the immune response. Furthermore, these observations leave the possibility open that direct presentation by LN stromal cells preferentially protects the intestine against CD8 T cell attack, although other mechanisms of mucosal tolerance may be also involved.
Altogether, these results provide new insights into the mechanisms of peripheral CD8 T cell tolerance, and tissue specific autoimmunity. A thorough understanding of these events is necessary to allow effective therapeutic intervention in autoimmunity and cancer.
A
BBREVIATIONS
AIRE Auto Immune REgulator
AMP Adenosin Mono-Phosphate
APC Antigen Presenting Cell
BM Bone Marrow
cDNA complementary DeoxyriboNucleic Acid
CNS Central Nervous System
CTL Cytotoxic T Lymphocyte
DC Dendritic Cell
dLN draining lymph node
DN Double Negative
DNA DeoxyriboNucleic Acid
DP Double Positive
DTg Double Transgenic
EAE Experimental Autoimmune Encephalomyelitis
EGC Enteric Glial Cell
GFAP Glial Fibrillary Acidic Protein
HA Hemagglutinin
IBD Inflammatory Bowel Disease
IEL Intra-Epithelial Lymphocyte
IFN Interferon
IL Interleukin
ILN Inguinal Lymph Node
i.p. intra-peritoneal
i.v. intra-venous
LN Lymph Node
RNA RiboNucleic Acid
RT-PCR Reverse Transcription – Polymerase Chain Reaction
s.c. sub-cutaneous
SP Single Positive
STg Single Transgenic
TCR T Cell Receptor
TEC Thymic Epithelial Cell
TGF Transforming Growth Factor
Th T helper or helper T cell
TNF Tumor Necrosis Factor
I.
T
HE IMMUNE SYSTEMThis chapter reviews notions of basic immunology (Kuby, 1994) with emphasis on T cells and recent findings on their different subsets, in the last paragraph.
Immunity plays a major role in maintaining our bodies’ integrity as it protects against microbial infections. The immune system also recognizes any damaged or “modified” cells as in the case of cancer. Immunity has both nonspecific and specific components. Both innate, or nonspecific, and adaptive, or specific, immunity depend on the ability of the immune system to distinguish between self and non-self molecules.
In this chapter, we will review general knowledge on hematopoeisis, organs of the immune system, innate immunity, and adaptive immunity.
A. H
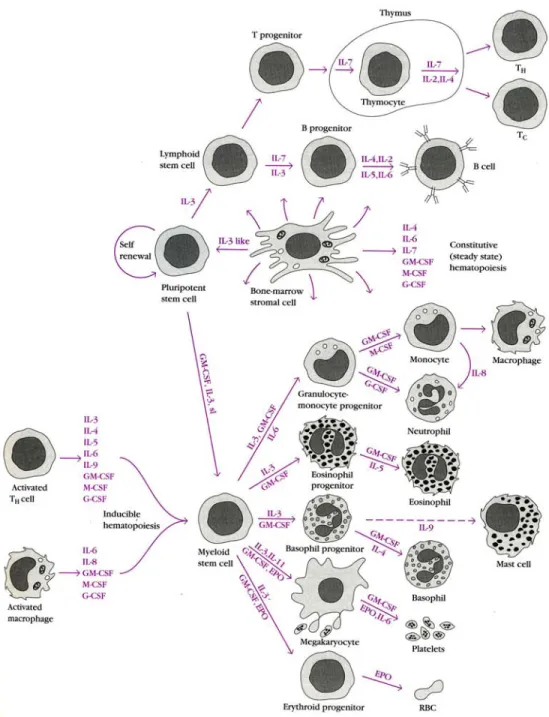
EMATOPOEISISHematopoeisis is the formation and development of platelets, red and white blood cells from stem cells. In developing embryos, blood formation occurs in aggregates of blood cells in the yolk sac, called blood islands. As development progresses, blood formation occurs in the spleen, liver and lymph nodes (LNs). When bone marrow (BM) develops, it eventually assumes the task of forming most of the blood cells for the entire organism. However maturation, activation, and some proliferation of lymphoid cells occur in primary (thymus) and secondary (spleen, Peyer’s Patches and LNs) lymphoid organs. While most haematopoiesis in adults occurs in the marrow of the long bones such as the femurs, it also occurs in spongy bone like ribs and sternum. In some cases, the liver, thymus, and spleen may resume their hematopoietic function if necessary (called extramedullary hematopoiesis). It is remarkable that every functionally specialized, mature blood cell is derived from a common stem cell. In contrast to a unipotent cell, which differentiates into a unique cell type, a hematopoeitic stem cell is pluripotent, able to differentiate along a number of
pathways and thereby generate erythrocytes, granulocytes, monocytes, mast cells, lymphocytes and megakaryocytes. These stem cells are few in number, occuring with a frequency of one stem cell per 104 BM cells. By virtue of their capacity for self-renewal, stem
cells are maintained at homeostatic levels throughout adult life; however, when there is an increased demand for hematopoeisis, stem cells display an enormous proliferative capacity. Early in hematopoeisis, a pluripotent stem cell differentiates along one of two pathways, giving rise to either a lymphoid stem cell or a myeloid stem cell (Fig. 1). Subsequent differentiation of lymphoid and myeloid stem cells generates commited progenitor cells for each type of mature blood cell. Progenitor cells have lost the capacity for self-renewal and are committed to a given lineage. The lymphoid stem cell generates T and B lymphocytes. The myeloid stem cell generates progenitor cells for erythrocytes, neutrophils, eosinophils, basophils, monocytes, mast cells and platelets. Progenitor commitment depends on the acquisition of responsiveness to particular growth factors. When the appropriate growth factors are present, these progenitor cells proliferate and differentiate, giving rise to the corresponding type of mature red or white blood cells. The types and amounts of growth factors present in the microenvironment in which a particular stem cell resides controls its differentiation.
In adult BM, the hematopoeitic cells grow and mature on a meshwork of stromal cells, which are non-hematopoeitic cells that support the growth and differentiation of the hematopoeitic cells. Stromal cells include fat cells, endothelial cells, fibroblasts and macrophages. Stromal cells influence hematopoeitic stem cell differentiation by providing a hematopoeitic-inducing microenvironment consisting of a cellular matrix and either membrane-bound or diffusible growth factors. As hematopoeitic stem cells differentiate in this microenvironment, their membranes acquire deformability, allowing the mature cells to pass through the sinusoidal wall into the sinuses of the BM, from whence they enter the circulation.
by programmed cell death or apoptosis. Each of the cells produced by hematopoeisis has a characteristic life span and then dies by apoptosis. This death, coupled with production of new cells, maintains steady-state levels of these cells. If apoptosis fails to occur, a leukemic state may develop. Some leukemias may also be resulting from abnormalities in the expression of hematopoeitic cytokines or their receptors.
