Transgenic Mice
Expressing the Human Heat Shock
Protein 70 Have Improved
Post-Ischemic Myocardial Recovery
J.-C. L.Plumier,* B. M. Ross,* R. W. Currie,* C. E.Angelidis,*H.Kazlaris,5 G.Kollias,OandG. N. Pagoulatost
*Department of Anatomy and Neurobiology, Dalhousie University, Halifax, Nova Scotia, Canada B3H 4H7; *Laboratoryof General
Biology, University of Ioannina Medical School, loannina, Greece 45332; and §Department of Molecular Genetics,Hellenic Pasteur
Institute,Athens, Greece 11521
Abstract
Heat shock treatment induces expression of several heat
shock proteins and subsequent post-ischemic myocardial
protection. Correlations exist between the degree ofstress
usedto induce the heat shock proteins, the amount ofthe
inducible heat shock protein 70 (HSP70) and the level of
myocardial protection. The inducibleHSP70has also been
shown tobe protective in transfected myogenic cells. Here we examined the role of human inducible HSP70 in transgenic mouse hearts. Overexpression of the human HSP70 does not appear to affect normal protein synthesis
or the stress response in transgenic mice compared with nontransgenic mice. After 30minofischemia,upon reperfu-sion, transgenic heartsversus nontransgenic hearts showed significantly improved recovery of contractile force (0.35+0.08versus0.16+0.05g,respectively,P <0.05),rate
ofcontraction, andrate of relaxation. Creatine kinase, an
indicator of cellular injury, was released at a high level (67.7+23.0 U/ml) upon reperfusion from nontransgenic hearts, butnot transgenic hearts (1.6±0.8U/ml). We
con-clude that high level constitutive expression of the human inducibleHSP70 plays adirect role in theprotection of the myocardium from ischemia andreperfusioninjury. (J. Clin.
Invest. 1995.95:1854-1860.) Key words: heat shock
protein-physiology *ischemia*myocardium-pathology *transgenic animal * hypoxia.
Introduction
It seems we are on the threshold of an era ofmolecular and
genetic therapeutics (1-3). Transgenic mice expressing high
levels of specific gene products have increased resistance to
cerebral ischemia(4, 5)orhaveenhancedmyocardialfunction (6). In fact, heat shock-induced changes in gene expression
have been associated with cellular protection and improved
This workwas presented inpart at the 1994 meeting on Biology of HeatShock Proteins & Molecular ChaperonesatColdSpringHarbor andatthe 67th ScientificSession of the American Heart Association.
Address correspondence to D. R. William Currie, Department of Anatomy andNeurobiology, DalhousieUniversity,Halifax,Nova Sco-tia, Canada B3H 4H7. Phone: 902-494-3343; FAX: 902-494-8008; e-mail, R.William.Currie@dal.ca.
Receivedforpublication 12 October 1994 and inrevisedform2 December 1994.
function formorethan adecade(7-9). Morerecently, in iso-lated andperfused heart studies, heat shock treatment and
ex-pressionof heat shockproteinswereassociated with improved
post-ischemic myocardialcontractile recovery (10-12). In in vivoexperiments, myocardial infarct size was significantly re-duced in heatshock-treated animals(13-15). Indeed, a direct correlation has now been shown between the amount of the
highlyinduciblememberof the heat shockprotein 70(HSP70)' family of stress-induced proteins and the degree of myocardial protection, as measured by reduction in infarct size (16).
Although heat shock treatment is associated with myocardial
protection, it is difficulttoknow whether theprotection isdue to theoverexpression of one or several proteins, or to some other unrelatedadaptiveprocess.OverexpressionofHSP70alone was
demonstratedtoprotectcells from thermal injuryand increase
cell survival(17, 18). Wedecided therefore to use transgenic
miceengineeredtoexpressconstitutively high levels of human inducible HSP70,toinvestigate the contribution of this protein
to myocardial protection.
Methods
Transgenic mice. Micewerecaredfor in accordance with the Guide to the Care and UseofExperimental Animals of the Canadian Councilon Animal Care.
Preparation of the DNAfragments for microinjection. The Apr-HS70expression vector wasconstructedasdescribedpreviously(17). Itcontains the human induciblehsp70gene under theregulation of the
,/-actin
promoter. A6.6-kb EcoRI-Hind III fragment containing the human 63-actinpromoter,IVS1 (intervening sequence1 of theP-actingene), and the humanhsp70genewasmade devoid ofvectorsequences by preparativeelectrophoresis, Genecleanpurification (RI0101, UK), and passagethroughanElutip-Dcolumn(Schleicher& Schuell, Ger-many). Fragments formicroinjection weredissolved in 10 mM Tris-HCl,pH7.6,0.1 mM EDTAto afinal concentration of 2-4,ug/ml.
Generationoftransgenicmice.OriginalinbredCBA,C57B1/6,and outbredNMRI micewereobtainedfrom IFFA-CREDO(France) and maintained in the HellenicPasteurInstitute'sfacilities. CBAxC57B1 / 6 hybrid mice(Fl) were used for the backcrossings throughout the study. NMRI vasectomized maleswereusedtopreparepseudopregnant Fl females for oviduct transfers. To obtain fertilized F2 zygotes for microinjection, 3-4-week-old F1 females were superovulated as de-scribed (19)and mated withF1 males.Microinjection wasperformed
essentially asdescribed elsewhere (20, 21). Viable eggs were
trans-ferred into oviducts ofpseudopregnantfemales and allowedtodevelop to term.
