Cardiovasc Intervent Radiol (1997) 20:204-210
Pictorial Essays
CardioVam
and Interventional
Radiology
9 Springer-Verlag New York Inc. 1997
Ablation of Hepatocellular Carcinoma by Percutaneous Ethanol
Injection: Imaging Findings
Christoph D. Becker, 1 M a r i a n n e Grossholz, 1 Gilles Mentha, 2 Arnaud Roth, 2 Emiliano Giostra, 3
Pierre-Alain Schneider, l Franqois Terrier 1
1 Division of Diagnostic and Intervenfional Radiology, University Hospital of Geneva, 24 Rue Micheli-du-Crest, CH-1211 Geneva, Switzerland
2 Department of Surgery, University of Geneva, 24 Rue Micheli-du-Crest, CH-1211 Geneva, Switzerland
3 Division of Gastroenterology, University Hospital of Geneva, 24 Rue Micheli-du-Crest, C H - 1211 Geneva, Switzerland
Percutaneous ethanol injection (PEI) is an accepted
treatment option for selected patients with hepatocel-
lular carcinoma (HCC). PEI induces local tumor necro-
sis as a result of protein denaturation, cellular
dehydration, and occlusion of small vessels. PEI allows
selective treatment of limited HCC without significant
side effects on the adjacent liver parenchyma, and is,
therefore, suitable even in advanced stages of cirrhosis
with impaired liver function [1-3]. PEI has also been
used to treat secondary hepatic neoplasms under certain
circumstances, but its role and efficacy for this purpose
remain to be defined [4].
Another well-established modality for treating un-
resectable HCC is intraarterial chemoembolization.
This is often performed with an emulsion of doxoru-
bicin and lipiodol which accumulates preferentially
within HCC nodules. The intraarterial technique can be
used as a regional or segmental treatment and is partic-
ularly suitable in the presence of daughter nodules or
multiple HCC. To combine the advantages of intravas-
cular segmental treatment and direct percutaneous local
treatment of tumor nodules it has been recommended
to use chemoembolization and PEI sequentially [5].
Our current treatment protocol for unresectable HCC
is as follows. Single nodules with a diameter < 3 cm
are treated with PEI alone. Single nodules < 5 cm and
up to three lesions < 3 cm are treated with a combi-
nation of chemoembolization and PEI. Patients with
nodules > 5 cm or with widespread tumor disease are
not treated with PEI but with chemoembolization alone
or with systemic chemotherapy, or are given no treat-
ment at all.
The technique of PEI has been well described [1 -
3]. The total volume of ethanol to be injected into a
Correspondence to: C.D. Becker, M.D.
neoplastic lesion may be determined according to the
formula V = 4/37r (0.5 + r) 3, where V is the volume in
milliliters, and r the radius of the lesion in centimeters.
Treatment protocols vary in terms of volume of abso-
lute ethanol administered per session, and in using PEI
either alone or combined with intraarterial chemoem-
bolization [2, 5, 6]. Although the response to PEI is
most promising in lesions < 3 cm, ablation of larger
tumors is also feasible.
Needle placement, monitoring of PEI, assessment
of the treatment effect, and follow-up are done with
cross-sectional imaging techniques. Image-guided fine-
needle aspiration biopsy enables direct cytologic proof
of residual or recurrent tumor, but negative sampling
errors may easily occur in treated, partially necrotic
lesions. Therefore, knowledge of the changes observed
on cross-sectional images obtained during and after PEI
greatly facilitates diagnostic and therapeutic decisions.
We have used PEI with ultrasound (US) and com-
puted tomography (CT) guidance, either alone or in
combination with chemoembolization, in 35 patients;
32 of these had histologically proven HCC and 3 had
secondary lesions of gastrointestinal neoplasms. We
describe the immediate, early, and late changes ob-
served on US, CT, and magnetic resonance (MR) im-
ages after PEI treatment.
