Université de Montréal
When pain offset induces pleasure: A psychophysical and fMRI study
Par Nathalie Bitar
Faculté de Médecine,
Programme de maîtrise en Sciences Biomédicales, Université de Montréal
Mémoire présenté
en vue de l’obtention de grade de Maîtrise (M.Sc) en Sciences Biomédicales
option Sciences psychiatriques
Université de Montréal
Faculté de Médecine, programme de maîtrise en Sciences Biomédicales
Ce mémoire intitulé
When pain offset induces pleasure: A psychophysical and fMRI study
Présenté par Nathalie Bitar
A été évalué par un jury composé des personnes suivantes
Pierre-Paul Rompré (Président-rapporteur) Stéphane Potvin (Directeur de recherche) Guillaume Léonard (Membre du jury)
Résumé
Introduction: Une stimulation nociceptive localisée peut produire une analgésie diffuse par un mécanisme endogène inhibiteur de la douleur (MEID). Des stimuli plaisants (e.g. musique) ainsi que le plaisir induit par l’interruption de la douleur peuvent également induire une analgésie. Pour cette raison, il est possible que l’analgésie causée par le plaisir (induite par l’arrêt de la douleur) soit un effet confondant dans le MEID. Objectifs: 1) Examiner la possibilité d’une relation entre le plaisir induit par l’arrêt de la douleur et le MEID et 2) Étudier l’interaction entre le plaisir et la douleur en examinant les activations/désactivations cérébrales pendant une stimulation nociceptive. Méthodologie: Étude 1) Le MEID a été mesuré (N=27) en administrant une chaleur nociceptive (thermode) avant et après le test de l’eau froide. Après une pause de 30 minutes, le test de l’eau froide a été réadministré pour mesurer le niveau de plaisir (0-100) induit par l’arrêt de la douleur (mesuré pendant 4 minutes). Étude 2) Un stimulus nociceptif (gel froid) a été administré (N=26) pendant une session d’IRMf. Résultats: Étude 1) L’arrêt du test de l’eau froide a induit une hypoalgésie avoisinant les 40%. Le MEID et le plaisir induit par l’interruption de la douleur n’étaient pas corrélés. Étude 2) Comparativement au stimulus neutre, le gel froid a induit une activation significative des régions de douleur (e.g. insula, precuneus) et une désactivation significative dans le gyrus frontal orbital moyen. Discussion: La désactivation du gyrus frontal orbital moyen illustre le débalancement de l’homéostasie pendant la stimulation douloureuse, qui est ensuite rétablit par l’augmentation du plaisir, suite à l’interruption de la douleur (effet compensatoire entre la douleur et le plaisir).
Abstract
Background: A localized painful stimulation can produce diffused analgesia through the inhibitory conditioned pain modulation system (ICPM). Analgesia can also be induced by pleasant stimuli (e.g. music) or by the interruption of a painful stimuli (pleasant pain relief). Because pleasure has analgesic benefits, the effect of pleasant pain relief could be a confounding factor in ICPM. Furthermore, pain offset induces activations in reward regions, though results showing the deactivation of reward regions during pain onset have been inconsistent. Objectives: 1) investigate the possible relationship between pleasant pain relief and ICPM using psychophysical measures and 2) investigate cerebral activations/deactivations during pain onset. This will allow a better comprehension of the pain/reward interaction. Methodology: In study 1, ICPM was measured (N=27) by administering noxious heat (thermode) before and after the cold pressor test (CPT). After a 30 minutes break, the CPT was re-administered to measure pleasant pain relief (0-100) for 4 minutes. In study 2, a modified CPT (gel) was administrated (N=26) during an fMRI session to investigate cerebral activations/deactivations during pain onset. Results: In study 1, interruption of the CPT induced a mean pleasant pain relief of almost 40%. ICPM and pleasant pain relief did not correlate. In study 2, we found significant activations in the insula, the precuneus and the middle frontal gyrus and a significant deactivation in the medial orbital frontal gyrus during pain onset, when compared to the neutral stimulus. Discussion: Deactivation of reward regions illustrates the disruption in homeostasis caused by pain onset, which is later reinstated during pain offset (pleasant pain relief), therefore showing a compensatory effect. This allowed an enhanced comprehension of the opponent process theory.
Table of Contents Chapter 1. Introduction ... 1 1.1 Problem ... 1 1.2 Types of Pain ... 3 1.2.1 Nociceptive pain ... 3 1.2.2 Neuropathic pain ... 4 1.2.3 Functional pain ... 5 1.3 Components of pain ... 5 1.3.1 ... 6 Sensory ... 6 1.3.2 Affective ... 7 1.3.3 Cognition ... 7 1.4 Pain Perception ... 9 1.4.1 Peripheral fibres ... 9 1.4.2 Ascending tracts ... 10 1.4.3 Sensory processing ... 11 1.4.4 Emotional Processing ... 13 1.4.5 Cognitive processing ... 15
1.5 Endogenous pain modulation system ... 17
1.5.1 The excitatory mechanisms ... 17
1.5.2 Inhibitory mechanisms ... 18
1.6 Pleasant pain relief ... 21
1.7 Objectives ... 25
Chapter 2. Article published in Pain Research and Management ... 28
Chapter 3: Methodology for study 2 ... 63
3.1 Participants ... 63 3.2 Clinical assessment ... 63 3.3 Stimulus ... 65 3.4 Experimental design ... 66 3.4.1 Stimulus presentation ... 66 3.4.2 Subjective measurements ... 67
3.5 MRI acquisitions parameters ... 68
3.6 Processing of fMRI images ... 69
3.7 Statistical analysis ... 70
3.7.1 Psychophysical data ... 70
3.7.2 fMRI analysis ... 71
Chapter 4: Results of study 2 ... 71
4.1 Demographic results ... 71
4.2 Psychophysical session ... 72
4.2.1 ... 72
4.2.3 Test-retest reliability ... 73
4.2.4 Correlations between pain perception and pleasant pain relief taken during the psychophysical session ... 74
4.2.5 Correlations between subclinical psychological symptoms and psychophysical measures taken during the psychophysical session ... 74
4.3 fMRI session ... 74
4.3.1 Pain perception of the modified cold pressor test during the fMRI session ... 74
4.3.2 Pleasant pain relief ... 75
4.3.3 Correlations between pain intensity and pleasant pain relief taken during the fMRI session ... 75
4.3.4 ... 76
Correlations between psychophysical measures taken during the fMRI session and subclinical psychological symptoms ... 76
4.3.5 fMRI BOLD activation ... 76
4.4 Correlational analyses with significant brain activations ... 78
4.4.1 Correlation between psychophysical results and beta values ... 78
4.4.2 Correlations between subclinical psychological symptoms and beta values ... 79
Chapter 5. Discussion ... 81
5.1 Study 1 ... 83
5.2 Study 2 ... 85
5.2.1 Psychophysical results ... 86
5.2.2 fMRI results ... 87
5.3 Theoretical and methodological implications ... 89
5.3.1 Theoretical implications ... 89 5.3.2 Methodological implications ... 91 5.4 Limitations ... 92 5.4.1 Participants ... 92 5.4.2 Stimuli ... 93 5.4.3 Correlational analysis ... 94
5.5 Recommendation for future studies ... 94
List of tables
Study1. Accepted by Pain Research and Management
Table 1. Characteristics of the participants ... 58 Study 2
Table 1. Participant characteristics ... 71 Table 2. Activation clusters ... 76
List of figures
Study 1 accepted by Pain research and Management
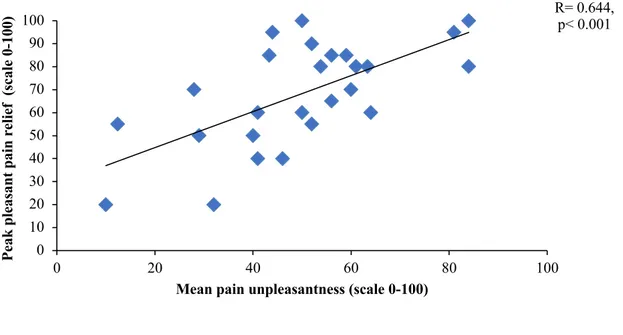
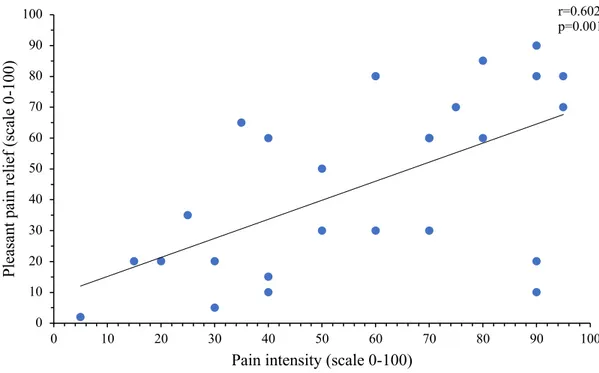
