PHYSIOLOGY OF LOW TEMPERATURE-MODULATED
POSTHARVEST NEEDLE SENESCENCE AND
ABSCISSION IN BALSAM FIR {ABIES BALSAMEA L.)
Thèse présentée
à la Faculté des études supérieures et postdoctorales de l'Université Laval
dans le cadre du programme de doctorat en biologie végétale
pour l'obtention du grade de Philosophiae Doctor (Ph.D.)
DÉPARTEMENT DE PHYTOLOGIE
FACULTÉ DES SCIENCES DE L'AGRICULTURE ET DE L'ALIMENTATION
UNIVERSITÉ LAVAL
QUÉBEC
2012
RÉSUMÉ
Le sapin baumier (Abies balsamea L.), espèce favorite des producteurs d'arbres et de couronnes
de Noël dans les provinces de l'Atlantique, est victime de chutes d'aiguilles sévères (abscission
prématurée) après la récolte. Une série d'expériences ont été réalisées pour : (i) déterminer le rôle
des racines, (ii) identifier la température et le temps d'exposition optimums permettant
d'augmenter la durée de rétention des aiguilles (DRA) en post-récolte; (iii) déterminer et
caractériser les changements de concentration en phytohormones en réponse à un traitement de
basse température (BT); (iv) élucider l'effet interactif des facteurs génotypiques et
environnementaux sur la DRA post-récolte; et (v) investiguer les dynamiques temporelles de
l'accumulation de l'acide abscissique (ABA) induite par les basses températures et évaluer l'effet
de l'ABA sur la DRA. Les semis de sapin baumier soumis à 5°C durant au moins 6 h ont présenté
une DRA supérieure de 11 jours et la présence de racines a eu un effet synergique retardant
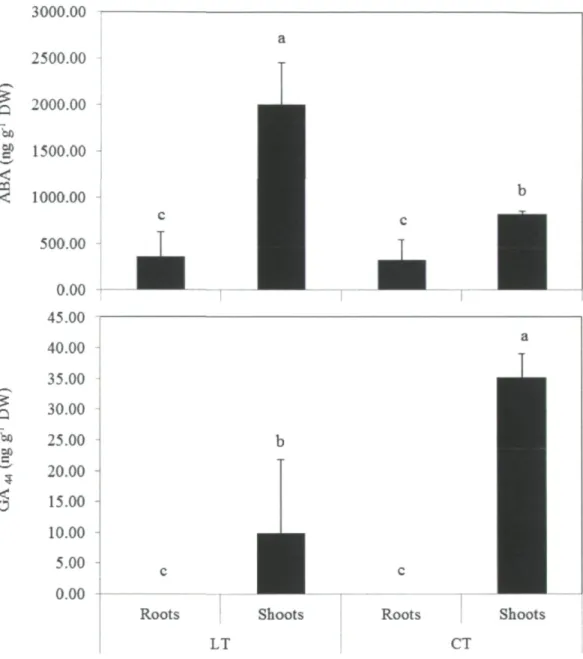
l'abscission des aiguilles. Lorsque soumis à 5°C durant 48 h, la concentration foliaire en ABA
des semis a augmenté de 2,5 fois (2007 ng g"
1DW) et l'acide gibbérellique GA44 a diminué de
3,5 fois (9,85 ng g"
1DW). Le traitement BT n'a eu aucun effet sur les phytohormones dans les
racines.
Parmi les deux génotypes (AB-NSD-184 et AB-NSD-004) étudiés dans le verger Debert
en Nouvelle-Ecosse, une corrélation négative fut observée entre la DRA post-récolte des
branches échantillonnées et les facteurs climatiques choisis (moyenne des heures de photopériode
quotidienne (PP) et moyenne des températures maximales quotidiennes) (R
2=0,75; P=0,001)
ainsi qu'avec les concentrations foliaires en ABA (R
2=0,38; P=0,001) chez le génotype
AB-NSD-184 seulement. Une DRA maximale de 180 jours et minimale de 41 jours furent observées
respectivement en octobre et juin. Les conditions environnementales ont fortement modulé les
effets du traitement BT sur la RDA post-récolte. La déshydratation a accéléré l'abscission des
aiguilles, et le traitement BT appliqué sous conditions lumineuses a compensé pour les effets de
la déshydratation et a augmenté la RDA de 30 jours. De tels avantages n'ont pas été observés
lorsque le traitement fut appliqué dans l'obscurité. Les bénéfices physiologiques découlant du
traitement BT ne furent présents que sous un faible déficit de pression de vapeur (DPV; < 0,87
kPa). Sous DPV élevé (> 1,30 kPa), BT a diminué la RDA de 35% (45 jours), réduit de moitié la
teneur en eau relative des aiguilles, et augmenté de 4 fois le potentiel hydrique du xylème. Les
changements temporels de la concentration foliaire en ABA ont différé entre les génotypes et
l'ABA a augmenté avec le temps après récolte. L'apport d'ABA via le xylème à des
concentrations > 95 uM a provoqué l'abscission des aiguilles. Cependant, une concentration
entre 2,5 et 7,5 uM d'ABA acheminée via le xylème durant 15 jours a prolongé la DRA de 20
jours. Ni les concentrations élevées d'ABA (12,5 uM), ni l'inhibition de la synthèse de l'ABA
n'ont augmenté la DRA. Les bénéfices du traitement BT sur la durée de rétention des aiguilles
implique des changements de l'ABA foliaire et cette réponse a varié en fonction des génotypes et
des conditions environnementales.
ABSTRACT
Balsam fir (Abies balsamea L.), the premier species of Atlantic Canada's Christmas tree and greenery industries suffers from severe needle abscission postharvest. Series of investigations were carried out to: (i) determine the role of roots, (ii) identify the optimum temperature and duration that prolongs postharvest needle retention duration (NRD), (iii) determine and characterize the phytohormonal changes in response to low temperature (LT) exposure, (iv) understand the interactive influence of genotypic and environmental factors on postharvest NRD and (v) investigate the temporal dynamics of LT-induced ABA accumulation and ascertain the effect of ABA on NRD. Exposure of balsam fir seedlings to 5°C for at least 6 h resulted in a 6% weight gain and presence of roots was beneficial. When balsam fir seedlings were exposed to 5°C for 48 h, the shoot ABA concentration increased by 2.5x (2007 ng g"1 DW) and Gibberellic acid (GA)44 declined by 3.5x (9.85 ng g"1 DW). Root phytohormones did not respond to LT treatment. Of the two contrasting genotypes investigated (AB-NSD-184 and AB-NSD-004), a negative correlation existed between the postharvest NRD and selected weather factors (average daily photoperiod hours (PP) and average daily maximum temperature) (R =0.75; f)=0.001). The needle ABA concentrations (R2=0.38; />=0.001) also correlated with NRD in genotype
AB-NSD-184. A maximum (180 days) and a minimum NRD (41 days) were observed in October and June, respectively. Environmental conditions strongly modulated LT effects on postharvest NRD. Dehydration accelerated needle abscission and LT compensated for dehydration effects and increased NRD by 30 days under light. No such benefits were noticed under dark. Physiological advantages induced by LT existed only under low vapor pressure deficit (VPD) (0.87 kPa). At high VPD (1.30 kPa and above), LT decreased NRD by 35% (45 days), lowered relative water content (RWC) by 2x, and increased xylem pressure potential (XPP) by 4x. Temporal changes in ABA differed with the genotypes and ABA increased with postharvest duration. Xylem feeding of ABA at concentrations above 95 uM promoted needle abscission. However, short-term (15 days) pre-loading of ABA at 2.5-7.5 uM prolonged NRD by 20 days when xylem-fed. Benefits of LT-induced changes in NRD involve alterations in shoot ABA and this response strongly varied with genotype, harvesting month and environmental conditions.
Selected chapters of this dissertation have been published in peer-reviewed, high impact journals and presented at various international scientific conferences/congresses. The following provides information on the manuscripts/scientific presentation and their corresponding reference sections in this document. I, Arumugam Thiagarajan (PhD candidate) was engaged in review of the literature, developing the hypotheses of the project, design and conduct of the experiments, statistical analyses and manuscript preparation. Co-supervisor, Dr. R. Lada contributed to the development of hypotheses and supervision of entire research at Christmas tree Research Centre, Nova Scotia Agricultural College and provided funding for this research. The committee members contributed to improvements in experimental design, review of manuscripts and encouraged synthesis of ideas.
Conference presentations and peer-reviewed publications
(i) Arumugam Thiagarajan and Rajasekaran Lada. 2010. Cold acclimation influence postharvest needle retention in root detached balsam fir. International Horticultural Congress, Portugal, Lisbon, August 22-27, 2010 (Poster presentation)
(ii) Arumugam Thiagarajan and Rajasekaran Lada. 2012. Cold acclimation influence post-harvest needle retention in root detached balsam fir (Abies balsamae (L.) Mill.). Acta Horticulturea [In Press].