Figure 1. Schematic view of hematopoeisis and its regulation by cytokines that stimulate the proliferation and/or differentiation of various hematopoeitic cells. In the absence of infection, bone marrow stromal cells are the major source of hematopoeitic cytokines. In the presence of infection, cytokines produced by activated
B. L
YMPHOID ORGANSA number of morphologically and functionally diverse organs have various functions in the development of an immune response. These organs can be divided on the basis of function into the primary (or central) and secondary (or peripheral) lymphoid organs. Immature lymphocytes generated during hematopoeisis mature and become commited to a particular antigenic specificity within the primary lymphoid organs. Only after a lymphocyte has matured within a primary lymphoid organ is the cell immunocompetent. In mammals, the primary lymphoid organs are the bone marrow, where B cell maturation occurs, and the thymus, where T cell maturation occurs.
A variety of peripheral lymphoid organs exists, each uniquely suited to trap an antigen from defined tissues or vascular spaces and to provide the structural microenvironment where mature, immunocompetent lymphocytes can interact effectively with that antigen. The LNs function to collect antigens from the intracellular tissue fluids, whereas the spleen filters blood-borne antigens. The respiratory and gastrointestinal tracts possess aggregations of mucosal-associated lymphoid tissue (MALT), including Peyer’s patches, tonsils, adenoids, and the appendix; these trap antigens entering through various mucous membrane surfaces.
1. Primary lymphoid organs
Thymus
T cell progenitors formed during hematopoeisis enter the thymus gland as immature thymocytes and mature there to become antigen-commited, immunocompetent T cells. The thymus is a flat, bilobed organ situated above the heart. Each lobe is surrounded by a capsule and is divided into lobules, which are separated from each other by strands of connective tissue called trabeculae. Each lobule is organized into two compartments: the outer compartment, or cortex, is densely packed with thymocytes, whereas the inner compartment, or medulla, is sparsely populated with thymocytes. It is generally thought that progenitor T cells enter the thymus and begin to multiply within the cortex. Here there is rapid proliferation of thymocytes coupled with a very high rate of cell death. A small subset of more mature thymocytes are then thought to migrate from the cortex to the medulla, where they continue to mature and finally leave the thymus via postcapillary venules. Both the
cortex and medulla are criss-crossed by a three-dimensional network of stromal cells composed of epithelial cells, interdigitating DCs, and macrophages, which make up the framework of the organ and contribute to thymocyte maturation. Many of these stromal cells physically interact with the developing thymocytes.
Thymocytes are subjected to positive and negative selection during their maturation process to ensure that only those thymocytes that recognize self-antigens are not released in the periphery. This is termed central tolerance (see II. A.). However, negative selection of autoreactive thymocytes is not 100% complete. Additional mechanisms of tolerance active in the periphery exist to silence these cells, such as anergy, deletion and regulatory T cells (see II. B.). If these peripheral tolerance mechanisms also fail, autoimmunity may arise.
Bone marrow
Bone marrow is the primary site of B cell ontogenesis. We will not extend on this topic as it is not the focus of this thesis.
2. Secondary lymphoid organs
Lymph nodes
LNs are encapsulated bean-shaped structures containing a reticular network packed with lymphocytes, macrophages, and DCs. Clustered at junctions of the lymphatic vessels, LNs are the first organized lymphoid structure to encounter antigens that enter the tissue spaces. As lymph percolates through a node, any particulate antigen that is brought in with the lymph will be trapped by the cellular network of phagocytic cells and reticular dendritic cells. The overall architecture of a LN provides an ideal microenvironment for lymphocytes to effectively encounter and respond to trapped antigens.
number of projections (trabeculae) into the interior to form a compartmentalized structure. The compartments are of two types, the red pulp and the white pulp, which are separated by a diffuse marginal zone. The splenic red pulp consists of a network of sinusoids populated with macrophages and numerous erythrocytes; it is the site where old and defective red blood cells are destroyed and removed. Many of the macrophages within the red pulp contain engulfed red blood cells or iron pigments from degraded hemoglobin. The splenic white pulp surrounds the arteries, forming a periarteriolar lymphoid sheath (PALS) populated mainly by T lymphocytes. The marginal zone, located peripheral to the PALS, is rich in B cells organized into primary lymphoid follicles. Upon antigenic challenge, these primary follicles develop into characteristic secondary follicles containing germinal centers where rapidly dividing B cells and plasma cells are surrounded by dense clusters of concentrically arranged lymphocytes.
Mucosal-associated lymphoid tissue
The mucous membranes lining the digestive, respiratory, and urogenital system, which have a combined area of about 400 m2, are the major sites of entry for most pathogens. The
defense of these vulnerable membrane surfaces is provided by organized lymphoid tissues known collectively as mucosal-associated lymphoid tissue (MALT). Structurally, these tissues range from loose clusters of lymphoid cells with little organization in the lamina propria of intestinal villi to organized structures such as the tonsils, appendix and Peyer’s patches. The functional importance of MALT in the body’s defense is attested to by its large population of antibody-producing plasma cells, whose number far exceeds that of plasma cells in the spleen, LNs and BM combined.
C. I
NNATE IMMUNITYInnate, or nonspecific, immunity refers to the basic resistance to disease that one possesses. Innate immune systems are found in all plants and animals. Innate immunity can be envisioned as comprising four types of defensive barriers: anatomic, physiologic, endocytic and phagocytic, and inflammatory.
1. Barriers of the innate immune system
Anatomic barriers
Physical and anatomic barriers to the entry of pathogens are an organism’s first line of defense against infection. They comprise the skin and the surface of mucous membranes. The skin consists of two distinct layers: a relatively thin outer layer, the epidermis, and a thicker layer, the dermis. The skin is maintained at an acidic pH comprised between 3 and 5, which is inhibitory to the growth of most microorganisms. Thus it prevents not only the penetration of most pathogens but it also inhibits most bacterial growth.
The conjunctivae and the alimentary, respiratory, and urogenital tracts are covered by mucous membranes, which consist of an outer epithelial layer and an underlying connective tissue layer. A number of nonspecific defense mechanisms serve to prevent the entry of pathogens through mucous membranes, such as tears, saliva, and mucous secretions that act to wash away potential invaders and also contain antibacterial and antiviral molecules such as lysozyme and mucoproteins.
Some organisms have evolved ways to escape this defense mechanism and thus are likely to invade the body. However, even when a pathogen eludes the anatomic defenses provided by the skin and mucous membranes, it still faces other types of innate defenses including various physiologic, phagocytic, and inflammatory barriers.
Physiologic barriers
Physiologic barriers include temperature, pH, oxygen tension, and various soluble factors. For example, the low pH of the stomach prevents most ingested microorganisms from surviving. Soluble proteins such as lysozyme, interferon, and complement also contribute to nonspecific immunity. Lysozyme is a hydrolytic enzyme found in mucous secretions and is able to cleave the peptidoglycan layer of the bacterial cell wall. Interferons are a group of proteins produced by virus-infected cells and other cells, and have the ability to bind to
controlled enzymatic cascade that results in membrane-damaging reactions, which destroy pathogenic organisms or facilitate their clearance.