Figure 1. Inhibitory conditioned pain modulation ... 59 Figure 2. Perception of PRP during 240 seconds ... 60 Figure 3. Correlation between pain intensity during the cold pressor test and mean pleasant pain relief ... 61 Figure 4. Correlation between pain unpleasantness during the cold pressor test and peak pleasant pain relief ... 62 Study 2
Figure 1. Ascending pain and pain modulation pathways ... 11 Figure 2. Stimulus presentation ... 68 Figure 3. Correlation between pain intensity and pleasant pain relief during the modified CPT in the fMRI session ... 75 Figure 4. Cluster activations ... 77 Figure 5. Mean beta ... 78 Figure 6 a. Correlation between the average beta value of the medial orbital frontal gyrus with the BPI questionnaire (pain interference subscale) ... 80 Figure 6 b. Correlation between the average beta value of the precuneus with the BPI questionnaire (pain interference subscale) ... 80 Figure 7. Graphical representation of the opponent process theory ... 91 Figure 8 a. Activations in the thalamus and the primary somatosensory cortex during pain onset ... 91 Figure 8 b. Deactivation in the nucleus accumbens during pain onset ... 91 Figure 8 c. Activation in the nucleus accumbens during pain offset ... 91
List of abbreviations
ACC = Anterior cingulate cortex
aMCC = Anterior middle cingulate cortex BDI = Beck depression inventory (BDI) BPI = Brief Pain Inventory
BOLD = Blood oxygenated level dependent CNS = Central nervous system
CPT = Cold pressor test
DLPFC = Dorsal lateral prefrontal cortex
fMRI = Functional magnetic resonance imaging GLM = general linear model
ICPM = Inhibitory conditioned pain modulation IAPS = International Association for the study of Pain MCC = Middle cingulate cortex
MFG= Middle frontal gyrus NAc = Nucleus accumbens NRM = Nucleus raphe magnus
pACC = Pregenual anterior cingulate cortex PAG = periaqueductal grey
PCC = Posterior cingulate cortex
pMCC = Posterior middle cingulate cortex PRP = Pleasant relief of pain
RVM = Rostral ventral medulla
sACC = Subgenual anterior cingulate cortex SEM = Standard error of the mean
SHIPS = Snaith-Hamilton Pleasure Scale
STAI-S = State and Trait Anxiety Inventor-State subscale SI = Primary somatosensory
Acknowledgments
Accomplishing this memoir would not have been possible without a few people, to whom I wish to thank.
I would like to thank Dr Stéphane Potvin for giving me the opportunity to learn and grown in his lab, for believing in me and for his consistent support and mentoring throughout my masters.
To my lab mates, Andras Tikasz, Jules Dugré and Laura Dellazizzo, I consider myself extremely fortunate to have worked in a lab with such exceptional students. Thank you for taking the time to share all your knowledge with me, for all the emotional support you gave me and a special thank you to Laura for always baking deserts for us, the lab would not be the same without you.
I would also like to thank my friends; Phillippe Desmarais, Felix Gilmore, Danika Laramée, Roxane Grégoire and Lauriane Guillot; thank you for all the advice, the constant support and for always reminding me of what I am capable of accomplishing. I am forever grateful to have such amazing friends.
Finally, and most importantly, thank you to my amazing family. You showed me love and support like no other. Thank you for understanding my passion, allowing me to pursue it far from home, motivating me and encouraging me to never give up and, of course, for helping me move three times. I am truly blessed to have all of you.
Chapter 1. Introduction 1.1 Problem
Pain is considered a vital component for survival as it allows us to be aware of possible tissue damage in our body (Garland & Ph, 2013). In fact, pain is generally viewed as an unpleasant experience, both emotionally and physically, that humans tend to avoid. In turn, it is the main reason people seek medical attention (Shi, Langer, Cohen, & Cleeland, 2007). In accordance with the International Association for the study of Pain (IASP), pain is currently defined as a stressful and unpleasant sensory and emotional experience, that involves either potential or actual tissue damage. The main components of pain are sensory, affective and cognitive (Williams & Craig, 2016).
Pain may be beneficial when it serves for awareness and survival, but it becomes problematic when it is persistent and unrelieved, such as in chronic pain states (Fenton, Shih, & Zolton, 2015). Chronic pain is defined as pain that persists for longer than 6 months (Cheng & Rosenquist, 2018). Three main factors have been identified in playing a role in the development of chronic pain: (i) environmental factors (e.g. family abuse, history of pain), (ii) psychological factors (e.g. depression, anxiety) and (iii) individual predispositions (e.g. gender, age) (Marchand, 2008). Chronic pain affects 20% of people worldwide and has an estimated prevalence of 29% amongst Canadians over the age of 18 (Bonakdar, 2017; Cheng & Rosenquist, 2018; Tracey & Mantyh, 2007; Velly & Mohit, 2018). Furthermore, the total cost of pain treatment is estimated at 16,636 CAD per patient (Lalonde et al., 2014). Chronic pain causes therefore a major social and economic burden to both patients and society.
2018; Sheng, Liu, Wang, Cui, & Zhang, 2017). Also, patients with chronic pain are 4 times more likely to have depression or anxiety when compared to non-chronic pain sufferers (Velly & Mohit, 2018). Because the cause of chronic pain remains unknown in many clinical cases, it is often accompanied by feelings of anger, helplessness and hopelessness (Tang & Crane, 2006). Consequently, the lifetime prevalence of suicidal ideation is common amongst these patients (Velly & Mohit, 2018).
Chronic pain also has physical consequences. Such consequences include muscles tension, difficulty in walking, loss of appetite, lack of energy and lack of sleep, which often all lead to the inability to work (Cheng & Rosenquist, 2018). A review composed of 43 studies on the functional consequences of pain over different chronic pain conditions, such as arthritis and fibromyalgia, has shown that 13 to 76% of chronic pain patients face loss of employment (Moore, Derry, Taylor, Straube, & Phillips, 2014).
Finally, patients with chronic pain tend to have a reduced quality of life (Abu Bakar et al., 2016; Lamé, Peters, Vlaeyen, Kleef, & Patijn, 2005). Quality of life questionnaires usually measure the impact of the illness on the emotional, social and physical functioning of the patient (Abu Bakar et al., 2016). Vast clinical observations show that chronic pain has major impacts on these three qualities of life domains (Lamé et al., 2005). Certain characteristics, such as pain intensity, pain frequency, duration of pain and the presence of other symptoms (e.g. nausea), are important predictors for reduced quality of life (Abu Bakar et al., 2016).
In brief, patients with chronic pain face psychological and physical consequences which may lead to disabilities in work, household and social functioning (Abu Bakar et al., 2016).
1.2 Types of Pain
One important step in diagnosis is identifying the type of pain the patient is experiencing. The three main categories of pain are nociceptive, neuropathic and functional pain (Marchand, 2008).
