BIO-INSPIRED CONJUGATED ORGANOMETALLIC DYADES, TRIADS
AND POLYMERS FOR PHOTOPHYSICAL STUDIES
By
Diana Bellows
Thesis submitted to the chemistry department in partial fulfilment for the degree of Master of Science in Chemistry (M.Sc.)
FACULTE DES SCIENCES UNIVERSITE DE SHERBROOKE
1*1
Library and Archives Canada Published Heritage Branch 395 Wellington Street OttawaONK1A0N4 Canada Bibliotheque et Archives Canada Direction du Patrimoine de I'edition 395, rue Wellington Ottawa ON K1A 0N4 CanadaYour file Votre reference ISBN: 978-0-494-53374-1 Our file Notre reference ISBN: 978-0-494-53374-1
NOTICE: AVIS:
The author has granted a
non-exclusive license allowing Library and Archives Canada to reproduce, publish, archive, preserve, conserve, communicate to the public by
telecommunication or on the Internet, loan, distribute and sell theses
worldwide, for commercial or non-commercial purposes, in microform, paper, electronic and/or any other formats.
L'auteur a accorde une licence non exclusive permettant a la Bibliotheque et Archives Canada de reproduire, publier, archiver, sauvegarder, conserver, transmettre au public par telecommunication ou par I'lnternet, preter, distribuer et vendre des theses partout dans le monde, a des fins commerciales ou autres, sur support microforme, papier, electronique et/ou autres formats.
The author retains copyright ownership and moral rights in this thesis. Neither the thesis nor substantial extracts from it may be printed or otherwise reproduced without the author's permission.
L'auteur conserve la propriete du droit d'auteur et des droits moraux qui protege cette these. Ni la these ni des extraits substantiels de celle-ci ne doivent etre imprimes ou autrement
reproduits sans son autorisation.
In compliance with the Canadian Privacy Act some supporting forms may have been removed from this thesis.
Conformement a la loi canadienne sur la protection de la vie privee, quelques
formulaires secondaires ont ete enleves de cette these.
While these forms may be included in the document page count, their removal does not represent any loss of content from the thesis.
Bien que ces formulaires aient inclus dans la pagination, il n'y aura aucun contenu manquant.
1*1
DYADES, TRIADES ET POLYMERES ORGANOMETALLIQUES
CONJUGUES BIO-INSPIRES POUR ETUDES PHOTOPHYSIQUES
Par
Diana Bellows
Memoire soumis au departement de chimie en vue de l'obtention du grade de maitre es sciences(M.Sc)
FACULTE DES SCIENCES UNIVERSITE DE SHERBROOKE
Le 30 avril 2009
lejury a accepte le memoire de Mme Diana Bellows dans sa version finale.
Membres dujury M. Pierre Harvey Directeur Departement de chimie M. Dale Wood Membre - Bishop's University M. YueZhao President-rapporteur Departement de chimie
SOMMAIRE
Les objectifs se rapportant a mes projets etaient la synthese et la caracterisation de tous les polymeres organometalliques et les materiaux hybrides de meme que la synthese des composes modeles pour la biomimetique de Punite B800-carotenoi'de-B850 present dans le dispositif LHII des bacteries photosynthetiques pourpre. La conception de nouveaux polymeres conjuges qui montrent des applications potentielles pour les cellules solaires, un appareil con9u par Fhomme qui converti de l'energie de la lumiere en electricite, ou des diodes electroluminescentes qui utilisent peu d'electricite pour produire de la lumiere, sera discutee ainsi que les transferts d'energie a travers un materiel ou un polymere en fonction de temps dans une maniere controlee. Ces dernieres proprietes, la nature des processus et les taux des transferts d'energie, sont les parametres cles qui definissent 1'efilcacite de tels materiaux.
Dans le chapitre 2, des materiaux hybrides composes de polymeres anioniques organiques conjugues et luminescents et de polymeres cationiques organometalliques electrostatiquement tenus ensemble ont ete prepares et caracterises. La polydispersite intrinseque des polymeres cationiques organiques conjugues et luminescents de depart, a porte une attention sur la possibilite qu'elle pourrait limiter la morphologie du materiau hybride resultant. En effet, ces materiaux de depart se sont averes a etre amorphes et qu'en consequent, les materiaux hybrides formes montraient un faible caractere cristallin. Ce parametre semble jouer un role important, voire crucial, dans le caractere d'organisation dans le solide influence grandement Pefficacite de la migration d'energie a travers le materiel. Les resultats de ce projet ont ete publies dans le journal de Inorganic Chemistry.
Dans le chapitre 3, des oligomeres mono-disperses (les monomeres, dimeres, et trimeres), ont ete synthetises et caracterises prenant en consideration deux parametres structuraux dans la conception. Premierement, le chromophore devait etre facile a synthetiser et aussi il devait contenir des proprietes chromophoriques semblables a celles des bacteriochlorophylles.
Deuxiemement, le contact-rc interchromophorique responsable pour la communication electronique a travers un materiel doit etre obtenu. Nous avons done choisi d'utiliser les metalloporphyrines comme macrocycle aromatique jouant le r61e des bacteriochlorophylles. Les deux consistent d'un centre cyclique tetrapyrole mais qui different quelque peu a cause 1' absence de deux doubles liens dans le macrocycle bacteriochlorophylle et les groupements autour de l'anneau. Nous avons choisi de relier ces chromophores ensemble de maniere covalente avec l'espaceur fraw^-bis^thynyl-benzene^is^-n-buty^platine^I) directement sur les carbones meso. La conjugaison assure la communication electronique et la formation du lien covalent assure l'integrite de l'assemblage en solution (en comparaison des liens-H par exemple). Nous avons aussi decide d'utiliser des porphyrines luminescentes contenant du palladium(II)- et zinc(II)- servant comme donneur ou accepteur d'energie, respectivement. Ce choix est base sur l'experience ou les transferts d'energies a l'etat singulet et triplet ont ete demontres par rapport au transfert d'electron possible. Les resultats de ce projet ont ete soumis au journal de Inorganic Chemistry.
Les interets pour l'amelioration de l'efficacite des appareils photoniques connu aujourd'hui, soit les DEL's ou les panneaux solaires ont augmente formidablement ces dernieres annees. Cet interet, ainsi que 1'efficacite des bact^ries photosynthetiques nous a inspire et incite a entreprendre ces projets. Notre hypothese de creer ou de garder une «communication» (comme le transfert d'energie et comme le transfert d'electron) entre les donneurs et les accepteurs par exemple, par l'addition d'un metaux lourds dans le squelette des polymeres qui d'une part augmentent la stabilite thermique par rapport a leurs congeneres organiques et le croisement d'intersysteme. Cette derniere propriete mene a une augmentation de la population des etats triplets et procure ainsi un deuxieme chemin de communication entre les deux chromophores.
SUMMARY
The objectives pertaining to my projects were the synthesis and characterization of all organometallic polymers and hybrid materials as well as the synthesis of the model compounds for the biomimicry of the B800-carotenoid-B850 unit in the LH II (light harvesting devices) of the purple photosynthetic bacteria. The design of new conjugated polymers that have potential applications in solar cells, a man-made device that converts light energy into electricity, or light emitting diodes which use little electricity to produce light will be discussed as well as the energy flow across a material or a polymer as a function of time in a controlled manner. The latter properties, rate of exciton and energy transfer processes, are the key parameters that define the efficiency of such materials. The full understanding of the relationship between the structure and the rate of energy migration is necessary for such design.
In chapter 2, electrostatically held polymer anion / polymer cation's were prepared and characterized. The intrinsic polydispersity of the starting ionic organic conjugated and luminescent polymers was believed to limit the morphology of the resulting hybrid, which in turn play an important, if not crucial, role in the efficiency of the energy migration across the material. The results from this project have been published in Inorganic Chemistry.
In Chapter 3, the mono-dispersed oligomers (monomers, dimers, and trimers), were synthesized and characterized taking into consideration two crucial structural parameters in the design. First, the chromophore had to be easy to synthesize and also exhibit chromophoric properties very similar to those of the bacteriochlorophylls. Secondly, the interchromophoric rc-contact responsible for the electronic communication across a material must be elucidated. We elected to use a porphyrin derivative as the aromatic macrocycle playing the role of the bacteriochlorophyll. Both consist of a cyclic tetrapyrol center but differ by the absence of the double bonds in the bacteriochlorophyll macrocycle and the substitution groups around it. We
chose to covalently link these chomophores together with ethynyl-phenyl spacers directly onto the /raw-positioned meso-carbons. Conjugation secures electronic communication and covalent bonding secures the integrity of the assembly in solution (in comparison with H-bonds for example). We also decided to use luminescent Pd- and Zn-containing porphyrins acting as the energy donor and acceptor molecules, respectively. This choice is based on experience where both the singlet and triplet energy transfers were demonstrated with respect to the possible electron transfer. The results of this project have been submitted to Inorganic Chemistry.
Interests in improving the efficiency of current photonic devices, whether it be LED's or solar panels, has increased tremendously over the past few years. This interest, along with the efficiency of photosynthetic bacteria, has inspired us to carry out these projects. Our hypothesis of creating or keeping a "communication" (such as energy transfer and electron transfer) between donors and acceptors, for example by the addition of a heavy metal, was proven to be correct. The key feature is that incorporation of a heavy metal in the backbone of the polymers can enhance intersystem crossing, hence leading to an increase in the population of the triplet states, allowing for a second path of communication between the two chromophores.