Endocytic and phagocytic barriers
Endocytosis and phagocytosis refer to the ingestion of extracellular macromolecules and particles respectively. Only specialized cells are capable of phagocytosis, whereas virtually all cells are capable of endocytosis. The specialized phagocytic cells include blood monocytes, neutrophils, and tissue macrophages.
In endocytosis, macromolecules are internalized by cells into endocytic vesicles, which fuse with each other and are delivered to endosomes. Endosomes then fuse with Golgi-derived primary lysosomes containing degradative enzymes to form secondary lysosomes. It is within the secondary lysosomes that the ingested macromolecules are digested into small breakdown products (e.g. peptides, nucleotides, and sugars), which eventually are externalized from the cell.
In phagocytosis, the plasma membrane of the phagocytic cell expands around the particulate material to form large vesicles called phagosomes. The phagosomes will then fuse with lysosomes and the ingested material will be digested similarly to endocytosis.
Barriers created by the inflammatory response
Tissue damage caused by a wound, invasion by a pathogenic microorganism or by cancerous cells induces a complex sequence of events collectively known as the inflammatory response. This response can be characterized by three major events: vasodilatation, increased capillary permeability, and an influx of phagocytic cells. Vasodilatation refers to an increase in the diameter of blood vessels. The increased capillary permeability facilitates the migration of various white blood cells, the majority of which are phagocytic cells, from the capillaries into the tissue. The emigration of phagocytes involves cellular adherence (margination) to the endothelial wall followed by emigration between the capillary endothelial cells into the tissue (diapedesis or extravasion) and, migration through the tissue to the site of the inflammatory response (chemotaxis). As the phagocytic cells accumulate at the site and begin to phagocytose bacteria, they release lytic enzymes, which can damage nearby healthy cells. The accumulation of dead cells, digested material, and fluid forms a substance called pus.
The events in the inflammatory response are initiated by a complex series of events. During the inflammatory response, many chemical mediators are released in response to tissue damage. They are called acute-phase proteins. The concentrations of these proteins increase dramatically in tissue-damaging infections. C-reactive protein is a major acute-phase protein produced by the liver. Histamine is another mediator of the inflammatory response released by a variety of cells in response to tissue damage. Histamine causes vasodilatation and increased permeability. Another important group of inflammatory mediators are called kinins. They are normally present in blood plasma in an inactive form. Tissue injury activates these peptides, which then cause vasodilatation and increased permeability. A particular kinin, called bradykinin, also stimulates pain receptors in the skin. Vasodilatation and the increase in capillary permeability in an injured tissue enable enzymes of the blood-clotting system to enter the tissue. These enzymes activate an enzyme cascade that results in the deposition of insoluble strands of fibrin. The fibrin stands wall off the injured area from the rest of the body and serve to prevent the spread of infection.
Once the inflammatory response has subsided and most of the debris have been cleared away by phagocytic cells, tissue repair and regeneration of new tissue begins. Capillaries grow into the fibrin of a blood clot. New connective tissue appears, called fibroblasts, capillaries accumulate, and scar tissue forms.
2. Cells of the innate immune system
Innate leukocytes include: natural killer cells, mast cells, eosinophils, basophils, and phagocytic cells such as macrophages, neutrophils and dendritic cells (DCs).
Mast cells
Mast cells reside in the connective tissue and in the mucous membranes, are intimately associated with pathogen defense and wound healing, and often play a role in allergy and
cells are responsive to chemokine (C-C motif) ligand 9 (CCL9), and require the sialomucin CD34 (and CD43) for efficient trafficking in vivo (Blanchet, Maltby et al. 2007). Mast cells have recently been identified as an essential hematopoeitic component for polyp and colon cancer development (Gounaris, Erdman et al. 2007).
Macrophages
Macrophages are large phagocytic leukocytes, which function is to engulf and digest cellular debris and pathogens either as stationary or mobile cells, and to stimulate lymphocytes, by presenting it antigens, and other immune cells, by the release of chemokines, to respond to the pathogen. The lifespan of a macrophage ranges from months to years. In tissues, organ-specific macrophages are differentiated from phagocytic cells called monocytes, present in the blood. Macrophages are the most efficient phagocytes, and can eat substantial numbers of bacteria or other cells. The binding of bacterial molecules to Toll-like receptors (TLRs) on the surface of a macrophage triggers it to engulf and destroy the bacteria through the generation of reactive oxygen species,superoxide radical and hydrogen peroxide. Binding of TLR leads to the production of inflammatory cytokines, including TNF-α and IL-12, and enhances the cell’s antimicrobial killing mechanisms and antigen-presenting capacity. Thirteen TLRs have been identified in mice and humans together (named TLR1 to TLR13). Each recptor recognizes a small range of conserved molecules from a group of pathogens. For example, TLR4 binds to lypopolysaccharide (LPS) produced by most Gram-negative bacteria (Poltorak, He et al. 1998) and TLR9 recognizes bacterial DNA rich in CpG dinucleotides (Hemmi, Takeuchi et al. 2000). When activated, TLRs recruit adapter molecules known as MyD88, Tirap, Trif and Tram (Yamamoto, Sato et al. 2002; Yamamoto, Sato et al. 2003; Yamamoto, Sato et al. 2003). The adapters in turn recruit protein kinases known as IRAK1, IRAK4, TBK1, and IKKi (Li, Commane et al. 2001; Sharma, tenOever et al. 2003), which activate transcription factors such as NFkappa B (Rhee and Hwang 2000). TLR signaling is one of the most important gateways for gene modulation, particularly genes involved in inflammation, but also orchestrates a cross-talk between tissue-resident macrophages and other immune cells such as NK cells (Tu, Bozorgzadeh et al. 2008).
Neutrophils
Neutrophils, along with two other cell types; eosinophils and basophils (see below), are known as granulocytes, due to the presence of granules in their cytoplasm, or as polymorphonuclear cells (PMNs) due to their distinctive lobe nuclei. Neutrophil granules contain a variety of toxic substances that act to kill or inhibit bacteria and fungi. The release of granules is called degranulation. There are three types of granules: azurophilic granules or primary granules (myeloperoxidase, defensin, elastase, cathepsin G), specific granules or secondary granules (lactoferrin and cathelicidin), and tertiary granules (cathepsin, gelatinase). Neutrophils are the most abundant type of phagocyte, normally representing 50 to 60% of the total circulating leukocytes, and are usually the first cells to arrive at the scene of infection. The average halflife of a non-activated neutrophil in the circulation is about 4-10 hours. Upon activation, they marginate and migrate into tissues where they survive for 1-2 days. Neutrophils, and more precisely cathepsins, have been shown to play an important role in cancer (Bell-McGuinn, Garfall et al. 2007). Neutrophils are also involved in animal models of inflammatory bowel diseases (Leach, Bean et al. 1996; Iqbal, Oliver et al. 2002) (see III. B. 2.).