1.2.1 Nociceptive pain
Firstly, nociceptive pain is one of the most common types of pain and is caused by the activation of pain receptors (nociceptors) (Cervero, 1999; Steeds, 2009). Indeed, nociceptive pain can have a protective role in the human body as it can be triggered when potentially harmful stimuli are detected by nociceptors (Marchand, 2008). Nociceptive stimuli can be mechanical, thermal or chemical and can be detected in various parts of the body, such as skin, muscles, bones or internal organs (C. J Woolf, 1995). The typical description for this type of pain is aching or throbbing, which tends to worsen when a patient moves or coughs (Cheng & Rosenquist, 2018).
Nociceptive pain is divided in three subcategories: somatic, visceral and inflammatory (Cheng & Rosenquist, 2018; Marchand, 2008). 1- Somatic pain can be either superficial (at the surface of the skin) or deep (e.g. muscle pain). These types of pain can be caused by lacerations or fractures (e.g. surgical wound or broken bone) (Marchand, 2008). 2- Visceral pain is related to pain that is located on the viscera (e.g. gallbladder, appendix or heart). One important characteristic of visceral pain is that it is frequently irradiated pain. In simpler terms, visceral pain is often perceived in a different area than the actual damaged tissue, that is, it is poorly located and diffuse (Sikandar & Dickenson, 2012). A common example consists of the symptoms of a heart attack, where pain is diffused in the left arm and neck (Cheng & Rosenquist,
2018). 3- Inflammatory pain is a phenomenon associated with the healing process of injured tissues and is characterized by hypersensitivity to the injured area (Marchand, 2008). The inflammatory response can cause a heightened perception of pain to nociceptive stimuli (hyperalgesia). The inflammatory response includes swelling and redness on the injured area (Gyurkovska et al., 2011).
1.2.2 Neuropathic pain
Neuropathic pain refers to pain caused by a lesion or a disease affecting the peripheral nervous system (e.g. diabetes) or the central nervous system (e.g. brain trauma from tumours or strokes) (Cheng & Rosenquist, 2018). Neuropathic pain can be both spontaneous (not elicited by a stimulus) or non-spontaneous (elicited by a stimulus) (Cruccu & Truini, 2009). The latter is triggered by mechanical, thermal or chemical stimuli (Marchand, 2008). Damages to the nervous system can cause hyperalgesia and allodynia (pain sensitivity to non-nociceptive stimuli) (Borzan & Meyer, 2009). Some clinical characteristics that arise from neuropathic pain are burning pain, shooting pain, sensory deficit or pain to a light touch to the skin (Borzan & Meyer, 2009).
Neuropathic pain can be assessed clinically based on symptomatology and physical characteristics (Borzan & Meyer, 2009). Hence, the presence of characteristics such a burning, painful cold and electric shock accompanying the pain and the presence of symptoms such as tingling, pins and needles, numbness and itching in the same area as the arising pain are indicative of neuropathy (Borzan & Meyer, 2009). Finally, using touch and pinpricks, hypoesthesia (diminished sensitivity to stimuli) can be detected and allodynia can be detected using a light touch to the skin (e.g. brushing) (Cruccu & Truini, 2009).
1.2.3 Functional pain
The last category of pain is functional pain. This category classifies pain that has no known medical cause (Marchand, 2008). Indeed, when physicians are unable to identify a disease based on the group of symptoms given to them by the patient, the pain experienced is classified as functional pain (Schechter, 2014). Some disorders that fall into this category are fibromyalgia and irritable bowel syndrome (IBS) (Marchand, 2008). Fibromyalgia is characterized by chronic widespread pain and includes several symptoms such as fatigue, decreased physical functioning and tenderness (Wolfe et al., 2016). A diagnosis of fibromyalgia requires chronic pain to be present in at least 4-5 different regions (e.g. left arm, right arm, lower back, left hip, right hip and abdomen) and for the symptoms to be present for at least three months (Wolfe et al., 2016). The pain experienced can be of aching or cramping nature (e.g. headaches or stomach cramps). IBS is characterized by abdominal pain that can be tolerable to severe (El-Salhy, 2012). Symptoms include constipation or diarrhea, and in some cases, a combination of both. IBS diagnostic requires abdominal pain to be present for at least 6 months and the presence of symptoms such as abnormal stool frequency, abnormal stool shape and bloating (El-Salhy, 2012).
1.3 Components of pain
Pain is a multifaceted phenomenon and factors such as context, cognition, mood and attention, all have an influence on pain perception (Tracey & Mantyh, 2007; Tracey et al., 2002; Wiech, Ploner, & Tracey, 2008). Even when nociceptive stimuli are the same, patients may perceive pain differently due to these factors (M. P. Jensen et al., 2006). As previously
mentioned, pain perception is comprised of three main components; sensory, affective and cognitive (Williams & Craig, 2016).
1.3.1 Sensory
The first component of pain that will be discussed is the sensory-discriminative component. This component refers to the patient’s ability to describe the intensity (mild to severe), the texture, the duration (for how long the pain has been ongoing) and the spatial characteristics of the pain (location) (Marchand, 2008). The latter two characteristics can be crucial in identifying the medical problem (M. E. Mendoza, Gertz, & Jensen, 2014). Localization is generally facilitated by asking the patient to perform different movements or by locating any tender areas that may increase pain perception. On the other hand, temporal characteristics allow the differentiation between acute, chronic, variable (pain always present but at different intensities) or intermittent pain (pain comes and goes) (M. E. Mendoza et al., 2014). Patients may use terms such as throbbing, aching, cramping or shooting to describe their experience (M. P. Jensen et al., 2006).
Pain can also be induced in experimental settings. Mechanical, thermal or chemical stimuli can be used to induce moderate pain to patients, who in turn are questioned about pain intensity (Marchand, 2008). These experiments can allow the identification of different sensory deficits such as hypoalgesia or hyperalgesia. Although the sensory-discriminative component allows patients to describe the pain, emotional components are important to take under consideration, as they may increase or decrease pain perception (Marchand, 2008).
1.3.2 Affective
In experimental and clinical settings, the affective component of pain is often referred to as pain unpleasantness (Leknes, Brooks, Wiech, & Tracey, 2008; Marchand, 2008). Emotional factors influencing pain perception include positive (e.g. happy) and negative (e.g. anxiety and depression) emotional states (M. E. Mendoza et al., 2014). Studies have shown that emotional factors alone can affect pain sensitivity and pain unpleasantness (Wiech & Tracey, 2009). However, positive and negative emotions tend to have a greater impact on the affective component of pain (pain unpleasantness) than on the sensory component of pain (pain intensity) (Villemure & Bushnell, 2002). Factors such as pleasant odours, music and emotionally pleasant pictures have been used to increase positive mood and thus decrease pain perception (Leknes & Tracey, 2008; Villemure & Bushnell, 2002). Conversely, factors causing a negative mood (e.g. viewing of negative images) has been shown to increase pain sensitivity and pain unpleasantness (Meagher, Arnau, & Rhudy, 2001; Villemure & Bushnell, 2002). Likewise, increased anxiety has also been found to cause increased pain intensity and pain unpleasantness (Ploghaus et al., 2001). Equally, treating anxiety has shown to reduce both pain and the need for analgesic medication (Hansen & Streltzer, 2005).