ACKNOWLEDGEMENTS
I would like to express my sincere thanks to my supervisor Dr. Pierre D. Harvey for his invaluable advice, encouragement and uninterrupted support throughout my studies. His helpful comments and opinion in the preparation of this thesis, at a time where he was very busy is gratefully appreciated.
I would also like to express my thanks to the various members of our research group which I have encountered throughout my studies, Jean-Philipe Morin-Tremblay, Jean-Francois Berube, Karl Gagnon, Christine Salomon, Etienne Bertrand, Shawkat Aly, Dr. Li Liu, Dr. Sebastien Clement, Katry Robert, Thomas Goudreault, Tommy Kenny, Simon Lamare, and Dr. Fenglei Jiang.
I would also like to take this opportunity to thank Dr. Daniel Fortin for his help and ideas throughout my laboratory experience, as well as for the crystallographic structures he resolved and his numerous calculations. I would also like to thank my collaborators, Dr. Mario Leclerc and Emilie Gingras, from the Universite de Laval, for their work on the project involving the hybrid materials. Thanks also to collaborators from France, Dr. Claude Gros and Dr. Roger Guilard, from the Universite de Bourgogne, for providing me with the porphyrin compounds necessary for my projects.
I would like to acknowledge the Universite de Sherbrooke, the Natural Sciences and Engineering Research Council of Canada (NSERC) and Le Fonds Quebecois de la Recherche sur la Nature et les Technologies (FQRNT), as well as the Centre d'Etudes des Materiaux Optiques et Photoniques de l'Universite de Sherbrooke (CEMOPUS) for their financial support.
Last but not least, my wordless and wholehearted gratitude must be dedicated to my friends and family for their care, comfort and continuous support throughout the course of my studies. Thank you for being there for me. I love you all.
TABLE OF CONTENTS
SOMMAIRE ii SUMMARY iv ACKNOWLEDGEMENTS . . ...vi
TABLE OF CONTENTS vii LIST OF ABREVIATIONS . ix
LIST OF TABLES xi LIST OF FIGURES.. xii INTRODUCTION .1 CHAPTER 1 Theory 21
1.1 Polymers . 21 1.1.1 Organometallic Coordination Polymers ...27
1.2 Photophysical Properties ..35 1.2.1 Absorption 36 1.2.2 Luminescence 44 1.2.3 Quantum Yield and and Lifetimes 47
1.2.4 Energy Transfers 49 1.3 Light Emitting Diodes (LED's) and Photovoltaic cells 51
CHAPTER 2 Organometallic and Conjugated Organic Polymers Held Together by
Strong Electrostatic Interactions to Form Luminescent Hybrid Materials *.. 58
2.1 Preface 58 2.2 Manuscript published in Inorganic Chemistry: Organometallic and Conjugated
Organic Polymers Held Together by Strong Electrostatic Interactions to Form
Luminescent Hybrid Materials 60 CHAPTER 3 Energy and Electron Transfers in Dimers and Trimers of Zinc and
Palladium Porphyrins Bridged by Rigid Pt-Containing Conjugated Organometallic Spacers 117
3.2 Manuscript submitted to Inorganic Chemistry : Energy and Electron Transfers in Dimers and Trimers of Zinc and Palladium Porphyrins Bridged by Rigid Pt-Containing
Conjugated Organometallic Spacers 119
GENERAL DISCUSSION 181
CONCLUSION 187 APPENDIX 189 BIBLIOGRAPHY 227
LIST OF ABREVIATIONS
dmb : 1,8-diisocyano-p-menthane.
dppm: bis(diphenylphosphino)methane.
LED : light emitting diode.
WLED: white light emitting diode.
OLED: organic light emitting diode.
XRD : X-Ray diffraction
IR: infrared.
MALDI-TOF : Matrix assisted laser desorption ionization - time of flight.
NMR : nuclear magnetic resonance.
MS : mass spectroscopy.
U V : ultraviolet.
DP : degree of polymerization.
Mw : the average mass in weight.
PI: polymolecularity index.
HOMO: the highest occupied molecular orbital.
LUMO: the lowest unoccupied molecular orbital.
MLCT: metal-to-ligand-charge-transfer
ITO: Indium Tin Oxide
BNPB:Mes2B(p-4,4'-biphenyl-NPh(l-naphtyl))
NPB:N,N'-di-l-naphthyl-N,N'-diphenylebenzidine
Alq3: Tris(8-hydroxyquinolato) aluminum)
ET: energy transfer
RC: reaction center
TCNQ: tetracyanoquinodimethane;
TDDFT: Time-dependent density functional theory
LH I: Light harvesting system I
LIST OF TABLES
1. Time scales for energy migration and energy transfers between B800 and B850 units
of the LHI and LHII rings in the purple photosynthetic membrane (22,25) 13
Manuscript 1: Organometallic and Conjugated Organic Polymers Held Together by Strong
Electrostatic Interactions to Form Luminescent Hybrid Materials
1. Codes employed for the hybrid materials... 70 2.298 K solid state spectroscopic and fluorescence lifetime data. 90
3. Phosphorescence lifetimes measured as solid suspended in frozen 2MeTHF at 77K
using Xex = 450 nm and monitored at X«m = 600 nm 93
Manuscript 2: Energy and Electron Transfers in Dimers and Trimers of Zinc and Palladium
Porphyrins Bridged by Rigid Pt-Containing Conjugated Organometallic Spacers
1. Absorption spectral data measured in 2MeTHF at 298K 139 2. Luminescence data for the metalloporphyrin chromophores 2-5 in 2MeTHF 142
3. Luminescence data measured in2MeTHF for 6-12 150
4. Emission lifetimes for 2-7 in 2MeTHF 151 5. Emission lifetime measured in 2MeTHF 152 6. Emission lifetimes of the trans-CelUO^CPttEt&QzCCetU spacers and Ti energy
transfer for spacer —• metalloporphyrin.3 156
LIST OF FIGURES
1. (Left) Model showing the chlorosome above the reaction centre. The photon penetrates the chlorosome and is absorbed by the bacteriochlorophyll c. Then the excitation energy is transported through the linear aggregation of the self-assembled bacteriochlorophyll c via an exciton process, then funnelled down (energy transfer) to a bacteriochlorophyll a and the reaction centre where the special pair of bacteriochlorophyll a is. (Right top). Recently proposed model of the chlorosomes. (Right bottom) Proposed 7t-stacking of the bacteriochlorophyll c securing the
energy migration across the chlorosome to the reaction centre (7,8) 4 2. A diagram of a basic light-emitting diode containing various layers 5 3. General structure of polyphenylenevinylene dervied polymers 6 4. Various orientations possible in a 3-D array of a polymeric material 6 5. A crystalline structure of the semi-conducting {[Ag(dmb)2]TCNQ2.5}n (11) material 7
6. A representation of the cationic and anionic mixing to form a hybrid material 8 7. (Top Left) A High resolution AFM image of the photosynthetic membrane belonging
to the purple photosynthetic bacteria (19). (Top Right) Two LH II sitting next to a LH I unit. The gray circles are polypeptides and the bars are rings of interacting bacteriochlorophyll a (B850). In the middle of LH I is located the reaction centre (RC). (Bottom) A LH II ring showing only the chlorophyll a for the B850 network,