Presented in Chapter 5.1 with modifications from the original manuscript to the Results and Discussion Section as this article directly addressed the fundamental part of investigation whether the low temperature and presence of root affected the postharvest needle retention characteristics in balsam fir.
(iii) Arumugam Thiagarajan, Rajasekaran Lada, Steeve Pepin, Charles Forney, Yves Desjardins and Martine Dorais. 2012. Low Temperature exposure increases the postharvest needle retention of balsam fir (Abies balsamea L.) genotypes. International Conference of American Society of Horticultural Sciences. Waikoloa, Hawaii, September 25-28, 2011 (Poster presentation)
(iv) Arumugam Thiagarajan, Rajasekaran Lada, Steeve Pepin, Charles Forney, Yves Desjardins and Martine Dorais. 2012. Characterization of phytohormonal and postharvest senescence responses of balsam fir (Abies balsamea (L) Mill.) exposed to short-term low temperature. Trees. DOI: 10.1007k00468-012-0728-1 [Available online].
Presented in Chapter 5.2 with certain modifications from the original manuscript. Introduction and Material and Methods sections were trimmed to avoid repetition. Discussion has been extended to ensure flow to the next Chapter. This manuscript includes information about the discovery of the baseline hormone concentrations alone with the specific hormonal changes that occurred in roots and shoots in response to low temperature with direct relevance to postharvest needle senescence and abscission.
(v) Arumugam Thiagarajan, Rajasekaran Lada, Steeve Pépin, Charles Forney, Yves Desjardins and Martine Dorais. 2012. High water vapor pressure deficit negates low temperature promoted needle retention in balsam fir (Abies balsamae (L.) Mill). Postharvest Biology and Technology - "Under review".
Presented in Chapter 6.0 with limited modifications from the original manuscript. Introduction and Material and Methods sections were altered to avoid redundancy. Discussion has been extended to ensure flow to the next Chapter.
(vi) Arumugam Thiagarajan, Rajasekaran Lada, Steeve Pepin, Charles Forney, Yves Desjardins and Martine Dorais. 2012. Temperature and photoperiod influence postharvest needle abscission of balsam fir (Abies balsamea L. (Mill)) in selected genotype modulating ABA levels. Journal of Plant Growth Regulation - "Under review".
Presented in Chapter 7.2 with modifications to the introduction and discussion chapters from the original manuscript.
Technology
i. Benefits of LT exposure will be advanced towards development of a technology/good management practice to extend the NRD of harvested trees.
ii. Short-term pre-loading of ABA is submitted for disclosure for exploring the possibilities of developing a needle abscission retarding agent.
ACKNOWLEDGEMENTS
My first note of thanks is due to Dr. Rajasekaran Lada, who believed in my abilities and encouraged me to pursue this daunting academic dream. He was a constant source of inspiration and a great mentor throughout this pursuit. His participation in this project was incomparable and I express my deep gratitude for sharing his experience and wisdom.
I sincerely thank Dr. Steeve Pepin, who trusted me as his PhD student. I recognize the timely guidance and constructive criticisms he offered on this project. Steeve has been an "advocate of great research", "scientist as impartial judge" and a "good friend". Without his support, accomplishing this task would have been difficult. I thank you, "Steeve".
I express my respect and thanks to the committee members, Dr. Charles Forney, Dr. Martine Dorais and Dr. Yves Desjardins for devoting their time in providing feedback on experimental planning, statistical analyses, preparation of manuscripts and dissertation despite their busy schedules.
I wish to express my thanks to fellow research team members, Ms. Azure Adams, Mr. Scott Veitch and Dr. Mason MacDonald for their time and support in the Christmas tree research center (CRC) laboratory tasks, field visits and technical inputs, respectively. I thank all the CRC members for their contributions.
Mere words cannot express my feelings for my wife, "Kala" towards achieving this milestone. She has endured long hours at lab corridors, missed weekend trips, sacrificed family time and shared my emotions all these years. The huge pile of research datasheets and hand-written notes came to me as neatly organized Excel sheets from her hands. With her on my side, I can pursue big dreams with no fear.
My daughter Harshini has always showed unabated enthusiasm in understanding my experiments and has been a driving force within me. I reminisce and cherish the days she helped me collect the data from the greenhouses and growth chambers. I express my love to Rishi, in giving up his "Daddy Time" for my research. I recognize the moral support offered by my father, mother and all my friends in India.
I acknowledge the funding support for this study from NSERC-CGS, NSERC to Dr. Lada, research funding from AgriFutures, Nova Scotia and New Brunswick Councils, Atlantic Innovation Fund from Atlantic Canada Opportunities Agency.
TABLE OF CONTENTS
1.0 INTRODUCTION 4 2.0 LITERATURE REVIEW 6 2.1 Cold acclimation and needle retention 6
2.2 Signal transduction in cold acclimation and de-acclimation 11
3.0 HYPOTHESES AND OBJECTIVES 17 4.0 GENERAL METHODOLOGY 18 4.1 Sample collection and postharvest protocol 18
4.2 Physiological parameters 19 4.2.1 Needle retention duration, needle abscission and senescence, percent needle loss and
percent biomass change 19 4.2.2 Average daily water use and cumulative water consumption 20
4.2.3 Xylem pressure potential 20 4.2.4 Membrane injury index 21 4.2.5 Relative water content 21 4.2.6 Stem capacitance 21 4.2.7 Controlled growth chamber conditions 22
4.2.8 Imposition of low temperature treatment 22 5.0 LOW TEMPERATURE INFLUENCES POSTHARVEST NEEDLE ABCISSION AND
SENESCENCE 23 5.1 The effects of different temperature and durations; and the role of roots on postharvest
needle retention 23 5.1.1 Résumé 23 5.1.2 Abstract 24 5.1.3 Introduction 24 5.1.4 Materials and Methods 25
5.1.5 Results 28 5.1.6 Discussion 35 5.1.7 Conclusions 38 5.2 Characterization of the endogenous hormonal and needle senescence responses of
balsam fir exposed to short-term low temperature 40
5.2.1 Résumé 40 5.2.2 Abstract 41
5.2.5 Results 48 5.2.6 Discussion 56 5.2.7 Conclusions 60 6.0. GENOTYPIC AND SEASONAL EFFECTS ON HORMONAL AND NAS CHANGES
61
6.1 Abstract 61 6.2 Introduction 62 6.3 Materials and Methods 64
6.4 Results 67 6.5 Discussion 78 6.5 Conclusions 80 7.0 ENVIRONMENTAL CONDITIONS MODULATE POSTHARVEST NAS INDUCED
BY LOW TEMPERATURE 82 7.1 Effects of hydration and PPFD on the low temperature-induced postharvest needle
abscission and senescence changes in balsam fir (Abies balsamea L.) 82
7.1.1 Abstract 82 7.1.2 Introduction 83 7.1.3 Material and Methods 84
7.1.4 Results 85 7.1.5 Discussion 90 7.1.6 Conclusions 91 7.2 Effects of postharvest vapor pressure deficit on low temperature-induced changes in