1.3.3 Cognition
Cognitive factors such as pain anticipation, pain catastrophizing, pain distraction and pain relief expectations, may modulate our painful experience by increasing or decreasing pain perception (Seminowicz & Davis, 2007). Firstly, pain anticipation has shown to cause increased pain perception (Fairhurst, Wiech, Dunckley, & Tracey, 2007). For instance, in a typical experimental procedure, participants received a warning cue before the application of the
noxious stimulus. This experiment showed a significative positive correlation between the level of pain anticipated during the anticipation cue and pain intensity during nociceptive stimulation (r=0.62, p=0.02) (Fairhurst et al., 2007). Likewise, several studies have observed a positive relationship between high levels of pain catastrophizing and increased pain intensity (Edwards, Bingham, Bathon, & Haythornthwaite, 2006; Tracey & Mantyh, 2007). Pain catastrophizing has been defined as an exaggerated perception of pain (threat, value or seriousness) that an individual attributes to their painful experience (Tracey & Mantyh, 2007). Furthermore, attention and distraction have opposite effects on pain perception. Although focused attention on pain has been shown to increase pain perception, pain distraction has been shown to be very useful in decreasing pain perception during painful procedures (Hansen & Streltzer, 2005; Palermo, Benedetti, Costa, & Amanzio, 2015). In fact, burn victims who were distracted using virtual reality reported significantly less pain during their treatment compared to patients receiving no distraction with their treatment (Hoffman, Patterson, Carrougher, & Sharar, 2001). This effect has also been tested in experimental settings. When participants were distracted by focusing their attention on a visual stimulus or a cognitive task (e.g. stroop task), sensory pain ratings were significantly reduced (Kenntner-Mabiala, Weyers, & Pauli, 2007; Moont, Crispel, Lev, Pud, & Yarnitsky, 2012). Finally, according to research on placebo analgesia, participants report reduced pain perception when they are informed a treatment will induce analgesia, even in the absence of actual treatment (Watson et al., 2009).
1.4 Pain Perception
As highlighted beforehand, mood and several cognitive factors can have an influence on the perception of pain (Tracey & Mantyh, 2007). On neurobiological grounds, the first step of pain perception lies in the activation of nociceptors in the periphery (Marchand, 2008).
1.4.1 Nociceptors and peripheral fibres
The trajectory leading to pain perception starts with the activation of the peripheral nociceptors. These nociceptors are free nerve endings of nerve fibres that are activated by nociceptive stimuli (Steeds, 2009). There are three main types of peripheral afferent fibres, Ab, Ad and C fibres (Marchand, 2008). Ab and Ad are both myelinated fibres and C fibres are unmyelinated. Ab fibres participate in the transmission of non-nociceptive signals, such as a light touch or a vibration. Ad and C fibres are the two fibres involved in the transmission of nociceptive signals. These sensory fibres are first order neurones (Kaiser, Haid, Shaffrey, & Fehlings, 2018). Ad fibres react to thermal and mechanical nociceptive information, whereas C fibres are activated by mechanical, thermal and chemical information (Marchand, 2008). Since Ad fibres are myelinated, they are responsible for the first pain response, a fast and sharp and pain sensation. Unmyelinated C fibres, on the other hand, are responsible for the second pain response. They transfer their information at a slower rate and produce the prolonged deep sensation of pain (Fenton et al., 2015; Marchand, 2008). From the periphery, nociceptive signals follow the ascending pathway to the superior centres of the brain (Marchand, 2008).
1.4.2 Ascending tracts
From the periphery to the spinal cold. When potentially harmful stimuli are detected by peripheral nociceptive fibres (Ad and C fibres), these first order neurons will send afferent signals to the spinal cord through the dorsal root ganglion. The first order neurones will then synapse with the second order neurones in the dorsal horn of the spinal cord (Marchand, 2008).
From the spinal cord to the thalamus. From the second order neurons in the spinal cord, the afferent signals will decussate immediately and ascend to the thalamus, where they synapse with the third order neurones (Farmer & Aziz, 2014; Marchand, 2008; Steeds, 2009). Hence, the nociceptive signals project to the thalamus on the contralateral side of the nociceptive simulation (Marchand, 2008).
From the thalamus to the cortex. Located in the centre of the brain, the thalamus is an important relay in pain perception and the gateway to the cortex. Nociceptive signals ascend from the spinal cord to the thalamus through the spinothalamic or the spinoreticular tract (Marchand, 2008). The former projects signals to the lateral thalamus. From there, thalamocortical fibres will ascend information to the primary and secondary somatosensory cortices (Marchand, 2008). This pathway determines the sensory components of pain (e.g. location and duration of pain) (Farmer & Aziz, 2014; Marchand, 2008; Steeds, 2009). The spinoreticular track leads nociceptive information to the medial thalamus (Marchand, 2008). From there, third-order neurones will ascend the information to the anterior cingulate cortex (ACC) and the insula (please see Figure 1). These brain regions determine the affective components of pain (e.g. pain unpleasantness) (Farmer & Aziz, 2014; Marchand, 2008).
Figure 1. Ascending pain and pain modulation pathways Extracted from Marchand (2008)
1.4.3 Sensory processing
The somatosensory cortex. Located posterior to the central sulcus, the somatosensory cortex is divided into the primary somatosensory cortex (SI) and the secondary somatosensory cortex (SII) (Marchand, 2008). The former receives projections from the ventral posterior lateral thalamus (Marchand, 2008). A review of positron emission tomography (PET) studies from Schnitzler & Ploner (2000) revealed that the repeated administration of heat stimuli to the dorsum of the hand and feet induce activations in the SI contralateral to the location stimulated and a somatotopic arrangement of pain in SI, suggesting that SI plays a role in the localization of pain (Schnitzler & Ploner, 2000). The medial part of the somatosensory cortex will receive information from rostral regions of the body such as the face or hands and the lateral regions of the cortex receive input from caudal regions such as the feet (J. C.W. Brooks, Zambreanu,
Godinez, Craig, & Tracey, 2005). Furthermore, a review showed that studies investigating the relationship between pain intensity and cerebral activations found a positive relationship between SI and pain intensity (r=0.69, p<0.005); however, pain unpleasantness did not correlate with SI activations (Coghill, Sang, Maisog, & Iadarola, 1999; Porro, Cettolo, Francescato, & Baraldi, 1998; Schnitzler & Ploner, 2000). Secondly, SII receives projections from the ventral posterior inferior thalamus and plays a role in tactile discrimination, which allows recognition of the type of stimulus (e.g. pressure or temperature), stimulus roughness or stimulus size (J. Brooks & Tracey, 2005; Schnitzler & Ploner, 2000). Taken together, both somatosensory cortices are involved in the sensory discrimination of pain; SI is involved in spatial discrimination and SII in tactile discrimination (Marchand, 2008).
Insula. The insula is located between the frontal and temporal lobe and can be subdivided into the posterior and the anterior insula (Petrovic, Petersson, Hansson, & Ingvar, 2002). While the posterior insula is involved in interoception (one’s own perception of their bodily state), the anterior insula is involved in emotional awareness (Craig, 2009).
The posterior insula allows the processing of thermal and painful stimulation (Craig, 2009). A review by Garcia-Larrea (2012), focusing on the role of the posterior insula in pain paradigms, has reported that patients with lesions to the posterior insula, caused by a stroke, suffered loss of pain and temperature sensations. These findings were also reported in a review by J. Brooks & Tracey (2005). Furthermore, functional neuroimaging meta-analyses have shown that the posterior insula is also involved in the processing of stimulus intensity and location (J. Brooks & Tracey, 2005; K. B. Jensen et al., 2016; Wiech et al., 2010).
1.4.4 Emotional Processing
It has been proposed that three major brain regions are involved in the affective processing of pain; namely the anterior insula, the amygdala and the ACC (Fenton et al., 2015).
Anterior insula. The anterior part of the insula is a limbic structure mainly involved in emotional awareness (Lamm, Decety, & Singer, 2011). A review on the structure and function of the insula by Uddin, Nomi, Herbert-Seropian, Ghaziri, & Boucher (2017) has reported significant activations in the anterior insula in participants viewing images of emotional facial expressions (fear, disgust or happy), compared to neutral facial expressions. In addition, the anterior insula has also shown activations in individuals receiving a noxious stimulus (Fenton et al., 2015). Meta-analyses on functional brain imaging in response to pain have shown consistent activations of the anterior insula during continuous noxious heat stimulation (e.g. thermode) or noxious cold stimulation (e.g. cold water bath) in healthy volunteers (Farrell, Laird, & Egan, 2005; Peyron, Laurent, & Garcia-Larrea, 2000). Notably, experimental studies have shown that viewing pictures of negative emotional faces while concurrently receiving noxious stimulation causes even greater anterior insula activations (Dunckley et al., 2005; Phillips et al., 2003). Furthermore, greater activation in the anterior insula was revealed in participants who were asked to give an affective evaluation of pain, comparatively to participants not attending to pain unpleasantness (Jonathan C.W. Brooks, Nurmikko, Bimson, Singh, & Roberts, 2002; Kong et al., 2006). Finally, a review on studies investigating lesions to the anterior insula has shown that
individuals with lesions in this region have reduced pain affect responses to nociceptive stimuli (Schnitzler & Ploner, 2000). Taken together, these findings strongly support the hypothesis that the anterior insula is involved in the integration of emotional states and interoceptive states
(Craig, 2009; K. B. Jensen et al., 2016; Schnitzler & Ploner, 2000). Thus, the role of the anterior insula has often been linked to the emotional processing of the painful experience (Farrell et al., 2005; Fenton et al., 2015; Peyron et al., 2000).