the non-interacting B800 bacteriochlorophyll, and the rhodopin glucosides (20) 9 8. Structure of Bacteriochlorophyll a (top left) and rhodopin glucoside (carotenoid)
(bottom) (20). Local geometry of the two bacteriochlorophylls a called B850 inside
the ap-apoprotein unit (21) 10 9. (Left) Absorption spectrum of the LH II supramolecular antenna of
Rhodopseudomonas acidophila stressing the various components belonging to the rhodopin glucoside, and the B800 and B850 chromophores (in the Qx and Qy
regions). The band at 380 nm is the Soret band also associated with B800 and B850 units. (Centre) The bacteriochlorin macrocycle indicating the orientation of the
transition moments associated with the Qx and Qy bands. (Right) The close C—C
contacts between one of the B850 units (in grey) and one neighbouring rhodopin
glucoside residues (in black; only a part of the chain is shown) (41) 11 10. (Left) Path of the ultrafast energy migration (exciton; fs time scale) from one LH II
complex to another, via the B850 rings. The bar with the star is the starting point where the B850 is excited, (centre and right) Typical state diagram for a diamagnetic molecule designated as "Donor" stressing all radiative (A = absorption, F = fluorescence, P = phosphorescence) and non-radiative processes (ic = internal conversion, isc = intersystem crossing, ip = intersystem crossing from the triplet state, ET = energy transfer from Si or Ti, if in the presence of an "Acceptor" molecule), and exciton phenomenon (reversible energy transfer) between identical coupled chromophores (here "Donor"). In this example, the exciton process is terminated by a down-hill energy transfer rendering exciton reversibility less
probable (41) 12 11. (Left) A drawing showing the presence of a contact between the ketone of B800 and
one of the neighbouring protons of the carotenoid in LH II. (Right) A drawing of the local environment of the carotenoid (in green) with respect to B800 and B850 units of LH II stressing the downhill Si energy transfers (red arrows) and the
possibility of slightly uphill transfers (45) 15 12. State diagram of the carotenoid along with the Qx, Qy and So levels of the B800 and
B850 chromophores in the LH II complex. The "hot Si" state is yet to be determined. Selected intramolecular relaxation and intermolecular Si energy transfer processes (i.e. energy flow) are indicated in grey arrows along with their corresponding time scale (based on data extracted from references (44,45). The
values in percent indicate the relative population that uses this relaxation path (46) 16 13. Drawings of the metalloporphyrin macrocycle (left) where R can vary from the
common aromatic group to the aliphatic one, and the bacteriochlorophyll a for
example (right) 18 14. Drawing of the target oligomers showing the exciton 19
16. A schematic representation of a linear polymer and a random coil 23 17. A schematic representation of various types of branched polymers 24
18. A schematic representation of reticulate polymers. .24 19. A schematic representation of a homopolymer and various copolymers 25
20. A schematic representation of a crystalline and semi-crystalline polymer 26 21. a) A unidimensional polymer with a dmb structural unit (118). b)
Palladium-containing polymers with the structural units Palladium-containing dmb and diphosphines
(119). c) Tetrameric platinum and palladium complexes (120) 28 22. Cyclic Voltammetric measurements of [Pt2(dppm)2(C=CFc)2] (top) and of
[Pt2((x-AuBr)(dppm)2(C=CFc)2] (bottom) which leads to the oxidation of ferrocenyls
(120). The dppm ligands are not shown for reasons of clarity 30 23. An example of an organometallic polymer with a metal-metal bond incorporated
into the polymer back bone. This polymer is an example of a d9-d9 system
coordinated with diisocyanide ligands. 31 24. Applications of polyplatinaynes in various domains of material science 32
25. Literature examples of studied organometallic polymers (101,130,131) 33 26. rra«5-bis(ethynylbenzene)bis(phosphine)platinum(II), where R is triethyl or tributyl
phosphines 33 27. Mechanism for the conventional synthesis of transition metal a-alkynyl complexes
using copper iodide 34 28. General skeleton of a transition metal polyyne polymer 34
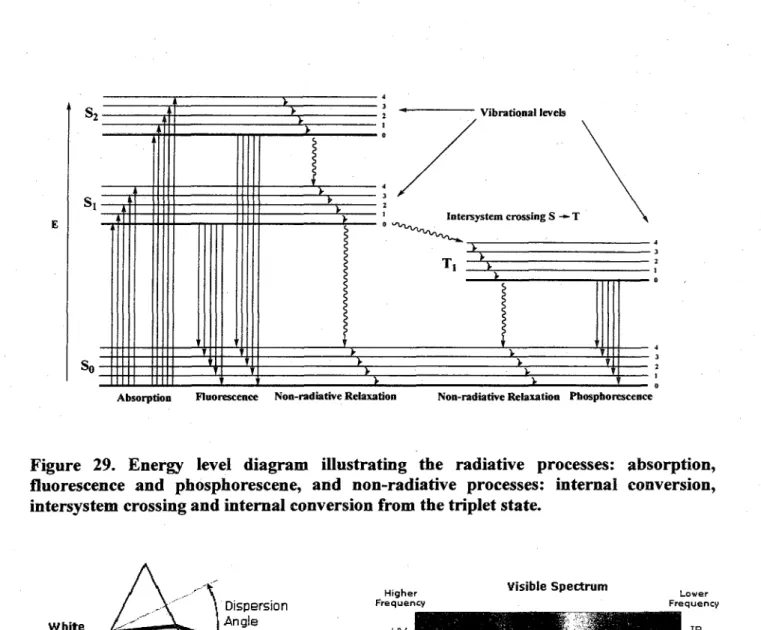
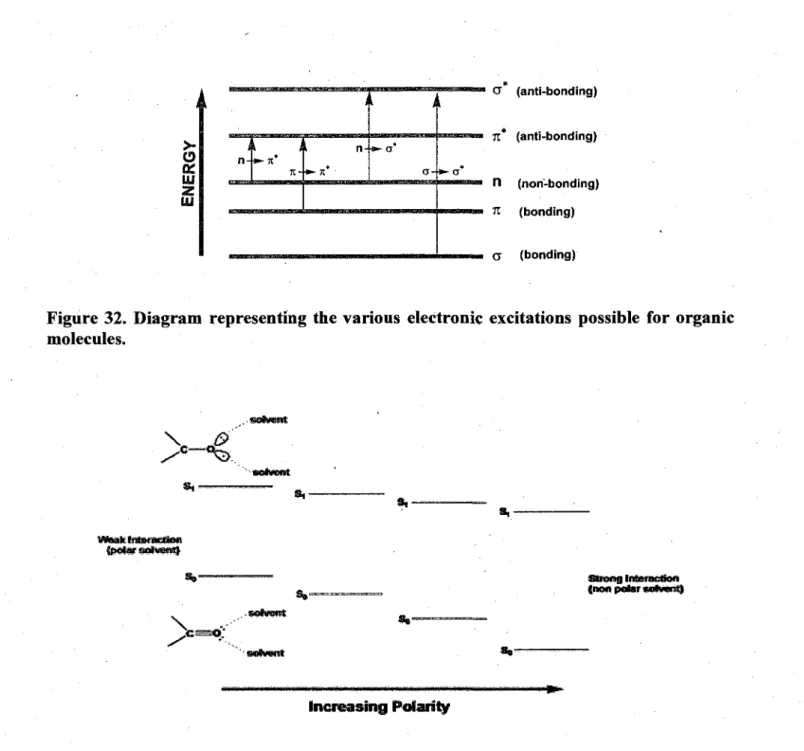
29. Energy level diagram illustrating the radiative processes: absorption, fluorescence and phosphorescene, and non-radiative processes: internal conversion, intersystem
crossing and internal conversion from the triplet state 37 30. A representation of the splitting of the visible spectrum of colors (left) and a
representation of the total visible spectrum with the indicative wavelengths with
their corresponding colors."modified from reference ". 37 31. Illustration of the ground and excited state arrangements of the electrons in the
32. Diagram representing the various electronic excitations possible for organic
molecules. 40 33. A diagram illustrating the blue shift resulting from the solvent effect (polarity of the
solvent) 40 34. Schematic representation of an electronic transition (135-137) 41
35. An example of an absorption spectra at 298 K and 77 K showing the disappearance
of the hot bands (143) , 43 36. A typical Jablonski Diagram illustration the radiative and non radiative processes
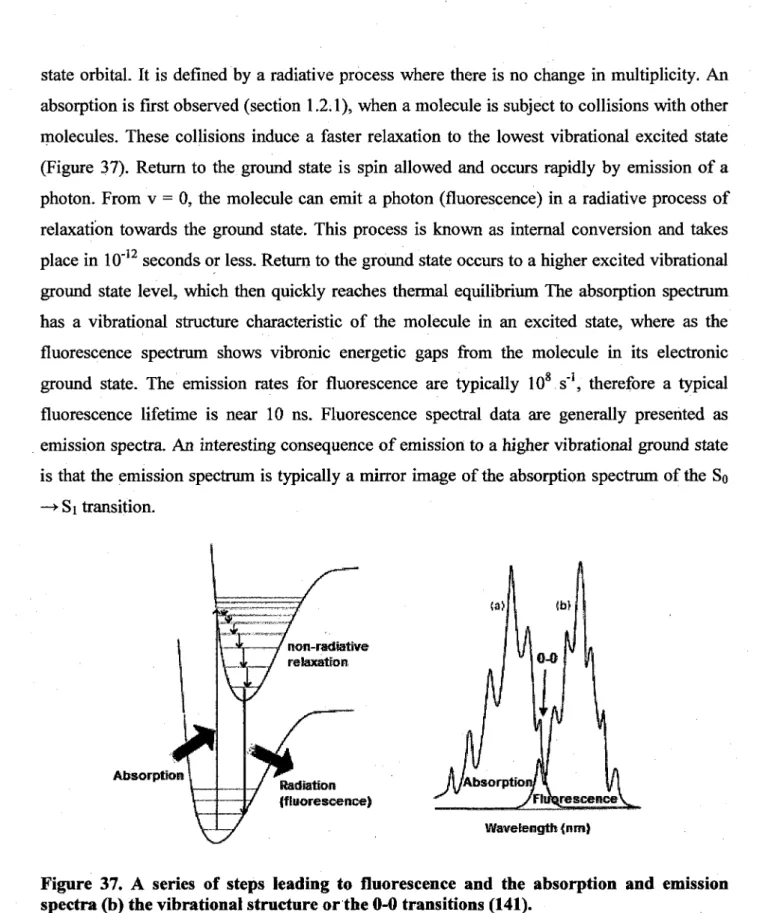
possible as seen above 44 37. A series of steps leading to fluorescence and the absorption and emission spectra (b)
the vibrational structure or the 0-0 transitions (143) 45 38. Series of steps leading to phosphorescence (141). 46 39. Series of transitions incorporated in the calculations for the quantum yields and the
lifetimes. Where kjC is the internal conversion, k,sc (ST) is the intersystem crossing
from singlet to triplet, kjSC (TS) is the intersystem crossing from triplet to singlet, kF
is the radiative fluorescence and kp is the radiative phosphorescence 48
40. Forster/Dexter Energy Transfers 51 41. Schematic representation of the hole and electron (exciton) transport illustrated on a
semiconducting material (149) 53 42. Diagram of the structure of an organic light emitting diode (150) 54
43. Diagram of a photovoltaic cell where the donor is a polyphenylenevinylene derivative and the acceptor is fullerene. (Reproduced with permission from Dr.
