O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 16639
To link to this article : DOI:10.1016/j.apsusc.2016.08.170
URL :
http://dx.doi.org/10.1016/j.apsusc.2016.08.170
To cite this version : Uhart, Arnaud and Ledeuil, Jean Bernard and
Gonbeau, Danielle and Dupin, Jean-Charles and Bonino, Jean-Pierre
and Ansart, Florence and Esteban, Julien An Auger and XPS survey
of cerium active corrosion protection for AA2024-T3 aluminum
alloy. (2016) Applied Surface Science, vol. 390. pp. 751-759. ISSN
0169-4332
Any correspondence concerning this service should be sent to the repository
administrator:
staff-oatao@listes-diff.inp-toulouse.fr
An
Auger
and
XPS
survey
of
cerium
active
corrosion
protection
for
AA2024-T3
aluminum
alloy
A.
Uhart
a,
J.B.
Ledeuil
a,b,
D.
Gonbeau
a,
J.C.
Dupin
a,∗,
J.P.
Bonino
b,
F.
Ansart
b,
J.
Esteban
c aIPREM-ECP-UMRCNRS5254,UniversitédePauetdesPaysdel’Adour,TechnopoleHélioparc,2AvenuePrésidentPierreAngot,64053PauCedex09,France bUniversitédeToulouse,UPS-INP-CNRS,InstitutCarnotCIRIMAT,118RoutedeNarbonne,31062ToulouseCedex09,FrancecMessier-Bugatti-Dowty,EtablissementdeMolsheim,3,rueAntoinedeStExupéry,67129Molsheim,France
Keywords: XPS Auger Cerium Conversioncoating AA2024alloy Corrosion 1. Introduction
Aluminumanditsalloysarewidelyusedinaerospaceindustry becauseoftheirlowdensityandmechanicalproperties.Despite the protective film ((hydro) oxide thin film) formed in mois-tureconditions,thehighreactivitycommonlyobserveddrastically increasesincontactwithsolutionscontainingcomplexingagents (e.g.,halides)[1–4].Thecorrosionprocessofaluminuminvolves theadsorptionofaggressiveionsonthe(hydr)oxidealuminum sur-face(e.g.,Cl−),thedissolutionofthisprotectivelayerandthedirect
attackofthemetal[5].Asolutiontoimprovethecorrosion resis-tanceistouseprotectivecoatings.Chromates(CrVI)compoundsare
themostcommonsubstancesusedduetotheirefficiencyinsevere atmosphereandtheirlowcost[6].Chromatescanbeintroduced bythreemainways:directincorporationintoconversioncoatings, onlyaddedinanodizingbathsandaspigmentsinpaintingprimers. However,theuseofchromatesincoatingsinvolvedserious envi-ronmentalproblemsforthesurfacetreatmentindustry,because hexavalentchromatesarecarcinogenicandhighlytoxic[5].Since environmentalregulationsgotstricterinthelastfewyears,withthe comingprohibitionuseofCrVIcompoundsincorrosionprotection
∗ Correspondingauthor.
E-mailaddress:dupin@univ-pau.fr(J.C.Dupin).
systems(REACH,2017),differentalternativesubstitution formu-lationshavebeenextensivelystudied.Oneofthemostpromising systemsisbasedonrare-earth(Ce,Nd,La,Y)impregnation solu-tions[7–9].Moreover,Hintonetal.haverevealedthehighpotential of ceriumions inthereduction of thecorrosionrate[7]. Some newcompositeformulations(e.g.,Ce-dopedsol-gelnetwork)offer bothactive(electrochemical)andpassive(barrier)protectionfor theunderlyingalloys[2,4,10,11].TheideaofCeconversion coat-ingsasactiveprotectionfilmsonaluminum alloysisstill akey parameterinthegeneralthoughtandrecentworkseven recog-nizedanenhancedactivitywithaprioracidsurfacepre-treatment
[12].Allthesepreliminarystudiesinspiredanincreasinginterest forresearchersandespeciallyaircraftmanufacturerswhowantto progressintheknowledgeoftheactiveprotectionfilmformation processtooptimizethelifecycleofthecoateddevices.The Cerium-basedconversionprocesshasalreadybeenundertakenwiththe differentceriumsalts[13]and,foreachofthem,withthe addi-tion(ornot)ofdifferentsodiumsalts.Mainobservationswerethe nitrateionprovedtobeastronginhibitorandthesulphateionhad nomarkedeffect(onlyveryslightinhibition).
Thepurposeofthispaperistogivecomplementary informa-tioninthetopicabouttheroleofceriuminhibitorviathestudy oftheconversioncoating(CC)compositionbyadualX-Ray pho-toelectron(XPS)/Augerspectroscopiesanalysis.Foraconvenient protectiveprocessusedintheindustry,suchaconversioncoating
isalwayspre-depositedonto themetalsupportbeforea primer (filler,UVabsorber,...)andtopcoat(againstmechanicalabrasion andenvironmentalerosion)[14].Then,specificpropertiesofthe CeriumCCdiscussedhereafterhavetobeconsideredinaglobal framework.Thecorrosionprotectionofthesomepartsusedinthe aeronauticsisthenthegoodalchemybetweentheactive(CC)and thepassive(primer/paint)assembliesofthewholeprotective sys-tem.Ourshortstudy,onlyfocusedontheactiveprotectiondeposit, presentsanoriginalsurvey,atthesub-micronscale,ofthechemical evolutioninthesurroundingofthecorrodedzones(pits,crevices). Withtheseadaptedtoolsofsurfacecharacterization,migrationof entitiesandchangesintooxidationstatesaredirectlyand simulta-neouslyrecorded.TherealroleoftheactiveCClayerscanbethen wellunderstood.