NAS 93 7.2.1 Abstract 93
7.2.2 Introduction 94 7.2.3 Materials and Methods 95
7.2.4 Results 97 7.2.5 Discussion 105 7.2.5 Conclusions 107 8.0 ABA HAS A PHYSIOLOGICAL ROLE IN MODULATING POSTHARVEST NAS
8.1 Effects of exogenous ABA application on postharvest needle abscission and senescence 109
8.1.1 Abstract 109 8.1.2 Introduction 109 8.1.3 Materials and Methods 111
8.1.4 Results 114 8.1.5 Discussion 119 8.1.6 Conclusions 121 8.2 Temporal dynamics of ABA accumulation during LT exposure and postharvest 122
8.2.1 Abstract 122 8.2.2 Introduction 122 8.2.3 Materials and Methods 123
8.2.4 Results 124 8.2.5 Discussion 127 8.2.6 Conclusions 128 9.0 GENERAL DISCUSSION 130 10.0 CONCLUSIONS 135 11.0 FUTURE DIRECTIONS 138 REFERENCES 139 APPENDIX 1 150 APPENDIX II 153
LIST OF TABLES
Table 1. Statistical P values for the main and interactive effects of root severance and acclimation temperature on the postharvest characteristics of balsam fir based on
ANOVA 30 Table 2. Statistical P values for the main effects of root severance and acclimation
temperature on the postharvest characteristics of balsam fir based on Kruskal-Wallis
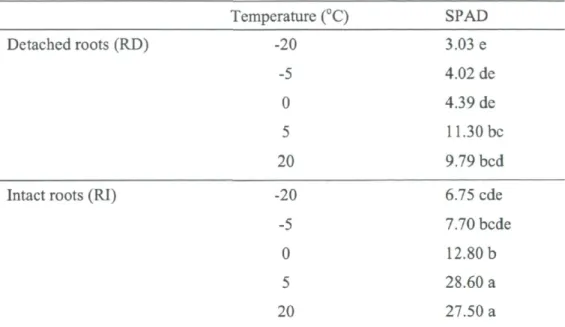
test 30 Table 3. Contribution of root and temperature on the XPP and relative chlorophyll index of
balsam fir needles. ANOVA tests proved that temperature and root exerted significant
effects on relative chlorophyll index (SPAD) and XPP at P<0.001 33 Table 4. The interactive effects of root severance, temperature and duration on percent
needle loss (PNL) of balsam fir seedlings 34 Table 5. Statistical P values for the LT and the tissue effect on the ABA, cytokinins and
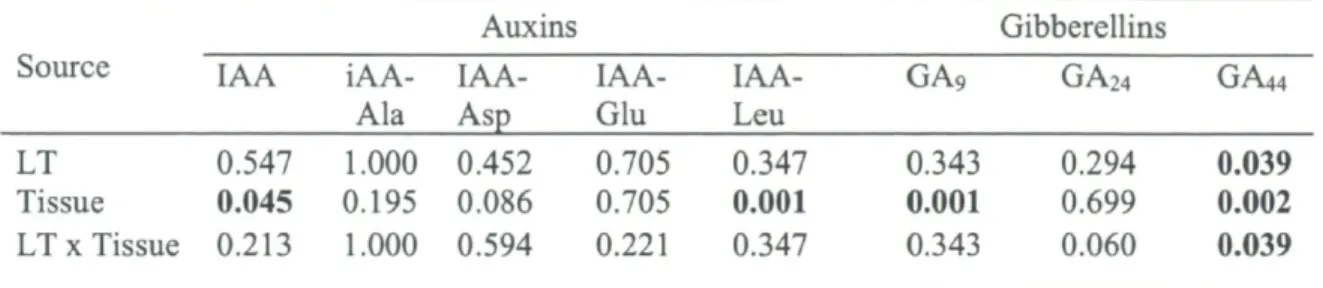
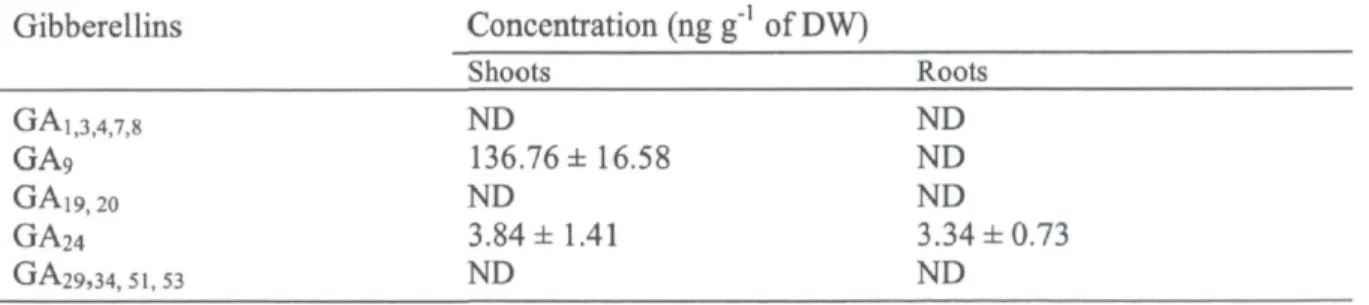
their metabolites in young balsam fir seedlings 50 Table 6. Statistical P values for the LT and tissue effect on the auxins and gibberellins in
young balsam fir seedlings 51 Table 7. Endogenous concentrations of abscisic acid metabolites (ng g"1 DW) in roots and
shoots of young balsam fir seedlings 51 Table 8. Endogenous concentrations of cytokinins (ng g"1 DW) in roots and shoots of young
balsam fir seedlings 52 Table 9. Endogenous concentrations of auxins (ng g"1 DW) in roots and shoots of young
balsam fir seedlings 52 Table 10. Endogenous concentrations of gibberellins (ng g'1 DW) in roots and shoots of
young balsam fir seedlings 52 Table 11. Statistical P values indicating the influence of genotype and seasonal impact on
the ABA concentration and postharvest needle characteristics of balsam fir (Abies
balsamea L.) 70 Table 12. Membrane injury index values of balsam fir (Abies balsamea L.) influenced by the
month of harvest 70 Table 13. The R2 and statistical P values for the relationship between average daily
photoperiod (PP), average daily maximum temperature (Tmax), average daily minimum
temperature (Tmi„) and NRD of balsam fir 71
Table 14. Statistical P values for the effect of LT and hydration on the postharvest needle
characteristics of balsam fir under dark conditions 86 Table 15. Statistical P values for the effect of LT and hydration on the postharvest needle
characteristics of balsam fir under lighted conditions 89 Table 16. Statistical P values for the effect of VPD and acclimation temperature on various
needle quality parameters 99 Table 17. Statistical P values for the effects of ABA feeding on the postharvest needle
abscission characteristics 114 Table 18. Statistical P values for the effects of short-term pre-loading of ABA on the
postharvest needle abscission characteristics 117 Table 19. Statistical P values for the effects of genotype, acclimation temperature and
LIST OF FIGURES
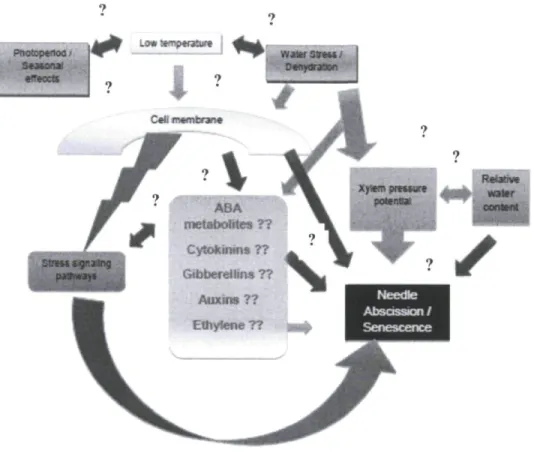
Figure 1. The biosynthetic pathway of synthesis and breakdown of ABA in higher plant cells as suggested by Cutler and Krochko (1999). The metabolic pathway predominantly occurs inside the plastids and the intermediate compounds are found inside the vacuole.... 10 Figure 2. Schematic diagram of current knowledge status in reference to relationship
between various environmental, weather and physiological factors and needle retention in conifers. The question marks represent the incomplete knowledge status that
currently exists with reference to balsam fir 16 Figure 3. (A) Percent biomass loss (%) and (B) Average daily water use (mL g'1 day"1) of
root intact (RI) and root detached (RD) balsam fir seedlings along with their standard error bars («=100). Any two means followed by same letters are not significantly
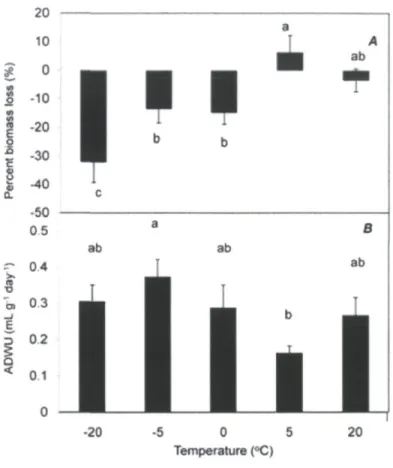
different (P<0.05) 31 Figure 4. Influence of low temperature on (A) percent biomass loss , (B) average daily water
use (mL g"1 day"1) and (C) membrane injury index (Mil) (%) of balsam fir seedlings
along with their standard error bars («=40) 32 Figure 5. Endogenous ABA (ng g"1 DW) (A) and GA44 (ng g"1 DW) (B) concentrations in
young balsam fir roots and shoots exposed to LT treatment. LT was imposed at 5°C for 48h. Vertical lines indicate the SEM (rc=3). Bars represented with same letters are not
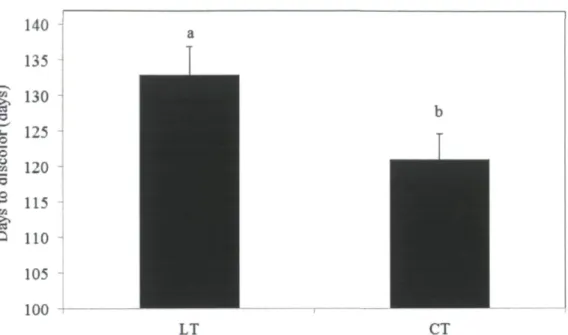
significantly different (P<0.05) 53 Figure 6. Days to discolor (days) in young balsam fir exposed to LT treatment. LT was
imposed at 5°C for 48 h. Vertical lines indicate the SEM (H=3). Bars represented with
same letters are not significantly different (P<0.05) 54 Figure 7. Photograph showing the needle senescence of balsam fir seedlings after 100 days
of harvest as influenced by LT treatment (Acclimated) imposed at 5°C for 48 h 55 Figure 8. The average daily photoperiod (hours), average daily maximum (Tmax) and
minimum (Tmjn) temperature (°C) that prevailed at the experimental site (Debert, NS)
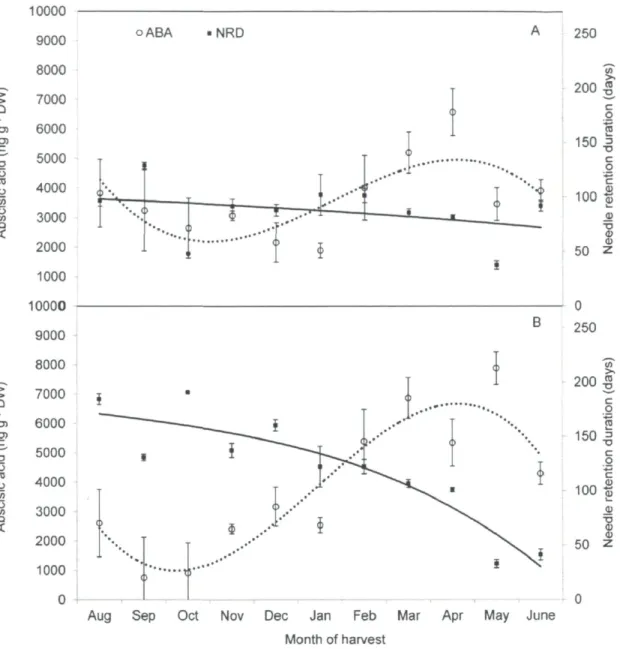
during the investigation period of August 2010 to June 2011 72 Figure 9. Seasonal effects on the Abscisic acid concentrations (ng g"1 DW) and postharvest
needle retention duration (NRD) (days) in the current-year shoots of balsam fir (Abies balsamea L.) genotypes, AB-NSD-004 (A) and AB-NSD-184 (B). Each point
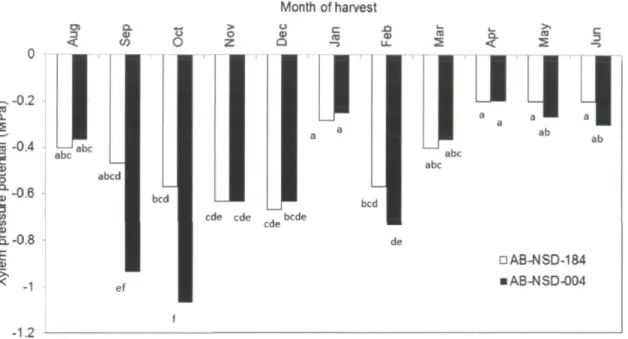
represents an average of three replicates (n=3) along with their SEM 73 Figure 10. Seasonal influence on the xylem pressure potential (XPPsum) (MPa) of balsam fir
(Abies balsamea L.) (n=3) genotypes AB-NSD-004 and AB-NSD-184. Bars followed
by same letters are not different at P<0.05 74 Figure 11. Relationship between the needle abscisic acid concentrations (ng g'1 DW) and the
postharvest needle retention (days) in the current year shoots of balsam fir (Abies
balsamea L.) genotypes, AB-NSD-004 (A) and AB-NSD-184 (B) 75 Figure 12. Relationship between the average daily photoperiod (hours) and the postharvest
needle retention (days) in the current year shoots of balsam fir (Abies balsamea L.)
genotypes, AB-NSD-004 (A) and AB-NSD-184 (B) 76 Figure 13. Photograph illustrating the contrast in the postharvest needle abscission