Dendritic cells
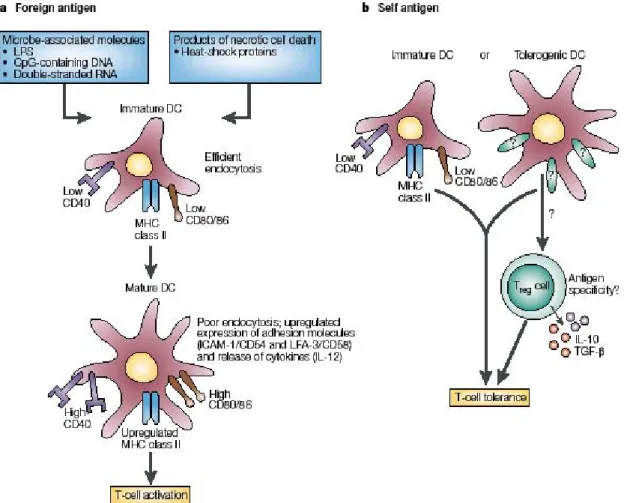
DCs are phagocytic cells present in tissues that are in contact with the external environment, mainly the skin (among which the Langerhans cells), the inner lining of the nose, lung, stomach and intestines. They are named for their resemblance to neuronal dendrites, but dendritic cells are not connected to nervous system function. Dendritic cells are very important in the process of antigen presentation, are also called professional antigen presenting cells (APCs), and serve as a key link between the innate and adaptive immune systems. DCs play a crucial role in the determination of immunogenicity vs. tolerance (see II. B. 4.).
toxic proteins and free radicals that are highly effective against bacteria and parasites but also responsible for most tissue damage occurring during allergic reactions. Activation and toxin release by eosinophils is tightly regulated to prevent any inappropriate tissue destruction. Natural killer cells
Natural killer cells, or NK cells, are a component of the innate immune system, and are distinctive in that NK cells attack host cells that have been infected by microbes, but do not attack the microbes themselves. NK cells attack and destroy tumor cells, and virally infected cells, through a process known as "missing-self", a term used to describe cells with low levels of a cell-surface molecule called MHC I (major histocompatibility complex). They were named "natural killer" because of the initial notion that they seemingly did not require activation in order to kill cells that are "missing self."
D. A
DAPTIVE IMMUNITYUnlike innate immunity, acquired immunity displays specificity, diversity, memory, and self/non-self recognition. The immune system is capable of generating tremendous diversity in its recognition molecules, allowing it to specifically recognize billions of uniquely different structures on foreign antigens. Antigens (short for antibody generators) are a class of non-self molecules and are defined as substances that bind to specific immune receptors and elicit an immune response. Once the immune system has responded to an antigen, it exhibits memory; that is, a second encounter with the same antigen induces a heightened state of immune reactivity. Because of this attribute, the immune system can confer life-long immunity to many infectious agents. A failure in self/non-self discrimination can lead to an inappropriate response to self-antigens with dramatic consequences, such as autoimmune diseases.
Acquired immunity does not occur independently of innate immunity. Cells of the phagocytic system are ultimately involved in activation of the specific immune response. At the same time, various soluble factors, produced during a specific immune response, have been shown to augment the activity of these phagocytic cells. For example, as an inflammatory response develops, soluble mediators are produced, attracting cells of the acquired immune system. The specific immune response will, in turn, serve to regulate the
intensity of the inflammatory response. Through the carefully regulated interplay of innate and acquired immunity, the two systems work together to effectively eliminate a foreign invader.
Cells of the adaptive immune system are called lymphocytes and comprise B and T cells.
1. B cells
B cells play a major role in the immune response mediated by antibody secretion, or humoral immune response. The abbreviation “B” comes from bursa of Fabricius, an organ in birds in which avian B cells mature. The principal function of B cells is to make antibodies against soluble antigens. B cells are produced in the bone marrow of most mammals. B cell development occurs in several stages, each stage representing a change in the genome content at the antibody loci. An antibody is composed of two light (L) and two heavy (H) chains. In the H chain loci there are three regions, V, D and J (each containing multiple copies of Vn, Dn or Jn gene segments), which recombine randomly, in a process called VDJ
recombination or rearrangement, to produce a unique variable domain in the immunoglobulin (Ig) of each individual B cell (Rajewsky, Forster et al. 1987). Similar rearrangement occurs for the L chain locus except that there are only two regions, namely V and J. VDJ recombination is carried out by critical lymphocyte specific enzymes called recombination activating gene-1 and-2 (RAG-1 and RAG-2).
The human body makes millions of different types of B cells each day that circulate in the blood and lymph and perform the role of immune surveillance. They do not produce antibodies until they become fully activated. Each B cell has a unique receptor protein, called the B cell receptor or BCR, on its surface that will bind to one particular antigen. The BCR is a membrane-bound immunoglobulin and is the main protein involved in B cell activation. B cells recognize their cognate antigen in its native form as a free or soluble antigen in the lymphoid organs. B cells can then become activated in a T cell-dependent or –independent
2. T cells
T cells play a central role in cell-mediated immunity. They are characterized by the presence of a special protein on their cell surface called the T cell receptor (TCR). The abbreviation “T”, in T cell, stands for thymus, as the thymus is the principal organ for their development. T cells can be categorized in different subsets: the αβ subset, which comprises CD8, CD4 and NK T cells; and the γδ subset. Naïve CD8 αβ T cells become cytotoxic T cells (CTLs) after activation, whereas naïve CD4 αβ T cells differentiate into helper T cells (Thcells). The differentiation of CD8 or CD4 T cells into effectors generates memory T cells that will persist long after an immune response has ended. The αβ T cell subset also comprises regulatory T cells (Tregs) that can be either CD8+ or CD4+.
Cytotoxic T cells
CTLs are capable of inducing the death of infected somatic or tumor cells; they kill cells that are infected with viruses or other pathogens, or are otherwise damaged or dysfunctional. CTLs are CD8+ T cells that have become activated. They express TCRs that recognize
specific antigenic peptides bound to MHC Class I molecules present on all nucleated cells. The activation of naive CD8 T cells into CTLs is dependent on several simultaneous interactions between molecules expressed on the surface of the T cell and molecules on the surface of the APC. There are two major signals leading to the activation of a naive CD8 T cell into a CTL. The first signal occurs when the TCR of the CD8 T cell strongly interacts with a peptide-bound MHC class I molecule on the APC. The antigens that bind to MHC Class I are 8-10 amino acids long. The interaction between the CD8 co-receptor and the MHC class I molecule serves to stabilize this signal. The second signal comes from an interaction between the CD28 molecule on the T cell and either CD80 or CD86 (also called B7-1 and B7-2) on the APC (Harding and Allison 1993). CD80 and CD86 are known as costimulators of T cell activation. The second signal can be assisted (or replaced) by stimulating the CD8 T cell with cytokines released from Th cells (Behrens, Li et al. 2004). Once activated, the CTL undergoes clonal expansion with the help of the cytokine IL-2, a growth and differentiation factor for T cells provided by CD4 helper T cells. This increases
the number of CTLs specific for the target antigen that can circulate throughout the body in search of cells expressing the specific MHC:peptide complex at their surface.