2. Materialsandmethods 2.1. Materialandtreatments
ThematerialstudiedwasAA2024-T3aluminumalloycomposed by(inwt%)3.8–5.0Cu,0.2–1.2Mg,0.4–1.0Mn,0.5–1.2Si,<0.7Fe, <0.1Cr.Eachsamplesurface(80mm×42mm×1mmpanel)was cleanedandpreparedusingseveralsteps;afteracetonedegreasing, achemicalpretreatmentwasperformedasitfollows:a 20-min immersionina NaOHsolution(pH=9)maintainedat60◦C,
fol-lowedbyarinsingwithdeionizedwater;anda5minneutralization inanaqueoussolutionofNaNO3atpH=0.95atroomtemperature.
Thesampleswerefinallywashedinethanolanddriedinair. Ceriumbasedconversioncoatings(CeCC)weredepositedatroom temperaturebyimmersionofthealloypanelinawatersolution containingCe(NO3)3,6H2O(Fluka, CAS:10294-41-4) ata
differ-entceriumconcentrations(0.01M,0.05M,0.1Mand0.5M).The pHoftheimmersionbathismaintainedto4andnoaccelerating agent(e.g.,H2O2)wasused.Onceimmersedduring300s(duetime
forahomogeneous/coveringfilmandbetterelectrochemical per-formance),sampleswererinsedfourtimeswithdeionizedwater anddriedatroomtemperatureinadesiccator.CeCCwerefinally about2.1±0.5mmthick(examinedbySEM)withyellow/brown colorization,aftera300simmersionintheconversionbath.
Aqueous corrosion solutions—Corrosion attack wasconducted on the samples in a 3.5wt.% NaCl solution at 25◦C. Time of
immersionwassetto1haccordingtotheappearanceoffirst cor-rosionpits. Asingleandspecific testof ioniccrosssectionwas achievedontheAA2024substratecoveredwithCe-basedcoating ([Ce]=0.1M)withaJEOLIB-0901crosspolisher.Analytical condi-tionsarereportedelsewhereinarecentwork[15].After96hunder airexposure,samplessurfaceandcrosssectionwereanalyzedwith XPSandAuger; this operationwasa firsttrytoinvestigatethe corrosionpropagationandtheeffectoftheinhibitordeepinthe material.
Theelectrochemicalbehaviorofthesystemswasevaluatedby electrochemicalimpedance spectroscopy (EIS)in a 0.05MNaCl staticsolution(pH=6.0)forthedifferentpanelsoriginalygotfrom severalimmersiontimes(1s,60sand300s)intheconversionbath. Fortheelectrochemicalmeasurements,athree-electrode electro-chemicalcellwasused,consistingofaplatinumcounterelectrode, asaturatedcalomelreferenceelectrodeandthesamplewasused asaworkingelectrode,withanexposedareaequalto15cm2.The
experimentalapparatususedfortheelectrochemicalinvestigation wasapotentiostat(AUTOLABPGSTAT30)andafrequencyresponse analyzer(FRA). EISmeasurementswere performedin potentio-staticmodeattheOCP,obtainedaftera1hstabilizationofthe potentialintheelectrolyte.TheamplitudeoftheEISperturbation signalwas10mV,andthefrequencystudiedrangedfrom100kHz to10mHz.Onlyvaluesofthechargestransferresistance(R)are
reportedelsewhereinthetextforanyconcentrationsofcerium testedintheconversionbath.
2.2. Surfaceanalysis
2.2.1. Morphologyofmaterialswithscanningelectron microscopy(SEM)
Themorphologyandthemicrostructureofthecoatings,before andaftertheimmersiontestsincorrosionsolutions,wereobserved by Scanning Electron Microscopy (SEM). High resolution high-energy images were obtained with a JEOL JAMP-9500F Auger spectrometer(JEOLLtd,Tokyo,Japan)workingunderprecise condi-tions(30keV,2nA,workingdistance=23mm,pressure<2.10−7Pa)
fittedwithaSchottkyfieldemissionelectrongunusinga conven-tionalsecondaryelectrondetector(SED)intheanalysischamber. Thepresentanalysismodepermitsahighdepthfieldfor nanopar-ticlesvisualization.
2.2.2. SurfacechemicalanalysiswithX-rayphotoelectron spectroscopy(XPS)
IntheaimtocharacterizetheCeCCcomposition,X-Ray Photo-electronSpectrometry(XPS)measurementswereperformedona ThermoK-alphaspectrometerwithahemisphericalanalyzerand amicrofocussed(analysis areawasc.a.400mm2) monochroma-tizedradiation(AlKa,1486.6eV)operatingat72Wunderaresidual pressureof1×10−9mbar.Thepassenergywassetto20eV.Charge
effectswerecompensatedbytheuseofa chargeneutralisation system(low energy electrons)which had the uniqueability to provideconsistentchargecompensation.Alltheneutraliser param-etersremainedconstantduringanalysisandallowonestofinda 285.0eVC1sbindingenergyforadventitiouscarbon.Spectrawere mathematicallyfittedwithCasaXPSsoftwareusingaleastsquares algorithmandanon-linearbaseline.Thefittingpeaksofthe exper-imentalcurvesweredefinedbyacombinationofGaussian(70%) andLorentzian(30%)distributions.Onlycorelevelsspectraforthe elementswithhighestphotoionizationcrosssectionwererecorded inordertoextractmorereliableinformation.
2.2.3. Surfacechemicalanalysiswithaugerspectroscopy(XPS) andchemicalmappingwithscanningaugermicroscopy(SAM)
TheAugeranalyseswerecarriedout withthepreviousJEOL JAMP9500FAugerspectrometerworkingunderUHVconditions (pressure<2.10−7Pa).The UHVequipmentwasa Schottky field
emissionAugerelectronspectrometer(FE-AES)dedicatedtovery highspatialresolution analysis andhigh brightness.The hemi-sphericalelectronanalyzercoupledwithamultichanneldetector (7channeltrons)offeredidealsettingsforenergyresolvedAuger analysis.