To identify transgenic foundermice, DNA wasisolated from tail biopsies at 10-15 d of age (19), digestedwith BglII, and analyzed by Southern blot (22) using the microinjected fragment as a probe. Approximatecopy numbers were estimated by comparison ofsignal
1. Abbreviations used in thispaper:HSF,heat shocktranscription fac-tor; HSP70,inducible 70-kDheat shockprotein.
J. Clin. Invest.
©TheAmericanSocietyfor ClinicalInvestigation,Inc. 0021-9738/95/04/1854/07 $2.00
intensitywith thatofanendogenous Thy-Isingle-copygene.Transgenic
progeny from founder micewereidentifiedbyeither Southernor slot-blothybridizationanalysis.
RNA analysis. Total RNA was prepared form animal tissues by
guanidinium thiocyanateextractionasdescribed(23).S1nuclease map-pingwas performed usingas aprobe a692-bp SalI-BglII fragment,
derived from the PAT-HS70 plasmid (24). This fragment contained 277bpofvectorsequences, 63bpof the humanhsp70gene5'
untrans-latedregion,and352bpof the humanhsp70gene5'codingsequences. Theprobewas5'endlabeledusing[y 32p]ATPand T4polynucleotide kinase, purified thoughaG-50spin column,denaturedby boiling,and allowedtohybridizewith30
Msg
of RNAat50'Covernight.S1nucleasedigestionwasperformedat370Cfor 1 h withanenzymeconcentration of 600 U/ml. Under theseconditions, onlythefully homologous
frag-ments wereprotected;theseweresubsequentlyanalyzedunder
denatur-ingconditionson5%sequencing gels (25).
Proteinanalysis. Hearts, livers, and kidneysfrom fourtransgenic and four nontransgenic mice were analyzed by one- and two-dimen-sionalgel electrophoresis. Hyperthermictreatment(42TCrectal
tempera-turefor 15 min)was appliedto twotransgenicandtwonontransgenic
mice. The hyperthermia-treated mice wereallowed to recoverfor 30 min before injection of radiolabel. Hyperthermia-treated andcontrol mice were givenan intraperitoneal injectionof 0.25 mCi ofL-
[35S]-methionine(Dupont-NEN,Boston, MA;spact> 1,000 Ci/mmol)and overdosed with sodiumpentobarbital2 hlater,and organswereexcised. Micereceiving hyperthermic treatmentwereinjected with radioactive label30 min after the end of the heat shock.
One-dimensionalgels.Proteins(40
sg
proteinperlane)were sepa-ratedby7.5%SDS-PAGE. Gelswerestained with Coomassiebrilliant blue R, destained, and photographed. Gels were then processedforfluorography and exposed toX-Omat AR film (Eastman Kodak Co.,
Rochester,NY).
Two-dimensionalgels and Western blotanalysis. Protein samples from heart ventricular musclewereanalyzed bytwo-dimensional SDS-PAGE(26)aspreviouslydescribed(27).For Westernblotting, proteins weretransferredtoImmobilon-P membranes(MilliporeCorp., Toronto,
Ontario) at 200 mAovernight, accordingto the method of Towbinet
al.(28).BlotswereincubatedinPBScontaining5% skim milkpowder toblock nonspecificbinding sitesonthemembranes(29). Blotswere immunoreacted first with a 1:7,500 dilution ofa rabbit(#799) anti-humaninducible 70-kDpolyclonalantibodygraciouslyprovided byDr. R. M. Tanguay (Universite Laval, Quebec). Second,blotswere incu-bated in a 1:500 dilution ofaperoxidase-conjugatedgoatanti-mouse
IgG.4-Chloro-1-naphtholwasusedasthesubstrate for the visualization of the reactionproduct.Blotswerecounterstained with amidoblackto show otherproteins.
Langendorif
perfusion protocol. Adultmice(25-35 g) wereanes-thetized with sodiumpentobarbital (50 mg/kg body wt
intraperitone-ally). Hearts were excised rapidly and immersed to ice-cold buffer, and theaorta wascannulatedon aLangendorffapparatus. Heartswere
retrogradely perfusedwith Krebs-Henseleit bufferconsistingof 120mM
NaCl, 20 mM NaHCO3, 4.63 mMKCl, 1.17 mM KH2PO4, 1.2 mM
MgCl2, 1.25mMCaCl2, and 8 mMglucose,pH7.4,at aflowof 3 ml/ min for 30 min. The perfusion system was regulated during the
pre-ischemic, pre-ischemic, and reperfusion periods at37°C, using a
water-jacketedchamber and coil. Heartswere madeischemicby turning off thebuffer flow for 30 min and thenreperfusedwith 3 ml/minbuffer for 30 min. Heartswerepacedat 390 beats per min(6.7 Hz, 3.3V,3 ms) with a stimulator(model S44, Grass Instruments, Quincy, MA). Mechanical activitywas measured throughouteach perfusion experi-mentaccordingtopreviouslydescribed methods(10, 11).Briefly, api-cobasal displacementwas obtained by attaching astrain gauge trans-ducer (model FT.03, Grass Instruments)totheheart apex. The trans-ducerwaspositionedtoyieldaninitialrestingtension of0.3 g. Perfusion pressure was measured using a pressure transducer(model P23 ID, Gould Inc., Cleveland OH). Recordings of mechanical activity and pressurewererecordedon apolygraph(model 7,GrassInstruments).