Amygdala and ACC. Other regions such as the amygdala and the ACC have also shown involvement in the affective processing of pain, although results are not as robust as in the case of the anterior insular (Stevens, Hurley, & Taber, 2009). Firstly, the amygdala is an almond shaped structure located in the temporal lobe and forms part of the limbic system (Carrasquillo & Gereau IV, 2008). This structure is mainly known for its role in emotional processing, such as fear and stress (Carrasquillo & Gereau IV, 2008; Corder et al., 2019). Precisely, by integrating sensory information, the amygdala provides an emotional value to the sensory input, either positive (e.g. happy) or negative (e.g. fear). By using a similar protocol as Jonathan C.W. Brooks et al (2002) and Kong et al (2006) (shown above), Kulkarni et al (2005) observed significant increased activations in the amygdala when participants attended to pain unpleasantness. Thus, the activation of the amygdala during nociceptive stimulation has been linked to the emotional processing of pain.
Lastly, the ACC, wrapped around the corpus collosum, can be subdivided into two; the dorsal ACC and the ventral ACC (Stevens et al., 2009). The ventral ACC, also known as the pregenual ACC, is implicated in the integration of the autonomic system and in the emotional processing of stimuli (Stevens, Hurley, & Taber, 2011; Sturm et al., 2013). In the context of pain, the ventral ACC has been linked to the affective processing of pain (e.g. pain unpleasantness, fear and stress) (Tracey & Mantyh, 2007; Wiech & Tracey, 2009). Indeed, increased pain unpleasantness is correlated with increased activity in the ventral ACC (L.
Becerra, Navratilova, Porreca, & Borsook, 2013; Kulkarni et al., 2005). The dorsal ACC is involved in cognitive control and will be further development in the following section.
In brief, activations in the amygdala and in the ventral ACC has been reported in studies investigating the affective component of pain. However, these findings are not consistent throughout all studies, implying that their role in pain perception may not be fully understood to date.
1.4.5 Cognitive processing
As mentioned earlier, several cognitive processes, such as pain anticipation and placebo analgesia, have an important influence on pain perception (Wiech et al., 2008). Empirically speaking, the prefrontal cortex, the dorsal ACC and the middle cingulate cortex (MCC) are the three brain regions that have the most consistently shown increased activity during cognitive processing (Stevens et al., 2009).
The dorsolateral prefrontal cortex. The prefrontal cortex is essential for decision-making, planning and plays a pivotal role in the cognitive processing of pain (Euston, Gruber, & McNaughton, 2012). In a functional magnetic resonance imaging (fMRI) study on placebo analgesia, subjects participated in two experiments, a first experiment applying shock pain and a second experimental applying thermal pain (Wager et al., 2004). In each experiment, subjects participated in a control trial, where they were told a lotion offered no relief, and in a placebo trial, where they were told a lotion would offer pain relief. Results of this study showed increased activity in the dorsal lateral prefrontal cortex (DLPFC) during pain relief anticipation, compared to the control trial. Moreover, this increase in activation was significantly correlated
with the magnitude of the reduction in reported pain between the control and the placebo trial. These correlations were found in both the shock study (r=0.62, p<0.005); and the thermal study (r=0.60, p<0.005) (Wager et al., 2004). Coherently, increased activity in the DLPFC during placebo analgesia and pain anticipation was also reported in two reviews (Tracey & Mantyh, 2007; Wiech et al., 2008).
Cingulate cortex. The cingulate cortex is thought to contain several specialized subregions which may hold unique functions (Vogt, 2016). The ACC was first discussed in the section above, however the role of the cingulate cortex may be further expanded.
The MCC shares connectivity with the prefrontal cortex and is involved in cognitive functions such as decision-making and cognitive control, and some authors have hypothesized that the MCC plays a key role in cognitive pain modulation (Stevens et al., 2011). In order to identify brain regions implicated in pain anticipation, functional neuroimaging studies performed analyses comparing groups receiving a pre-stimulation cue indicating the level of pain of the stimulus, with a group receiving no pre-stimulation cue (Wiech et al., 2010). Both groups received the same nociceptive stimulus. Results showed increased activity in the MCC when the stimulus was anticipated to be painful. Furthermore, stronger MCC activations also correlated with stronger pain perception (Wiech et al., 2010). Importantly, several meta-analyses have shown activation in the MCC following nociceptive stimulation and following attention and anticipation of pain (Porro, Cettolo, Francescato, & Baraldi, 2003; Wiech et al., 2010). These results indicate the implication of the MCC in the cognitive processing of pain.
The dorsal ACC is adjacent to the MCC (Stevens et al., 2009). Based on the well-known involvement of the dorsal ACC in cognitive control (e.g. ability to flexibly adjust behaviour)
and decision making, some authors have hypothesized that the dorsal ACC may play a key role the cognitive processing of pain (e.g. attention to pain) (Shenhav, Cohen, & Botvinick, 2016; Tracey & Mantyh, 2007; Wiech & Tracey, 2009). Yet, results of fMRI research have been inconsistent thus far, and additional research is needed to fully understand the role of the dorsal ACC in pain modulation.
1.5 Endogenous pain modulation system
Pain perception is a dynamic phenomenon that involves the modulation of nociceptive signals at multiple levels of the CNS (Marchand, 2008). These endogenous pain modulation systems involve either excitatory (increasing the nociceptive response) or inhibitory (inducing analgesia) mechanisms.
1.5.1 The excitatory mechanisms
Central sensitization in the spinal cord. Central sensitization is characterized by an augmented response to nociceptive stimuli (hyperalgesia) or a pain response to non-nociceptive stimuli (allodynia) (Marchand, 2008). At the mechanistic level, a high frequency stimulation of C fibres at the same intensity will trigger a progressive increase of action potential discharge in the spinal cord (Marchand, 2008). The prolonged firing of C fibres will allow the release of glutamate, which will in turn bind to N-methyl-D-aspartate (NMDA) receptors, found in the spinal cord, and will induce spinal sensitization. In humans, this reaction evokes an increase in sensitivity to noxious stimuli (Bennett, 2000; Marchand, 2008; Potvin, Grignon, & Marchand, 2009). In experimental settings, two distinct psychophysical paradigms are used to study central
sensitization, namely temporal summation and spatial summation (Marchand & Arsenault, 2002).
Temporal and spatial summation. Temporal summation is defined as repeated stimulation to the same surface area at the same intensity for a prolonged time. The high frequency of painful stimulation causes a temporal stimulation of the C fibres due to their slow conduction rate, resulting in increased pain perception (pain intensity and pain unpleasantness) (Marchand, 2008). Spatial summation, on the other hand, can be defined as the effect of the size of the surface area stimulated on pain perception (pain intensity, pain unpleasantness and pain threshold) (Marchand & Arsenault, 2002). A larger stimulated area will increase the number of nociceptors recruited, resulting in increased pain perception (Marchand, 2008). However, prolonged spatial stimulation may eventually cause pain inhibition (Marchand & Arsenault, 2002). Consequently, spatial summation paradigms may also be used to study pain inhibitory mechanisms.