3. Resultsanddiscussion 3.1. AA2024-T3surfacepreparation
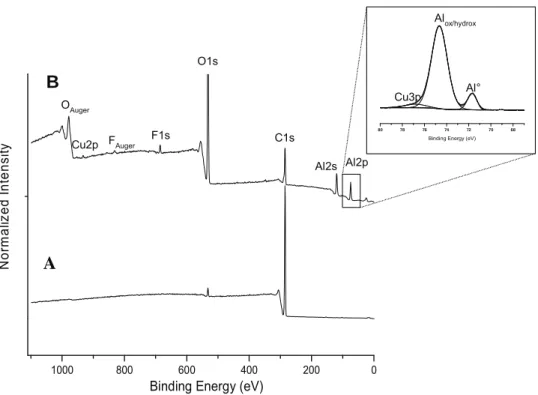
TheAA2024-T3alloysurfacewasinitiallycleanedbeforeCeCC asreportedintheMaterialsandtreatmentsection.Thechemical surfacestateiscontrolledwiththeXPSandresultsshowahigh initialcontentofhydrocarbonentitiesattheAAl2024-T3surface (Fig.1A).
Thesurfaceisfullcontaminatedasnoalloyelementsarevisible. Oncechemicallytreatedwithacidandalkalinebaths(Fig.1B),the nearalloysurfaceexhibitsacleardecreaseofthecarboncomponent (Table1)andanaluminumsignalwithtwodistinctcomponents: –at71.8eVassociatedwiththemetallicAl◦oxidationstate,–and
at74.6eVcorrespondingtoAl-O(H)environment;thethinnative (hydro)oxideoverlayerisapotentialanchorageareaforthefuture conversioncoating.CopperCu2psignalattestsofthebaremetal
80 78 76 74 72 70 68
Binding Energy (eV)
1000 800 600 400 200 0
N
o
rm
a
liz
e
d
I
n
te
n
s
it
y
Binding Ene
rgy (eV)
B
A
C1s O1s OAuger Al2s Al2p F1s Cu2p FAuger Al° Alox/hydrox Cu3pFig.1. LargescaleXPSspectrumofAA2024-T3alloy:(A)beforeand(B)aftersurfacepreparation(degreasing/acidcleaning).
Table1
SurfacecompositionoftheAA2024-T3substrate:beforeandafterdegreasing/acid treatmentandcoveredwithCeCC([Ce]=0.1M)after300sofimmersioninthe conversionbath.
Element(at.%) C O Al Ce N Cu F Beforesurfacepreparation 96.5 3.5 – – – – Aftersurfacepreparation 27.1 46.3 25.5 – 0.2 0.9 AA2024-T3/CeCC([Ce]=0.1M) 31.0 46.8 3.8 12.2 6.2 – –
detectionandtheefficiencyofthecleaningtreatment. Effective cleaningpre-treatmentsareoftendemonstratedtobeassociated withthegenerationofsurfacedefaults,grainedtopologyfavoring redoxreactionsnecessarytotheCeCCformation[16].
Fluorine components oflow intensityobserved in theclean samplespectrumcomefromaresidualpollutionintheanalysis chamberintheperiodoftheexperiment.Itdoesnothaveanyeffect ontothesurfacechemistryasithasbeenmainlyfound,inverylow concentration(around0.9at.%),bondedtoresidualhydrocarbon entities.
3.2. Ce-basedconversioncoating(CeCC)overAA2024-T3before corrosion
TheCeCCcoveringisexpectedtobequitehomogeneouswhich isfundamentalforthefutureindustrialapplications.SEMimagesof theCeCCpanelssurfaceweresystematicallyrecordedinthe differ-entimmersionconditions([Ce],immersiontime).Beforecorrosion, theappearanceofthesurfacecoatingisquitehomogeneousfor ceriumconcentrationsrangingfrom0.01Mto0.1Mwhateverthe immersiontime.OnfigureESI1,onecanobserveinthecaseofthe Ce(0.1M)CCcoating(300sofimmersion)asurfacerarelyaffected bytopographydefects(holes,excrescences,...).Whenincreasing theceriumconcentration(e.g.,[Ce]=0.5M),rightfromthefirst sec-ondsofimmersion,thecoatingiscrackedandcanpeeloffforlonger stayintheconversionbath.Thenthehigherthecerium concentra-tionis,themoredefectivethecoatingsurfacebecomes.
XPSanalysisforthecoatedpanelshasshownwell-identified chemical entities from the coating but also from the AA2024 substrate.For instance, after300sof immersionin the conver-sionbath,thelargescalesurvey (Fig.2A) ofAA 2024-T3/CeCC (e.g.,[Ce]=0.1M)systemdisplaysemergenceofthemain differ-entorbitals(Al2p,Al2s,C1s,O1sandCe3d)withintensesignals (Table1).Thecoating,veryfewmicronsthick,appearstobequite homogeneousandleveling(Fig.2B)buttheobservationofsome substrateelementswiththeXPStechniquewouldattestofa cer-tainporosityofthedeposit.After1sofimmersion,TheXPSdata give,atthesubstratesurface,aCe/Alratiobetween0.4and0.5for [Ce]=0.1Mwhenitis6timeshigherforthe[Ce]=0.5Min rela-tionwitharealdifferenceofdepositthickness.Forlongertimes ofimmersion(e.g.300s),theCe/Alratioisquitestablefor0.1M whereasitdrasticallydropsfor0.5M.Thiscouldconfirmthe dam-agingincreaseofthecoatingathighceriumconcentrationwiththe immersiontime.
Differentareasofanalysiswerepointedoutandnodifference wasfoundforthechemicalcompositionoftheCeCCcoating traduc-ingagoodhomogeneity.Theceriumcorrosioninhibitorhasbeen recordedinquiteimportantat%between2.6%(for[Ce]=0.01M) to13.7%for[Ce]=0.5M).Forallthecoatings,thechemicalsurvey attestsofamixtureofceriumspecies(Ce+IIIandCe+IV,seefigure ESI2)asreportedelsewhere[17].Histogramsshowamain propor-tionofCe+IVforpanelscoveredupatlowceriumconcentration whenCe+IIIisinthemajorityforhigherceriumconcentration.