When exposed to infected or dysfunctional somatic cells, CTLs release the cytotoxins perforin and granulysin. These form pores in the target cell’s plasma membrane, causing ions and water to flow into the target cell, making the cell expand and eventually lyse. CTLs also release granzyme, a serine protease that can enter target cells via the perforin-formed pore and induce apoptosis by activation of cellular enzymes called caspases. Another way to induce apoptosis is via cell-surface interactions between Fas-ligand (FasL) on the CTL and Fas on the target cell (Kojima, Shinohara et al. 1994).
Helper T cells
Th cells play an important role in establishing and maximizing the capabilities of the immune system. These cells do not have any cytotoxic or phagocytic activity. They are involved in activating and directing other immune cells. They are essential in determining B cell antibody class switching, in the activation and growth of CTLs and in maximizing bactericidal activity of phagocytes such as macrophages. It is this diversity in function and their role in influencing other cells that give T helper cells their name.
Th cells differentiate from naive CD4+ T cell precursors. CD4 T cells have TCRs with an
affinity for MHC class II molecules, which are generally found only on professional APCs. Professional APCs are primarily DCs, macrophages and activated B cells, although DCs are the most efficient APCs on a per cell basis, due to their high expression of MHC Class II and costimulatory molecules. The peptides that bind to MHC Class II molecules are up to 25 amino acids long.
During an immune response, DCs endocytose foreign material, which undergoes processing, then travel from the site of infection to the LNs. Once in the LNs, DCs can present peptides bound to MHC class II to CD4 T cells, allowing CD4 T cells expressing specific TCRs against the peptide-MHC complex to activate.
activate each other via phosphorylation. With the assistance of another phosphatase present on the intracellular domain of CD45 (common leukocyte antigen), these molecules activate the major biochemical pathways in the cytosol of the CD4 T cell (Zamoyska 2007). Together with the antigen-MHC complex, TCR complex and CD4 interactions, the protein LFA-1 on the T cell and ICAM on the APC are the primary molecules of adhesion in this T cell-APC interaction. It is unknown what role the relatively bulky extracellular region of CD45 plays during cell interactions, but CD45 has various isoforms that change in size depending on the Th cell's activation and maturation status. For example, CD45 shortens in length following Th activation (CD45RA+ to CD45RO+), but whether this change in length influences
activation is unknown (Holmes 2006). It has been proposed that the larger CD45RA+ may
decrease the accessibility of the TCR for the antigen-MHC molecule, thereby forcing an increase in the affinity (and specificity) of the T cell required for activation (Altin and Sloan 1997). Once the activation has occurred, however, shorter alternatively spliced variants of CD45 allow easier interactions and activation as an effector T helper cell. The second signal needed to ensure a full CD4 T cell activation is provided by the co-stimulatory interaction between CD28 on the T cell and CD80 or CD86 on the professional APC.
Once the CD4 T cell has received both activation signals, it proliferates. It achieves this by releasing IL-2. Activated T cells also produce the alpha subunit of the IL-2 receptor (CD25 or IL-2Rα), enabling an autocrine loop with the binding of IL-2, which in turn promotes T cell proliferation. The function of IL-2 released by activated CD4 T cells is not solely for auto-regulation purposes but also to bind to other T cells in the vicinity, including activated CTLs awaiting instruction to proliferate. Other molecules get up-regulated or down-regulated on the cell surface of an activated T cell, such as CD69, CD44 and the lymph node homing receptor CD62L respectively. The loss of CD62L, which is a naive T cell marker, allows T cells to migrate from the LNs into tissues.
Proliferating helper T cells that develop into effector T cells differentiate into 3 major subsets: Th1, Th2, and the recently identified Th17 cells. These subtypes are defined on the basis of the cytokines they produce:
-Th1 cells produce interferon-gamma (IFN-γ) and tumor necrosis factor-beta (TNF-β, also known as lymphotoxin)
-Th17 cells produce IL-17, IL-22 (Zheng, Danilenko et al. 2007), and IL-21 (Wei, Laurence et al. 2007)
Th1 cell lineage has evolved to enhance eradication of intracellular pathogens (e.g. intracellular bacteria, viruses and some protozoa). Th1 differentiation is initiated by the coordinate signaling through the TCR and STAT1-associated cytokine receptors (Fig. 2a). Both type I and type II IFNs activate STAT1, as can the IL-12 family member IL-27, receptors for each of which are expressed on naive T cells (Hibbert, Pflanz et al. 2003; Lucas, Ghilardi et al. 2003). STAT1 signaling induces the transcription factor T-bet, which is a master regulator of Th1 differentiation (Szabo, Kim et al. 2000). T-bet potentiates expression of the Ifnγ gene and up-regulates the inducible chain of the IL-12 receptor (IL-12Rβ2), the expression of which enables IL-12 signaling through STAT4. The IL-12-driven component of Th1 development, which is downstream of STAT1-induced early differentiation, results in mature effector cells that can produce IFN-γ through either TCR-dependent or –inTCR-dependent pathways (Robinson, Shibuya et al. 1997; Yang, Murphy et al. 1999). Thus, IL-12, elicited from innate immune cells activated by pathogen recognition, affects Th1 development in the later stages, i.e. in maturation and/or expansion of effector cells rather than initial commitment.
Th2 cells have evolved to enhance the elimination of parasitic infections (e.g. helminths) through the activation of B cell IgE production, eosinophil recruitment and mucosal expulsion mechanisms (mucous production and hypermotility). Th2 differentiation is initiated by TCR signaling in concert with IL-4 receptor signaling via STAT6 (Fig. 2b), which in turn up-regulate the low-level expression of GATA3, a master regulator of Th2 differentiation (Zheng and Flavell 1997). GATA3 autoactivates its own expression and drives epigenetic changes that enable expression of the Th2 cytokine cluster (Il4, Il5 and Il13 genes), while suppressing factors critical to the Th1 pathway, such as STAT4 and the IL-12Rβ2 chain (Yamashita, Ukai-Tadenuma et al. 2004). Thus, early IL-4 signaling rapidly
Th2 responses can cause allergy and asthma. The cellular sources of the polarizing cytokines responsible for Th1 and Th2 development in vivo have been the subject of considerable debate. Although Th1 and Th2 cells can themselves provide IFN-γ or IL-4 for the recruitment of Th1 or Th2 differentiation respectively, investigators have not yet definitely determined which cells initiate effector T cell differentiation in primary versus secondary responses. Plasmacytoid DCs, NK cells, or NKT cells appear to be involved in early production of type I and II IFNs for induction of Th1 cells, but which cells initiate Th2 development is less clear. Basophils, eosinophils, mast cells, and NKT cells are sources of IL-4 that may be important for Th2 differentiation (Yoshimoto, Bendelac et al. 1995; Min, Prout et al. 2004; Voehringer, Reese et al. 2006), and each of these cell populations may be important for initiating Th2 responses to distinct pathogens or in distinct settings.