characteristics as influenced by genotypes harvested in the month of June (A) and
Figure 14. Main effect of hydration on the postharvest plant biomass loss (%) (A), xylem pressure potential (MPa) (B) and needle retention duration (days) (C) of balsam fir clone AB-NSD-184 (in dark). Bar represents average values («=10) with their corresponding SEM. Values followed by same letter are not significantly different
(P<0.05) 87 Figure 15. Effect of LT and hydrated (A) and unhydrated (B) conditions on the cumulative
water consumption (CWC) (mL g'1) and PNL of balsam fir clone AB-NSD-184 in the dark. Each point represents an average (n=5) for each treatment. The continuous lines indicate the PNL of the treatments. The broken lines indicate the CWC trend observed
with the treatments 88 Figure 16. Acclimation temperature and hydration effects on needle retention duration
(days) of balsam fir clone AB-NSD-184 (dark). Each point represents average value (n=5) along with their SEM. Values followed by same letter are not significantly
different (P<0.05) 89 Figure 17. Main effect of acclimation temperature on xylem pressure potential (MPa) of
balsam fir clone AB-NSD-184 (Light). Each point represents average value («=10) along with their SEM. Values followed by same letter are not significantly different (P
<0.05) 90 Figure 18. The interactive effects of acclimation temperature (LT and CT) and postharvest
VPD (0.22, 0.87, 1.30, 1.86 kPa) on needle retention duration (days) (A), membrane injury index (%) (B) of balsam fir seedlings. Each point represents an average of three
replicates. Same letters indicate no significant difference at P <0.05 100 Figure 19. The interactive effects of acclimation temperature (LT and CT) and postharvest
vapor pressure deficits (0.22, 0.87, 1.30, 1.86 kPa) on xylem pressure potential (MPa) (A) and relative water content (%) (B) of balsam fir seedlings. Each point represents
average of three replicates. Same letters indicate no significant difference at P<0.05 101 Figure 20. The VPD effect (0.22, 0.87, 1.30, 1.86 kPa) on postharvest stem capacitance (pF),
of balsam fir seedlings. Each point represents average of six replicates. Same letters
indicate no significant difference atP<0.05 102 Figure 21. Cumulative water consumption (mL g"1) of balsam fir as influenced by
acclimation temperature (LT and CT) and postharvest vapor pressure deficits (0.22 (A), 0.87 (B), 1.30 (C) and 1.86 kPa (D)). Each point represents average of three replicates... 103 Figure 22. Relationship between the relative water content (%), xylem pressure potential
(MPa) and needle retention duration (days) in balsam fir 104 Figure 23. Effect of continuous ABA feeding on the percent weight change (%) (A), needle
retention duration (days) (B) and percent needle loss (%) (C) in balsam fir (Abies balsamea L.) clone AB-NSD-184. Each point represents average values («=4) along with their SEM. Values followed by same letters are not significantly different
CP<0.05) 115 Figure 24. Cumulative water consumption (mL g"1) and the percent needle loss (%) of
balsam fir (Abies balsamea L.) clone AB-NSD-184 as influenced by the continuous xylem feeding of ABA at concentrations of 0, 95, 180 and 360 uM. The vertical lines indicate the SEM («=4) for the cumulative water consumption values observed every
Figure 25. Effect of short-term xylem feeding or foliar application of ABA on the xylem
pressure potential (MPa) (A), percent weight change (%) (B) and needle retention
duration (days) (C) in balsam fir (Abies balsamea L.) seedlings. Each point represents
an average of four replicates with SEM. Values followed by same letters are not
significantly different (P<0.05) 118
Figure 26. The percent needle loss (PNL) (%) of the balsam fir branches as influenced by
the days after harvest. The vertical bars indicate the SEM («=12). Bars followed by
same letters are not significantly different (P<0.05) 125
Figure 27. The ABA concentrations (ng g'
1DW) of balsam fir needles as influenced by the
genotypes (AB-NSD-184 and AB-NSD-004) and postharvest duration (days). Each
point represents an average of six replicates. Bars indicate SEM 126
Figure 28. Photograph of the clone AB-NSD-184 exhibiting the lack of LT influence on
postharvest needle abscission and senescence postharvest (Day 27) 126
Figure 29. Schematic diagram representing the nature of relationships between selected
weather and physiological factors on needle abscission in balsam fir 134
Figure 30. Example of one of the standard curves illustrating the relationship (R
2= 0.97)
between the % Binding (transformed value) and the standard ABA (pmol/mL) based on
the absorbance readings obtained from the microplate reader at a frequency of 405 nm... 152
2iP Isopentenyl adenine
7'-OH ABA 7'-Hydroxy ABA
ABA Abscisic acid
ABA-GE Abscisic acid glucose ester
ADWU Average daily water use
ANOVA Analysis of variance
CBF Cold binding factors
CWC Cumulative water consumption
CK Cytokinin
COR Cold regulated genes
DD Days to discolor
dhZ Dihydro zeatin
dhZR Dihydro zeatin riboside
DPA Dihydro phaseic acid
DREB Drought responsive element binding factors
DW Dry weight
EC electrical conductivity
FW Fresh weight
GA Gibberellin
HPLC-ESI-MS/MS High performance liquid chromatography-electrospray ionization tandem mass spectrometry
IAA-Asp IAA-Glu IAA-Leu iP iPA LSD LT Mil MRM NAR NAS neoPA NRD PA PBL PNL PP PPFD RD RJ RIA ROS RWC SPAD
Indole acetic acid- Asparagine Indole acetic acid- Glucoside Indole acetic acid- Leucine Isopentenyl
Isopentenyl adenosine Least significant difference Low temperature
Membrane injury index Multiple reaction monitoring Needle abscission resistance Needle abscission and senescence Neophaseic acid
Needle retention duration Phaseic acid
Percent biomass loss Percent needle loss Photoperiod hours
Photosynthetically active photon flux density root-detached
root intact
Radio immune-assay Reactive oxygen species Relative water content
t-Z Trans-zeatin
t-Z-0-Glu Trans-zeatin-0-glucoside
t-ZR Trans-zeatin riboside
VPD V apor pressure deficit
XPP Xylem pressure potential
1.0 INTRODUCTION
It is a deep-rooted tradition to buy a Christmas tree to celebrate the spirit of winter
solstice. Balsam fir (Abies balsamea L.), is a Christmas tree species preferred worldwide owing
to its characteristic fragrance, needle color, soft needles and unique tree architecture. Demand for
this popular ornamental tree species is increasing worldwide (CTCNS 2009). Currently, balsam
fir is cultivated in more than 25,000 ha in Atlantic Canada and approximately two million trees
are exported annually to markets in US, Mexico, Puerto Rico and Japan which is worth $72
million annually (Statistics Canada 2011). Increased demand for these trees combined with
consumers' desire to purchase trees by US-Thanksgiving holiday has resulted in early harvesting
practices (mid-October versus late November) to ensure timely delivery to distant markets.