In the high resolution Ce3d spectrum, a same structured experimentalprofileisrecorededwhateverthedifferentcerium concentrationoftheCCbathandimmersiontimes.Onlyintensities varyaccordingtothecontentofCe+IIIandCe+IVspecies.InFig.3A andtheTable2(e.g.,Ce(0.1M)CCimmersed300s),fourmainpeaks correspondingtothepairsofspin-orbitdoubletscanbeidentified, ingoodagreementwithotherauthors[20,21]and thepresence ofa+IIIoxidationstate.Thehighestbindingenergypeaks,u1and
u’1respectivelylocatedatabout885.8±0.2eVand904.3±0.2eV
aretheresultofaCe3d94f1O2p6finalstate.Thelowestbinding
energystatesu0andu’0respectivelylocatedat881.8±0.2eVand
Fig.2.Cerium-basedconversioncoating(with[Ce]=0.1M;300sofimmersionintheconversionbath)overAA2024-T3alloy:(A)largescaleXPSspectrumand(B)SEM micrographofthecrosssection.
R e la ti v e i n te n s it y 870 880 890 900 910 920 930
Binding Energy (eV)
A
u0 u’0 u1 u’1 v’2 v2 v1 v’0 v0 v’1B
u0 u1 u’1 u’0 870 880 890 900 910 920 930Binding Energy (eV)
Fig.3.XPSCe3dspectrumoftheCe(0.1M)CC(300simmersiontimeinthe con-versionbath)/AA2024-T3:(A)as-preparedand(B)after180sofcompucentricAr+
erosion(rastersize:0.8mm,IE:3000eV).
Table2
Labeling,meanpositionofthe10componentsofamixedCe(III)/Ce(IV)sample accordingto(*)meanpositionofpeaksforthedifferentCe(0.1M)CC(300s immer-siontime)ontoAA2024-T3substrate.
Spin-orbit component electronicstate attribution (*)peakposition (eV)±0.5eV Ce(III) u0 3d5/2 Ce(III)3d94f1 O2p6 880.7 u0′ 3d3/2 901.1 u1 3d5/2 Ce(III)3d94f2 O2p5 885.6 u1′ 3d3/2 904.6 Ce(IV) v0 3d5/2 Ce(IV)3d94f2 O2p4 882.5 v0′ 3d3/2 903.4 v1 3d5/2 Ce(IV)3d94f1 O2p5 886.8 v1′ 3d3/2 907.5 v2 satellite Ce(IV)3d94f0 O2p6 898.8 v2′ satellite 916.7
Someminorcomponentsowingtohybridizationwithligand orbitalsand partialoccupancyofthevalence 4forbitalarealso observed.Sixnewpeakslabeledv0,v1,v2(for3d5/2)andv0′,v1′,
v2′(for3d3/2)referringtothethreepairsofspinorbitdoubletsare
characteristicofCe4+3dfinalstates[22,23].Thelowestbinding
energystatesv0(v’0),v1(andv’1)respectivelylocatedat882.5eV
(903.4eV),886.8(and907.5)aretheresultofCe3d94f2O2p4and
Ce3d94f1O2p5finalstates.Thehighestbindingenergypeaks,v2
andv’2respectivelylocatedatabout916.7and898.8±0.2eVare theresultofaCe3d94f0O2p6finalstate.Thesatellitepeakv2
asso-ciatedtotheCe3d3/2ischaracteristicofthepresenceoftetravalent
Ce(+IVoxidationstate)duetoapartialoxidationofceriuminthe referencematerial.Thisfactisprettyinterestingasthis compo-nentisisolatedfromtheothersatapprox.916–917eV,asamatter offact,onecanusethispeakasaquantitativeprobeoftheamount ofCe(IV)[18,24–26].
InsuchacoatingtheminorCe+IVspeciesseemtobelocated inthetoplayerpartasdemonstratedbysoftAr+ionerosionwhich
revealsonlyaCe+IIIstateafter180sofsurfaceerosion(Fig.3B). SuchobservationswereevenreportedinafurnishedworkofL.S. Kastenandcoll.[18].Ce+IV(depositionofCeO2-2H2OorCe(OH)4
layer)isfoundtobepredominantunderdifferentexperimental conditions:whenthecerium-basedconversioncoatingisachieved using,forinstance,chlorideceriumsaltbasedconversionbathin presenceofhydrogenperoxide[19].AtlowCeconcentration,the smallerthicknessofthedepositinvolvestheoxidationofmost dis-persedCe+IIIspecieswhichislessevidentforthickercoating(high Ceconcentration)forwhichonlytoplayersofCe+IIIareoxidized. TheoxidationeffectismorepronouncedforCe(0.1M)CCcompare toCe(0.5M)CCcertainlyduetoamoreimportantporosityforthis lastone.
Indeed,ithasbeendemonstratedthattheintegralareaofv2′
peakwithrespecttothetotalCe3dareacouldbetranslatesinto percentageofCe4+[24,27].Shyuandcoll.[28]havedemonstrated
thattheintegralareaofthev2′peakwithrespecttothetotalCe3d
areacouldbetranslatedintopercentageofCe4+withtherelative
errorofbeingintherangeof10%.InthecaseofpureCe4+,the
v2′peakshouldconstitutearound14%oftotalintegralintensity.
A
B
Rel a ti v e i n te n s it y 72 76 80 84 88Binding Energy (eV)
72 76 80 84 88 Binding Energy (eV)
Fig.4.XPSAl2pspectrumof:(A)Ce(0.1M)CC(300simmersiontimeinthe con-versionbath)/AA2024-T3and(B)AlCeO3referencematerial.
Ce4+reportedintheliterature,percentageofCe4+wascalculated
by: Ce4+=
′ 2%
14 ×100 (1)
wherev2′%isthepercentageofv2′peakareawithrespecttothe
totalCe3darea.