Creatine kinaseanalysis. Creatine kinase release from hearts was
measured in the effluent buffer.Sampleswere collectedduringthe last minute of pre-ischemic perfusion and at 1, 5, 10, 20, and 30 min of reperfusion.Creatine kinase content was determinedbythe spectropho-tometric method of Rosalki(30) usingcreatine kinasekits fromSigma Chemical Co. (St. Louis,MO).
Catalaseassay. Catalaseactivitywasdetermined inheartsusinga modification ofpreviously described methods(31, 32). Briefly,100mg of heart was homogenized in 0.5 ml of ice-cold Tris sucrose buffer containing0.25 M sucrose, 10 mMTris-HCl, 1 mMEDTA,0.5 mM DTT, and 0.1 mMPMSF, pH7.5.Samples werecentrifuged at3,000 gfor 15min,and thesupernatantswere recovered.Protein concentration of thesupernatants was determinedbythe method ofLowryet al.(33). Samplesof heart supernatants(40
I.g
ofprotein)werebroughtto1-ml volume with anassay buffer of 35 mM phosphatebuffer, pH 7.2,0.02% Triton X-100. The enzymaticreaction was startedby adding30p1
of 1% H202tothe assaymixture.Catalase activity was estimatedby mea-suring the rateof H202 consumption at 240 nm in aspectrophotometer (model DU 50, Beckman Instr., Fullerton,CA). Optical density was recorded at 15, 25, 35, 45, and55 s, and the velocity ofthe reaction (slope) was determined.Statistical analysis. All values expressed aremean±SEM. Differ-ences were determined by comparison of the calculated test statistic with the two-tailed significance limits of Student's distribution.
Results
Transgene expression. Four transgenic mouse lines (namely 2906, 2633, 2702, and 2710) were obtained as determined by Southern blothybridization analysis of DNA from tailbiopsies
(data not shown). Expression of the transgene was monitored using an S 1 nuclease mapping assay. To distinguish expression of the transgene from that of the endogenous mouse hsp70,
wetook advantage of a divergence in the sequences of the 5' untranslatedregion in these two genes and used a692-bp probe containing human HSP70 coding sequences plus 63 bp of the human 5' untranslatedregion. Under stringent conditions of S 1 digestion, different length fragments of this probe were pro-tected withmouse andhumanhsp70mRNAs.Using this assay, hsp70 mRNA levels in the four transgenic mouse lines were tested in heart tissues (Fig. 1). We found that the 2906 transgenic line expressed large amounts of humanhsp70mRNA at normal temperature, compared with the barely detectable levels of theendogenous mouse hsp70 mRNA. We estimated the copy number of the transgene to be - 50. Offspring of
founder mouse 2906 were selected by Southern blot or slot-blot hybridizationfor transgene expression.Nontransgenic lit-termates as well asnormal miceof the same strain were used ascontrols.
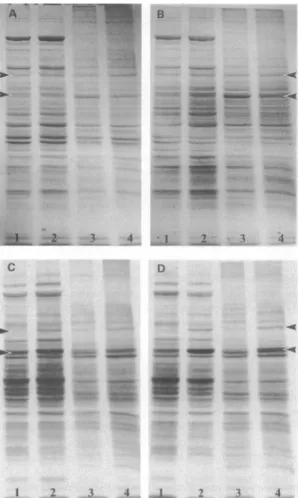
Protein analysis. One-dimensional gels stained with Coo-massiebrilliant blue R and fluorograms of livers andkidneys of transgenic and nontransgenic mice and of heat-shocked
transgenicandnontransgenicmice arepresentedinFig.2.
Com-parison of liver orkidney fromnontransgenic (Fig. 2 A) and
transgenic (Fig. 2 B) mice revealed no apparent difference in thesteady-state level ofproteinsseparated byone-dimensional
gelelectrophoresis. Afterhyperthermictreatment (lanes 2 and
4), there appears to be a similar increased accumulation of
severalprotein bands intheliversandkidneys of nontransgenic andtransgenic mice (compare Fig.2 Awith B).De novoprotein synthesis was assessed by L-[35S]methionineradiolabeling of
heat-shocked and non-heat-shocked transgenic and
non-transgenic mice. L-
["5S]
methionine incorporation was similarfor livers and kidneys of transgenic and nontransgenic mice
H E A - C 6A CD 622- _ 439-430 * l 370- _ NRT CD N-CI) CJ 0
~
4 _-mHS70Figure 1.Specific
ex-pression of the hsp70
transgenein heart
tis-sues.Total RNA from
hearttissueswas pre-* pared from control (Fl)
andtransgenic(2906, 2633, 2702, 2710) ani-malsand subjectedtoSI nuclease protection anal-ysis. Positions of hsp70 ( 4) and (3-actin (*) probesareindicated. The
protectedexogenous
hu-manhsp7O (hHS70) and
endogenousmouse
hsp70(mHS70)
tran-scriptsareshown by
arrows. Aladder of end-labeled HaeIl digest of pBR322wasused for
-mnAcTIN sizeestimation, and
,/-actin levelswereusedas aninternalassaycontrol.
in protein synthesisbetween thenontransgenic (Fig. 2 C)and
transgenic (Fig. 2D)mice. Bothnontransgenicandtransgenic
miceappearedtorespondinasimilar fashiontothe hyperther-mic treatment (lanes 2 and 4). Several protein bands have
increased incorporationofL-[35S]methioninein liver and
kid-ney (compare Fig. 2 CwithD).