In recent years, balsam fir suffers from aggravated needle drop, loss of needle softness
and needle discoloration postharvest. Such deterioration in the aesthetic qualities of natural
balsam fir trees has deterred consumers towards artificial trees (Statistics Canada 2011). While
needle drop is a common phenomenon in natural stands (Kennelly 2008), recent occurrences of
needle abscission postharvest are uncharacteristic and atypical. Thus, needle drop has been
identified as the foremost issue affecting the postharvest quality of balsam fir trees. Owing to this
problem and reduced marketability of these trees, the Christmas tree industry faces significant
economic challenges.
Presence of several long-distance signaling factors has been demonstrated by many
researchers in both woody and herbaceous species (Chen and Gupta 1983; Mantyla et al. 1995) in
relation to moisture stress and cold stress. Abscisic acid (ABA), ethylene, jasmonates, salicylates,
pH, cytokinins, phytins, mitogen activated protein kinases and malate (Schachtman and Goodger
2008) are some of the extensively documented signals that arise from roots in response to cold
acclimation and/or abiotic stresses. Although, the presence of such signals has been
acknowledged, consensus on the origin, nature and identity of these signals have still not been reached with regard to cold acclimation and needle abscission.
Cold acclimation invoked by low temperature and shorter photoperiods is well established to predispose plants to unfavorable environments (Welling et al. 2002). Absence of cold acclimation due to early harvest practices has been anecdotally linked as a potential cause for the needle abscission. In a study conducted with naturally habituated Nordmann fir trees, Chastagner and Riley (2003) have established that warm fall temperatures in combination with early harvesting practices can delay the onset of cold acclimation and subsequently reduce needle retention. Indirect evidence exists for beneficial effect of cold acclimation on needle retention in natural and root-intact conditions. For instance, trees that are not adequately frost hardened have been reported to shed needles swiftly and dry faster after harvest (Hinesley 1984). Also, low temperatures prevailing at higher altitudes have positively influenced needle life of Pinus sylvestris trees (Reich et al. 1996). Furthermore, research findings have shown that cold acclimation is physiologically manifested through alteration in cell membrane fatty acids (Sangwan et al. 2001), regulation of moisture losses (Smit-Spinks et al. 1984) and enhancement in light energy quenching activities (Huner et al. 1998), which can directly impact the needle retention characteristics of balsam fir. Exposure to a single stress has been found to trigger a cascade of metabolic responses that confer tolerance to several other environmental stresses since there is a common network of signaling networks (Pastori and Foyer 2002). Our current knowledge on the metabolic response that affects the postharvest abscission physiology is very limited. Accordingly, the proposed research attempts to uncover the nature and physiology of the low induced phytohormonal signal(s), the genotypic plasticity to low temperature-induced changes, seasonal effects and influence of various postharvest conditions on needle retention/abscission in balsam fir.
2.0 LITERATURE REVIEW
2.1 Cold acclimation and needle retention2.1.1 Cold acclimation
Cold acclimation determines the survival of several perennial species growing in the temperate regions of the planet Earth. Cold acclimation refers to the adaptive mechanism, where the plants acquire their ability to withstand freezing temperatures in response to milder temperatures during the onset of winter (Gusta et al. 2005). Cold acclimation is suggested to be one of the complex biological adaptations that affect almost every single cellular process (Xin and Browse 2000). They suggest that more than 25% of plant genome is affected by cold acclimation and several characteristic metabolic and morphological changes are established to occur due to cold acclimation. Apart from freezing tolerance, empirical evidence suggests that needle longevity of conifers increases with cold acclimation (Reich et al. 1996; Xiao 2003).
2.1.2 Environmental factors influencing cold acclimation
Temperature, photoperiod and light intensity are the three major environmental factors that orchestrate cold acclimation in temperate plants. Although either one of these environmental factors are known to invoke or mimic cold acclimation responses in herbaceous and woody species, it has been shown that low temperature primarily manifests cold acclimation processes with moderate influence of low light intensity (Greer et al. 2000). The difference between the temperature that invokes beneficial cold acclimation and temperature that inflicts adverse cold injury can be very narrow. For instance, white pine sustains minor injuries at -7°C but suffers severe injuries at a slightly lower temperature of -9°C (Li et al. 2005). Furthermore, the benefits attained from cold acclimation are species-specific and in some cases, vary even within the same species. For instance, Weng and Parker (2008) studied cold acclimation of aspen trees and found
that provenance level differences exist within the same species. Differences in nutrition and other growth conditions alter the acclimation benefits. The rate of cold acclimation depends on the intensity and duration of the environmental cues. For example, a rapid onset of cold acclimation was noticed in apple trees when they were exposed to shorter photoperiods followed by severe frosts (Howell and Weiser 1970). It is clear that the duration of acclimation has to be established at the species level.
2.1.3 Physiological and morphological adaptations of cold acclimation
The primary adaptive benefit of cold acclimation is the protection of cell membranes from cold injury. This characteristic phenomenon has been demonstrated in plant species like winter wheat and rye. A series of physiological changes such as those in membrane fluidity, reorganization of cytoskeleton and sudden influx of Ca2+ ions are found to occur during cold acclimation (Orvar et al. 2000). Reduction in water uptake, transpiration rates, and hydraulic conductivity are characteristic features of cold acclimation (Parsons 1978). Cold temperature induces closure of stomates either directly (Honor et al. 1995) or indirectly, through regulatory ions such as potassium (Ilan et al. 1995) in order to maintain the osmotic potential inside the guard cells to conserve moisture. The rapid closing of stomata prevents water losses from leaves when water becomes unavailable at low temperatures (Davies et al. 2002). Nevertheless, the potential role of ABA in regulating this action under LT is still not fully understood. The reduced stomatal conductance results in diminished CO2 exchange rates. Consequently, during winter, leaves absorb more solar energy than they could process through photosynthesis (Oquist and Huner 2003). If this excessive energy is not quenched properly, the photosystems could become excessively reduced and result in the production of reactive oxygen species, (ROS) (Kalberer et al. 2006). Interestingly, cold acclimation has been found to induce accumulation of malate, which
acts as a dissipating system against ROS. In a study conducted with Scots pine, the
photosynthetic activity was found to decline during cold acclimation and resumed to high levels
after acclimation (Repo et al. 2006). Although photosynthesis is one of the significant
physiological processes that are altered during cold acclimation, the knowledge on the effects of
cold acclimation on photosynthesis is still limited (Kalberer et al. 2006).
