Publisher’s version / Version de l'éditeur:
Official Digest, 36, 470, pp. 232-243, 1964-04-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
The water absorption and water vapor permeability of clear organic
coatings
Ashton, H. E.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=fd52e780-ff1c-454e-bd15-889796699bc1 https://publications-cnrc.canada.ca/fra/voir/objet/?id=fd52e780-ff1c-454e-bd15-889796699bc1
Ser
T H l
N21r2
no. 217
c.2
BLDC
NATIONAL
RESEARCH
COUNCIL
C A N A D A
DIVISION O F BUILDING RESEARCH
THE WATER ABSORPTION
AND WATER VAPOR PERMEABILITY OF CLEAR
ORGANIC COATINGS
BY H. E. ASHTON REPRINTED FROM O F F I C I A L DIGEST. VOL. 3 6 . N O . 470. M A R C H 1964. P. 2 3 2-
243. PRICE 2 5 CENTSRESEARCH PAPER NO. 217
OF THE DIVISION OF BUlL
OTTA
T h i s p u b l i c a t i o n i s being d i s t r i b u t e d by t h e Division of Building R e s e a r c h of the N a t i o n a l R e s e a r c h Council. It s h o u l d not be r e p r o d u c e d in whole o r In p a r t , w i t h o u t p e r m i s - s i o n of t h e o r i g i n a l p u b l i s h e r . T h e D i v i s i o n would b e glad t o b e of a s s i s t a n c e i n o b t a i n i n g s u c h p e r m i s s i o n . P u b l i c a t i o n s of the D i v i s i o n of Building R e s e a r c h m a y b e o b t a i n e d by m a i l i n g the a p p r o p r i a t e r e m i t t a n c e , ( a Bank, E x p r e s s , o r P o s t Office Maney O r d e r o r a c h e q u e m a d e p a y - a b l e a t p a r in O t t a w a , to t h e R e c e i v e r G e n e r a l of Canada, c r e d i t National R e s e a r c h Council) t o the N a t i o n a l R e s e a r c h Council, Ottawa. S t a m p s a r e not a c c e p t a b l e .
A coupon s y s t e m h a s b e e n i n t r o d u c e d t o m a k e p a y - m e n t s f o r p u b l i c a t i o n s r e l a t i v e l y s i m p l e . C ~ u p o n s a r e a v a i l - a b l e i n d e n o m i n a t i o n s of 5, 2 5 a n d 50 c e n t s , and m a y be ob- t a i n e d by m a k i n g a r e m i t t a n c e a s indicated a b o v e . T h e s e c o u p o n s m a y b e u s e d f o r the p u r c h a s e of a l l N a t i o n a l R e s e a r c h C o u n c i l p u b l i c a t i o n s including s p e c i f i c a t i o n s of t h e Canadian G o v e r n m e n t S p e c i f i c a t i o n s B o a r d .
The Water Absorption
And
Water Vapor Permeability
Of
Clear Organic Coatings
By HARRY E. ASHTON National Research Council*
Canada
T h e three factors which degrade a clear coating o r the wood substrate are light, water, and oxygen. T h e paper covers the trans- mission and absorption by clear organic coatings of one of the agents-water. T h e range of materials tested include pure phenolic varnishes, air-drying alkyds, cold-cured epoxies, and two types of urethanes. Absorption was found not to vary with composition and was extremely low for all the ~ ~ n p i g m e n t e d materials. Both wet and dry cup permeabilities were measured. At e q ~ ~ a l f i l n ~ thickness permeability was not affected by the number of coats. Permeability correlated very well with conlposition and increased with increasing oil content of phenolic varnishes and alkyd resins. It decreased with increasing molecular weight of a series of varnishes. There was, however, no correlation between permeability and durability. An amide-cured epoxy which had tlie lowest permeability also h a d a low durability rating. T u n g oil-p. phenyl phenolic i.arnis11es which gave the best exterior exposures were intermediate in pernleability. T h e ratio of wet cup to tlry cup permeability was close to o n e in- dicating the materials are not water sorbing. T h e results show that pern~eability and water nbsosptioi~ are not related.