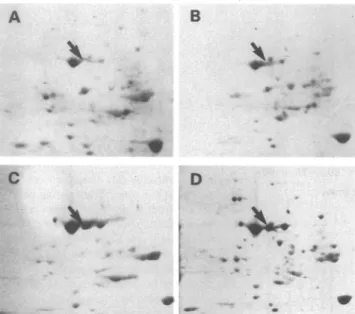
L-[35S ]methionine-labeledproteinsfrom heartswere
sepa-ratedbytwo-dimensionalgelelectrophoresis andprocessedto reveal theproteins synthesizedduring the 2-hlabeling period. Fluorograms of L-[355]methionine-labeled proteins revealed
nodetectable basal level ofsynthesis of eithermouseinducible
HSP70 or human HSP70 (transgene product) in the
non-transgenicortransgenic mousehearts(Fig. 3,AandB).After thehyperthermictreatment,mouseinducibleHSP70waseasily
detectable in thenontransgenic mousehearts(Fig.3C).Inthe
transgenic mouse hearts there was also anincrease in mouse
inducible HSP70, whereas there was little or no increase in humanhsp70geneproduct (Fig. 3D;comparewithFig.4D).
Western analysis of two-dimensional gels of normal and
hyperthermia-treated nontransgenicandtransgenichearts is
pre-sented in Fig. 4. A small amountof mouse inducible HSP70 was detectable in the nonstressed nontransgenic mouse hearts
(Fig. 4,AandA').Afterhyperthermictreatment,the accumula-tion ofmouse inducible HSP70 wasvisibly increased (Fig. 4,
B and B') innontransgenic mouse hearts. Interestingly, from the nonstressed transgenic mouse hearts, mouse inducible
HSP70and humanHSP70fractionated into discretespots(Fig.
2- 3 4 'N 1 2 3 4 D lip 11M _A -1 2 3 4 4-...I...3 4
Figure 2.SDS-polyacrylamide gelsofL-[35S] methionine pulse-labeled
liversand kidneys. Nontransgenic and transgenic mice with and without heat shocktreatmentweregivenanintraperitoneal injection of 0.25 mCi ofL-[35S] methionineandwerekilled 2 hlater. Micereceiving
hyperthermictreatment (lanes2and4)wereinjectedwithradioactive
label 30minafter the end of the15-minheat shock.Labeledproteins (40Mgproteinperlane)fromliver(lanes Iand2)andkidney (lanes 3 and4)wereseparated by SDS-PAGE. Coomassie brilliant blue
R-stainedgels (AandB)wereexposedtoautoradiography film(Cand D). (AandC)Nontransgenicmouse;(B andD) transgenicmouse.The toparrowpointstothe 90-kD heat shockprotein,and the bottomarrow
pointstoHSP70.
4,C andC').A smallaccumulation ofmouseinducibleHSP70 was detectable adjacent to a larger accumulation of human
HSP70.Afterhyperthermictreatment,in the transgenicmouse
hearts,the accumulation ofmouseinducibleHSP70wasvisibly
increased, whereas there was no discernible change in the
amountof humanhsp70transgeneproduct (Fig. 4,DandD'). Contractility. Contractile data for isolatedhearts from both
groupsof animals is illustrated inFig.5. None of theparameters differed duringthe equilibration period (t = 0-30 min). The
restingtensionwasadjustedto0.3gduringandatthe end ofthe
equilibrationperiod.Ischerniaproducedacompletereductionin
contractility within 15 min (t = 45 min), with aprogressive
increase inresting tension. Theresponsesduringischemia did not differ significantly between the nontransgenic and
transgenic hearts, although therate of relaxation and therate of contractionappearedto decrease more slowlyin transgenic hearts. However,substantial differences in contractilerecovery
_ =!_.
A":.R
!;'
wj *. .:_
:.,.X;9ffi
.'.L .,, .BF...
:'._.
>.:r.:...
.: ...,,_.,,:.^
:dFuN ..,* __ .... -B ---hHS70 227- _181-N<
N...-.11"W....o -.., ;..,..4-.i..
ndW Aka *NOUN,A
B
A
A'
0 & .S,W 0B
C
B'
D
C.
cl
a.Figure 3.SDS-polyacrylamide gels ofL-[35S ] methionine pulse-labeled hearts.Proteins were labeled asdescribedinFig.2.Two-dimensional gelelectrophoresis-separatedradiolabeledproteins (1mg) extracted fromheartsof controlnontransgenic(A), controltransgenic(B), heat shockednontransgenic(C), and heat-shockedtransgenic (D)mice.The arrowin eachpanel pointsto mouseHSP70.
wereevident after restoration of thenormal flow. After 30 min ofreperfusion (t = 90 min), hearts of transgenic animals had
significantly increased recovery of force (0.35±0.08 versus
0.16±0.05 g),rate of contraction (+dF/dt; 90.91±9.98 ver-sus 47.08±13.99 g/s), and rate of relaxation (-dF/dt; 94.09±10.34 versus 52.33±13.04 g/s) when compared with nontransgenic hearts.Relative to pre-ischemic values, the recov-ery of forcewas70versus 29%, +dF/dtwas 87versus48%, and -dF/dtwas83versus50% for thetransgenicversuscontrol hearts.Inaddition, the differences tendedtoincreaseaccording to the reperfusion time. Resting tension changed in a similar fashion for both groups.