Alteration in carbohydrate metabolism is a well-established phenomenon of cold
acclimation. In Scots pine, the cold hardiness plant exhibited after cold acclimation was linearly
correlated to the amount of sugars accumulated (Ogren et al. 1997). Similar relationships between
the soluble sugar contents and cold acclimation responses were observed in Monterey Pine
(Pinus radiate L.) and Aleppo Pine (Pinus halepensis L.) (Tinus et al. 2000). Apart from simple
sugars (glucose, fructose), oligosaccharides such as galactose and raffinose also accumulate in
Petunia (Pennycooke et al. 2003). While contributing to osmotic potential, the accumulation of
sucrose and other simple sugars also contribute to the protection of plasma membrane from
freezing injury and potentially improve needle retention. Addition of more solutes to the cell sap
decreases the freezing point to more negative values and thus offers protection against extremely
low temperatures. Evidence on the alteration of sugar metabolism induced by cold acclimation in
balsam fir is limited to none.
2.1.4 Water balance and hydraulic implications on post-harvest needle retention
The xylem pressure potential (XPP) represents the tension at which the water is held
within its xylem vessels. Higher negative values indicate high levels of stress in conducting
moisture throughout the plant system. Water relations in conifers are traditionally monitored by
XPP of the branches. The XPP values above damage threshold levels are found to be associated
with severe adverse consequences such as, accelerated needle loss, discoloration and defoliation
even when rehydrated (Hinesley and Snelling 1997). The threshold XPP and their relation to
needle loss in several intact pine and spruce species are well documented and the threshold levels
are species specific. For instance, Chastagner and Riley (2003) studied moisture and needle
retention characteristics of Noble and Nordmann fir trees and found that Nordmann firs lose
needles at a relatively higher XPP (-3 MPa) than noble firs (-6 MPa), indicating that drought
tolerance differ between species.
Nevertheless, no consistent relationship between XPP and needle retention has been
observed in several other investigations. Rajasekaran et al. (2005) investigated average daily
water uptake and needle retention in young balsam fir seedlings and concluded that water uptake
is negatively related to needle retention. Despite large initial uptake, constant consumption of
water indicates normal water status maintenance activity. On the contrary, very low and
excessive consumption of water indicates irreversible drying of the trees and enhanced
transpiration losses, respectively. Proper hydration ensures adequate supply of moisture to meet
the demand of transpiration and basic physiological functions. From our recent experiments,
while XPP is fairly consistent among all treatments irrespective of their needle retention qualities,
water consumption has been high for the ones that lost large amounts of needles indicating that
the needle loss is triggered by factors other than the biophysical process such as XPP
(Rajasekaran et al. 2005). Bates et al. (2004) investigated the impact of trimming, resident time in
field after harvest and effect of hydration in Canaan and Fraser fir. Their results revealed that,
XPP values did not correlate with needle retention. It is agreed that multitude of factors govern
needle retention and color characteristics beyond proper hydration. From previous reports, it is
obvious that the relationship between XPP and needle retention characteristics is still
controversial and requires further investigation at the species level. Again, it is not conclusive
whether hydraulic signals are involved in triggering needle abscission.
2.1.5 ABA and protein changes due to cold acclimation
Since its discovery by Addicott et al. (1968), abscisic acid has been implicated in several physiological roles such as, abscission, biotic and abiotic stress signaling and dormancy. Excellent progress has been made in furthering the understanding on the synthesis and breakdown of ABA in higher plant cells (Cutler and Krochko 1999) (Figure 1). Furthermore, this hormone has been widely reported for its role in inducing cold acclimation processes in many plant species. Cold acclimation induced freezing tolerance was repeatedly associated with increased levels of ABA and external application of artificial analogs of ABA have resulted in increasing freezing tolerance in brome grass cell cultures (Robertson et al. 1994). Similarly cold hardened winter wheat was reported to have increased concentrations of ABA (Taylor et al.
1990).
(+>-ABA
(+.I-ABA :>!«_■ > *ij_ Stkra
Figure 1. The biosynthetic pathway of synthesis and breakdown of ABA in higher plant cells as suggested by Cutler and Krochko (1999). The metabolic pathway predominantly occurs inside the plastids and the intermediate compounds are found inside the vacuole and intercellular spaces.
Several changes in gene expression occur during cold acclimation. It is well established that cold acclimation is a multi-trait manifested mechanism. A gene family, called as Cold Regulated genes (COR) is found to be overexpressed (>100 fold) within the first 6h of cold acclimation (Xin and Browse 2000). The functions of the COR gene transcripts are not fully known yet. Majority of these COR proteins are hydrophobic and act as cryoprotectants. These functions are similar to dehydrin proteins, which act as drought tolerant proteins. The binding proteins that act on the cis elements on the COR genes are named as drought responsive element binding factors (DREB) or CBF (cold binding factor) proteins. The involvement of ABA is still indisputable yet its role is not fully understood. Both ABA-independent and ABA-dependent pathways of COR gene expressions are documented in model organisms (Gilmour and Thomashow 1991; Nordin et al. 1993). In many cases addition of exogenous ABA has elicited DREB gene products and subsequently cold acclimation responses (Kobayashi et al. 2008). However, in some cases the cold acclimation response was not attained with just ABA (Reed 1993). It is now widely agreed that ABA regulates some of the genes involved in cold acclimation process (Gusta et al. 2005). The information on ABA's involvement in COR proteins in particular reference to fir or conifer species is very limited. Also, unknown is the relationship between these proteins and needle abscission in balsam fir.
2.2 Signal transduction in cold acclimation and de-acclimation
2.2.1 Cell membrane changes and Ca + in cold acclimation
It is agreed that cell membrane acts as the primary sensory mechanism and activates secondary relay systems in response to cold signals. Heino and Palva (2004) have suggested that receptor-like protein kinases act as cold sensors on the cell membrane. These proteins could be activated by a temperature-induced conformational change in their extracellular domains, which
subsequently induces the kinase activity on the cytoplasmic side of the receptors. Nevertheless, none of these receptors are characterized yet. A large body of evidence supports that different stress stimuli including cold temperatures induce sudden influx of Ca2+ in the cytoplasm arriving from intracellular spaces (e.g., Sanders et al. 1999). Ca2+ is also suggested to act as secondary signal inducing several other hormonal changes related to cold acclimation. When calcium was supplemented to white poplar (Populus alba) trees, the level of unsaturated fatty acid content increased in cell membrane, cell electrolyte leakage values decreased and their winter hardiness increased (Percival et al. 1999). They suggested that Ca2+ ions participate directly in mediating cold hardiness through altering fatty acid composition of cell walls. Similarly, when Anjou pears were supplemented with calcium nitrate, their cold hardiness increased (Raese et al. 1996). Calcium has been attributed towards better hardiness of the trees. Also, several dehydrin proteins (25 kDa) are identified to accumulate in dogwood species in response to cold acclimation (Sarnighausen et al. 2002). They also suggested that these proteins are directly correlated with cold acclimation of the woody plants. These proteins are suggested to be involved in several roles including changes in saturation of cell membrane fatty acids. However, our knowledge on the role of Ca2+ in mediating cold acclimation of balsam fir is very limited. If cold acclimation increases cell membrane integrity (Rajasekaran et al. 2005), predominant role of Ca +, cold acclimation induced proteins, and negating needle abscission can be expected.
2.2.2 Chemical signaling
ABA is well known as a signal for several abiotic stresses especially water deficit (Christmann et al. 2006) and recently have been established to be a broad spectrum signaling hormone for common abiotic stress pathways (Pastori and Foyer 2002) including cold acclimation. A direct relationship between accumulation of ABA and cold temperatures has been
established in many herbaceous and woody plants (Li et al. 2002; 2003). Application of ABA has also been proved to induce synthesis of de novo proteins manifesting cold acclimation responses (Li et al. 2003). Nevertheless, recent molecular investigations have shown that ABA acts independently, invokes the downstream cold responsive pathways and elicits cold acclimation responses. Traditionally, ABA is suggested to be a long-distance signal molecule that transmits water stress signal from root to shoot and cause closure of stomata. Nevertheless, controversies exist with the amount of xylem sap ABA and the closure of stomata in various plant systems (Munns and Sharp 1993; Wilkinson and Davies 2002). Also, recent experiments conducted with ABA deficient rootstocks of Arabidopsis sp. showed that ABA from roots is not necessary for closure of the stomata and shoot ABA levels were sufficient to invoke stomata closure (Christmann et al. 2006). However, this hypothesis is not yet tested in root detached woody plants. Also, Wilkinson et al. (2001) have demonstrated that ABA is not necessary for closure of stomata in tobacco plants, which were cold tolerant. They concluded that Ca2+ was sufficient to relay the stress signals. Therefore, it appears that the role of ABA in signal transduction of cold acclimation is inconclusive and requires investigation at species level. Furthermore, in a needle retention perspective, regulation of stomatal functions is central in extending the needle life. Owing to the proven upstream role ABA plays in mediating cold acclimation and to its historical role in signaling of water stress, more investigation is needed to understand ABA's role in needle retention especially under root-detached conditions.