INTRODUCTION
For several years the Division of Building Research, Natio~lal Re- search Coullcil, has been working on a project on clear coatings for ex- terior wood. This project had its inception in the cooperative testing program that led to the develop~llent of ASTM Method Dl641 : Test for Exterior Durability of Varnishes. T h e work was continued and ex- panded because of the great interest in clear coatings o n the part of architects, builders, and homeowners. Many users had approached the Division for advice because they consideretl that clear finishes had not
*Organic Materials Section, Division of Building Research, Ottawa, Canada.
WATER ABSORPTION AND PERMEABILITY OF ORGANIC COATINGS
given adequate performance. T h e project includes both exterior ex- posures and laboratory investigations.
A broad range of alkyd a n d phenolic varnishes was exposed at Ottawa in 1955. Based on the results obtained, selected alkyds and phenolics were exposed in 1960, together with other types such as ure- thanes, epoxies, and pigmented stains. A report on their exterior dura- bilities will be published at a later date. Laboratory work has been designed to provide a better understanding of the materials a n d of the properties which contribute the most to their durability.
T h e three chief factors which cause coatings, or in some cases the wood beneath them, to degrade are light, water, and oxygen. Other factors such as ozone, temperature, or sudden changes in temperature might have some influence but the first three are consiclered to be the most important. All three agents have to be transmitted partially or com- pletely through the film in order to cause sufficient degradation to clis- r u p t the film or the substrate. Consequently knowledge of transmission rates through coatings can be of value in explaining the mechanisms of protection and degradation.
With clear coatings light transmission is generally consiclered to be the dominant factor and it is now being investigated in the laboratory program. Although the importance of oxygen transmission is recognized, no work on this factor has yet been undertaken. T h e study of the trans- mission and the absorption of water by clear films has been completed and this work is now reportecl.
Water absorption by free films was examined first. T h e different materials absorbed only very small quantities of water and the perme- ability of a range of materials was then checked to see whether there were differences in this property. When it was shown that the films did transmit water a t different rates, the program was broadened to cle- termine the effect of film thickness, number of coats, and composition of the coatings. Finally, attempts were inacle to correlate permeability with clurabili ty.
PREVIOUS WORK
Much of the work on absorption and permeability has been carried out on pigmented coatings. T h e pre-eminent name in the field of be- havior of paint films upon immersion i n water is that of Browne who has investigated most of the different factors which affect t h e swelling of the films.ls223 A few unpigmented films were included in some of the investigations a n d Dr. Browne reported a considerable variation be- tween different binders in water absorption as per cent by volume of the film.
I n two papers concerned with corrosion of steel, the Pittsburgh Society for Paint Techriology reported studies of water absorption,
water permeability and water vapor permeability of seven different types of clear coating^.^ T h e water absorptions were very similar with a range, excepting one material, of only 1
%
by weight of the film while the water permeabilities differed by a factor of 4. T h e water vapor permeabilities were not reported because they did not correlate with corrosion. I t was concluded that oxygen permeability was a t least as important as waterin the rusting of steel.
MATERIALS AND METHODS
Materials
I n T a b l e 1 are listed those materials the permeabilities of which were measured. T a b l e 1 includes most b u t not all of the coatings that have been studied in the over-all project. T h e phenolic varnishes were cooked in a stainless steel beaker by the usual open-kettle process. T h e desired viscosity was B-D at 50% solids except where certain factors were being examined. T h e alkyds were obtained commercially and all the phthalic resins were from the same manufacturer. T h e viscosity was ad- justed as close to B-D as possible without reducing the solids content too much. T h e urethanes and epoxy solutions were made to contain 50% solids after mixing.
Film Preparation
Free films were used for both the absorption and vapor permeability. I n this laboratory such films are obtained from photographic paper ac- cording to the method of Harris.5 Because water is involved in this procedure a n d also in the subsequent tests, preliminary experiments were made comparing tin foil, tin plate, and photographic paper methods to see whether the water used in the last-mentioned methocl would affect the absorption results. All other tests were made on film strippetl from photographic paper. Attempts were also made to obtain free films of lin- seed oil in order to compare results with those of Browne. None of the procedures producecl linseed films that could be hantlled o r tested.
A mechanical drawblade apparatus with vacuum plate was used to apply material to all substrates. T h e photographic sheets were removed from the vacuum plate and taped to flat panels to prevent curling. Films were dried seven days at 23
+
2°C and 50+.
2% R. H . before stripping. They were conditioned at least another seven clays before testing. Film thickness was measured by micrometer.Water Absorption
Films were cut into squares of 2 sq. in. (13 s q cm)
.