1.5.2 Inhibitory mechanisms
The inhibitory conditioned pain modulation system. The inhibitory conditioned pain modulation (ICPM) theory suggests that a localized painful stimulation will cause inhibition of spinal neurons, which in turn will produce diffused analgesia (pain inhibition over the whole body) (Marchand 2008). According to this theory, diffused analgesia will occur when an intense nociceptive stimulus is administered for a prolonged time on a large surface area (e.g. the forearm). This in turn will cause reduced pain perception, a phenomenon known as pain inhibits pain (Potvin et al., 2009). When testing ICPM in healthy subjects, the results generally show an
hypoalgesic effect (Marchand, 2008; Potvin et al., 2009). However, this phenomenon appears to be absent or reduced in many chronic pain patients (Edwards, Ness, Weigent, & Fillingim, 2003; Staud, Robinson, Vierck Jr, & Price, 2003).
The descending pathway. The mechanisms underlying the ICPM phenomenon involve descending pathways at the brainstem level (Marchand, 2008). These mechanisms start a cascade of reactions beginning with the recruitment of endogenous opioids in the periaqueductal grey (PAG) (Steeds, 2009). The PAG is a brainstem structure, located precisely in the midbrain, containing both opioid and cannabinoid receptors (Behbehani, 1995; Steeds, 2009). The stimulation of these receptors will then activate cells in the nucleus raphe magnus (NRM) (Steeds, 2009). The latter is located in the rostral ventral medulla (RVM). When the cells in the NRM are activated, they cause a release of serotonin in the spinal cord, which in turn blocks the transmission of pain signals, causing diffuse analgesia and blocking both hyperalgesia and allodynia effects (Ossipov, Morimura, & Porreca, 2014; Pud, Granovsky, & Yarnitsky, 2009a; Steeds, 2009).
Located in the pons, the locus coeruleus also plays a role in pain inhibition and is comprised of a large population neurones producing noradrenaline (Llorca-Torralba, Borges, Neto, Mico, & Berrocoso, 2016; Ossipov et al., 2014). The locus coeruleus receives inputs from the PAG and the RVM and projects noradrenaline into the spinal cord, causing the suppression of nociceptive signals (Muta, Sakai, Sakamoto, & SUzuki, 2012; Ossipov et al., 2014; Schwarz & Luo, 2015).
In brief, the PAG, the NRM and the locus coeruleus are engaged in the ICPM phenomenon. Their activation leads to the release of neurotransmitters including opioids,
cannabinoids, serotonin and noradrenaline that induce diffuse analgesia. This diffuse analgesia observed during the ICPM phenomenon can be shown during experimental procedures.
Experimentally inducing ICPM. In experimental settings, ICPM can be measured using two stimuli that induce pain; a test stimulus and a conditioning stimulus (Marchand & Arsenault, 2002). The most commonly used test stimulus and conditioning stimulus are respectively a contact thermode generating heat and the cold pressor test (CPT) (consisting of a cold-water bath) (Pud et al., 2009a). The CPT has been preferred over other stimuli as it involves both temporal and spatial summation (immersion of the whole arm into a water bath) (Marchand & Arsenault, 2002). Indeed, there are three main factors allowing the activation of ICPM: spatial summation, temporal summation, and the intensity of the conditioning stimulus (the stronger the conditioning stimulus, the stronger the analgesia measured will be) (Marchand & Arsenault, 2002). During the experimental procedure, the pain response to the test stimulus was measured twice, each time on a different surface of the skin to avoid peripheral sensitization. The experimental temperature is individually adapted. Although the experimental temperature used for both administrations is the same, participants typically report decreased pain perception during the second test stimulus, suggesting that endogenous pain inhibition mechanisms have been recruited (Kennedy, Kemp, Ridout, Yarnitsky, & Rice, 2016a; Pud et al., 2009a).
Importantly, ICPM can be measured through two different paradigms: the sequential paradigm and the parallel paradigm (Kennedy et al., 2016a). During the sequential paradigm, the test stimulus is measured once before the conditioning stimulus and once after the conditioning stimulus. As for the parallel paradigm, the test stimulus is measured firstly before the conditioning stimulus and secondly at the same time as the application of the conditioning
stimulus (Kennedy et al., 2016a). With the parallel paradigm, there is a possibility that the conditioning stimulus is acting as a distraction stimulus because it is applied concomitantly with the test stimulus (Kennedy et al., 2016a). Therefore, it is still unclear if the parallel paradigm truly measures the effect of pain modulation or of pain distraction. As mentioned earlier, distraction of a painful experience leads to a decrease in pain perception. For this reason, many have opted to use the sequential paradigm (Kennedy et al., 2016a). However, an important problem may also arise when using the sequential paradigm that has not been discussed in the literature until recently. Precisely, some articles have shown that the interruption of pain causes an increase in pleasure induced by pain relief (Leknes et al., 2008; Leknes & Tracey, 2008). Consequently, it is uncertain if the sequential paradigm is truly measuring ICPM (e.g. pain inhibits pain phenomenon), or if the sequential paradigm is measuring pain inhibition caused by pleasant pain relief.
1.6 Pleasant pain relief
Over the last decade, several experimental studies have shown that pain can be downregulated by positive emotional states induced by rewarding stimuli such as emotionally positive pictures, pleasant odours and pleasurable music (Kut et al., 2011; Leknes & Tracey, 2008). Pleasure induced hypoalgesia has been defined as reduced pain perception when concurrently receiving a pleasant and a nociceptive stimulus (Navratilova & Porreca, 2014). In a fMRI study, participants received noxious heat stimulation with a thermode while concurrently looking at images of their romantic partner (Younger, Aron, Parke, Chatterjee, & Mackey, 2010a). This research found significant decreases in key regions of the pain matrix (e.g. thalamus and posterior insula) in participants looking at images of their partner in comparison
to those who did not view pictures on their loved ones (Younger et al., 2010a). In a similar study conducted on 22 healthy individuals, participants viewing pleasant emotional pictures before receiving noxious heat stimulation from a thermode had increased pain tolerance compared to the control group, suggesting a potential role of reward-analgesia in pain modulation (Kut et al., 2011). Reward analgesia has also been tested in animals. When conducting experiments on reward-analgesia on rats, pain perception is measured as the time taken to withdraw from a painful stimulus. In fact, studies conducted on male and female Sprague-Dawley rats receiving noxious heat on their hind paw during voluntary drinking observed a significant increase in the time taken to remove their paw from the noxious surface when rats where receiving a sucrose solution as compared to solely water (Davies et al., 2019; Ren, Blass, Zhou, & Dubner, 1997). Notably, to further understand the reward system, these studies have also investigated its associated neurobiology.
More specifically, the reward paradigm induces pain-relieving effects primarily through dopamine, a catecholamine neurotransmitter (Potvin et al., 2009). The midbrain dopamine neurons exert their modulatory role through the mesocorticolimbic pathway, composed mainly of limbic, striatal and pre-frontal brain structures (Lidstone, de la Fuente-Fernandez, & Stoessl, 2005). External cues, such as pleasant stimuli, rewarding drugs or reward-predicting stimuli (e.g. placebo analgesia) can induce positive states in humans causing stimulation of the mesolimbic reward pathway. Once stimulated, dopamine neurons, which project from the ventral tegmental area to the nucleus accumbens (e.g. ventral striatum), the amygdala and the orbital frontal cortex, cause decreased pain (Altier & Stewart, 1999; Lidstone et al., 2005; Navratilova, Atcherley, & Porreca, 2015). In addition, a review has highlighted that there is a positive relationship between the amount of pain reduction (caused by pleasant stimuli) and
increased activation in the ventral striatum (reward region) (Navratilova & Porreca, 2014). Taken together, these results show the existence of a relationship between pain and pleasure.