Monitoring of PEI
PEI may be done with US guidance whenever the le-
sion to be treated is sufficiently well visualized. In ad-
dition to its low cost and availability, US monitoring
offers the advantage of visualizing the distribution of
ethanol directly because microbubbles injected with the
ethanol are hyperechogenic (Fig. 1). However, when
C.D. Becker et al.: Imaging of PEI 205
Fig. 1. US-guided percutaneous ethanol injection (PEI) of hepatocellular carcinoma
(HCC) and follow-up with CT. A Contrast enhanced CT scan (venous phase) shows a hypoattenuating mass 5 cm in diameter within the right hepatic lobe corresponding to a single HCC nodule. B On US, the mass is hyperechogenic and well defined. A special 21 gauge needle with multiple side holes but no end hole (Ago Pia, Milan, Italy), has been inserted under US guidance; the position of the needle tip close to the posterior border of the lesion (arrow) indicates that the side holes are in an appropriate position. C Injection of ethanol causes echogenic artifacts within the lesion and acoustic shadowing due to microbubbles, thus allowing real-time monitoring of the distribution of ethanol. D After injection of 20 ml of ethanol, the lesion becomes obscured by these artifacts. E Contrast enhanced spiral CT (venous phase) obtained 24 hr later demonstrates the ef- fect of PEI. The smooth, regular, enhancing rim around the nonenhancing tumor persist- ing in the venous phase was interpreted as reactive hyperemia rather than residual tumor. No further treatment was given. There was no evidence of residual tumor or local tumor recurrence on follow-up.
Fig. 2. CT-guided PEI of large HCC. HCC lesion 5 cm in diameter was treated with a total volume of 170 ml of absolute ethanol administered
in six PEI sessions. The distribution of ethanol is well visualized because it is hypoattenuafing compared with tumor and liver tissue. Note that some ethanol is visible in the liver tissue adjacent to the tumor (arrow).
Fig. 3. Periportal tracking of ethanol during CT-guided PEI of a recurrent HCC nodule in the left hepatic lobe after fight hepatectomy.
Hypoattenuating ethanol is not only seen within the treated lesion but also follows a linear track along the portal system of the left hepatic lobe (arrowheads). l a r g e v o l u m e s o f e t h a n o l a r e i n j e c t e d , t h e l e s i o n e v e n - t u a l l y b e c o m e s o b s c u r e d b y a c o u s t i c s h a d o w i n g a n d r e v e r b e r a t i o n artifacts (Fig. 1D). C T g u i d a n c e is p r e - f e r r e d f o r P E I w h e n e v e r U S d o e s n o t p e r m i t s u f f i c i e n t v i s u a l i z a t i o n o f t h e l e s i o n to e n a b l e t h e n e e d l e to b e p l a c e d c o r r e c t l y , p a r t i c u l a r l y in l a r g e t u m o r s that o f t e n r e q u i r e m u l t i p l e P E I s e s s i o n s (Fig. 2). C o n t r a s t - e n - h a n c e d d y n a m i c C T and, e s p e c i a l l y , spiral C T is su- p e r i o r to U S f o r v i s u a l i z i n g t h e e x t e n t o f n e c r o s i s i n d u c e d b y P E I . P E I - i n d u c e d n e c r o s i s r e s u l t s in a l a c k o f e n h a n c e m e n t , a n d t h e h y p o d e n s e , n e c r o t i c a r e a s a r e w e l l d e l i n e a t e d o n C T , u s u a l l y w i t h a t t e n u a t i o n v a l u e s o f 3 0 - 3 5 H U (Fig. 1E). I n j e c t e d e t h a n o l m a y b e v i - s u a l i z e d o n C T as it is h y p o a t t e n u a t i n g c o m p a r e d w i t h t u m o r a n d l i v e r t i s s u e a n d e v e n m o r e h y p o a t t e n u a t i n g t h a n n e c r o s i s ( < 2 0 0 H U ) (Fig. 2). E x t r a t u m o r a l t r a c k - i n g o f e t h a n o l is s e e n a f t e r i n j e c t i o n o f l a r g e v o l u m e s a n d u s u a l l y o c c u r s a l o n g t h e p o r t a l triad (Fig. 3). I f P E I
206 C.D. Becker et al.: Imaging of PEI
Fig. 4. Combined treatment of HCC with chemoembolization and PEI: immediate CT findings. A Contrast-enhanced spiral CT shows a HCC with a diameter of 3 cm before treatment with chemoembol- ization with doxorubicin and iodized oil. B Unenhanced scan ob- tained 4 weeks after selective intraarterial chemoembolization and immediately before PEI. The lesion is hyperattenuating due to reten- tion of iodized oil. C Unenhanced scan obtained immediately after PEI. A mixed-density pattern is seen owing to the hypoattenuating ethanol. D Early follow-up. Contrast-enhanced spiral CT scan ob- tained for follow-up several days after PEI. The hypoattenuating areas have disappeared, but some residual hyperattenuating areas due
to iodized oil are still visible. E, F Follow-up study obtained 10 months after treatment because of rising alpha-fetoprotein levels. Dual-phase spiral CT, same study. E Arterial phase. The treated le- sion within the right lobe shows no enhancement (large arrows). Some residual hyperattenuating lipiodol is visible within the lesion. However, there are now multiple hyperattenuating lesions visible mainly throughout the left hepatic lobe (small arrows). F Venous phase. The treated lesion shows no enhancement (large arrows). The small nodules are less well visualized than in the arterial phase be- cause they have become almost isoattenuating or slightly hypoatten- uating with the surrounding liver parenchyma (small arrows).