They were weighed and immersed in clistilletl water. At the end of the specified time they were blottetl between sheets of absorbent tissue paper to re- move surface water ancl immediately reweighed. I n the tests for effect of substrate, films were immersed for 1, 2, 4, 6, 8, 16, and 24 hours a n dWATER ABSORPTION AND PERMEABILITY OF ORGANIC COATINGS
T a b l e 1 - C o a t i n g s U s e d in P e r m e a b i l i t y a n d A b s o r p t i o n T e s t s
Number Composition Phenolic Varnishes*
I'ara p h e n y l phenolic P a r a p h e n y l phenolic l'ara p h e n y l phenolic I'ara p h c n y l phenolic I'ara p h c n y l phcnolic I'SI-a p h c n y l phenolic I'ara p h c n y l phcnolic P a r a p h e n y l p h e ~ t o l i c I'ara p h c n y l phenolic I'ara pllenyl p h e ~ ~ o l i c P a r a p l i c ~ i y l phenolic I'ar;~ plicny 1 phenolic I'ara p h c n y l phenolic I'ara p h e n y l phenolic Reactive plicnolict Noii-reactive p h e n o l i c t NOII-reactive p l i e ~ ~ o l i c t I,, '11 .. a I ~ ~ l t y l p h e ~ ~ o l i e 50% T u n g o i l 66.7v0 T u n g o i l 80% T u n g oil 75% T u n g oil - n o r m a l cook 75Yo T u n g oil - varnish n o t cooked 75v0 T u n g o i l - varnish u n d e r cooked
?.
-
/ > y o T u n g o i l - n o r m a l cook
75% T u n g o i l - varnish sl. ove~cooked 75% T u ~ i g oil - varnish m o d . overcooked 75% T u n g oil - varnish overcooked 66.7% Linseed oil
75yo Linseed oil 80% Linseed oil
46% T u n g , 23.7% linseed, 2.25% castor 73.8% T u n g o i l
73.8% T u n g oil
6G.3y0 T n n g oil, 7.6% bodied linseed oil 49.5% T u n g oil, 16.8% bodied linseed oil
Alkyd Solutions**
91 1 lsoplithalic alkyd 85% Linseed-Soya oil 55% solids 912 L o w viscosity p1ith;ilic alkyd 62.5% Soya o i l 50.2q0 solids 913 M e t l i u ~ n viscosity plitlialic alkytl (i?.5% Soya oil 50.2% solids 914 M e t l i u ~ t t viscosity plitlialic alkycl 56y0 Soya oil 50.2% solids 91.5 H i g h viscosity plitlialic alkytl 48Yo Soya oil 42% solicls !)l(i H i g h viscosity p l i ~ l i a l i c alkycl 38.5% Soya oil 40% solids 9 17 hlctliurn viscosity p h t h a l i c alkyd 51 % Linseed oil 43.2% solids
Urethanes .. -~ - ~.. . -- ~ 8.1-I Oil ~ ~ ~ o t l i l i e d u r e t h a n e 850 Castor oil c ~ ~ r e t l u r e t h a n e 11.451) C o ~ i i ~ i ~ c r c i ; ~ l oil nlotlifietl u r e t h a n e Epoxies 851 A m i ~ ~ e c u r e d 852 A n i i u c - ; ~ t l t l ~ ~ c t c ~ i r c t l 853 Poly;~nlitlc cnrctl
I n order lo comp;lre wit11 thc alkyds and bccause of the differcnce between the Canadian
~ I I I ~ U . S. gallo~~s, the var~lisl~es are calculated as percent oil rather than the usual oil length. They corrcspollcl approxi~liatcly to 10, 20, 30 and 40 gals. Imp. or 12, 24, 36 and 48 gals. U. S.
t Believed to Lx para tcrt butyl phenolic.
* * Oil content c;llculated from reported Fatly acids content according to Payne (reference 6)
H a r r y
E.
Ashton
was graduated from the University of British Columbia with First Class Honors. For eight years h e was engaged in development work, paint analysis, production statistics a n d costing for General P a i n t Corp. of Canada, Ltd. Since 1956, h e has been with the Paint Laboratory of the Divi- sion of Building Research, National Research Council, where his work has consisted mainly of development and improvement of paint test methods, special product de- velopment for government departments, a n d assistance with specification writing.