Pain and pleasure are two states that appear to fall on opposite sides of a hedonic continuum (pleasant or unpleasant sensations) (Leknes et al., 2008). According to the opponent process theory, when a negative stimulus, such as noxious heat, is abruptly terminated, a feeling of the opposite hedonic state will be felt (e.g. pleasure) (Leknes et al., 2008). In theory, it has been proposed that pain relief may induce a pleasant feeling (Ellingsen et al., 2013; Leknes et al., 2008). To test this model, a psychophysical study induced noxious thermal heat pain using a thermode in healthy participants and found a significant positive correlation between pain intensity and pain relief (r=0.82, p=0.012), suggesting that the greater the intensity of the noxious stimulus, the greater the intensity of the relief will be (Leknes et al., 2008). The intensity of the noxious stimulus is individually determined and must reach a minimum pain rating of 50/100 (0 no pain- 100 most intense pain imaginable) in order for relief from pain to be measured (Leknes et al., 2008). Finally, the higher the value of a pleasant stimulus the more this stimulus will able to reinstate our bodies homeostasis (bodily equilibrium) (Leknes et al., 2008). Equally, similar findings have been observed in fMRI studies.
Neuroimaging studies have investigated cerebral activations during pain onset/offset (or pleasant pain relief) (L. Becerra et al., 2013; Lino Becerra & Borsook, 2008; Sprenger, Bingel, & Büchel, 2011). These studies have generally used thermal noxious pain induced with a thermode while participants lie supine in a functional scan. During pain onset, the studies showed increased activations in pain-related regions such as the insula, SI and SII but one research team found decreased activations in the nucleus accumbens (L. Becerra et al., 2013; Lino Becerra & Borsook, 2008; Sprenger et al., 2011). However, these results regarding the
nucleus accumbens should be taken prudently as other research have failed to show a deactivation in the nucleus accumbens (K. B. Jensen et al., 2016; La Cesa et al., 2014). The role of the nucleus accumbens may therefore be more complex and need further research. Contrariwise, during pain offset, studies noted decreased activation in the insula and increased activations in the nucleus accumbens and the orbital frontal cortex, which are regions shown to encode positive hedonic states (Lino Becerra & Borsook, 2008; Leknes et al., 2012). Relief from pain can therefore be viewed as pleasurable and may even contain rewarding benefits (L. Becerra et al., 2013; Leknes, Lee, Berna, Andersson, & Tracey, 2011; Younger et al., 2010a).
It is noteworthy that the fMRI studies mentioned until now have used similar stimuli to induce pain and measure brain activations during a painful stimulation, that is, thermal noxious stimulation using a thermode. We have previously mentioned that pleasant pain relief increases with greater pain intensity (Marchand & Arsenault, 2002). That being said, the CPT may be better suited to measure pleasant pain relief, as it is composed of both spatial and temporal characteristics. In fact, studies using the CPT to induce pain on healthy individuals have found significant activations in the thalamus and the insula (La Cesa et al., 2014; Lapotka, Ruz, Ballesteros, & Hernández, 2016). Moreover, the CPT has been used as a conditioning stimulus in the sequential paradigm in studies investigating the ICPM phenomenon (Kennedy et al., 2016a; Marchand & Arsenault, 2002; Pud et al., 2009a). However, to our knowledge, none of these studies have precisely looked at pleasant pain relief induced by the CPT or at the possible relationship between ICPM and pleasant pain relief.
In this sense, our limited knowledge on the pleasant pain relief phenomenon raises a methodological problem when using the sequential paradigm to measure ICPM. In the sequential paradigm, the test stimulus is measured once before and once after the conditioning
stimulus (Kennedy et al., 2016a). In healthy individuals, studies have observed reduced pain perception between the two test stimulus (Potvin & Marchand, 2016a; Tousignant-Laflamme, Pagé, Goffaux, & Marchand, 2008). However, this pain reduction may be due to pleasant pain relief, meaning that the sequential paradigm may be measuring pain reduction induced by pleasant pain relief, rather than the ICPM phenomenon.
1.7 Objectives
Psychological investigations have suggested that relief from aversive stimuli can be perceived as pleasurable (Navratilova & Porreca, 2014). Even with the growing interest in this field, several aspects still remain understudied. Precisely, studies have used thermodes to induce pain and thus measure pleasant pain relief. However, the CPT may be better suited than the thermode because of its ability to induce greater pain and as a result causing greater pleasant pain relief. Consequently, the main objective of this memoir was to further our understanding on pleasant pain relief. In order to do so, two separate studies have been conducted. The first study sought out to investigate the possible relationship between pleasant pain relief, ICPM and subclinical psychological symptoms. This psychophysical article was published, and the corresponding article is found in the following section. In a second study, we conducted both psychophysical and fMRI testing with the objective of investigating the relationship between pleasant pain relief and brain activations and de-activations during pain onset. This study will be explained in detail in chapter 3 of this memoir.
Study 1. This study is a psychophysical study testing the relationships between ICPM, the pleasant pain relief phenomenon and subclinical psychological symptoms. Investigating the
relationship between ICPM and pleasant pain relief by using the sequential paradigm will allow us to determine if the reduction in pain perception between the first and second administration of the test stimulus is confounded by the pleasant pain relief phenomenon. We evaluated this relationship by inducing ICPM using a thermode (test stimulus) and the CPT (conditioning stimulus) and by measuring pleasant pain relief using the CPT. Moreover, to our knowledge, the relationship between pleasant pain relief and negative emotional states still remains largely unstudied in this field. For this reason, the second objective of this paper was to evaluate the possible relationship between pleasant pain relief and anxio-depressive subclinical symptoms. We hypothesized that there may be a positive relationship between ICPM and pleasant pain relief and a negative relationship between anxio-depressive subclinical symptoms and pleasant pain relief.
For this study, my contributions were the following; clinical testing of all participants, data analysis and writing the article shown in the following section.
Study 2. Studies investigating brain activations and de-activations during pain onset and pain offset have observed opposite results, that is, increased activations in pain-related regions during pain onset and decreased activations in pain-related regions and increased activations in reward regions during pain offset (L. Becerra et al., 2013; Lino Becerra & Borsook, 2008; Sprenger et al., 2011). Similarly, in this fMRI study, we sought to further extend the first study by, firstly, observing all cerebral activations/deactivation during pain onset. More specifically, past researches have failed to consistently show deactivation in reward regions when using a thermode to induce noxious pain. In our research, we opted to use the CPT to determine if this stimulus would be better suited to observe such deactivations in brain reward
regions during pain onset. Secondly, we sought to investigate all possible relationships between brain activations and de-activations during pain onset with pain perception, pleasant pain relief and subclinical psychological symptoms. In order to do so, noxious pain was induced to participants during fMRI scanning with a modified CPT. This modified CPT consisted of frozen gel that was placed on participants' right foot.
We hypothesized that the modified CPT would induce activations in pain related regions and deactivations in reward regions during its administration.
Chapter 2. Article published in Pain Research and Management
Pleasant Pain Relief and Inhibitory Conditioned Pain Modulation: A Psychophysical Study
Nathalie Bitar, BSc 1,2; Serge Marchand, PhD 3,4; Stéphane Potvin, PhD 1,2
1 Centre de recherche de l’Institut Universitaire en Santé Mentale de Montréal; Montreal,
Canada
2 Department of psychiatry, Faculty of medicine, Université de Montréal; Montréal,
Québec, Canada
3 Centre de recherche du Centre Hospitalier de l’Université de Sherbrooke;
Sherbrooke, Canada
4 Department of Surgery, Faculty of Medicine and Health Sciences, Université de
Sherbrooke; Sherbrooke, Québec, Canada
Corresponding author
Stéphane Potvin, PhD ; Centre de recherche de l'Institut Universitaire en Santé Mentale de Montréal ; 7331 Hochelaga ; Montréal, Canada ; H1N 3V2 ; Email : stephane.potvin@umontreal.ca
Abstract
Background Inhibitory conditioned pain modulation (ICPM) is one of the principal endogenous pain inhibition mechanisms and is triggered by strong nociceptive stimuli. Recently, it has been shown that feelings of pleasantness are experienced after the interruption of noxious stimuli. Given that pleasant stimuli have analgesic effects, it is therefore possible that the ICPM effect is explained by the confounding effect of pleasant pain relief. The current study sought to verify this assumption. Methods Twenty-seven healthy volunteers were recruited. Thermal pain thresholds were measured using a Peltier Thermode. ICPM was then measured by administering a tonic thermal stimulus before and after a cold-pressor test (CPT). Following the re-administration of the CPT, pleasant pain relief was measured for 4 minutes. According to the opponent process theory, pleasant relief should be elicited following the interruption of a noxious stimulus. Results The interruption of the CPT induced a mean and peak pleasant pain relief of almost 40% and 70%, respectively. Pleasant pain relief did not correlate with ICPM amplitude, but was positively correlated with pain level during the CPT. Finally, a negative correlation was observed between pleasant pain relief and anxiety. Discussion Results show that the cessation of a strong nociceptive stimulus elicits potent pleasant pain relief. The lack of correlation between ICPM and pleasant pain relief suggests that the ICPM effect, as measured by sequential paradigms, is unlikely to be fully explained by a pleasant pain relief phenomenon.