Pierre D. Harvey) 54 44. The principle chemical reactions involved in a LED and a photovoltaic cell. A
represents the acceptor and D the represents donor. 55 45. An example of a LED with only one product (BNPB =
Mes2B(p-4,4'-biphenyl-NPh(l-naphtyl))) which undergoes oxidation and reduction on the same molecule
while also emitting light (151) 56 46. This diagram represents the various energetic levels of a LED containing three
Tris(8-hydroxyquinolato) aluminum) which allows for a better exciton transport
(153) 57 47. (Left) Representation of a segment of the LH II of the purple photosynthetic
bacteria. (Reproduce with the permission from Dr. Pierre D. Harvey)(Right) A dyad
donor-spacer-acceptor synthesized to resemble the LH II segment on the left 183 48. X-ray structures of A and B at 30 % probability (according to reference 162) 183 49. A scheme showing various types of model compounds which were compared to one
another throughout this research project. 184 50. Illustration of the representative model compounds synthesised throughout the
course of this master's thesis (left) and illustration of the polymers of interest using the model comounds illustrated above and discussed in the bulk of this thesis
(right) 185 51. Diagram representing the target polymers and in the various coloured boxes are the
different model compounds which have been synthesised and characterized 185 52. Examples of porphyrin polymers synthesized so far in Dr. Pierre D. Harvey's
laboratory, structures of the various polymers synthesized by Dr. Li Liu in parallel with my work (top), photoprocesses involving the excitation of the spacer followed by a triplet energy transfer which in turn an excitonic process takes place (energy
derealization along the multiple metalloporphyrin) 186
Manuscript 1: Organometallic and Conjugated Organic Polymers Held Together by Strong
Electrostatic Interactions to Form Luminescent Hybrid Materials
1. XRD traces for polymers 10-13 76 2. Top: drawings of the cationic ([AgCdmb^]^ and ([Pt2(dppm)2(CNC6Me4NC)]2+)„
polymers stressing the distance between repetitive units containing 2 positive charges. Bottom: space filling model based on the X-ray structure of the ([Ag(dmb)2]+)n polymer showing the head-to-tail symmetry of the rigid rod induced
3. IR and Raman spectra of Hybrids B and D in the solid state in the [ FWHM are for Hybrid B 50 and 100, and for Hybrid D 42 and 90 cm"1, for the
Raman and IR peaks, respectively 81 4. Comparison of the *H (left) and 13C (right) NMR MAS spectra of solid ([Pt2(dppm)2
-(CNC6Me4NC)](BF4)2)n, polymer 11 and Hybrid D at 298 K as an example 82
5. Energy diagram for the hybrid materials based on the observed fluorescence and phosphorescence spectra of the polycarbazole materials and the literature data for the ([Ag(dmb)2]+)„ (top) and ([Pt2(dppm)2](CNC6Me4NC)2+)n (bottom)
organometallic polymers. The exact location of the Si state is not known with certainty, but arguments are provided in the text in favour of this diagram where Si
is an optically silent state (from a forbidden transition) 84 6. Solid state excitation (red) emission spectra (blue) of Polymer 10 and Hybrids A and
B at 298 K. The excitation and emission wavelength are indicated in the spectra ....85 7. Solid state excitation (red) emission spectra (blue) of polymer 11 and Hybrids C and
D (left) and polymer 12 and Hybrids E and F at 298 K. The excitation and emission wavelengths are 350 and 500 nm. The intensities were adjusted so they fit in the frame. The emission intensity of the fluorescence and phosphorescence of Hybrids
D and F is very low 86 8. Time-resolved emission spectra of polymer 10 (top) and Hybrid A (bottom) in the ns
time scale. The delay times labelled 42 and 43 ns are located on the short-time side of the laser pulse where the laser has not fired (FWHM = 1 . 4 ns). The 45 ns is located at the maximum of the pulse, so the 43-45 ns delay range represents the rise time of the laser pulse. The feature is the 515-nm band associated with the emission
of the ([Ag(dmb)2]+)n .87
9. Fluorescence lifetime of iV-hexyl-3,6-bisethynylcarbazole (left) and iV-butylcarbazol-3,6-diyl trimer (right) as solid at 298 K measured using A^m = 420 nm and XeX = 350
nm 89 10. Distribution of fluorescence lifetimes for thin films of polymers 10, 11 and 13 at
51. Comparison of the JH (left) and 13C (right) NMR MAS spectra of solid
([Ag(dmb)2]BF4)„, polymer 10 and Hybrid A at 298 K 101
52. Comparison of the lH (left) and 13C (right) NMR MAS spectra of solid
([Pt2(dppm)2-(CNC6Me4NC)](BF4)2)„, polymer 10 and Hybrid B at 298 K 101
53. Comparison of the *H (left) and 13C (right) NMR MAS spectra of solid
([Ag(dmb)2]BF4)„, polymer 11 and Hybrid C at 298 K 102
54. Comparison of the 'H (left) and 13C (right) NMR MAS spectra of solid
([Ag(dmb)2]BF4)n, polymer 13 and Hybrid E at 298 K 102
55. Comparison of the *H (left) and 13C (right) NMR MAS spectra of solid
([Pt^dppmMCNCeMe^C^^F^n, polymer 13 and Hybrid F at 298 K 103
56. XRD pattern of Hybrids B and E 104 57. Solid state emission (blue) and excitation (red) spectra of polymer 10 at 77 K 105
58. Solid state emission (blue) and excitation (red) spectra of Hybrid A at 77 K 105 59. Solid state emission (blue) and excitation (red) spectra of polymer 11 at 77 K 106 S10. Solid state emission (blue) and excitation (red) spectra of Hybrid C at 77 K 106 SI 1. Solid state emission (blue) and excitation (red) spectra of polymer 13 at 77 K. 107 512. Solid state emission (blue) and excitation (red) spectra of Hybrid E at 77 K 107 513. Solid state emission (blue) and excitation (red) spectra of Hybrid F at 77 K 108 514. Time-resolved emission spectra of polymer 11 (top) and Hybrid C (bottom) at 298
K in the ns time scale. The delay time labelled 43 ns is located on the short-time side of the laser pulse where the laser has not fired (FWHM =1.4 ns). The 45 ns is located at the maximum of the pulse, so the 43-45 ns delay range represents the rise
time of the laser pulse 109 SI 5. Time-resolved emission spectra of polymer 13 (top) and Hybrid E (bottom) at 298
K in the ns time scale. The delay time labelled 43 ns is located on the short-time side of the laser pulse where the laser has not fired (FWHM =1.4 ns). The 45 ns is located at the maximum of the pulse, so the 43-45 ns delay range represents the rise
time of the laser pulse 110 SI6. Phosphorescence decay traces of JV-hexyl-3,6-bisethynylcarbazole measured as
SI 7. Phosphorescence decay traces of Hybrids A-F and polymer 10, 11, 13 measured at
77K using the solid suspension in 2MTHF using Xem = 600 nm and A.ex = 450 nm 114
SI8. Distribution of fluorescence lifetimes for Hybrids A-F measured as solid at room
temperature using Xem = 460 nm and Xex = 400 nm 116
Manuscript 2: Energy and Electron Transfers in Dimers and Trimers of Zinc and
Palladium Porphyrins Bridged by Rigid Pt-Containing Conjugated Organometallic Spacers
1. Emission (blue), excitation (red) and absorption (black) spectra of the parent
compound PhC=CPt(PEt3)2C=CPh in 2MeTHF at 77 K 141
2. Emission (blue and green), excitation (red) and absorption (black) spectra of Zn(P)-C6H4C=CPtL2C=CC6H5 (bottom, 6) and Pd(P)-C6H4C-CPtL2C=CC6H5 (top, 7) in
2MeTHF at 77 K (L = PEt3). 144
3. Emission (blue and green), excitation (red) and absorption (black) spectra of Zn(P)-C6H40CPtL20CC6H4-Zn(P), 8 (bottom), and Pd(P)-C6H4C=CPtL2C=CC6
H4-Pd(P), 9 (top) in 2MeTHF at 77 K (L = PEt3) 146
4. Emission (blue and green), excitation (red) and absorption (black) spectra in
2MeTHF at 77 K of Pd(P)-C6H4C=CPtL2<>CC6H4-Zn(P) (L = PEt3) 147
5. Emission (blue and green), excitation (red) and absorption (black) spectra in 2MeTHF at 77 K of Zn(P)-C6H4C=CPtL2C=CC6H4-Zn(P)-C6H4C=CPtL2C=CC6
H4-Zn(P) (bottom) and Pd(P)-C6H4C=CPtL2C=CC6H4-Zn(P)-C6H4CsCPtL2C=CC6
H4-Pd(P) (top) (L = PEt3) 148
6. Transient absorption spectrum of fra«5-PhC=CPt(PEt3)2C=CPh in 2MeTHF at 77 K
(A-exc = 355 nm, delay = 0.1 jxs). The negative signal at 430 nm is due to
phosphorescence , 157 7. Transient absorption spectra of 2, 4, 6, 8, 10, and 11 in 2MeTHF at 298 K (^-exc =
SI 1. Emission (blue), excitation (red) and absorption (black) spectra of
Zn(P)-C6H4C=CH (top, 2) and Pd(P)-C6H4OCH (bottom, 3) in 2MeTHF at 77 K 171
SI 2. Emission (blue), excitation (red) and absorption (black) spectra of Zn(P)-C6H40CPtL2Cl (top, 2) and Zn(P)-C6H4C=CPtL2Cl (bottom, 3) in 2MeTHF at 298
K 172 SI 3. Emission (blue, green), excitation (red) and absorption (black) spectra of
Zn(P)-C6H4C=CPtL2Cl (top, 4) and Zn(P)-C6H40CPtL2Cl (bottom, 5) in 2MeTHF at 77
K 173 SI 4. Emission (blue), excitation (red) and absorption (black) spectra of
Zn(P)-C6H4C=CPtL2Cl (top, 4) and Zn(P)-C6H4(>CPtL2Cl (bottom, 5) in 2MeTHF at 298
K 174 SI 5. Emission (blue and green), excitation (red) and absorption (black) spectra of
Zn(P)-C6H4C=CPtL2C=CC6H5 (top, 6) and Zn(P)-C6H4C=CPtL2C=CC6H5 (bottom,
7) in 2MeTHF at 298 K 175 SI 6. Emission (blue), excitation (red) and absorption (black) spectra of
Zn(P)-C6H4C=CPtL2CsCC6H4-Zn(P) (top, 8) and Zn(P)-C6H4C=CPtL2C=CC6H4-Pd(P)