IL-17 producing CD4 T cells were first characterized in 2003 (Aggarwal, Ghilardi et al. 2003; Murphy, Langrish et al. 2003) and have been widely accepted as a new lineage of Th cells distinct from Th1 and Th2 cells in 2005 (Harrington, Hatton et al. 2005; Park, Li et al. 2005). IL-23 is one of the critical factors for the promotion of the Th17 pathway. The first clear evidence for an IL-23/IL-17 cytokine axis in autoimmunity, as opposed to the previously implicated IL-12/IFN-γ axis, came with studies of murine models of autoimmunity, namely experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA) (Cua, Sherlock et al. 2003; Murphy, Langrish et al. 2003). In addition to the link of the IL-23/IL-17 axis to EAE and CIA, recent studies have extended the correlation with this pathway and autoimmunity to models of inflammatory bowel disease (IBD) (Rennick, Fort et al. 1997; Uhlig, McKenzie et al. 2006; Yen, Cheung et al. 2006). IL-23 is not required for the generation and commitment of Th17 cells but rather for their expansion and/or maturation, similar to the role of IL-12 for Th1 cells (Fig. 3). So far, three independent studies have found that TGF-β and IL-6 but not IL-23 are required for Th17 commitment (Bettelli, Carrier et al. 2006; Mangan, Harrington et al. 2006; Veldhoen, Hocking et al. 2006). The requirement for TGF-β in Th17 differentiation is shared with adaptive Tregs, which default to Foxp3 induction in the absence of IL-6 (Fig. 3). Thus, IL-6, elicited by pathogen-induced activation of innate immune cells via toll-like receptors (TLRs), is a critical switch factor that diverts antigen-activated naive T cells toward a pro-inflammatory rather than an anti-inflammatory adaptive response (Weaver, Harrington et al. 2006). The master regulator of Th17 cells has recently been identified as retinoic orphan receptor (ROR)γt (Ivanov, McKenzie et al. 2006). Unlike T-bet and GATA3, however, RORγt is a nuclear receptor whose putative ligand remains unidentified, and it is unknown whether RORγt activity is regulated by a ligand or is ligand-dependent. It also remains to be determined how Smad and STAT signals emanating from TGF-β and IL-6 might cooperate with RORγt signals to specify Th17 commitment. Potential inhibitors of Th17 cell development have been attributed to the IL-12 family member IL-27 (Batten, Li et al. 2006; Stumhofer, Laurence et al. 2006), which in turn promotes Th1 differentiation, and to IL-25 (Owyang, Zaph et al. 2006), which in turn promotes Th2 differentiation.
Figure 3. Model for divergent differentiation of Th17 and adaptive regulatory T cell (Treg) lineages. This model emphasizes distinct pathways leading to mature Th17 effector cells or Foxp3+ adaptive Tregs,
contingent upon a common requirement for TGF-β but differential effects of IL-6 and IL-23. Naive CD4 T cells activated by antigens presented on an immature dendritic cell (iDC) are induced by TGF-β to express Foxp3 and develop into Tregs (top). Naive CD4 T cells activated by a mature dendritic cell (mDC) produce IL-6, which acts in concert with activated TGF-β to induce expression of the retinoic orphan receptor (ROR)γt and to upregulate IL-23R, thus becoming competent for IL-17A and IL-17F production and IL-23 signaling. IL-23 signaling can act synergistically with IL-23 to induce Th17 cytokine production independently of TCR stimulation. Alternatively, TCR stimulation by antigen can induce Th17 cytokine production directly, without a requirement for IL-23, IL-1, or IL-18. G-CSF [and GM-CSF (not shown)] produced by mature Th17 cells acts to stimulate granulopoiesis and mobilization of neutrophils. Dotted lines indicate possible positive feedback loops by which cytokine products of Th17 (IL-6) or Treg cells (TGF-β) may reinforce lineage development (Weaver, Hatton et al. 2007).
Memory T cells
Memory T cells are a subset of antigen-specific T cells that persist long-term after an infection has resolved. They quickly expand to large numbers of effector T cells upon re-exposure to their cognate antigen, thus providing the immune system with "memory" against past infections. Memory T cells comprise two subtypes: central memory T cells (TCM cells) and effector memory T cells (TEM cells). TCMs express L-selectin (or CD62L) and the chemokine receptor CCR7; they secrete IL-2 but not IFN-γ or IL-4 (Willinger, Freeman et al. 2005). TEMs do not express L-selectin or CCR7 but produce effector cytokines such as IFN-γ and IL-4 (Willinger, Freeman et al. 2005). Memory cells may be either CD4+ or CD8+. Regulatory T cells
Treg cells, formerly known as suppressor T cells, are a specialized subpopulation of T cells that act to suppress activation of the immune system and thereby maintain immune system homeostasis and self-tolerance. The existence of a dedicated population of suppressor T cells was the subject of significant controversy among immunologists for many years. However, recent advances in the molecular characterization of this cell population have firmly established their existence and their critical role in the immune system. Interest in regulatory T cells has been heightened by evidence from experimental mouse models demonstrating that the immunosuppressive potential of these cells can be harnessed therapeutically to treat autoimmune diseases and facilitate transplantation tolerance, or specifically eliminated to potentiate cancer immunotherapy.
One of the most convincing series of studies demonstrating the importance of thymus-derived cells in the prevention of organ-specific autoimmunity was performed in the late 1960s and early 1970s (Nishizuka and Sakakura 1969; Gershon and Kondo 1970). In these experiments, mice that were thymectomized on the third day of life were shown to develop organ-specific autoimmune diseases. The idea of the existence of a population of suppressor T cells was first born. However, it was not until 1972, with the publication of an article called “Suppressor T cells” that people began to recognize that there was more to immunity than clonal selection, and that T cells might be capable of both enhancement and inhibition of immunity (Gershon, Cohen et al. 1972). CD8+ T cells were the initial focus of suppressive
T cells with regulatory activity. An early finding indicated that the Ib Qa-1 surface protein was a potential target of suppressive CD8+ cells (Cantor, Hugenberger et al. 1978). The
discovery that T lymphocytes are responsible for the lymphoproliferative disorder in scurfy mice was made in 1991 (Godfrey, Wilkinson et al. 1991). This group subsequently showed that the disease is mediated by CD4+ T cells. The finding that suppressor activity is enriched
in CD4+ CD25+ cells has permitted further phenotypic and functional characterization of
this elusive subset of T cells (Sakaguchi, Sakaguchi et al. 1995), which comprises 5-10% of the mature CD4+ helper T cell subpopulation in mice and about 3-8% CD4+ helper T cells
in humans. In addition to the diseases seen post-day 3 thymectomy, it has also been shown that the CD4+ CD25+ subset is involved in the regulation of IBD (Read, Malmstrom et al.