2, 5, 8, and 12 days. T h e immersion times for the range of materials were
1; 2, 4, 8, and 24 hours. Weighings were made on a single pan analytical balance.
Permeability
ASTRI E96-53T procedure A, dry cup, and procedure B, wet cup, were used. T h e conditions for the test were as follows:
Temperature
External relalive humidity, yo
Internal relative humidity, %
Vapor pressure difference ill. mercury
Dry Cup Wet C u p
73°F (23°C) 73°F
50 50
Of l o o t
0.4090 0.4090
'Desiccant-calcium chloride. tDistilled water inside cup.
Filnls were cut to fit the permeability cups and mounted using a mixture of paraffin and micro-crystalline waxes to prevent leakage. Results were obtained in perms, i.e., grains per hour, per square foot, per inch of mercury vapor pressure difference.
TEST RESULTS
Absorption
EFFECT OF SUBSTRATE: A long oil varnish ( o n ~ a i n i n g 80';1, Lung oil antl 20% 11. phenyl-phenolic resin was used. I t was thought that i t woultl absorb more water than a short oil varnish. Films were stripped from tin plate and tin foil by mercury amalgamation antl from photographic paper by moistening with water. Films could not be obtained from aluminum panels and aluminum foil because amalgamation did not take place. T h e free films from the three substrates weighed about 43 mg. After immersion for the various times the weight changes ranged from +0.5 to -0.6 mg indicating little if any waler absorption.
WATER ABSORPTION AND PERMEABILITY OF ORGANIC COATINGS
In spite of the magnitude of the weight changes, Student's "t" test showed that the tin foil method was statistically different from the other two. It was noted during the experiment that a slight residue of tin was left on films stripped from foil. Since this substrate generally resulted in weight losses i t was believed that some of this residue had fallen off dur- ing- immersion. T h e other two substrates gave equivalent results. Because of hazards in the use of mercury with tin plate and the ease of the pho- tographic paper method, the latter was used in all subsequent work.
EFFECT OF COMPOSITION: Three films of each material for each time
were immersed in distilled water. Because of differences in film thickness, the films varied in weight from 40 to 50 mg for the phenolic varnishes to 80 to 100 mg for the isophthalic alkyd. T h e largest weight changes were
t 2 . 6 mg and -0.6 mg. T h e largest gains were restricted to one material, the short oil alkyd, while the maximum for the other 12 samples was 1.6 mg. This is rather surprising in view of the composition.
T h e vast majority oE changes were ahout9.3 mg. Such small amounts could he due entirely to weighing techniques since even a desiccated flask will vary by that amount at different weighings. There was no constant trend with time and the average for each period varied erratically from those before or after. Each material followed approximately the same er- ratic cycle. This also indicates that most of the changes were due to differ- ences in weighing technique and not to actual absorption. T h e mean per
Type
Phenolics
88'2 5070 Tung oil-phenolic
894 759" Tung oil~phenolic
895 75% Tung oil-phenolic not cooked 900 75% Tung oil-phenolic overcooked 903 75% Linseed oil-phenolic
'907 75% Tung oil-reactive phenolic 910 66.3% Oil cold~mixed varnish Alkyds
911 Long oil isophthalic 912 Long oil soya-phthalic 914 Medium soya-phthalic 916 Short soya-phthalic Urethanes
844 Oil modified 850 Castor oil cured
Epoxies
851 Amine cured
853 Polyamide cured
Mean A b s o ~ p t i o n
cent absorption by weight for the different types are given below. T h e results are also calculated in per cent by volume (g/100 cc), the same units used by Browne i n his studies.