Key words
1. Introduction
Chronic pain affects approximately 22% of the adult population (Tamburin, Paolucci, Smania, & Sandrini, 2017), and is a complex phenomenon resulting from biological, psychological and social factors. Among these factors, the importance of central mechanisms, such as the activity of endogenous pain excitatory and inhibitory systems, are increasingly acknowledged (DeSantana & Sluka, 2012; Kwon, Altin, Duenas, & Alev, 2014; Tousignant-Laflamme et al., 2008). Indeed, growing evidence suggests that endogenous pain modulation mechanisms are impaired in nearly every type of chronic pain disorders, and that alterations are particularly significant in neuropathic and functional pain syndromes (Lewis, Heales, Rice, Rome, & McNair, 2012; Clifford J. Woolf, 2011; Yarnitsky, 2015).
Inhibitory conditioned pain modulation (ICPM) is one of the principal endogenous pain inhibition mechanisms (Lewis, Rice, & McNair, 2012a; Moont, Crispel, Lev, Pud, & Yarnitsky, 2011; Nahman-Averbuch et al., 2013). The ICPM theory postulates that a nociceptive stimulation will reduce another nociceptive stimulation if it occurs on a body surface distant from the pain surface (Le Bars, Dickenson, & Besson, 1979a, 1979b). Pre-clinical studies have shown that the ICPM effect is mediated by brain stem and bulbo-spinal mechanisms (Basbaum & Fields, 1978; Marchand, 2008; Millan, 2002; Willer, Bouhassira, & Le Bars, 1999). When triggered, ICPM causes a diffuse diminution of pain throughout the body.
From an experimental point of view, two types of paradigms are used to measure ICPM: in the parallel ICPM paradigm, a noxious stimulus (test stimulus) is applied before and at the same time as a heterotopic conditioning painful stimulus, while in the sequential paradigm, the test stimulus is applied before and after a heterotopic conditioning painful stimulus (Kennedy, Kemp, Ridout, Yarnitsky, & Rice, 2016b). Considering that it is unclear if the parallel ICPM
paradigm truly measures the ICPM effect or a distracting effect, some investigators prefer the sequential paradigm which removes the potential effect of distraction (Olesen, Van Goor, Bouwense, Wilder-Smith, & Drewes, 2012; Valencia et al., 2014; Valencia, Kindler, Fillingim, & George, 2012). It is indeed well known that pain experience is reduced when individuals are engaged in cognitive tasks (e.g. arithmetic, working memory, etc.) (Moont et al., 2011). This raises the possibility that the conditioning stimulus actually distracts participants from their pain when it is concomitantly administered at the same time as the test stimulus. Conversely, some laboratories have made mention of their preference of the parallel ICPM paradigm over the sequential one, considering that ICPM effect gradually fades over time and that the precise duration of this effect remains uncertain (Pud, Granovsky, & Yarnitsky, 2009b).
Another potential limitation of sequential ICPM paradigms that has gone unnoticed is that the pain reduction observed using these paradigms may be confounded by the pleasant pain relief phenomenon. According to the opponent process theory, when a stimulus causing deviation from homeostasis is terminated, the opposite sensation will be felt (Andreatta, Mühlberger, & Pauli, 2016). Consistently with this theory, recent research has shown that the interruption of a noxious stimulus causes a feeling of pleasantness (Leknes et al., 2008), similar to the feeling often observed in reaction to analgesic drugs (Leknes et al., 2008). Given that pleasant stimuli (e.g. music, odors, attractive faces, etc.) are well-known for producing analgesic effects (Dobek, Beynon, Bosma, & Stroman, 2014; Prescott & Wilkie, 2007; Younger, Aron, Parke, Chatterjee, & Mackey, 2010b), it is therefore possible that the interruption of the conditioning stimulus elicits a pleasant feeling, which decreases in turn pain perception when the second test stimulus is re-applied. If so, the reduction in pain perception observed during the
second test stimulus would not reflect a pure ICPM effect but rather a pleasure-induced analgesia effect, at least partially.
In the past, our research team has pursued several studies on ICPM using a sequential paradigm, consisting in the application of a tonic noxious heat stimulation to the left forearm of participants eliciting moderate pain, administered before and after the immersion of their right arm in a bath of cold water. This paradigm has allowed us, among others, to show that pain perception is reduced during the second application of the test stimulus, relative to the first one, indicating that endogenous pain inhibition mechanisms have been recruited (Normand et al., 2011; Potvin & Marchand, 2016b). In the current study, we sought to examine a hypothetical association between ICPM and pleasant pain relief, using our validated ICPM procedure (Tousignant-Laflamme et al., 2008). Thus far, most studies on pleasant pain relief have used heating thermodes to elicit the phenomenon (Leknes et al., 2008; Mohr et al., 2009). The current study differed in that we measured pleasant pain relief after the interruption of the cold-pressor test, given that it is the conditioning stimulus used in our sequential paradigm to trigger the ICPM effect. The secondary objective of the current study was to examine the potential associations between pleasant pain relief and anxio-depressive sub-clinical symptoms. Although several experimental studies have shown that anxiety and depression influence pain perception in experimental settings (De Heer et al., 2014; Defrin, Schreiber, & Ginzburg, 2015; Zambito Marsala et al., 2015), the influence of these variables on pleasant pain relief is unknown.
2. Method 2.1 Participants
We recruited a total of 27 (14 women) healthy participants, aged between 18 and 35 years old (mean age 25.1 years ± 4.27, mean; standard error of the mean (SEM)) (Table 1). Exclusion criteria were the following: (1) any DSM-V Axis psychiatric disorder (including substance use disorders); (2) centrally-acting medications; (3) neurologic disorders; and (4) any unstable medical condition. In particular, none of the participants suffered from chronic pain and none had significant acute painful symptoms as determined with the Brief Pain Inventory (mean pain= 0.9 ±0.4) (Atkinson et al., 2010; Poundja, Fikretoglu, Guay, & Brunet, 2007). Sub-clinical psychological symptoms (e.g. depression, anxiety, anhedonia and pain) were evaluated, respectively, with the French versions of the Beck Depression Inventory-II (BDI-II) (Lahlou-Laforêt, Ledru, Niarra, & Consoli, 2015), the State and Trait Anxiety Inventory-state subscale (STAI-S) (Barnes, Harp, & Jung, 2002; Gauthier & Bouchard, 1993) and the Snaith-Hamilton Pleasure Scale (SHPS) (Ameli et al., 2014; Loas et al., 1997). Recruitment was made via word of mouth and through online advertisement (Kijiji). Each participant signed a detailed consent form, and the local ethics committee approved the research.
2.2 Inhibitory conditioned pain modulation (ICPM) paradigm
2.2.1 Heat pain threshold and tolerance. Thermal pain threshold and tolerance were measured by applying a 3 cm2 Peltier thermode on the left forearm of participants (TSA II, Medoc, Advanced Medical Systems, Ramat Yishai, Israel) (Potvin & Marchand, 2016b).This heating plate was connected to a computer and allowed a precise control of temperatures. Experimental temperatures were initially set at 32°C and gradually increased at a rate of 0.3°C per second. To