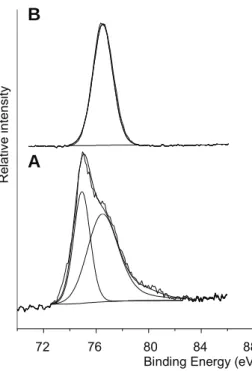
For alltheanalyzed panelsunder differentpreparation con-ditions, the Al2p experimental is quite the same with a two componentsconvolution.Fig.4AofCe(0.1M)CCimmersed300s
showstwochemicalenvironments:thecomponentat74.8eVcan beassociatedwiththenativeoxidelayerontopAA2024-T3 sub-stratewhiletheoneonhighenergyside(76.9eV)isattributedtoa Al/O/Cemixedchemicalenvironmentasdetectedinacommercial stoichiometricAlCeO3material(CAS12014-44-7,Sigma-Aldrich)
analyzedinsameexperimentalconditions(Fig.4B).Thenpartofthe ceriumentitiesseemstobelocatedinthenearsurfaceofthealloy (neartheAloxide/hydroxidetoplayer)whichcouldbeakeypoint inthecorrosionprotection,intermsofproximitywiththemetal surface.Thefactthatonecanobservethepresenceofaluminum, evenifthecoatingisaround2.0mmthick,would alsosignifya certainporositydegreeofthedeposit.
Whatevertheconditionsofimmersiontime,acomplementary surveyofthesurfacesamplewassystematicallydonewithanAuger mapping(SAM)analysistocontrolthespatialchemical homogene-ityofchemicalelements.
InFig.5,thecaseoftheCe(0.1M)CCcoatingimmersed300sin theconversionbathispresentedandfewsurfacedefects(cracks, holes,...)arenoticedasdiscussedelsewhere,attestingofagood levelingdeposit inthepresent conditionsofdeposit.TheAuger mappingallowsonetoconsideragooddispersalofceriumwiththe experimentaldepositprocessusedinthiswork.Theobservationof thealuminumandoxygeninquitegoodrelativecontentconfirms thenativealuminum(hydro)oxideoverlayerintheproximityof thecerium.
3.3. Ce-basedconversioncoating(CeCC)overAA2024-T3after corrosion
3.3.1. Corrosionresistance
Theelectrochemicalresults(Fig.6),indicate,afteronesecondof immersionina0.05MNaClstaticsolution(pH=6.0),thatin con-trolledproportions,theadditionofceriumintheconversionbath leadstotheincreaseof theanti-corrosionproperties.However, itseemsthereisacerium concentrationthreshold(0.1M)over whichthecorrosionresistancefails.Thecoatingdopedwith0.5M Ce(NO3)3showsthelowestresistanceandthehighestcapacitances
Fig.5.Ce(0.1M)CCcoating(immersiontime=300sintheconversionbath)as-prepared:SAMimagesforCe,AlandOelements(RVBscale)andSEMmicrographofthesame area(bottomright).
Fig.6.ChargestransferresistanceevolutionofAA2024T3panelsimmersedin someconversionbathsofdifferentceriumconcentrationsandfordifferenttimes ofimmersion.
Fig.7.(A)SEMmicrographofthecorrodedCeCC(Ce=0.1M)appliedontoAA 2024-T3(300simmersiontime).
attheendofthetestperiod,suggestingthattheincreaseofthe ceriumconcentrationpromotestheformationofmoredefective coatings.Moreover,thecorrosionresistanceisclearly enhanced after300sofimmersion.
Theseeventsareprobablyduetothephysico-chemical struc-turationoftheCeCCwhichcouldchangewiththeceriumcontent (internalconstraints).Actually,inrelationwiththeeffectiverole ofCe+IIIionsextensivelyreportedfortheactiveprotectionofAA 2024-T3alloy[6,29],thepresentstudywillonlyfocusonto cerium-basedconversioncoating(with[Ce]=0.1M)forwhichthebetter corrosionresistanceisreachedafter300sofsubstrateimmersion intheconversionbath.
3.3.2. Chemicalanalysisofthecorrosionattack
OnthecoatedCeCCpanels,whatevertheirinitialimmersion timeandCeconcentrationproceededintheconversionbath,first corrosionpitsappearedbefore1hofimmersionintothe3.5wt.% NaClsolution.After96hunderairexposuredrying,panelswere thenanalyzedwithXPS,SEMandSAM.AglobalSEMmicrograph forthetreatedsubstrateinthe3.5wt.%NaClsolutionispresented inFig.7.Itdisplaysthepresenceofholesrandomlydispersedatthe surfaceandsomezoneshighlydamagedwithalocalizedpeeloff phenomenon.Thewhitenodulesarewell-knownCu-richdeposited particles(e.g.,Al2CuMgmetallicagglomerates...)asdetectedwith
Augerspectroscopyandreportedelsewhere[30–32].Theclose-up SEMmicrographofa“zoomin”corrosionpit(Fig.8)allowsone tobetterappreciatedetailsofthecoatingpeeloffstateandareal changeofcolornuanceinthedamagedareasuggestingachangein
thechemicalcomposition(differentsecondaryelectronsemission recorded).
TheAugerchemicalmappingachievedonthecoatingsurface showscleardifferencesinthespatialdistributionofceriumbefore (fig.5)andafter(fig.8)thecorrosionattacksofthecoatedpanel. Astheceriumisprimarilywelloverlaidontothesubstrateafter theCeCCimmersion,itseemstomovetothepitsareasduringthe corrosionevent.Actuallyarelativehighcontentisobservedinthe hollowringofthecorrosionpitandinitscenterpart.Color inten-sitygradient(inagreementwiththerelativeconcentrationofthe element)attestsofamigrationofceriumfromsurroundedzones tothecorrosionpit.Inthedamagedzone,therelative concentra-tionofceriumincreasesrelatetothesurroundingzones.Thisinflux iscertainlyexplainedfromthemigrationofceriumwhichensures theactivesubstrateprotectionagainstcorrosion.Intheattacked haloofthecoating,ceriumbecomesmoreconcentratedwhen rela-tivealuminumandoxygenamountsfalldown.Thisresultisoriginal andsignificantforthedirectvisualizationofthecorrosioninhibitor behaviorandtheprotectiveactionofCeCC.