Creatine kinase release. Creatine kinase releaseduringthe pre-ischemic perfusion was undetectable in hearts from transgenic and nontransgenic mice. Upon reperfusion, a high level of creatine kinasewasreleasedfrom nontransgenic hearts (67.7±23.0U/ml)ascomparedwithtransgenichearts(1.6±0.8 U/ml)(Fig. 6).At5minofreflow, the level of creatine kinase
wasconsiderably reduced in nontransgenic hearts and was simi-lartothat intransgenic hearts (13.7±8.8 versus 19.9±10.1 U/ ml, respectively).
Catalase activity. Catalaseactivitywas notsignificantly dif-ferent in the transgenic and nontransgenic hearts (1.75±6.46 [4] versus 0.83±4.84 [6] U per mg of protein; mean ±1 SD [n];P = NS, respectively).
Perfusion pressure. Fig. 7 illustrates pressure data from transgenic and nontransgenic mouse hearts. Before ischemia,
no significant difference was noted between the two groups, although nontransgenicheartsappearedtohave ahigher perfu-sion pressure(107±12mmHg)than transgenic hearts (73±14 mmlHg) (t = 30 min). Upon reperfusion (t= 60 min), perfu-sion pressure appeared to be higher for nontransgenic hearts than transgenic hearts, and the two experimental groups (non-transgenic versus transgenic hearts) differed significantly
D
\,rFigure 4. Western Blotanalysis of 70-kDheatshockproteinsinmouse hearts.Two-dimensional gel electrophoresis-separated proteins (1 mg) extractedfrom hearts of controlnontransgenic (A), heat-shocked non-transgenic (B), controltransgenic (C),andheat-shocked(D)mice. Aftertheproteinsweretransferredtothemembrane,the membranewas immunoreactedwith arabbitpolyclonalanti-humaninducible 70-kD heat shockproteinantibody.Thesecondaryantibodywasa peroxidase-conjugatedgoatanti-rabbitIgG.4-Chloro-l-naphtolwasused as the substrate for theimmunoreaction (right).Blots were counterstained withamido black(left)toshow otherproteins.Thearrowsindicate mouseHSP70(left)and humanHSP70(right).
(109±12 versus 69±11 mmHg; respectively) after 10 min of reflow (t= 70 min).
Discussion
Persistentoverexpression of the human hsp7Ogeneproductin mice does not result in observable changes in basal protein
content or synthesis. After hyperthermic treatment, the transgenicmouseliver, kidney, and heart haveasimilar increase insynthesisofasmallnumber ofproteinsasthenontransgenic
mouse tissues. However, overexpression of the human hsp7O geneproductconveysasignificant protectiontoisolated hearts after ischemicinjury, during reperfusion. The transgenic hearts had significantly improved post-ischemic contractilerecovery
andsignificantlylower release of creatine kinase.
Inprevious studies,elevatedlevelsof the highly inducible member of the HSP70 family of stress-induced proteins has been associated with improved post-ischemic recovery (10-12) and with reduction in infarct size in hearts (13-15). As
mentioned earlier, there appearstobe a direct correlation be-tween theamount of the inducibleHSP70 and theamountof myocardial protection (16). With transgenic mice
overex-pressinginducible human HSP70, assessmentof the
contribu-I i
0 9 ,
A
15 30 45 80 75 90 Time(min)
B
I II 15 30 45 80 75 90 lirm(min)
120-v.100-
80-10-a-
40-20 -0-D
120 - 100-80 - 60-40 - 2 0-1 I I 15 30 45 S0 75 90 15315 80IW 75F %FlF*
-Figure
5. Contractile data of isolated non-Time(min) transgenic and transgenic hearts duringperfu-sion. Hearts from nontransgenic (open circles) andtransgenic(closed circles)micewere retro-* *
gradely
perfused
withKrebs-Henseleit bufferat370(.Heartsweremade ischemicby turningoff thebuffer flow for 30min(horizontal bar)and thenreperfusedfor30min.Apicobasal
displace-ment wasmeasuredbyagauge transducer attached to the heart apex and positioned to yield aninitialresting tension of 0.3 g. (A) Contractile force,(B) restingtension,(C)+dFldt,and(D)
,,, , , , -dFldt.Eachpointrepresents themean±SEM
15 30 45 60 75 90 of at least IIanimals. *P < 0.05 from non-transgenicvalues using Student's t test for
un-lime (miii) paired
data.
tionofinducible HSP70tocellularprotection could be
exam-ined.
Overexpression of a single protein may induce drastic changes in cell morphology, physiology, and cell and animal
survival (34, 35). However, in the case of the mice studied
here, there was no obvious developmental or morphological
differences betweennontransgenic and. transgenic mice.Atthe
biochemicallevel, protein analysisrevealednocleardifferences
inprotein profilesof tissues fromnontransgenicandtransgenic
mice. Even after heat shock treatment, the transgenic tissues
respond in a similar fashion as the nontransgenics, with the
increasedsynthesis of severalheat shockproteins (Figs. 2 and 3).Comparison ofthe two-dimensional gel fluorograms (Fig. 3)and the Western blots(Fig. 4)revealed thatafterheatshock treatment, the humantransgeneisnotinduced whereas the
en-dogenousmouse gene isinduced. It should also be noted that
a small amount ofmouse inducible HSP70 is present in the non-heat-shockednontransgenic andtransgenicmousehearts. Theprimary antibodyused forthe Westernanalysisisextremely
E Figure6.Creatine kinase
releaseineffluentbuffer c 120- * of isolated hearts during
100- perfusion.Creatine
ki-§80- release
60- suredatthe last minute
1°
40 ofthepre-ischemicpe-, 20 n-o I n riodandduring the
reper-IIi0-fusionof heartsfrom
' ' nontransgenic (open
cir-15 30 45 60 76 90 cles)and transgenic
Time(nfun) (closedcircles)mice.