(bottom, 9) in 2MeTHF at 298 K. 176 SI 7. Emission (blue), excitation (red) and absorption (black) spectra of
Pd(P)-C6H4C=CPtL2C=CC6H4-Zn(P) (10) in 2MeTHF at 298 K 177
SI 8. Emission (blue), excitation (red) and absorption (black) spectra of Zn(P)-C6H4C=CPtL2C=CC6H4-Zn(P)-C6H4C=CPtL2C=CC6H4-Zn(P) (top, 11) and
Pd(P)-C6H4C=CPtL2C=CC6H4-Zn(P)-C6H4C=CPtL2C=CC6H4-Pd(P) (bottom, 12) in
2MeTHF at 298 K 178 SI 9. Typical example of fluorescence decay of compounds 10 and 12 in 2MeTHF at 77
K (red) against the lamp profile 179 SI 10. Transient absorption spectra of 3, 5, 7, 9, and 12 in 2MeTHF at 298 K (X^ =
INTRODUCTION
Nature took hundreds of millions of years to perfect its "art of survival" of photosynthetic organisms which live in all kinds of environments ranging from mild to harsh conditions. The living environment for these light-dependant organisms is highly variable. The well-known cyanobacteria live within the first foot of water in lakes, whereas the purple photosynthetic bacteria live nearly seven meters below the surface of the water where light is more scarce and the availability of wavelengths is limited. Even under minimal light conditions, one finds the green sulphur photosynthetic bacteria which survive in mud or at the bottom of the ocean near volcanic vents "black smokers". Photosynthesis is a metabolic pathway that converts light energy into chemical energy via a primary electron transfer process. This process is one of the most important biochemical pathways (1), since all life on earth either directly or indirectly depends on it as a source of energy. It is a complex process occurring in plants, and algae, as well as photosynthetic bacteria, such as green sulphur bacteria and purple photosynthetic bacteria. These living organisms are equipped with photosynthetic membranes or other mechanisms to collect light in a specific available window, and channel this light energy towards the reaction center where the primary photo-induced electron transfer takes place, with an amazing efficiency (i.e high quantum yield). The yield for the number of photons absorbed by the photosynthetic membrane of the purple photosynthetic bacteria and converted into an electron transfer in the reaction center is 99% and near 100% for green sulphur bacteria. Scientists are envious of such efficiency because it is unequalled by man-made photonic devices such as photovoltaic cells. The crystal structures of many of these protein-containing photosystems have been determined and allow one to analyze the role of each component of the complex photosynthetic machinery such as molecular solar panels (chlorophyll a, b, or c, bacteriochlorophyll a and b, and carotenoids), energy migration relays (via energy transfer and excitons) and electron transfer devices (special pair, phyophytin, plastoquinone...). Over the years, chemical and biochemical physicists and theoreticians have investigated the excited state dynamics of several bio-devices, namely the photosynthetic membranes of the cyanobacteria, green sulphur bacteria and the purple photosynthetic
bacteria, and have provided a good understanding of how these organisms work so well. The question one may ask is: is it possible to synthesize some of the key components of such energy relays between donors and acceptors, cyclic, acyclic (i.e. polymeric) and dendrimeric molecular solar panels with excellent excitonic (energy migration) properties and special pairs (responsible for the primary electron transfer) equipped with its own dendrimeric or polymeric solar panels? One can even go one step further by designing dynamic supramolecular devices that can mimic the electron transfer relay between cytochrome f and photosystem I via the plastocyanin protein in plants and the cyanobacteria. Numerous works have reported the preparation of organic dyads and multiads in order to investigate energy and electron transfer processes in relation with biological systems, but to our knowledge no investigation involving organometallics with the specific goals of mimicking nature has appeared in the literature. This idea is extremely appealing for two reasons. First, modern research on photonic devices such as photovoltaic cells and light emitting diodes (LED's) has recently turned towards organometallics but has not yet found applications since these are only at the early stages of development. Secondly, the presence of heavy metals in the polymer chain of the material promotes intersystem crossing which populates the triplet states giving the opportunity to explore the triplet excited state dynamics, in parallel with the singlet state for such metal-containing materials. In fact, nature avoids the presence of heavy metals for the survival of the organism since molecules sitting on the triplet states promote the formation of singlet oxygen, which is very toxic to living cells and energy transfer rates are significantly slower in these excited states in comparison with the singlet excited states.
The antenna effect (absorption of light and energy migration across a material such as a cell membrane) in plants and photosynthetic bacteria is the primary photophysical process that occurs in these living organisms. A good capture of the photon and a rapid migration of this light energy towards the reaction center where the primary electron transfer occurs are obviously crucial for survival. This is particularly true for green sulphur bacteria and purple photosynthetic bacteria that are found in environments with little light. In such environments, every photon must be captured and the corresponding energy should be transferred quickly to the reaction center with maximum quantum efficiency. These bacteria can not afford to waste
this rare energy. This is why they are equipped with a very efficient antenna network. Early green photosynthetic bacteria have developed a unique organelle for light-harvesting in deep waters where they can scavenge photons even at 100 m under the water's surface (2). Interestingly, the chlorosome (Figure 1) present in the green sulphur bacteria (Chlorobiaceae) is the most efficient photosynthetic antenna complex found in nature. Indeed, it is believed that this efficiency is near 100% (ratio of number of primary electrons transferred vs. number of absorbed photons). The antenna organelles of these green bacteria are different from the light-harvesting complexes of, for instance, the purple bacteria, cyanobacteria, algae or plants, where proteins organize the proactive pigments such as bacteriochlorophylls and carotenoids into precise orientations enabling an energy funnelling from the peripheral antenna systems to the core where charge separation occurs within the reaction center (3). Because of their much simpler genomes, these bacteria can avoid using a protein scaffold by self-assembling bacteriochlorophylls c, d and e forming nanostructures called "chlorosomes" to reflect their "green sac" nature, with a large photon capture cross section (4). A careful examination of the proposed structure of the antenna device of the chlorosome in these bacteria is relevant. In spite of considerable research efforts, in the absence of crystallographic proof, the exact chlorosomal structure remains unknown. Several contradicting models have been proposed recently (2,3,5,6). Initially, a tubular structure was proposed based on X-ray diffraction data, but more recently a linear self-assembled aggregation of bacteriochlorophyll c dimers was demonstrated by high resolution images. These linear aggregates stack side by side to form lamellar structures separated by 20A. The 7t-stacking aggregation also secures a good electronic communication among the bacteriochlorophylls c similar to graphite, a semi-conducting material. Based on kinetic data for other photosynthetic bacteria, the time scales for these events are in the fs for excitpns (energy derealization between identical chromophores) and fs and ps for energy transfers (transfer between two different chromophores).
Figure 1. (Left) Model showing the chlorosome above the reaction centre. The photon penetrates the chlorosome and is absorbed by the bacteriochlorophyll c. Then the excitation energy is transported through the linear aggregation of the self-assembled bacteriochlorophyll c via an exciton process, then funnelled down (energy transfer) to a bacteriochlorophyll a and the reaction centre where the special pair of bacteriochlorophyll a is. (Right top). Recently proposed model of the chlorosomes. (Right bottom) Proposed ^-stacking of the bacteriochlorophyll c securing the energy migration across the chlorosome to the reaction centre (7,8).
Based on the concept of these bacteria we can address a challenge in today's society. We are faced with a vast demand for clean energy in order to address environmental issues. The main incentive of this research is driven by the urgent need to harvest and save energy, knowing that fossil fuels are not renewable and are heavy pollutants contributing to the green house effect. There are different types of "green energy", such as wind turbines, light-emitting diodes, solar panels, etc.