2.2.3 Hydraulic, pH and other signaling molecules
The capillary water of the xylem is an essential part of a continuous hydraulic conduit system that connects absorbing surfaces of the roots to evaporating surfaces in the leaves, and therefore, hydraulic forces are rapidly transmitted from roots to shoots (Steudle 2001). Since low
temperature causes freeze-induced water deficit, cold stress is closely similar to moisture stress and the hydraulic principle applies directly to cold acclimation reactions. Many authors have supported this hypothesis (Eamus and Wilson 1983; Lalk and Dôrffling 1985). Interestingly, many of the conifers, which are found to be cold adaptive were also found to be drought tolerant. The mechanism behind the co-existence of these beneficial attributes is still not understood. Recently, Christmann et al. (2006) investigated the stomatal conductance and hydraulic signal transduction in ABA mutants of Arabidopsis as well as in Acer pseudoplatanus and Fagus sylvatica and concluded that hydraulic signals close stomata independent of ABA and these responses are rapid and precede to stomatal closure. They also hypothesize that electric signaling may also be involved in this response. It is now clear that the presence of roots is essential for eliciting hydraulic signaling to modulate stomatal conductance and maintain positive turgor pressure in plant cells. Since balsam fir trees are severed off roots, the changes in hydraulic signals may have a severe impact on water relations and consequently on the needle retention characteristics.
Increase in the pH of xylem sap is associated with several stress responses and is strongly considered as an alternative or additional stress signal. Wilkinson (1999) has shown that gravimetric water deficit and xylem sap pH change had an inverse relationship based on observations from several plant species. He suggested that pH is involved in long-distance signaling of stress responses; pH and ABA act in concert to regulate the stomatal conductance in combating stress stimuli. He hypothesizes that an increase in pH or alkalinization decreases the diffusion of ABA from apoplastic spaces causing stomata to close. Felle et al. (2005) studied pH changes in stomatal spaces of barley plant and found that in response to low temperature the pH is increased by 0.2 to 0.3 units and this alteration disappeared once the low temperature stimulus was removed. However, they argued against involvement of pH signaling from root-to-shoot.
Although evidence exists for moisture stress responses, there is no direct evidence of pH changes
in response to cold acclimation.
Evidence on involvement of root-sourced cytokinins and auxins on growth and
development of plants is substantial. For example, Li et al. (2000) have shown that zeatin-0
glycosyltransferase increases in beans (Phaseolus vulgaris L.) and corn (Zea mays L.) in response
to cold stress. Based on their work and recent evidence it can be assumed that absence of roots
can result in absence of cytokinin levels and thereby potentially result in partial cold acclimation.
The dynamic and interactive role of cytokinin metabolites with ABA and ABA conjugates and
IAA in regulation of the growth and development of Douglas fir at different stages (Kong et al.
2009) provides strong evidence that there is complex cross-talk mechanism between these key
hormones rather than independent routes of signal processing. Also, ABA and cytokinins are
known to increase due to cold acclimation. Based on the fact that ABA and cytokinins have
antagonistic roles in pine seedlings (Jelic and Bogdanovic 1990), their nature of interaction in
mediating cold acclimation becomes very intriguing to explore. Current understanding of
hormone action is based on the assumption that the signaling systems construct a network and
mutually regulate signaling and metabolic systems. Therefore, analysis of ABA, cytokinins and
auxins will provide a more comprehensive understanding on the hormonal changes that occur due
to root severance and/or cold acclimation postharvest. Although several knowledge gaps exist
regarding the cold acclimation of woody species, the proposed research will focus on
environmental and hormonal signals associated with cold acclimation (Figure 2. Schematic
diagram of current knowledge status in reference to relationship between various environmental,
weather and physiological factors and postharvest needle retention in conifers. The question
marks represent the incomplete knowledge status that currently exists with reference to balsam
fir.).
pOWntSf p»3vwa,« Cytokinms ?? Gibberellins 7? Auxins ?? Ethylene??
▼, •
Figure 2. Schematic diagram of current knowledge status in reference to relationship between various environmental, weather and physiological factors and postharvest needle retention in conifers. The question marks represent the incomplete knowledge status that currently exists with reference to balsam fir.
3.0 HYPOTHESES AND OBJECTIVES
(i) Low temperature influences the postharvest NAS
To examine the effects of different temperature and duration treatments; and the role of roots on postharvest needle retention.
To identify and characterize the endogenous hormonal status and NAS responses of balsam fir exposed to low temperature.
(ii) Genotypic and seasonal conditions influence endogenous hormones resulting in NAS changes
To determine the relationship between endogenous hormone(s) and environmental factors on postharvest NAS in selected genotypes.
(iii) Environmental conditions modulates postharvest NAS induced by low temperature To determine the nature and the effects of hydration and PPFD during low temperature on the postharvest NAS.
To uncover the effect of postharvest vapor pressure deficit on low temperature-induced changes in NAS.
(iv) ABA has a physiological role in modulating the postharvest NAS To understand the role and effects of ABA on postharvest NAS.
4.0 GENERAL METHODOLOGY
4.1 Sample collection and postharvest protocol
Balsam fir branches were obtained from the Tree Breeding Centre, Department of Natural
Resources located in Debert, NS, Canada (45° 25' N, -63° 28' W) at an elevation of 37.5 m
above sea level. The site is composed of imperfectly drained gleyed humo-ferric podzols and
gleyed ortatein or fragic humo-ferric podzols with gentle slopes. The stand consisted of 21 rows,
each with 75 trees. Each row was spaced 3 m apart and trees were spaced 2 m apart within a row.
Seedling populations were collected from a nursery located in Debert, NS (45° 25' N,-63° 28' W).
This nursery raised seedlings from the seed material that originated from various tree nurseries
that are located in the provinces of Nova Scotia and New Brunswick and thus represented a
diversified seedling population.
Branches (~0.50 to 0.75 cm in diameter) were always collected at an elevation of 1 m
above the ground level and branches that exhibited signs of insect damage, disease infestation or
abnormal qualities were avoided. Once harvested, the branches were transported to the laboratory
within 1 to 2 h. Sterile distilled water was used to keep the branches hydrated while
transportation. A fresh cut (at least 2.5 cm from the bottom) was given to the branches with an
aseptic scalpel under water prior to experimentation to avoid blockage and/or development of
embolism. The specific protocols on the treatments are provided in the corresponding
methodology sections.
The branches or seedlings were placed in an amber colored 150 mL bottles containing 100
mL of sterile double distilled water. The dark color of the bottles arrested the penetration of light
that promote the growth of algae and sterile double distilled water provided a microbe and
ion-free growth medium for branches. The collar region of the branches was secured with inch wide
polycotton wool at the bottle mouth. This setup was used to avoid any direct transpiration losses that might occur from the bottle and also provided support to the branches.
4.2 Physiological parameters
4.2.1 Needle retention duration, needle abscission and senescence, percent needle loss and percent biomass change
The postharvest senescence is predominantly manifested either through abscission in clonal branches or through discoloration in seedlings. Nevertheless, occurrences of both these processes in either seedlings or branches are not uncommon. Therefore, two different parameters were used to evaluate the postharvest quality of the experimental material. Every two days, the mass of the fallen needles and degree of discoloration were recorded. Ten needles randomly selected from the branches were used to measure the discoloration. To avoid human errors, a SPAD 502 (Soil Plant and Analysis Development) meter was used to measure the degree of discoloration (Martinez and Guiamet 2004). This instrument measures the reflectance of light and estimates the relative chlorophyll a and b content of the needles. SPAD values less than 10 were normally discolored (brownish) and a value around 50 was dark green. In balsam fir, needles typically account for 50-60% of the branch fresh weight (MacDonald and Lada 2008). Therefore, the period until the branches lose 80-100% of their needles was referred to as needle retention duration (NRD). Similarly, the period when the needles discolor with or without exhibiting abscission was referred to as needle abscission and senescence (NAS). NAS was used to measure postharvest quality in seedlings as the discoloration was the dominant senescence phenomenon.
At the end of the experimental period, a "run-through-fingers" procedure was performed to ensure a complete detachment of loosely held needles. To perform the "run-through-fingers" test, each branch was gently passed three times through the index and middle fingers and the mass of the fallen needles was added to the respective needle fall data induced during the
experimental period to arrive at the total needle mass lost and expressed as mg g"1 of the initial fresh weight. Percent needle loss (PNL), the biomass (g) of the needles that fell on each day was recorded and expressed cumulatively as a percent of biomass lost from the initial fresh weight (FW), Percent biomass loss (PBL), the change in the fresh biomass of the branches was calculated as the difference between the initial and final biomass and expressed in percent change.