Eachpointrepresentsthe mean±SEMofatleast11 animals. *P<0.05fromcontrol values using Student's ttestforunpaireddata.
sensitive and detects very small accumulations of the protein. Although mouse inducible HSP70 is detectable by Western analysis, itssynthesis,as revealedby fluorography,is belowa
clearlydetectablelevel in thenon-heat-shocked hearts. At the
protein level, itseems thattransgenic andnontransgenic mice
donotdifferexceptforthepresenceofhuman HSP70.
HSP70has beenreportedtoautoregulateitsownexpression
inanegativefeedbackloop (36). hsp7O transcription depends on the availability of heat shock transcription factor (HSF). HSP70binds to HSF, thereby maintainingHSF in aninactive form. Inourtransgenic model, however, high levels of human
HSP70did notalter mouse HSP70constitutive expressionin hearts(Figs.3and4).Moreover,synthesisofmouseinducible
HSP70 after heat shock appeared in transgenic and
non-transgenicmice. Itmaybethat humanHSP70wasnotableto bind mouse HSF and regulate mousehsp7O transcription.
In-deed,mouseHSP70and humanHSP70arenotidentical. These
proteinscanbe resolved into discretespotsbytwo-dimensional
gelelectrophoresis.
The contractile data revealednosignificantdifference
dur-140- * ** * Figure7.Perfusion
pres-120
H
I
surefromisolated hearts E 100 duringperfusion.Hearts60 fromnontransgenic
i 40- (opencircles)and
20- transgenic (closed
cir-0 cles)mice were
sub-| | jectedtoischemia for 30 15 30 45 60 75 90 min(horizontal bar).
Time(min) Heartswereperfusedat a
fixedflowof 3.0ml/min duringpre-andpost-ischemicperiods.Eachpointrepresents the mean±SEMofatleast11 animals.*P< 0.05from control values
using Student'sttestforunpaireddata.
Ob U. 1L 0.8 -0.8 -0.4 -0.2 -0.0 -0.8 -0.8 -0.4 -0.2 -0.0 -C aC 0
C
ingthepre-ischemic perfusion period.Therewere nosignificant differencesincontractility duringtheischemicperiod;however, the contractility of the transgenic hearts appeared to decline moreslowly comparedwiththenontransgenichearts.Although the temperature of the perfusion apparatus was regulated at
370C, the temperature of the hearts may havedecrease slightly duringtheischemic period. However,it isunlikelythat such a
decrease in temperature would have contributedtotheapparent differenceincontractility duringtheischemicperiodsince
con-ditionsweresimilar for the
transgenic
andnontransgenic
hearts.Significant
differences betweentransgenic
andnontransgenic
hearts appeared upon reperfusion. The earliest indication of
protectionwasat1min ofreperfusion.Thenontransgenichearts released alargeamount of creatinekinase, indicatingcelland
membrane
disruption,
whereas the transgenic hearts released almost no creatine kinase(Fig. 6).
The contractile force and±dF/dt recovered stronger in mice
overexpressing
humanHSP70 (Fig. 5). Almost all hearts in both groups recovered
contractility
with 1 minofreperfusion. Forboth groups, initial contractileforcewasstrongand then declinedat5minof reper-fusion. This decline in contractile force between 1 and 5 min ofreperfusion
may be indicative of free radicalinjury.
The transgenic heartsclearly
had better recovery of contractility, bothinitially
andoverthe30-minreperfusion
period.Perfusion pressure,
although
notsignificantly
different dur-ing the pre-ischemic perfusion period, appeared to be higherin nontransgenic thanin transgenic mice(Fig. 7). During the reperfusion period, when flow rate was regulated at 3.0 ml/ min, thetransgenic hearts had a significantly lower perfusion pressure. At presentwe are uncertain whetherthe lower perfu-sion pressure isaresultof the transgene
product
inthe cellsof themicrovessels.However,itwaspreviously
reportedthatprior heat shock treatmentdid tend to lower theperfusionpressure inisolatedrathearts( 11).Ontheotherhand,otherexperiments suggest that heat shock pretreatment elevated the product of heartratemultiplied bythemeanarterial pressure(rate -pres-sure
product)
in rabbits(14).
The presence of human HSP70 in the transgenic hearts
seemstoplayarole in theimproved post-ischemiccontractile recovery. Withreperfusionand the introduction of oxygen, oxy-genfree radicals are generated, and membranes are disrupted
by lipid
peroxidation.
The release of creatinekinase appearstobe the result of suchinjury. AlthoughhumanHSP70seems to beinvolved in
reducing
thisinjury,it is unclear whether human HSP70 is involveddirectly
orindirectly.