The latter two devices are of interest in Dr. Pierre D. Harvey's laboratory and also have application toward the understanding of the previously described bacteria in that they exhibit similar light harvesting mechanisms. The invention of light emitting diodes (LED's) dates
back to the 1960's when Nick Holonyak, Jr. (General Electric) developed the first LED emitting in the visible region (GaAs) (9). In 1990, Friend, Burroughes and Bradley published an article on the first LED built using organic polymers (PLED) (10). The general structure of a commercial LED is shown in Figure 2 and will be discussed in further detail later on.
Cathode Emissive Layer
Figure 2. A diagram of a basic light-emitting diode containing various layers.
In 1991, Cambridge Display Technology developed the first organic light-emitting diode (OLED) ( 3 x 5 pixels). Later, polymeric light-emitting diodes (PLED) were developed using organic polymers based on polypheriylenevenylene and polyfluorenes which are good hole transporters arid are also electroluminescent. Other less efficient materials exist such as l-(3-methylphenyl)-1,2,3,4-tetrahydroquinoline-6-carboxylaldehyde-1, V -diphenylhydrazone
which is a charge transport material. There are two limiting factors to the improvement in the luminosity performance, the polyphenylenevinylene and polyfluorene derived polymers are better hole transporters than electron transporters. The electroluminescent process occurs at the polymer and cathode interface (or type-p electrode) where the polymer makes a junction. The general structure of the polyphenylenevinylene is seen in Figure 3, where the R groups are generally alkyl chains (CH2)nCH3 of various lengths. These groups aid in the solubility of the
polymers and therefore the synthesis of high molecular weight polymers necessary for long derealization of the holes throughout the material.
On paper these conjugated polymers appear to be linear and planar but the three-dimensional (3-D) organization is not necessarily regular. Polymer films are generally deposited by a
method know as spin-coating, followed by heating when necessary. Occasionally, the X-ray diffraction patterns reveal that the films are amorphous indicating that there is a lack of organization in the structure of the material.
Where R can = -(CH2)20(CH2)5CH3; Aldrich)
Figure 3. General structure of polyphenylenevinylene denied polymers.
These defects in the bulk occasionally make large gaps for both hole and charge migration and energy derealization, causing the electron or hole distances to be farther apart, slowing down the excitonic processes and making them less efficient. To address this problem, one can apply a larger tension to the bonds, though this leads to a loss of energy. A parallel orientation in conjugated 3-D chain polymers would be ideal (Figure 4). It would allow for an average distance between the hole or electron transfer sites (as well as energy derealization), and a smaller or larger number of chain linking bridges, as depicted in Figure 4. The organized structure should have a more efficient excitonic transport system. This orientation can also open the door for studies on electroluminescent systems.
Unorganized 3-D structure of a material Organized 3-D structure of a material (
(
I
It was suggested by Dr. Pierre D. Harvey that we synthesize new electroluminescent and conducting materials which have an organized array in the solid state. In Chapter 2, the work using supermolecular ionic interactions to enforce the orientation of the chains into an organized manner was demonstrated earlier by Harvey et al. This strategy is inspired by the crystallographic structures of organometallic polymers and cationic coordination where the polymers form 2-D polycationic layers, and the counter-ions form the other layers which alternate with one another. Some notable examples in relation to this proposal are the polymeric materials of the {type [M (dmb)2] (TCNQ)X}„ (M = Cu, Ag; dmb =
1,8-diisocyano-p-menthane; TCNQ = tetracyanoquinodimethane; x = 2, 2.5) (11).
Figure 5. A crystalline structure of the semi-conducting {[Ag(dmb)2]TCNQ2.5}n (11) material.
The structure in Figure 5 shows the regular alternate stacking of the anionic and cationic layers. The {TCNQs2"},, unit is responsible for the semi-conducting properties. The {Ag
(dmb)2+}n chain forms a rigid-rod as demonstrated by X-ray crystallography and do not
intervene in the electronic properties of the material, as verified by electrochemical methods according to reduction and oxidation potentials.
We have assembled novel conducting materials by supermolecular assembling between anionic and cationic polymers as shown in Figure 6. The cationic polymers are coordination or rigid structure organometallic polymers. These will be described in detail in Chapter 2.
The advantage of these materials is that they exist as oligomers where the average number of units varies between 4 and 14 and they are in equilibrium among themselves (i.e. fragments exchange). The cationic coordination polymer can adapt to accommodate the volume of the organic anionic polymer during the assembly. The second consideration in this ionic assembly is the difference between the distances of the positive-positive charges vs. negative-negative charges. It thus becomes important to choose polymers which will have similar distances. According to our experience (Figure 5), a small rotation of a plane with respect to the other can fix a small mismatch. The results and conclusion pertaining to this project are discussed in the published article found in Chapter 2.
anionic polymer
counter-amons .
/ ' V > • •
2 2 2 2 1 1 1 1 ®©@@©@@© .^^-©-^©-©jt.©^
( o 0 )• V \ / / hybrid materials cationic polymer counter-cations
Figure 6. A representation of the cationic and anionic mixing to form a hybrid material.
Related to solar panels and a focus of chapter 3 of this thesis is the function of purple photosynthetic bacteria and their ability to act as molecular solar panels in nature. These bacteria have been studied in detail and are well understood, making it easier to attempt to mimic their efficient systems.
Since early reports of the crystal structure of the light harvesting device II in purple photosynthetic bacteria (Rhodopseudomonas acidophilic! and Rhodopseudomonas
molischisnum), (12-14) experimental and theoretical studies on the photophysical properties
and excited state dynamics of this supramolecular biological antenna increased rapidly. Thus, both the supramolecular biostructure and ultrafast excited state dynamics are fairly well understood. Using X-ray (12-14), AFM methods (15-17) and synchrotron small-angle X-ray scatterings (18), many circular and ellipsoid structures for light harvesting devices I and II (LH I and LH II) were elucidated (Figure 7). The photosynthetic membrane of the purple bacteria is composed of numerous small phospholipid-filled rings called light harvesting
device, LH II and several larger dissymmetric rings, LH I stacked like a honey comb (Figure 7). Inside LH I is placed a protein called the reaction center (RC) where the primary photo-induced electron transfer arising from a special pair of bacteriochlorophylls (Figure 8) takes place. These doughnut-shaped bio-architectures are set at right angles with respect to the cytoplasmic membrane.
Figure 7. (Top Left) A High resolution AFM image of the photosynthetic membrane belonging to the purple photosynthetic bacteria(19). (Top Right) Two LH II sitting next to a LH I unit. The gray circles are polypeptides and the bars are rings of interacting bacteriochlorophyll a (B850). In the middle of LH I is located the reaction centre (RC). (Bottom) A LH II ring showing only the chlorophyll a for the B850 network, the non-interacting B800 bacteriochlorophyll, and the rhodopin glucosides (20).
The LH II of approximately a 10 nm diameter is made of nine aP-apoproteins (B800) and 18 bacteriochlorophyll a (B850) forming a ring. Each of them carries three of them and are placed in a circle (Figure 7, right) designated by their absorption Q-bands at 800 and 850 nm.(Figure 9) (22) Similarly, LH I is composed of 36 to 38 B875 units. These B800, B850, B875 and
carotenoid units secure efficient absorption of light and energy migration via energy transfers and energy derealization (exciton). These non-radiative processes operate at 99% efficiency (ratio of number of primary electrons transferred vs. number of absorbed photons, or quantum yield) (26) where essentially every absorbed photon leads to an electron transfer at the reaction center.
Figure 8. Structure of Bacteriochlorophyll a (top left) and rhodopin glucoside (carotenoid) (bottom) (20). Local geometry of the two bacteriochlorophylls a called B850 inside the a(3-apoprotein unit (21).
In the first X-ray report (14), one ail-trans carotenoid was found per ap-apoprotein (Figure 7). This carotenoid exhibits a twisted geometry and stretches across the whole apoprotein. Because the sugar fragment is deeply anchored in one of the a-helice and the carotenoid chain runs through the neighbouring a|3-apoprotein, the rhodopin glucoside molecule acts as an attaching device holding the structure of LH II in place. The carotenoid exhibits close van der Waals contacts with all three bacteriochlorophyll a's (Figure 9; right). A close contact is also observed between the ketone of the B800 and one of the carbon atoms of the carotenoid chain. This organization also allows the carotenoid to act as an efficient photo-protective accessory against singlet oxygen formation. Importantly, this long 7t-conjugated organic chain also acts
as a "solar panel" and can contribute to the antenna effect of LH I and LH II, notably because of the close proximity with the neighbouring pigments.
Figure 9. (Left) Absorption spectrum of the LH II supramolecular antenna of Rhodopseudomonas acidophila stressing the various components belonging to the rhodopin glucoside, and the B800 and B850 chromophores (in the Qx and Qy regions).
The band at 380 nm is the Soret band also associated with B800 and B850 units. (Centre) The bacteriochlorin macrocycle indicating the orientation of the transition moments associated with the Qx and Qy bands. (Right) The close C—C contacts between one of the
B850 units (in grey) and one neighbouring rhodopin glucoside residues (in black; only a part of the chain is shown) (21).