Thechemicalmappingofoxygenrelativecontentshowsless drasticchangefromtheundamagedcoatingtothecorrosionpit. Thiscouldcorrespondtotheexistenceofceriumoxide/hydroxide inbothareaswithslightly stoichiometrydifferences.Inanother hand,aluminumisclearlywithdrawnfromtheattackedzone.Some additionalmeasurementswereachievedwithAugerspectroscopy indifferentpointsatthepanelsurface(figureESI.3)beforeand after1hand5hofimmersioninthecorrosiveNaClbath. Quantifi-cationdata,extractedfromtheconvolutionofAugerlinesOKLL (510.0eV),AlKLL(1387.0eV)andCeMNN(625.0eV),clearly con-firmthedisplacementofceriuminthecorrodedpartsofthepanel withanenhancementofitsrelativecontentduringthecorrosion process:18.6at.%intheaspreparedcoatingandupto27.3at.% (1hof immersion)and 39.4at.%(5hofimmersion)inthe cen-terdamagedzone.TheAugerspectroscopyandmappingisgreat complementtoSEMsurveysasthesoleSEMimagesinterpretation cannotexplainthewholerealityofthecoatingsurfaceevolution. Forinstanceinthecaseofthepanelimmersedduring1hinthe corrosivebath,itiseasytomonitortheCemigrationwiththeCe contentfrompoint1towardspoints2and3.Thisobservationgives arealsensetothe“active”protectionoftheinhibitor.
Togofurtherintheunderstandingofchemicaleventsduring thecorrosionprocess,aXPSsurveyhasbeenrunintwospecific pointsofthecorrodedpaneldisplayedinFig.7(labelled1and2). Thisspecificzonewaschosenbecauseoftheimportantsizeofthe observedpit(around60mm×130mm)which fitswiththeused ofthefocusedX-raybeam(30mmdiameter).TheaimoftheXPS analysiswastoprecisethepossiblechemicalchangesduringthe ceriummigration.Forzone1,theCe3dspectrum(Fig.9A)fitsinto twodoubletsassignedtoaCe+III(hydro)oxidizedenvironment withpreviouslyreportedbindingenergies(U:880.8eV–901.3eV) and(V:885.9eV-904.8eV)forsuchoxidationstateofcerium.As thesurfacehasbeenexposedtothetreatmentsolution,thenear surfacemorphologyhasnecessarychangedtomoreroughnessand thedissolutionofthethinCe+IVlayer.Thephysicaland chemi-calperturbationofthesurfacealsoinfluencedtheCe3delectron emissionwhichdisplaysanoisysignal.Lossofcarbonatomic per-centagecombinedwiththeenhancementofcopperandaluminum elementssignalsofthesubstrateputsintoevidencetheNaClattack onthecoating.Thisphenomenonismorepronouncedinthevisible corrosioncrack(zone2)forwhichtheCecontentstronglyincreased (Ceat.%2timesmoreimportantthaninzone1,seeTable3)and substrateismorevisibleasthecoatingpeeledoffinthiszone.In thecorrosionpitzone(2),asignificantevolutionoftheCe3d spec-trumisnoticedwiththeappearanceCe+IVfeaturesandthespecific satellitepeakaround917eV(Fig.9B).
Fig.8. Ce(0.1M)CCcoating(immersiontime=300sintheconversionbath)aftercorrosionattack:SAMimagesforCe,AlandOelements(RVBscale)andSEMimageofthe samearea(topright).
U
0V
1V
0A
B
870 880 890 900 910 920 930Binding Energy (eV)
+III +III +III +III +III +III +III +III +IV +IV +IV +IV +IV +IV
V
2U
1Fig.9. XPSCe3dpeakoftreatedCe=0.1McoatingappliedonAA2024-T3:(A)zone 1and(B)zone2ofFig.7.
Then, it seems the cerium played its inhibitor role in the woundedzonewithagreatkineticanditsmigrationis accompa-niedwithachemicalstatetransformation(Ce+III→Ce+IV).The observationoftheO1sspectrum(Fig.10)givesthesametrend withthe top heights ofthe experimentalprofile which shifted
Table3
XPSSurfacecompositionofzones1and2ofthetreatedCe=0.1Mcoating(300s immersiontime)appliedontoAA2024-T3.
Element(at.%) Cu Ce C O Al N Na Cl Zone1 0.1 7.7 29.8 50.6 6.4 3.8 0.8 0.8 Zone2 0.4 15.0 25.3 43.1 14.5 1.2 0.3 0.2 H2O/NO3 -hydroxides Ce2O3 CeO2 526 528 530 532 534 536 538
Binding Energy (eV)
526 528 530 532 534 536 538 Binding Energy (eV)
A
B
Fig.10.XPSO1speakoftreatedCe=0.1McoatingappliedonAA2024-T3:(A)zone 1and(B)zone2ofFig.7.
asthe damageincrease attesting ofnewelectronicdistribution aroundoxygenatoms.Forzone1(fig.10A),anintensecomponent (BE=533.3eV)associatedwithresidualtracesofnitratesfrom ini-tialceriumsaltandwaterfromthetreatmentbath,isobserved.
Fig.11.CrosssectionofCerium-basedconversioncoating(with[Ce]=0.1M,immersiontime:300s)overAA2024T3alloyafter5hcorrosioninto3.5wt.%NaClsolution:(A) SEMviewand(B)Augerspectroscopyanalysisinthecoatingsection(point1)andwithinthecrevicecorrosionflaw(point2).