Studies of humanhsp7O-transfected
myocytes haveshownincreased resistancetohypoxic or metabolic stresses mimicking ischemia (37, 38). One
possibility
is that humanHSP70, byitsabilitiestorenatureprotein,is modifyingthe activity of enzymes such as
antioxi-dants,
whichcanprotectcells from free radicalinjury. Althoughthere isnodirect evidence that heat shockproteinscanchange the enzymatic
activity
of antioxidants, catalase activity (11) and reducedglutathione (39)arereportedtobeincreased after heat shocktreatment.Inthepresentexperiments,catalaseactiv-ity
didnotappear to beincreased in the transgenic compared withnontransgenichearts.However,conclusions about this low catalaseactivity
should be made cautiously, since thesemea-surements were determined in transgenic and nontransgenic hearts thatwerestored at- 80°C for several weeks.
Insights
on the role of human HSP70can besuggested byanalyzing
themyocardial contractility during reperfusion.First,the
apparent
stronger
contractile force recovery at 1 min ofreperfusion suggests that there are more myocytes surviving after the 30-min ischemic interval. Second, contraction rate
(+dF/dt) and relaxation rate (-dF/dt) of transgenic hearts progressively increased during reperfusion, whereas the
non-transgenic hearts progressively decreased. This suggests that reperfusion injury is less in the transgenic hearts. One might
argue that human HSP70 protects myocytes against anoxia-induced celldeath and increases the resistance ofmyocytes to
oxidativeinjury. HSP70maybindtoproteins denaturedduring ischemia andpromoterefoldingorrenaturationtonormal
con-figurations uponreperfusion.
These experiments with transgenic mice strongly suggest
thatanelevated level of inducible HSP70 plays arole in cell survival andrecoveryafterischemic injury. Inaddition, itseems
that alterations in gene expression, whether induced or engi-neered (as in this study), can play an important role in the
survivalof cells. Understanding theroleplayed bygene
expres-sionand how toregulate such expressionafter infarction and
other ischemic injuries will lead to new ways ofintervening therapeutically in cardiovascularinjury.
Acknowledgments
We thank Dr. SylviaCraig, Director of AnimalCare,Facultyof Medi-cine, for heradvice and assistance on theimportation of the miceto DalhousieUniversity. This work wasfundedby grants from theNew Brunswick Heart and Stroke Foundation (R. W. Currie), from the NATO International Scientific Exchange Programmes(G. N.Pagoulatos and R. W. Currie), andfromthe General Secretariat of Science and Technologie of Greece (G. N. Pagoulatos) and by a HumanCapital and Mobility grant of the European Community (G. N. Pagoulatos).
References
1. Chien, K. R. 1993. Molecular advances incardiovascular biology. Science (Wash DC). 260:916-917.
2.Mulligan,R.C. 1993. The basic science of gene therapy. Science (Wash. DC). 260:926-931.
3.Zhu, N.,D.Liggit,Y.Liu,and R. Debs. 1993.Systemic geneexpression afterintravenous DNAdeliveryinto adult mice. Science (Wash. DC). 261:209-211.
4.Yang, G., P. H.Chan,J.Chen, E.Carlson, S. F. Chen, P. Weinstein, C. J. Epstein,and H. Kamii.1994. Human copper-zinc superoxide dismutase transgenic mice arehighlyresistant toreperfusion injuryafter focal cerebral ischemia. Stroke. 25:165-170.
5.MacMillan,V., D.Judge,A.Wiseman,D.Settles, J. Swain, and J. Davis. 1993. Miceexpressing abovine basicfibroblast growth factor transgene in the brain showincreased resistance to hypoxemic-ischemic cerebral damage.Stroke.. 24:1735-1739.
6.Milano,C. A., L. F.Allen,H.A.Rockman, P. C. Dolber, T. R. McMinn, K. R.Chien,T. D.Johnson,R. A. Bond, and R. J. Lefkowitz. 1994. Enhanced myocardialfunction in transgenic mice overexpressing the,62-adrenergicreceptor. Science(Wash DC). 264:582-586.
7.Mitchell,H.K.,G. Moller, N. S. Petersen, and L. Lipps-Sarmento. 1979. Specific protectionfromphenocopyinduction by heat shock. Dev. Genet. 1:181
-192.
8.Li,G.C.,and Z. Werb. 1982. Correlation between thesynthesis of heat shockproteins and the development ofthermotolerance in Chinese hamster fibro-blasts.Proc.Nati.Aca~dSci. USA.79:3918-3922.
9.Landry,J., and P.Chretien. 1983. Relationship between hyperthermia in-ducedheatshock proteins and thermotolerance in Morris hepatoma cells. Can. J. Biochem.61:428-437.
10.Currie, R. W., M. Karmazyn, M. Kloc, and K. Mailer. 1988. Heat-shock responseis associated with enhanced post-ischemic ventricular recovery. Circ. Res.63:543-549.
11.Karmazyn,M., K.Mailer, and R. W. Currie. 1990. Acquisition and decay of heat-shock-enhanced post-ischemic ventricular recovery. Am. J. Physiol. 259:H424-H431.
12.Yellon,D.M., E.Pasini,A.Cargnoni, M. S. Marber, D. S. Latchman, and R. Ferrari. 1992. Theprotective role of heatstress in the ischaemic and reperfusedrabbitmyocardium.J.Mol. Cell.Cardiol.24:342-346.
13. Donnelly, T. J., R. E. Sievers, F. L. J. Vissern, W. J. Welch, and C. L. Wolfe. 1992. Heat shock protein induction in rat hearts. A role for improved myocardial salvage after ischemia and reperfusion? Circulation. 85:769-778.