When one bacteriochlorophyll a in the LH II unit absorbs light, a series of non-radiative photophysical processes take place to efficiently channel the excitation energy from the absorption site to the special pair in the reaction center. These photophysical events are either an energy migration (exciton) or an energy transfer. These fast events occur in the singlet state (Si) of the bacteriochlorophyll, but the role of the carotenoids was only recently elucidated. The carotenoids' role is important particularly with respect to the non-radiative energy transport event. It serves as an energy relay as well as a triplet state sensitizer. Figure 10 (left) shows an example of a path for energy migration across the LH II-containing membrane. The "hopping" of energy from one B850 chromophore to another inside and outside the LH II is in fact a series of reversible energy transfer processes in the Si state. Ultimately, this excitation energy efficiently ends up in the special pair of the reaction center prior to triggering the primary photo-induced electron transfer. The rates for energy migration via exciton and
energy transfers (ET) occur in the femto- and picosecond timescale, respectively. These two processes are described in Figure 10 (right) as well as the energy derealization along LH II units (left). The time scales for these events are presented in Table 1. This energy migration across the photosynthetic membrane is terminated inside the reaction center where the primary electron transfer takes place.
Figure 10. (Left) Path of the ultrafast energy migration (exciton; fs time scale) from one LH II complex to another, via the B850 rings. The bar with the star is the starting point where the B850 is excited, (centre and right) Typical state diagram for a diamagnetic molecule designated as "Donor" stressing all radiative (A = absorption, F = fluorescence, P = phosphorescence) and non-radiative processes (ic = internal conversion, isc = intersystem crossing, ip = intersystem crossing from the triplet state, ET = energy transfer from Si or Ti, if in the presence of an "Acceptor" molecule), and exciton phenomenon (reversible energy transfer) between identical coupled chromophores (here "Donor"). In this example, the exciton process is terminated by a down-hill energy transfer rendering exciton reversibility less probable (21).
Using ultrafast flash photolysis techniques (transient absorption spectroscopy; UV-Vis; luminescence; IR), the time scales for all inter-bacteriochlorophyll a pigments in the LH I and LH II complexes were recently determined (Table 1) (22). These fs and ps events were subjects of numerous theoretical investigations, notably with respect to the Forster theory which describes the amplitude of interchromophore couplings and molecular orbital properties and which is described in more detail in referneces (23-26).
Energy Transfers
ET isc\
Energy Migration (exciton)
Curiously, the rate for energy migration across the B800 units in LH II appears particularly fast (time scale of ~ 0.5 ps) when considering the rather long Mg—Mg separation of 21.3 A as compared with other data summarized in Table 1. Although these experimentally evaluated rates were also calculated and agreed relatively well with this type of time scale (25-27), some discrepancies were noted throughout the literature (28-31). Perhaps, the largest discrepancy comes from the B800 —> B850 energy migration. In this case, mixing with B800-B850 exciton levels were proposed to explain these discrepencies (29). In search of another possible explanation, the role of the carotenoid in the antenna effect was necessary.
Table 1. Time scales for energy migration and energy transfers between B800 and B850 units of the LH I and LH II rings in the purple photosynthetic membrane (22,25).
Non-radiative Time scale Non-radiative Time scale
photophysical event (ps) photophysical event (ps) B850 -> B85G (exciton in LH II) 0.1-0.2 B800 -> B850 (ET in LH II) 0.8-1.2 B800-»B800 (exciton in LH II) -0.5 LH II -* B875 (ET in LH I) 3-5
LH II -»• LH II (exciton) ~7 B875 -> special pair 20-40 B850 -* B850 (exciton in LH I) 0.1-0.2
All in all, the light energy is converted into electricity where a flow of electrons through and across the membrane occurs and these organisms can be considered as having their own photovoltaic cells. Considering the exceptional efficiency of the photosynthetic membrane in the purple bacteria, because it has been perfected over hundreds of millions of years, it appears evident to us that the mimicry of these antennae for photovoltaic cell applications could be a most promising avenue of research.
Photovoltaic materials are used to ecologically convert light into electric energy. The solar energy is free and renewable ("green energy"). Many commercially available devices are built upon polyphenylvinylene (Figure 3), a conjugated organic polymer that upon light irradiation, and in the presence of an electron acceptor, is involved in a photo-induced electron transfer
from the polymer to the acceptor. Then an excitonic process, migration of the positive and negative charges towards the anode and cathode, respectively, occurs. By connecting the electrodes, the circuit is closed and electrons flow, hence the harvesting of energy under the form of electricity. One of the limitations is the back electron transfer at the interface, which is also a problem in molecular-based photovoltaic cells, explaining why commercial cells still operate at 10-20% efficiencies. Nature figured out a way to render this energy wasting process inoperative by placing a series of electron acceptors in a downhill energy cascade (32). The photosynthetic machinery is also composed of efficient antennas (chlorophylls or bacteriochlorophylls), a photoactive redox center (special pair), a downhill energy cascade device (special pair—»pheophytin—>quinone A —>quinone B) and a chemical electron-proton carrier (plastoquinone). The large antenna network has the role of constantly "feeding" the redox centre. Nature converts light energy into chemical energy via a primary electron transfer with such efficiency that one can only be inspired by such machinery. Modern research on photovoltaic cells focuses on multilayer technologies in order to address the problem of charge migration across the materials. The back electron transfer at the interface still remains a problem and is something which needs improvement.
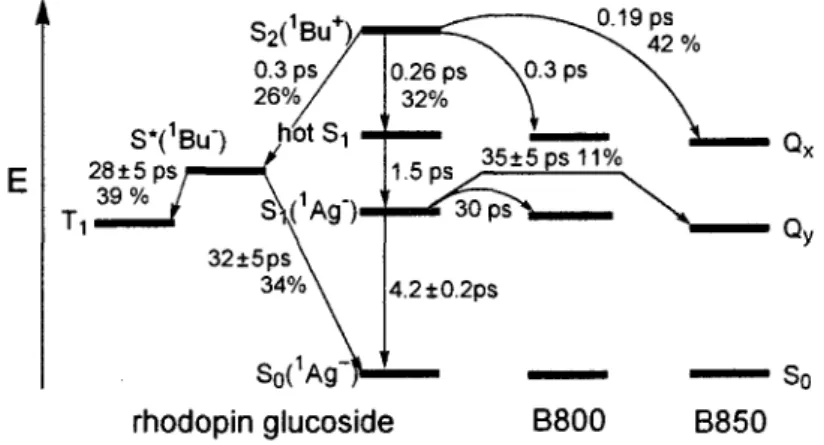
The carotenoids (rhodopin glucoside) photophysical behaviour in LH I and LH II was the subject of recent investigations (30,33-39).The key feature is the presence of a close contact (C- O = 3.4 A) between the B800 unit and a C-H group of the carotenoid (Figure 11). Evidence for its exact role in the Si energy transfer carotenoid—*B800 was addressed (40). The binding energy was estimated to be about 2 kcal/mol. Time-dependent density functional theory calculations (TDDFT) of the B800-carotenoid dyad indicate a red-shift of ~2 nm of the B800 Qy transition, along with a substantial increase in its oscillator strength in comparison
with the isolated B800 showing interactions.
The through space Si energy transfer mechanism from the carotenoid to B800 and B850 units is strongly suspected from theoretical calculations. The absorption spectrum of this chromophore has a range from 400 to 550 nm, between the Soret and the Qx bands of the
radiative process ('Ag-—^B/), whereas the symmetry-forbidden S0—>Si analogue
('Ag-—>lAf) is optically silent in the single-photon absorption spectra due to the C2h local
symmetry. The exact location of this "dark" Si state (12550 ± 150 cm"1; i.e. 797 ± 10 nm) was
determined by fs transient and two photon absorption spectroscopy (41,42). There is only a 3 nm difference (excluding the uncertainties) between the B800 absorption and that evaluated for the "dark" Si state of the carotenoid. If the absorption band for this symmetry-forbidden transition is strong, then the spectral overlap between the two bands would be large. The through space rates for Si energy transfer from the rhodopin to B800 and B850 as well as the internal excited state dynamics (i.e. internal conversion) are compared in Figure 12 (43,44). The rates for intermolecular through space Si energy transfers are fast (fs and ps), notably from the *Bu+ state for which more than 40% of the S2 population results in transfer to B800
and B850 Qx manifolds. Such rates are consistent with the relatively close proximity of the
chlorophyll and carotenoid chromophores shown in Figure 9 (right) and Figure 11. Concurrently, the rates for energy transfer from the Si state ('Ag~) to B800 and B850 are two orders of magnitude slower.
B800 ' carotenoid
carotenoid
Figure 11. (Left) A drawing showing the presence of a contact between the ketone of B800 and one of the neighbouring protons of the carotenoid in LH II. (Right) A drawing of the local environment of the carotenoid (in green) with respect to B800 and B850 units of LH II stressing the downhill Si energy transfers (red arrows) and the possibility of slightly uphill transfers (21).
The very complex and sophisticated, yet highly efficient photonic machineries and electron and energy relays described above (just to state a few examples), can only leave us amazed about what nature has accomplished to secure the survival of these organisms. The conversion
of solar energy into chemical energy via a primary electron transfer followed by an electron flow resembles that of the man-made photovoltaic cells and solar panels. However, nature has accomplished this design with unequaled performance. Professor Harvey suggested that it was a good idea to copy some of the well-established concepts of nature, improved over several billions of years through evolution for human kind applications such as photovoltaic cells.
rhodopin glucoside B800 B850
Figure 12. State diagram of the carotenoid along with the Qx, Qy and So levels of the B800
and B850 chromophores in the LH II complex. The "hot Si" state is yet to be determined. Selected intramolecular relaxation and intermolecular Si energy transfer processes (i.e. energy flow) are indicated in grey arrows along with their corresponding time scale (based on data extracted from references (43,44). The values in percent indicate the relative population that uses this relaxation path (21).