2000) and insulin-dependent diabetes mellitus (IDDM) in the NOD mouse (Salomon, Lenschow et al. 2000). Thus, CD4+ CD25+ T cells are regulators of almost all of the animal
models for human organ-specific autoimmune diseases and have also been found in human thymus, cord and peripheral blood (Baecher-Allan, Brown et al. 2001; Taams, Vukmanovic-Stejic et al. 2002; Wing, Ekmark et al. 2002).
CD4+ CD25+ cells are not the only subset of suppressor T cells. Lafaille and coworkers
(Olivares-Villagomez, Wensky et al. 2000) have described an immunoregulatory CD4+
CD25- T cell population that prevents the induction of EAE in animals that bear a
transgenic TCR specific for myelin basic protein (MBP). Similarly, Stephens and Mason (Stephens and Mason 2000) have identified both CD25+ and CD25- immunoregulatory T
cells in the rat. Most importantly, the suppressor activity of the CD25- T cells only becomes
manifest when CD25- recent thymic emigrants are removed from the animal. It is not known
whether the CD25- T cells in both of these models acquire their suppressor phenotype in the
thymus or in the periphery. On the other hand, it has been possible to generate potent populations of IL-10 secreting immunoregulatory T cells in vitro, named Tr1, by repeated stimulation of presumably CD25- T cells in the presence of IL-10 (Groux, O'Garra et al.
in vivo (Liu, Tugulea et al. 1998; Najafian, Chitnis et al. 2003). Another study found suppressive activity within the CD8+ CD122+ T cell pool (Rifa'i, Kawamoto et al. 2004).
Finally, CD8+ CD45RClow cells have been studied in an in vivo model of graft versus host
(GvH) (Xystrakis, Cavailles et al. 2004; Xystrakis, Dejean et al. 2004), and more recently, a novel population of Qa-1 restricted CD8αα+ TCRαβ+ T cells has been shown to prevent
the induction of EAE (Tang, Maricic et al. 2006). However the contribution of these populations to self-tolerance and immune homeostasis is less well defined than that of CD4+
CD25+ Treg cells.
We will address in chapter II. B.1. the mechanism of thymic selection of Foxp3+ Tregs and
the mechanism of suppression by Tregs. Natural killer T cells
NKT cells are a heterogeneous group of T cells that share properties of both T cells and natural killer cells. Many of these cells recognize the non-polymorphic molecule CD1d, an MHC class-I-like molecule that binds self- and foreign lipids and glycolipids. They constitute only 0.2% of all peripheral blood T cells (Godfrey, MacDonald et al. 2004). The best known subset of CD1d-dependent NKT cells expresses an invariant TCRα chain. These are referred to as type I or invariant NKT cells (iNKT) cells. These cells are conserved between humans and mice and are involved in many immunological processes. Upon activation, NKT cells are able to produce large quantities of IFN-γ, IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF), as well as multiple other cytokines and chemokines (such as IL-2 and TNF-α) (Vivier and Anfossi 2004). NKT cells seem to be essential for several aspects of immunity because their dysfunction or deficiency has been shown to favor the development of autoimmune diseases (such as diabetes or atherosclerosis) and cancers (Sakuishi, Oki et al. 2007). NKT cells have recently been implicated in the progression of human asthma (Sakuishi, Oki et al. 2007). The clinical potential of NKT cells lies in the rapid release of cytokines (such as IL-2, IFN- γ, TNF-α, and IL-4) that promote or suppress different immune responses.
γδ T cells
γδ T cells represent only 5% of total T cells and are found at their highest abundance in the gut mucosa, within a population of lymphocytes known as intraepithelial lymphocytes (IELs) (Holtmeier and Kabelitz 2005). The antigenic molecules that activate γδ T cells are still largely unknown. However, γδ T cells are peculiar in that they do not seem to require antigen processing and MHC presentation of peptide epitopes, although some recognize MHC class IB molecules. Furthermore, γδ T cells are believed to have a prominent role in the recognition of lipid antigens.
Like other 'unconventional' T cell subsets bearing invariant TCRs, such as CD1d-restricted NKT cells, γδ T cells exhibit several characteristics that place them at the border between the more evolutionarily primitive innate immune system, allowing a rapid beneficial response to a variety of foreign agents, and the adaptive immune system, where B and T cells coordinate a slower but highly antigen-specific immune response leading to long-lasting memory against subsequent challenges by the same antigen. On the one hand, γδ T cells may be considered a component of adaptive immunity in that they rearrange TCR genes to produce junctional diversity and will develop a memory phenotype. On the other hand, the various subsets may also be considered part of innate immunity (Born, Reardon et al. 2006) where a restricted TCR may be used as a pattern recognition receptor (Morita, Mariuzza et al. 2000). For example, according to this paradigm, large numbers of Vγ9/Vδ2 T cells respond within hours to common molecules produced by microbes, and highly restricted intraepithelial Vδ1 T cells will respond to stressed epithelial cells bearing sentinels of danger (Morita, Mariuzza et al. 2000).
Clearly, the complexity of γδ T cell biology spans definitions of both innate and adaptive immune responses.
II.
M
ECHANISMS OF IMMUNE SELF-
TOLERANCEThe immune system is delicately balanced between self-antigen-driven tolerance and pathogen-driven immunity. Imposition and regulation of self-tolerance within the T cell repertoire is exerted at two levels. First, the development and selection of T cells in the thymus strongly biases the naive T cell repertoire against self-reactivity by a mechanism called central tolerance. Second, mature T cells are subject to secondary selection in lymphoid and non-lymphoid organs via passive, or recessive, mechanisms such as ignorance, deletion and anergy or via active, or dominant, mechanisms such as Treg-mediated suppression. These mechanisms of tolerance are globally called peripheral tolerance.