9
Absorption by weight 1s quite low except for the short oil alkyd. Gen- erally the phenolics are lower than the other types. Although the volume figures appear to be significantly larger in some cases, it must be re- membered that the figures are based on the same very small weight changes. They are still much smaller than the results of 1.5% for a phen- olic varnish and 7.7% for a n alkyd given by Browne.2 T h e weight results are considerably closer to those given by the Pittsburgh Society.*
Permeability
Water vapor permeability as distinct from water permeability was also measured. This property is hereafter referred to simply as "perme- ability." Because of the relatively small differences in, and small amounts of, water absorption hy the different kinds of films, the permeabilities of a selection of materials were determined first. I t was shown that the dif- ferent films transmitted water vapor a t rates sufficiently different to justi-
fy
further tests.FILM THICKNESS AND NUMBER OF COATS: Since varnishes are generally
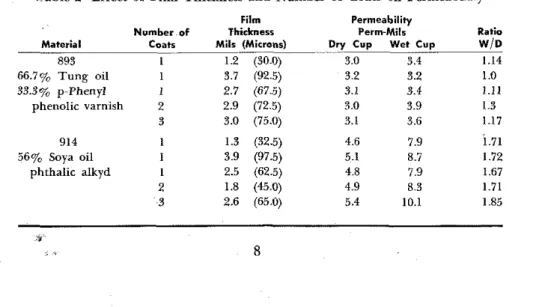
applied in three coats whereas the permeability films are prepared from single coats, a few tests were run to check whether the number of coats affected the results. Conceivably pinholes in a single coat could increase the permeability. Also, because the three-coat system is much thicker than the one-coat pelmeability film, the effect of thickness in a single coat was determined. This was performed to ensure that the permeability measure- ments were related to the thickness of service films and that permeance is inversely related to film thickness. T o compare results on the same basis they were calculated as perm-mils, i.e., the number of perms a 1-mil (25 microns) film would pass (Table 2).
Table 2-Effect of Film Thickness and Number of Coats on Permeability
66.7% Tuns 011 1 33.376 p Phenyl 1 phenolic varnish 2 3 914 1 56% Soya oil 1 phthalic alkyd I 2 3 Film Thirlmerr Mils (Micrenr) --- 1.2 (30.0) 3.7 (92.5) 2.7 (67.5) 2.9 (72.5) 3.0 (75.0) 1.3 (32.5) 3.9 (97.5) 2.5 (62.5) 1.8 (45.0) 2.6 (65.0) Permeability Perm.Mil. Dry Cup Wet Cup
3.0 3.4 3.2 3.2 3.1 3.4 3 0 3.9 3.1 3.6 4.6 7.9 5.1 8.7 4.8 7.9 4.9 8.3 5.4 10.1 Ratio J'J 1.14 1 .o
WATER ABSORPTION AND PERMEABILITY OF ORGANIC COATINGS
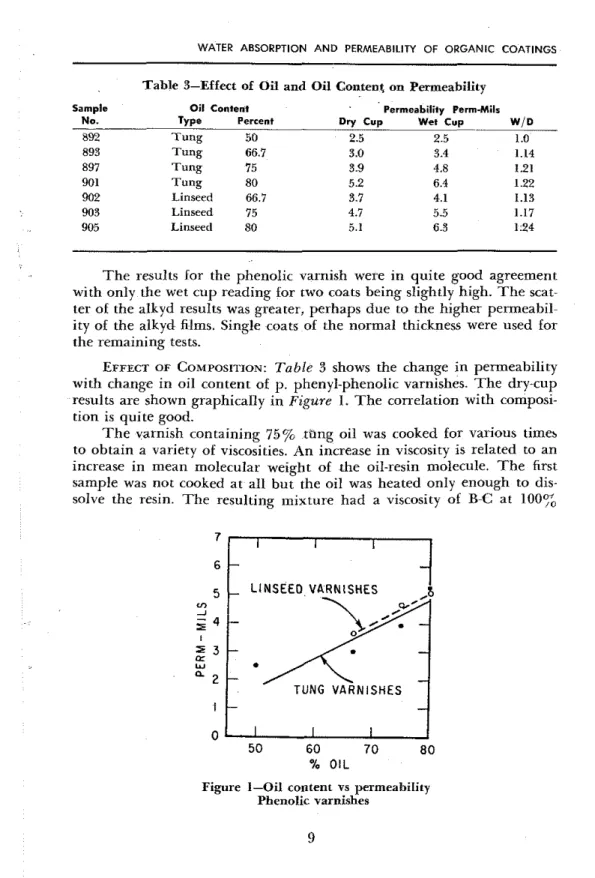
Table 3-Effect of Oil and Oil Content on Permeability
Sample Oil Content Permeability Perm.Mil. No. Type percent Dry Cup Wet Cup W I D
892 Tung 50 2.5 2 5 1 0 893 Tung
-
66.7 3.0 3.4 1.14 897 Tung 75 3 -9 4.8 1.21 901 Tung 80 5.2 6.4 1.22 902 Linseed 66.7 3.7 4.1 1.13 903 Linseed 75 4.7 5.5 1.17 905 Linseed 80 5.1 6.3 1.24T h e results for the phenolic varnish were i n quite good agreement with only the wet cup reading for two coats being slightly high. T h e scat- ter of the alkyd results was greater, perhaps due to the higher permeabil- ity of the alkyd films. Single coats of the normal thickness were used for the remaining tests.