Thecomponentat532.1eVcorrespondstothehydroxidespecies (hydroxilizedcarbon,Al(OH)3....)whilethenarrowcomponentat
530.2eVcaneasilybeassociatedtoceriumsesquioxide[33].Inthe areaofthecorrosionpit(zone2),theoxygenpeakonlowenergy side(fig.10B),shiftsto529.6eVwhichitissignificantofaCe+IV stateasfoundinCeO2[32].Ashoulderaround530.2eVisstill
vis-ibleasexpectedintheCe+III/Ce+IVmixturereportedintheCe3d spectrum(fig.9B).Inviewoftheseresults,adirectoverviewofthe chemicalchangesoccurringunderthecorrosiveNaCleffectcanbe screened.Ceriumactsasaself-healingagentintheCeCCcoating withappropriate migrationtotheboundedzonesandthe initi-ationofredoxprocesstoblockthecathodiczones.Actually,the releaseofhydroxylsinthecathodiczonesleadstothe precipita-tionofceriumspeciesmainlyintointermediatehydroxidesthen intoceriumdioxide.
Inthisrespect,Aldykiewiczetal.[34]proposedthatthecathodic depositionofCeO2filmswasduetotheoxidationinthesolution
ofCe3+ionstotetravalentCe(OH)
22+ionsinsolution(whichcan
diffusereachinglocaldefects)followingthePourbaixdiagram[35]:
4Ce3++6H2O+O2→4Ce(OH)22++4H+ (2)
andprecipitateaccordingtothereaction:
Ce(OH)22+→CeO2+2H+ (3)
Tocorroboratethemigration ofceriumspeciesfromthe Ce-basedcoatingtothedamagedzonesoftheAA2024alloy,anoriginal surveywasfocuseddeeperinthesubstrateafter5hofcorrosion attack(Fig.11A).Substratewascross-cutintheareaofawell iden-tifiedcrevicecorrosionflaw.TheSEMmicrographrevealstheinner morphologyinthedirectionofthesection,theunstructured Ce-basedcoatingoverthesubstrateinaccordancewiththegeneral degradationundercorrosiveconditions.Inthecenterpartofthe micrograph,thesubstrateisconsumedandalargecrevice (under-cutpit)extendsoverfewmicrons.AlocalAugerchemicalanalysis inbothzones(labeled1and2onFig.11A)wasachievedtoprecise thechemicalentitiesbehaviorduringthecorrosionmechanism.We focusedtheexperimentona300eV−1500eVenergyscaletoget betterresolutionofthedetectedtransitions.TheAugerspectrum ofthedamagedCe-basedcoating(Fig.11B,point1)mainlyexhibits theAugertransitionsofaluminumandoxygen.Theabsenceofany
ceriumsignalissignificantofthecompletedissolutionofthe Ce-basedlayerorthemigrationofceriumentitiesdeepinthematerial. Thethicknessoftheobservedtop-layerovertheAA2024-T3 sub-strategotthinner(0.9mm±0.2mm)andwouldonlybemadeof nativealuminum(hydr)oxidephasesandhydrocarbon(forcarbon element,CKVVtransitionat263eVcan’tbedetectedintheenergy scaleofthestudy).Withinthecrevicecorrosionflaw,sameanalysis wasrun(Fig.11B,point2)andanewsetoftransitionsisdetected: CeMNN(largecomponentwithtop-heightat630eV)andCuLMM (seriesoftransitionsbetween710eVand902eV).Theco-presence ofcerium,copperandaluminumwasfoundindifferentother ana-lyzedzoneswithinthecrevice.Thismainobservationsuggeststhe precisebehaviorofthecorrosioninhibitorwhichwouldblockthe cathodiczones.
4. Conclusion
Onthewayoftheknowledgeandunderstandingofthecorrosion protectionofAA2024alloy,thepresentpapergivesa complemen-taryviewofthechemicalevolutionsatthesurfaceofthesubstrate. Ceriumconversioncoatingonprepared2024aluminumalloy con-sistedofAloxide,andmainlyofCe+IIIoxideandhydroxide.TheCe stateexhibitedamixtureofCe+IIIandCe+IVdependingonthesalt concentrationintheconversionbath.Theouterlayerofthecoating isCe+IVrichanditprobablyindicatestheCe+IIIoxidizedtoCe+IV duringcoatingformationintheinitialsolution.Thecomparison ofCeconcentrationsoftheconversionhasshownbettercharges transferresistance(thenbetterrelativecorrosionprotection)for apanelimmersedduring300sina[Ce]=0.1Mbath.Forhigher Ceconcentrationsorimmersiontime,thedepositbecomesmore powderedandunsuitabletoaircraftapplications.ThedetailedXPS studyhasallowedonetoconsideraclose-upinteractionbetween ceriumandtheAloxidelayerontopalloywiththeexistenceof Al/O/Cemixedchemicalenvironment. Microscopicand spectro-scopictoolswereevenpreciousindicatorstoappreciatethecerium inhibitorbehaviorintermofdisplacementandchemical modifica-tion.Thelocationofceriumhasbeenmonitoredatanytimewitha preciseSAMsurveywhichiscomplextosetupbutessentialforan elementalscreeningwithinconfinedorverysmallzonesof inter-est.Actually,undercorrosiveconditions,ceriumtendstomove,
in a shorttime, towardswoundedzones leavingthe surround-ingcoatingareaalmostfreeofinhibitorcontent.Thisdirectaction involvesachemicalchangewiththeformationofCe+IVoxideand hydroxide.Sameobservationsweredoneatthesubstratesurface (corrosionpits)andsub-surface(crevices).Theeffectivenessofthe CeCCcoatingbeforethedepositofaprimerand/orafinaltopcoat isdeterminedbythesynergyofmanyexperimentalparameters. Evenifceriumalonecannotguaranteethewholeprotectionofa metalpanelagainstcorrosion,itactsasareallyactiveagentwitha quickkineticforhealingwoundedzones.
Acknowledgments
Thanks to the french aeronautics consortium (AESE pole SOLGREEN©)andtheDGA(DirectionGénéraledel’Armement)for theirtechnicalandfinancialsupport.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,athttp://dx.doi.org/10.1016/j.apsusc.2016.08. 170.