4.2.2 Average daily water use and cumulative water consumption
The average daily water use (ADWU) (mL g"1 day"1) of the branches was measured through the changes in the mass of the whole assembly over a unit period of time adjusted for weight losses due to abscission.
ADWU = ((Initial mass of the assembly - Final mass of the assembly + Needle abscission mass) /Time) / Initial fresh weight of branch.
The cumulative water consumption (CWC) (mL g"1) is the cumulative values of the ADWU over a specific period of time.
4.2.3 Xylem pressure potential
The xylem pressure potential (XPP) of the branches was measured using a Plant Moisture System Pressure Bomb (PMS Instrument Co., Corvallis, OR, USA). Mounting the seedlings upside down inside a pressure chamber, the pressure inside the chamber was increased slowly at a rate of 0.1 MPa per minute. The exposed cut ends were monitored for the appearance of water. The minimum pressure required to release water bubbles was recorded as the XPP in MPa.
4.2.4 Membrane injury index
Membrane injury index which measures the electrolyte leakage of the cell membranes as
described by Rajasekaran and Blake (1999) was performed on 0.1 g of needles. Needles from each
branch were soaked in 25 mL of distilled water overnight at 20°C. The electrolytes that were
leaked by the samples were measured for their electrical conductivities (EC) using a conductivity
meter (Con 5 series, Oakton, Singapore) to establish the baseline EC. The tubes were then placed
in a heat air oven for 3 h maintained at 80°C to enforce complete lyses of cell tissues. The solutes
were measured for their electrical conductivities (EC
a). Membrane Injury Index (Mil) was
calculated by using the following formula:
MI-
(EC
a-EC
EC,
xlOO
4.2.5 Relative water content
For determination of relative water content (RWC), 0.05 g of needles were removed and
immediately weighed for fresh weight (FW). Needle RWC was determined as follows:
RWC = [(fresh weight - dry weight) / (turgid weight - dry weight)]* 100
Turgid weight was determined by placing needles in distilled water for at least 3 hours. Dry
weight was obtained after placing the samples in an oven at 70°C for 48 h.
4.2.6 Stem capacitance
The stem capacitance (SC) was measured using the BK Precision 830C® capacitance meter
(B&K Maxtec Intl. Corp., Chicago, IL, USA) and expressed in pF (pico Farad). The unit was
custom fitted with sharp metal electrodes to ensure a consistent capacitance measurement at a 50
mm width. The electrodes were gently pushed into the stem (depth of 5 mm and consistently at 5
cm above the cut end) before the measurements were made. An average of three readings per
sample was taken to arrive at the SC for each branch. The SC is directly proportional to the
moisture content and ions present within the measured column of the stem and therefore, low SC
readings (<5 pF) indicate a poor moisture status and vice versa.
4.2.7 Controlled growth chamber conditions
The postharvest needle abscission was observed in a controlled environment chamber
where a temperature of 22°C, a photosynthetically active photon flux density (PPFD) of 100
umol m"
2s"
1and a relative humidity of 40% were maintained. The light source was provided by a
combination of fluorescent and incandescent bulbs with a 17 h of daylight. These environmental
conditions were chosen to mimic a typical household condition.
4.2.8 Imposition of low temperature treatment
(Wt.
The low temperature treatments (LT) were always imposed inside a Sanyo Phytotron
chamber which is capable of controlling the internal temperature, light intensity and relative
humidity at desired rate and levels. Initially the temperature was set to 22°C and the experimental
units were left at this temperature for about 1 hour. Later, the temperature inside the chamber was
gradually decreased from 22°C to 5°C in steps of 2°C h"
1. Once the internal temperature reached
5°C it was maintained for 48 h. This duration of acclimation was chosen from previous studies
conducted by Thiagarajan and Lada (2012). The light intensity and relative humidity were
maintained at 40 umol m'
2s"
1and 95%, respectively, in LT.
5.0 L O W TEMPERATURE INFLUENCES POSTHARVEST NEEDLE
ABCISSION AND SENESCENCE
5.1 The effects of different temperature and durations; and the role of roots on postharvest
needle retention.
The following poster and peer-reviewed article have been produced from this section
Arumugam Thiagarajan and Rajasekaran Lada. 2011. Cold acclimation influence
postharvest needle retention in root detached balsam fir. International Horticultural
Congress, Portugal, Lisbon, August 22-27 (Poster presentation)
Arumugam Thiagarajan and Rajasekaran Lada. 2012. Cold acclimation influence
postharvest needle retention in root detached balsam fir [Abies balsamea L.]. Acta
Horticulturae [In Press]
5.1.1 Résumé
Cette étude a examiné l'influence de différents traitements à basses températures sur la rétention
post-récolte des aiguilles chez le sapin baumier (Abies balsamea L.) et exploré le rôle
physiologique des racines et des basses températures. Lors d'un essai sous environnement
contrôlé, des semis âgés de 18 mois avec ou sans système racinaire ont été soumis à une gamme
de températures (-20°C à 20°C) et différents temps d'exposition, puis les réponses physiologiques
furent mesurées. L'acclimatation au froid des semis avec racines a diminué la chute des aiguilles
de 36%, les pertes de masse fraîche de 76% et la consommation en eau de 45% comparativement
aux semis endurcis au froid sans racines. Une température de 5°C a mené à l'utilisation de l'eau
quotidienne moyenne (0,16 mL g"
1jour"
1) et la chute d'aiguilles (7,1 mg g"
1) les plus faibles, ainsi
qu'à un gain de poids (4%) comparativement aux températures -20°, -5°, 0° ou 20°C. Une
température d'acclimatation de -20°C a réduit le contenu foliaire en chlorophylle de 9 fois par
rapport à 5°C. Le potentiel hydrique du xylème ne fut pas relié à la chute des aiguilles. Nous
supposons que les racines exercent un rôle clé dans l'acclimatation au froid et la rétention des
aiguilles en augmentant la stabilité des membranes cellulaires, préservant les fonctions
stomatiques et maintenant le contenu en chlorophylle par le biais de signaux inconnus à ce jour.
5.1.2 Abstract
This study investigated the influence of low temperature treatments on post-harvest needle
retention in balsam fir (Abies balsamea L.) and explored certain physiological roles of roots and
low temperature. In a controlled environment study, 18 month old seedlings with either detached
or intact roots were exposed to a range of temperatures (-20 to 20°C) for different durations and
selected physiological responses were measured. Exposure of seedlings to 5°C with intact roots
resulted in 36% less needle losses, 76% lower fresh weight losses and 45% less water
consumption when compared to those exposed to those with no roots. A temperature of 5°C
registered the lowest average daily water use of 0.16 mL g"
1day"
1, lowest needle loss of 7.1 mg
g"
1and a positive weight gain (4%) when compared to -20, -5, 0 or 20°C. Exposure to a
temperature of -20°C reduced needle chlorophyll index (SPAD values) by 9 fold when compared
to the 5°C. Xylem pressure potential did not relate to needle loss. We hypothesize that roots play
a key role in cold acclimation and needle retention by increasing membrane stability, preserving
stomatal functions and maintaining chlorophyll content through yet unknown signals.
5.1.3 Introduction
Cold acclimation invoked by low temperatures and shorter photoperiods has been
established to be beneficial towards extreme environments (Welling et al. 2002). There is indirect
evidence of benefit from cold acclimation on needle retention in root-intact conifers growing in
forests or woodlots. For instance, trees that are not adequately frost hardened have been found to
shed needles swiftly, and dry faster following harvest (Hinesley 1984). Also, low temperatures
prevailing at higher latitudes have prolonged the needle life of root-intact Pinus sylvestris (Reich
et al. 1996) and Pinus tabulaeformis (Xiao 2003) implying that low temperature indirectly favors
needle retention. Thus far, the key physiological events governed by cold acclimation are
identified as follows: reduction in stomatal conductance, increasing stability of plasma membrane
and down-regulation of photosynthesis (Chinnusamy et al. 2006; Welling et al. 2002; Welling
and Palva 2006; Xin and Browse 2000). Long distance signaling factors transported from roots
have been implicated in triggering a cascade of reactions promoting cold acclimation (Chen and
Gusta 1983; Mantyla et al. 1995). Until now, cold acclimation has been largely investigated in
root-intact systems. Understanding the physiological role of roots during cold acclimation is
central to enhance post-harvest quality of Christmas trees. There is, however, little or no
information on cold acclimation in root-detached (RD) balsam fir or the role of roots in needle
retention. Accordingly, this study was conducted with the following objectives: (i) to establish
the nature of the relationship between low temperature (pre-treatment) and role of roots in
post-harvest needle retention, (ii) to identify the optimum temperature and duration that improves
post-harvest needle retention and (iii) to elucidate the effect of low temperature on post-harvest
water use, chlorophyll content, and membrane stability.
5.1.4 Materials and Methods
*r\A