This thesis is therefore directly related to some of the issues which are of concern worldwide; energy and the environment. This project is "planting new seeds for the long term goal of this research to design better performing photonic devices generating electricity with minimal waste (under the form of heat) by using a clean and renewable source of energy (light). Photovoltaic cells and light emitting diodes (LED) are essentially of the same design. In the former light is used to generate electricity, while in the latter light is generated from electricity. Current research on LED technology focuses on development of the white LED (WLED) for indoor and outdoor lighting. In this case, the major limitation is the
photo-stability and thermal aging of the devices. Since an electric current is passing through a material (multi-layered materials to be exact), the electric resistance which is proportional to the current intensity, induces an increase in temperature, accelerating aging. The quest for more stable materials and polymers is ongoing and recent work has shown that organometallics may be the solution, notably those incorporating platinum (45).
The main question we are faced with is: can we copy some basic concepts of nature such as the relationship between efficient energy migration (exciton) and structure, or using and placing molecular building blocks for energy and electron transfers across materials in thermodynamic cascade fashion leading to irreversibility (i.e. faster rates)?
The long term objectives of this project will continue to be developed in Dr. Pierre D. Harvey's laboratory, and will aid in answering the above question. The two main objectives of this project are the design of new conjugated polymers that have potential application in solar cells, a man-made device that converts light energy into electricity, and is to monitor the energy flow across a material or a polymer as a function of time in a controlled manner. The latter property, rate of exciton and energy transfer processes, is the key parameter that defines the efficiency of such materials. The full understanding of the relationship between the structure and the rate of energy migration is necessary for such design.
The focus of this thesis is the synthesis and investigation of bio-inspired polymers. More particularly, mono-dispersed linear oligomers and polymers with addressable structures were prepared. Two crucial structural parameters had to be taken into account in the design. First, the chromophores needed be easy to synthesize and exhibit chromophoric properties very similar to that of the bacteriochlorophyll. Second, the interchromophoric Tt-contact responsible for the electronic communication across a material had to be secured. We elected to use a porphyrin as the aromatic macrocycle playing the role of the bacteriochlorophyll (Figure 13). Both consist of a cyclic tetrapyrol centre but differ by the absence of two double bonds in the bacteriochlorophyll macrocycle and the substituent groups around it. We chose to link the
chromophores together with ethynyl spacers which provide a path of conjugation and secure electronic communication and also provides structural integrity to the assembly in solution (in comparison with H-bonds for example). Most likely due to this covalent bonding, the formation of oligomers and polymers exhibited weak solubility as seen in the model compounds (Chapter 3). To overcome this challenge, soluble chains will be incorporated onto the backbone of the polymer. We also decided to use luminescent Pd- and Zn-containing porphyrins acting as the energy donor and acceptor molecules, respectively. This choice is based on experience where both singlet and triplet energy transfers were demonstrated with respect to the possible electron transfer which is not observed in this work.
Figure 13. Drawings of the metalloporphyrin macrocycle (left) where R can vary from the common aromatic group to the aliphatic one, and the bacteriochlorophyll a for example (right).
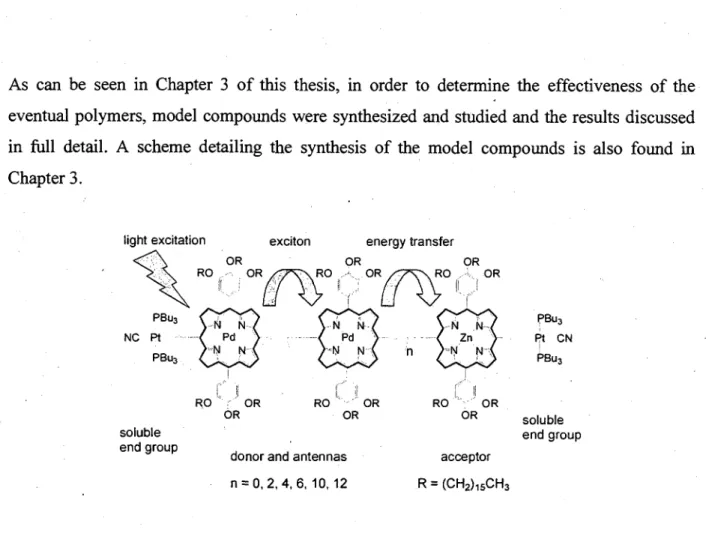
The eventual target oligomers (Figure 14) consist of three components: end of chain donor, middle of chain donor responsible of the exciton migration, and the acceptor end of chain. The R group will be a long alkyl chain such as (-(CH2)isCH3) which should improve the solubility of these materials. All the Pd-containing macrocycles act as antennas absorbing the light and transferring this energy to the acceptor. For synthesis simplicity, the end groups will be soluble Pt(PBu3)2CN centres. The C-C coupling will be performed using the common method: 2 RC=CH + catalyst —> RC=C-C=CR, explaining why n will be an even number.
As can be seen in Chapter 3 of this thesis, in order to determine the effectiveness of the eventual polymers, model compounds were synthesized and studied and the results discussed in full detail. A scheme detailing the synthesis of the model compounds is also found in Chapter 3.
light excitation exciton energy transfer
i a • L. it L I]
RO •.'• OR RO OR RO ~"y' OR
soluble end group end group , ,
donor and antennas acceptor 11 = 0 , 2 , 4 , 6 , 1 0 , 1 2 R = (CH2)i5CH3
Figure 14. Drawing of the target oligomers showing the exciton.
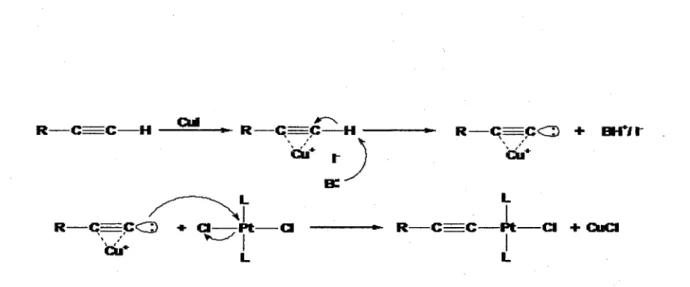
The design of organometallic and coordination polymers where the metallic fragment is incorporated into the backbone has become a field of growing interest in the recent years (46). Among these new materials, platinum containing oligomers and polymers form an interesting family of conjugated compounds, investigated for their optical, luminescent, and conductive properties (45). The investigation of model compounds containing platinum in the backbone are discussed in Chapter 3 and 4. Well characterized examples of the incorporation of metal-metal bonds in the backbone of organometal-metallic/coordination polymers are relatively rare (47,48) and most of them are nonconjugated. There have been reports of diplatinum complexes containing bridging bis(diphenylphosphino)methane (dppm) ligands and these have been extensively studied. A well studied complex demonstrating electronic communication is the [Pt2(dppm)2(C=CFc)2J (Fc = ferrocene) synthesized and studied by Vittal et al. This work showed that the Pt-Pt bond in conjunction with the C=C bonds mediates ground state electronic interactions mostly via inductive and/or magnetic exchange. It is shown that a
system that allows for a more direct interaction between the Fe centers and the C=C-Pt-Pt-C=C leads to a larger extent of electron derealization, hence better electron communication. Knowing the extent of the electronic communication, we decided to try an alternative approach to the monoplatinum research described in Chapter 3 and compare the results.
CHAPTER 1
Theory
1.1 Polymers
A polymer is a large molecule (macromolecule) composed of repeating structural units connected together by various types of chemical bonds. The word is derived from the Greek word noXv (poly), meaning "several or numerous"; and /uepog (meros), meaning "part or unit" (49). The smaller subunits making up a polymer are known as monomers, they are the smallest repetitive unit in the macromolecule. Polymers typically form long chains and if there are only a few units this small chain is called an oligomer.
Polymers can exhibit various conformations; the simplest form is a straight or linear chain polymer, with a main backbone. There also exist polymers which are folded upon themselves. The free rotation about single bonds allows for the flexibility of the polymer. The flexibility of an unbranched polymer chain is characterized by its rigidity, which is influenced by double bonds, steric hindrance and bridging ligands. These factors all influence the linearity of the polymer and will be discussed below.
The synthesis of polymers may be carried out by various methods. Regardless of the method used, there is chain growth which is completely random. As a consequence, the bulk polymer consists of chains of different lengths and therefore we must discuss the distribution of the molecular mass. The number of monomer units in a polymer is proportional to the molecular mass of the chain, the number of units is known as the degree of polymerization (DP). For oligomers, the degree of polymerization varies between 2 and ~ 25. If it is stated that polymers are a distribution of molecular mass, we must therefore discuss the average values for the molecular masses. There are two average molecular masses which are of importance: the
![Figure 5. A crystalline structure of the semi-conducting {[Ag(dmb)2]TCNQ2.5}n (11) material](https://thumb-eu.123doks.com/thumbv2/123doknet/2737763.65160/30.899.96.826.88.792/figure-crystalline-structure-semi-conducting-ag-tcnq-material.webp)