References
[1]K.A.Yasakau,M.L.Zheludkevich,O.V.Karavai,M.G.S.Ferreir,Prog.Org.Coat. 63(2007)352–361.
[2]S.M.Tamborim,A.P.Z.Maisonnave,D.S.Azambuja,G.E.Englert,Surf.Coat. Technol.202(2008)5591–6001.
[3]S.S.Pathak,A.S.Khanna,T.J.M.Sinha,Prog.Org.Coat.60(2007)211–218.
[4]V.Palanivel,Y.Huang,W.J.V.Ooij,Prog.Org.Coat.53(2005)153–168.
[5]R.T.Foley,Corros.Sci.42(1986)277–288.
[6]M.W.Kendig,A.J.Davenport,H.S.Issacs,Corros.Sci.34(1993)41–49.
[7]B.R.W.Hinton,D.R.Arnott,N.E.Ryan,Mater.Forum7(4)(1984)211–217.
[8]D.Zhao,J.Sun,L.Zhang,Y.T.J.Li,J.RareEarths28(1)(2010)371–374.
[9]A.KumarMishra,R.Balasubramaniam,Corros.Sci.49(3)(2007)1027–1044.
[10]L.E.M.Palomino,P.H.Suegama,I.V.Aoki,Z.Pászti,H.G.deMelo,Electrochim. Acta52(27)(2007)7496–7505.
[11]W.Pinc,P.Yu,M.O’Keefe,W.Fahrenholtz,Surf.Coat.Technol.203(23–25) (2009)3533–3540.
[12]K.Brunelli,ManueleDabalà,IreneCalliari,MaurizioMagrini,Corros.Sci.47 (4)(2005)989–1000.
[13]A.Decroly,J.-P.Petitjean,Surf.Coat.Technol.194(2005)1–9.
[14]G.P.Bierwagen,D.E.Tallman,Prog.Org.Coat.41(2001)201–216.
[15]J.B.Ledeuil,A.Uhart,S.Soule,J.Allouche,J.C.Dupin,H.Martinez,Nanoscale (2014),http://dx.doi.org/10.1039/c4nr03211j.
[16]C.M.Rangel,T.I.Paiva,P.P.daLuz,Surf.Coat.Technol.202(2008)3396–3402.
[17]X.Yu,G.Li,J.AlloysComp.364(2004)193–198.
[18]L.S.Kasten,J.T.Grant,N.Grebasch,N.Voevodin,F.E.Arnold,M.S.Donley,Surf. Coat.Technol.140(2001)11–15.
[19]R.G.Buchheit,S.B.Mamidipally,P.Schmutz,H.Guan,Corrosion58(2002) 3–14.
[20]J.P.Holgado,R.Alvarez,G.Munuera,Appl.Surf.Sci.161(2000)301–315.
[21]A.Pfau,K.D.Schierbaum,Surf.Sci.321(1994)71–80.
[22]M.F.Montemor,A.M.Simoes,M.G.S.Ferreira,M.J.Carmezim,Appl.Surf.Sci. 254(2008)1806–1814.
[23]Y.A.Teterin,A.Y.Teterin,A.M.Lebedev,I.O.Utkin,.Electron.Spectrosc.Relat. Phenom.88–91(1998)275–279.
[24]J.Z.Shyu,K.Otto,W.L.H.Watkins,G.W.Graham,J.Catal.114(1988)22.
[25]A.E.Hughues,J.D.Gorman,P.J.K.Paterson,Corros.Sci.38(1996)1957–1976.
[26]-K.Bak,L.Hilaire,Appl.Surf.Sci.70–71(1993)191–195.
[27]A.Kotani,T.Jo,J.C.Parlebas,Adv.Phys.37(1988)37–85.
[28]J.Z.Shyu,K.Otto,W.L.H.Watkins,G.W.Graham,Catalysis114(1988)23–33.
[29]P.Campestrini,H.Terryn,A.Hovestab,J.H.W.deWit,Surf.Coat.Technol.176 (2004)365–381.
[30]L.E.M.Palomino,I.V.Aoki,H.G.deMelo,Electrochim.Acta51(2006) 5943–5953.
[31]Z.Liu,P.H.Chong,A.N.Butt,P.Skeldon,G.E.Thompson,Appl.Surf.Sci.247 (1–4)(2005)294–299.
[32]J.A.DeRose,T.Suter,A.Bałkowiec,J.Michalski,K.J.Kurzydlowski,P.Schmutz, Corros.Sci.55(2012)313–325.
[33]M.Dabala,L.Armaelao,A.Buchberger,I.Calliari,Appl.Surf.Sci.172(2001) 312–322.
[34]A.J.Aldykiewiczs,A.J.Davenport,H.S.Isaacs,J.Electrochem.Soc.143(1) (1996)147.
[35]S.A.Hayes,PuYu,T.J.O’Keefe,M.J.O’Keefe,J.O.Stoffer,J.Electrochem.Soc. 149(12)(2002)C623–C628.
![Fig. 2. Cerium-based conversion coating (with [Ce] = 0.1 M; 300 s of immersion in the conversion bath) over AA2024-T3 alloy: (A) large scale XPS spectrum and (B) SEM micrograph of the cross section.](https://thumb-eu.123doks.com/thumbv2/123doknet/3108001.88214/5.892.204.675.121.457/cerium-conversion-coating-immersion-conversion-spectrum-micrograph-section.webp)


![Fig. 11. Cross section of Cerium-based conversion coating (with [Ce] = 0.1 M, immersion time: 300s) over AA2024T3 alloy after 5 h corrosion into 3.5 wt.% NaCl solution: (A) SEM view and (B) Auger spectroscopy analysis in the coating section (point 1) and w](https://thumb-eu.123doks.com/thumbv2/123doknet/3108001.88214/9.892.208.674.120.467/conversion-immersion-corrosion-solution-spectroscopy-analysis-coating-section.webp)