EFFECT OF COMPOSITION: Table 3 shows the change i n permeability with change in oil content of p. phenyl-phenolic varnishes. T h e dry-cup results are shown graphically i n Figure 1. T h e correlation with composi- tion is quite good.
T h e varnish containing 7570 tong oil was cooked for various times to obtain a variety of viscosities. An increase i n viscosity is related to an increase in mean molecular weight of the oil-resin molecule. T h e first sample was not cooked at all but the oil was heated only enough to dis- solve the resin. T h e resulting mixture had a viscosity of B C at 100yo
I n Y
.
TUNG VARNISHES 50 60 70 80 % OILFigure 1-Oil content vs permeability
V I S C O S I T Y I N STOKES
Figure 2-Viscosity vs permeability of 30 gallon tung-phenolic varnish
Figure 3-Oil content vs permeability Soya alkyd resins
WATER ABSORPTION A N D PERMEABILITY OF O R G A N I C COATlNGS
solids. T h e solids content was reduced to 65y0 so that it would be closer to the cooked varnishes and any difference in durability would not be due to film thickness. Fifty per cent butyl acetate had to be added to xylene to keep the resin in solution. There is thus evidence that in var- nish cooking the resin reacts with the oil and is not merely dispersed since even the under-cooked varnidhes were soluble in mineral spirits contain- ing only 10% xylene. T h e change in dry-cup-permeability with increasing molecular weight is shown in Figure 2. Again the corwlation is quite good.
In Figure 3 tke dry-cup results for the series of soya-phthalic alkyds are shown. With the alkyds both the effects of oil content and viscosity are shown together. The permeability of the low viscosity long oil alkyd was measured at a different time which may account for some of the large differences in results.
The permeabilities of the remaining materials are given in Table 4. T h e dry-cup results of a number of different materials are ananged be- low in order of inueasing permeability. If this property is directly related to clear coating durability, the first material shown should have the best durability. Unfortunately this is not the case.
The same materials were applied to red cedar siding cut to a 30-in. (76.2 cm) length with an exposed face of 6% in. (16.5' cm)
.
They were mounted at the Ottawa site in a vertical position facing south. Three sid- ing panels for each coating were prepared. The first coat-was reducedTable 4-Permeabilities of Different Matuials
Phenolic varnishes
p-Phenyl phenolic to TT-V-119 Reactive phenolic tung Alkyl phenolic tung Alkyl phenolic tung-linseed Rutyl phenolic cold mix varnish
Alkyds Long oil isophthalic
Medium linseed phthalic
Urethanes
Oil modified Castor oil cured
Commercial oil modified coating
EPO-Y
Amine cured Amine-adduct cured Polyamide cured
Dry Cup -wet Cup
-Ratio
with the appropriate thinner to A-2 viscosity. T h e two succeeding coats were applied a t approximately C-D viscosity and 50% total solids a t 2 4 hr. intervals. T h e spreading rate was 800 sq. ft. (74.32 sq m) per gal. Each panel was assigned a position on the test fence using a random num- ber table. T h e panels were examined at regular intervals and rated ac- col-ding to state of degradation, designated as follows:
10 No change 8 Slight 6 Moderate 4 Marked 2 Severe 0 Complete failure
When the exposure ratings after two years of exterior exposure in Ottawa are compared with the permeabilities there is no correlation.
N O . Tree 853 Polyamide-epoxy
. .