A. C
ENTRAL TOLERANCEDuring postnatal life, ~10-100 hematopoeitic precursors on average enter the thymus from the bloodstream per day. Upon commitment to the T lineage, these cells undergo approximately 20 divisions, mostly within the double-negative (DN) stage of T cell development that extends over about two weeks. This massive expansion of the precursor pool results in the generation of about 5×107 T cells daily, a figure in remarkable contrast to
the estimated 1-2×106 mature T cells that are actually released daily into the circulation. The
loss of over 95% of thymocytes reflects the stringent selection processes that shape the developing T cell repertoire. The first checkpoint, so-called beta-selection, is contingent upon pre-TCR signaling and ensures that only those DN thymocytes that have successfully rearranged their TCRβ locus progress to the CD4+ CD8+ double-positive (DP)
compartment. After progression to the DP stage and rearrangement of the TCRα locus, all subsequent developmental decisions of thymocytes are dictated by interactions with peptide/MHC ligands on stromal cells within the thymic microenvironment. Failure of a TCR to interact with peptide/MHC (pMHC) ligands within a certain window of affinity /avidity is interpreted as reflecting a useless specificity, i.e. lack of self-MHC restriction, resulting in apoptosis of the DP thymocytes. This process is called positive selection. Most
apoptotic cell death in the thymus has been attributed to failure of positive selection, indicating that the quantitative effect of selection for self-MHC restriction, or positive selection, far exceeds that of selection against potentially dangerous auto-reactivity, or negative selection (Surh and Sprent 1994). Thus, an estimated 90-95% of thymocytes are lost due to failure of positive selection (Huesmann, Scott et al. 1991). Approximately 50-70% of positively selected T cells are thought to be subject to negative selection (Ignatowicz, Kappler et al. 1996). Negative selection occurs when interactions between the TCR and pMHC ligands are too strong and could thus result in the release of potentially autoreactive T cells. Negative selection occurs mainly in the medulla (Hogquist, Baldwin et al. 2005) where the microenvironment provides a unique representation of self-antigens otherwise restricted to specific peripheral tissues. Peripheral tissue antigens (PTAs) are promiscuously expressed by medullary thymic epithelial cells (mTECs) (Derbinski, Schulte et al. 2001) and can also be cross-presented by thymic DCs (Gallegos and Bevan 2004). The promiscuous expression of PTAs by mTECs plays a major role in tolerance induction and protection against auto-immune diseases. For example, it was shown that expression of PLP, the main protein of the myelin sheath, in mTECs was sufficient to confer T cell tolerance and protection against experimental autoimmune encephalomyelitis (EAE), a mouse model for multiple sclerosis (Klein, Klugmann et al. 2000). The array of promiscuously expressed self-antigens appears to be random rather than selected (Derbinski, Schulte et al. 2001). Several transcription factors control expression of PTAs in mTECs. AIRE (Auto Immune REgulator) is the major transactivator identified so far (Pitkanen, Doucas et al. 2000) being expressed in mTECs and controlling a large array of PTAs, and plays an essential role in deletion of autoreactive developing T cells. In addition, it was recently shown that an IRF8-binding promoter variant plays an important role in regulating quantitative expression of the auto-antigen CHRNA1, indicating that AIRE and the interferon signaling pathway might work in concert to set the threshold for self-tolerance vs. autoimmunity (Giraud, Taubert et al. 2007). AIRE-/- mice develop multi-organ autoimmunity (Anderson, Venanzi et al. 2002;
characterized by numerous organ inflammatory infiltrates, display mutations on the AIRE gene (Bjorses, Aaltonen et al. 1998).
Mechanisms of tolerance induced in the thymus not only include deletion of autoreactive thymocytes with high avidity/affinity for self-antigen but also the development of naturally occurring CD4+ (CD25+) Foxp3+ regulatory T cells (Fig. 4 and II.B.1.), that are able to keep
autoreactive cells that have escaped negative selection in check in the periphery.
Figure 4. Recessive and dominant mechanisms cooperatively mediate tolerance toward PTAs. Various experimental models suggest a division of labor during central tolerance induction, in that Treg induction is efficiently mediated by TECs, whereas DCs are specialized for T cell deletion (Kyewski and Derbinski 2004). Constitutive cross-presentation by thymic DCs ensures that both tolerance modes operate concomitantly for PTAs, most of which are restricted in their expression to mTECs. Note that the apparent redundancy of antigen presentation is limited by the unidirectional flow of antigenic material from mTECs to DCs. Four possible routes of intercellular transfer of antigens are indicated. Uptake of apoptotic fragments from neighbor cells has been demonstrated for lymph node DCs. Antigen transfer via exosomes, capture of small portions of cytoplasm/membrane (“nibbling”), or gap junctions has been reported in particular systems in vitro, but their relevance in vivo remains to be established. From (Kyewski and Klein 2006).
B. P
ERIPHERAL TOLERANCEThere are severe limitations on the ability of central tolerance to delete all potentially autoreactive T cells. Despite the activity of AIRE, not all self-antigens have access or are expressed in the thymus at levels sufficient to eliminate all autoreactive T cells. Indeed, although negative selection deletes T cells of high avidity for self-antigens, low avidity T cells that have much less chance of initiating autoimmunity are spared (Liu, Fairchild et al. 1995). Other peripheral mechanisms of tolerance are thus needed to keep those auto-reactive T cells in check in order to prevent autoimmunity. These mechanisms are also required in order to avoid the development of an immune response against the myriad of innocuous environmental antigens to which we are continuously exposed, from both our diet and our environment (Mowat 2003). They include dominant mechanisms that involve suppression by regulatory T cells and recessive mechanisms including immunologic ignorance, anergy and deletion (Fig. 5).
1. CD4+ CD25+ Foxp3+ regulatory T cells
Molecular and functional characterization of CD4+ CD25+ Tregs: role of Foxp3 in function and
maintenance of Tregs
Tregs are defined by the expression of the forkhead family transcription factor FOXP3 (forkhead box p3), a key control gene in their development and function (Fontenot, Gavin et al. 2003; Hori, Nomura et al. 2003; Khattri, Cox et al. 2003), which was discovered during efforts to understand the genetic basis for a rare X-linked fatal autoimmune disease in humans known as IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome and an analogous lymphoproliferative disease observed in the spontaneous mouse mutant “scurfy” (Chatila, Blaeser et al. 2000; Bennett, Christie et al. 2001; Brunkow, Jeffery et al. 2001; Wildin, Ramsdell et al. 2001). Typically, high levels of CTLA-4 (cytotoxic T lymphocyte-associated molecule 4) (Salomon, Lenschow et al. 2000; Takahashi, Tagami et al. 2000) and GITR (glucocortucoid-induced TNF receptor) (McHugh, Whitters et al. 2002; Shimizu, Yamazaki et al. 2002) are also expressed on Tregs; however, the functional significance of this expression remains to be defined. The use of CD25, CTLA-4, and GITR as surface markers for Tregs is problematic as they are also expressed on activated effector T lymphocytes (Takahashi, Tagami et al. 2000; Shimizu, Yamazaki et al. 2002). Foxp3 is not expressed on activated T cells in mice and the Treg cell population is more accurately defined by Foxp3 expression. There is a great interest in identifying cell surface markers that are uniquely and specifically expressed on Foxp3-expressing Tregs. However, to date, no such molecule has been identified. Foxp3 is also highly expressed in CD25+ CD4+ T cells
with suppressor function in humans (Ziegler 2006), however it is also transiently expressed in activated conventional T cell populations in humans (Walker, Kasprowicz et al. 2003). Tregs are unable to produce pro-inflammatory cytokines such as IL-2, are hyporesponsive to antigenic stimuli in vitro and are thus called “anergic” (Gavin, Clarke et al. 2002; Sakaguchi, Ono et al. 2006). Consistent with this, the key molecular targets of Foxp3 have recently been identified by independent groups as suppressors of the activation of target genes on T cell stimulation (Marson, Kretschmer et al. 2007; Zheng, Josefowicz et al. 2007). The action of Foxp3 in regulating Treg cell function is in a dose-dependent, non-binnary manner, and decreased Foxp3 expression causes immune diseases by subverting the suppressive function of Treg cells and converting Treg cells into effector cells (Wan and Flavell 2007). Consistent