14.Currie, R. W., R. M. Tanguay, and J. G. Kingma, Jr. 1993. Heat-shock response and limitation of tissue necrosis duringocclusion/reperfusion in rabbit hearts. Circulation. 87:963-971.
15. Marber, M. S., D. S. Latchman, J. M.Walker, and D. M. Yellon. 1993. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardialinfarction. Circulation. 88:1264-1272.
16. Hutter, M. M., R. E. Sievers, V. Barbosa, and C. L.Wolfe. 1994. Heat-shockprotein induction in rat hearts: a directcorrelation between the amount of heat-shock protein induced and the degree ofmyocardial protection. Circulation. 89:355-360.
17. Angelidis, C. E., I.Lazaridis, and G. N. Pagoulatos. 1991. Constitutive expressionof heat-shockprotein 70in mammalian cells confers thermotolerance. Eur. J. Biochem. 199:35-39.
18. Li, G. C., L. G. Li, Y. K. Liu, J. Y. Mak, L. L. Chen, and W. M. Lee. 1991. Thermal response of rat fibroblasts stablytransfected with thehuman 70-kDa heat shockprotein-encodinggene. Proc. NatI.Acad. Sci. USA. 88:1681-1685.
19. Hogan, B.,F.Constantini, and E. Lacey. 1986. Manipulating the Mouse Embryo. A Laboratory Manual. ColdSpringHarborLaboratoryPress,ColdSpring Harbor, NY.332pp.
20. Brinster, R. L., H. Y. Chen, M. E. Trumbauer, M. K. Yagle, and R. D. Palmiter. 1985. Factors affecting the efficiency of introducing foreign DNA into miceby microinjectingeggs. Proc. Natl. Acad. Sci.USA. 82:4438-4442.
21.Kollias, G., N. Wrington, J. Hurst, and F. Grosveld. 1986. Regulated expressionof humanAy_,f-,and hybridyf3-globingenes intransgenic mice: manipulationof the developmentalexpressionpatterns. Cell. 46:89-94.
22.Southern,E. M. 1975.Detection ofspecificsequences among DNA frag-mentsseparated by gelelectrophoresis. J. Mol. Biol. 98:503-517.
23. Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isola-tionbyacidguanidinium thiocyanate-phenol-chloroformextraction.Anal. Bio-chem 162:156-159.
24.Hunt, C., and R. I. Morimoto. 1985.Conserved features of eukaryotic hsp70genes revealed bycomparison with the nucleotide sequence of human hsp70.Proc.Natl.Acad. Sci. USA. 82:6455-6459.
25. Maxam, A. M., and W. Gilbert. 1980.Sequencing end-labeled DNA with base-specificchemical cleavages. MethodsEnzymol.65:499-560.
26. O'Farrell, P. H. 1975. High resolutiontwo-dimensional electrophoresis of proteins. J.Biol. ChemL 250:4007-4021.
27. Currie, R. W. 1986. Synthesis of stress-induced protein in isolated and perfusedrathearts.Biochem. Cell.Biol. 64:418-426.
28.Towbin, H., T. Staeheln, and J. Gordon. 1979. Electrophoretic transfer of proteins frompolyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc.Natl.Acad. Sci. USA.76:4350-4354.
29. Johnson, D. A., J. W.Gautsch, J. R. Sportsman, and J. H. Elder. 1984. Improved techniqueutilizing nonfat dry milk for analysis of proteins and nucleic acids transferred to nitrocellulose. Gene Anal. Tech. 1:3-8.
30.Rosalki,S. B. 1967. Animproved procedurefor serum creatine phosphoki-nasedetermination. J. Lab.Clin.Med.69:696-705.
31. Beers, R.F., andI. W. Sizer. 1952. Aspectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195:133-140.
32.Cohen,G.,D.Dembiec,and J. Marcus. 1970. Measurement of catalase activityin tissue extracts.Anal. Biochem. 34:30-38.
33. Lowry,0. H., M. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurementwith the Folinphenol reagent. J. Biochem. 193:265-272.
34. Discroll, M. 1992. Moleculargenetics ofcell death in the nematode Caenorhabditiselegans.J.Neurobiol. 23:1327-1351.
35.Gagliardini, V., P.-A.Fernandez,R. K. K.Lee, H. C. A.Drexler, R. J. Rotello,M. C.Fishman, andJ. Yuan. 1994. Prevention of vertebrate neuronal deathby the crmA gene. Science(Wash. DC). 263:826-828.
36.Morimoto,R. I. 1993. Cells in stress:transcriptionalactivationof heat shockgenes. Science(Wash. DC).259:1409-1410.
37.Mestril, R., S.-H. Chi, M. R. Sayen, K. O'Reilly, and W. H. Dillman. 1994.Expressionof inducible stressprotein 70 in rat heart myogenic cells confers protection against stimulated ischemia-inducedinjury. J. Clin. Invest. 93:759-767.
38.Williams,R.S., J. A. Thomas, M.Fina,Z.German,andI. J.Benjamin. 1993. Human heat shockprotein 70 (hsp7o)protects murine cells frominjury duringmetabolic stress. J. Clin. Invest.92:503-508.
39.Yellon,D.M., E. Pasini,A.Cargnoni, M. S. Marber, D. S. Latchman, and R. Ferrari. 1992. The protective roleof heat stress in the ischaemic an reperfusedrabbitmyocardium.J.Mol. Cell.CardioL 24:895-907.