851 Amine-expoxy 850 Castor oil-urethane 892 50% T u n g phenolic 893 66.7% T u n g phenolic 844 Oil modified urethane 907 73.87, T u n g reactive phenolic 910 Cold mixed phenolic915 48Y0 Soya alkyd 914 ?6% Soya alkyd 906 TT-V-119 phenolic 11459 Commercial urethane 911 Isophthalic alkyd 'Men" of S panels. Exposurc Rating: Mean* Lowest 3 2 5- 2 7 i 6 6 5 8 8 5+ 3 3- 2 7.5 7 5- 2 6+ 6 7.5 6 7- 9 5- 4
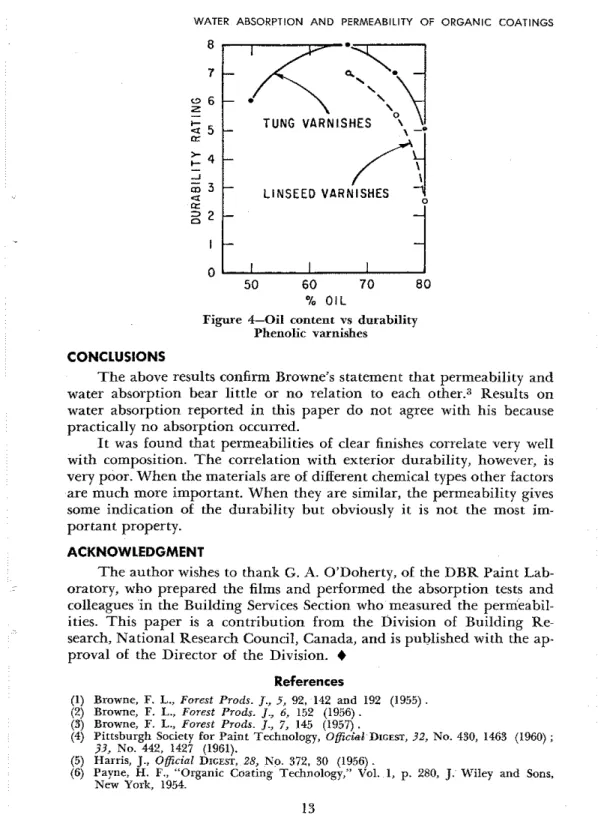
T h e ratings were made independently by two observers and averaged for three panels. T h e mean may be misleading because in some cases one or two of the panels failed badly. This was true of samples 851, 814 and 915. I n F i g w e 4 the durability ratings for the different p. phenyl-phenolic varnishes have been plotted for comparison with the results given in
Fig-
ure 1. Here the correlation is somewhat better. Disregarding the short oil varnish, the durability increased with decreasing permeability. T h e al- kyds, however, did not show any correlation.
WET CUP: DRY CUP RATIO: Materials of moderate to low permeahil- ity which are water sorbing will often show a ratio of wet cup to dry cup permeability of 3. T h e ratio for non-water-sorbing materials such as poly- ethylene is about 1. T h e values obtained with the clear finishes range from 1.1 to 1.8. This agrees with the findings that there was little or no absorption upon water immersion. T h e phenolic varnishes and epoxies were 1.1 to 1.2 while the alkyds were much higher at 1.5 to 1.8. This could indicate different mechanisms for the transmission of water.
WATER ABSORPTION AND PERMEABILITY OF ORGANIC COATINGS n
m
3 L I N S E E D VARNISHES 0 5 0 60 7 0 80 % O I LFigure 4-Oil content vs durability Phenolic varnishes
CONCLUSIONS
The above results confirm Browne's statement that permeability and water absorption bear little or no relation to each other.3 Results on water absorption reported in this paper do not agree with his because practically no absorption occuned.
I t was found that permeabilities of clear finishes correlate very well with composition. The correlation with exterior durability, however, is very poor. When the materials are of different chemical types other factors are much more important. When they are similar, the permeability gives some indication of the durability but obviously it is not the most im- portant property.
ACKNOWLEDGMENT
The author wishes to thank G. A. O'Doherty, of the DBR Paint Lab- oratory, who prepared the films and performed the absorption tests and colleagues i n the Building Services Section who measured the permeabil- ities. This paper is a contribution from the Division of Building Re- search, National Research Council, Canada, and is published with the ap- proval of the Director of the Division.
+
References
(1) Browne. F . L., Forest Prods. J., 5, 92, 142 and 192 (1955).
(2) Brome, F. L., Forest Prods. J , 6, 152 (1956)
.
(3) Browne, F . L., F o ~ e s t Prods. I., 7 , 145 (1957).
(4) Pittsburgh Society for Paint Technology, Oficiel DIGEST, 32, No. 430, 1463 (1960) ;
33, No. 442, 1427 (1961).
(5) Harris, J., Oficial DIGEST, 28, KO. 372, 30 (1956).
(6) Payne, H. F., "Organic Coating Technology," Vol. 1, p. 280, J . M'iley and Sons.