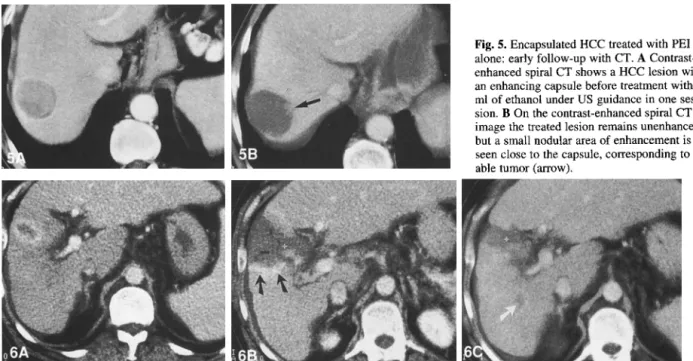
is d o n e after c h e m o e m b o l i z a t i o n , a m i x e d - d e n s i t y pat- tern m a y be o b s e r v e d due to hyperattenuating, retained, i o d i z e d oil and h y p o a t t e n u a t i n g ethanol (Fig. 4). Tu- m o r s that are w e l l defined, such as H C C with a capsule, are w e l l suited for P E I b e c a u s e the ethanol distributes r e l a t i v e l y h o m o g e n e o u s l y within the lesion (Figs. l , 2, 4, 5). PEI o f p o o r l y defined, infiltrating t u m o r s is m o r e difficult to c o n t r o l (Fig. 6).
E a r l y
Follow-up After
P E IF o l l o w - u p studies at defined intervals are n e c e s s a r y in o r d e r to e v a l u a t e the effect o f PEI and to d e t e c t resid- ual, recurrent, or n e w t u m o r manifestations. R o u t i n e controls s h o u l d i n c l u d e b i o c h e m i c a l t u m o r markers, such as a l p h a - f e t o p r o t e i n levels, and i m a g e - g u i d e d bi- opsies. H o w e v e r , t u m o r m a r k e r s m a y fail to b e e l e v a t e d b e f o r e PEI, and c y t o l o g i c e x a m i n a t i o n m a y b e false n e g a t i v e after PEI in a c o n s i d e r a b l e n u m b e r o f cases. Therefore, the findings on d i a g n o s t i c i m a g i n g studies m u s t also b e t a k e n into c o n s i d e r a t i o n .
C o n t r a s t - e n h a n c e d , d y n a m i c , i n c r e m e n t a l or spiral C T is c u r r e n t l y the m o s t c o m m o n i m a g i n g t e c h n i q u e for f o l l o w - u p after PEI. The t y p i c a l b e h a v i o r o f H C C nodules on C T is h y p e r a t t e n u a t i o n c o m p a r e d with the s u r r o u n d i n g liver p a r e n c h y m a in the arterial phase, of- ten f o l l o w e d b y h y p o a t t e n u a t i o n in the v e n o u s phase. Therefore, C T is b e s t p e r f o r m e d in the f o r m o f dual- p h a s e spiral C T with an arterial and v e n o u s p h a s e (Fig. 4E, F). C T studies o f large t u m o r s o b t a i n e d within 2 4 - 48 hr after PEI m a y show small gas collections. A l - t h o u g h these are often due to small injected air bubbles, gas f o r m a t i o n m a y also o c c u r due to tissue necrosis [6]. A s with arterial e m b o l i z a t i o n , these c h a n g e s u s u a l l y d i s a p p e a r s p o n t a n e o u s l y and should not be m i s t a k e n for abscess formation. S e v e r a l i n v e s t i g a t o r s h a v e shown that c i r c u m s c r i b e d , n o d u l a r e n h a n c e m e n t in the area o f the treated lesion is a r e l i a b l e i n d i c a t o r o f re- sidual t u m o r [ 6 - 8 ] (Figs. 5B, 6B). O c c a s i o n a l l y , smooth, regular, p e r i p h e r a l e n h a n c e m e n t m a y be seen c o r r e s p o n d i n g to reactive h y p e r e m i a (Fig. 1E). E b a r a et al. [8] h a v e shown that residual t u m o r shows en- h a n c e m e n t in the early but not in the late phase,
Fig. 5. Encapsulated HCC treated with PEI alone: early follow-up with CT. A Contrast- enhanced spiral CT shows a HCC lesion with an enhancing capsule before treatment with 40 ml of ethanol under US guidance in one ses- sion. B On the contrast-enhanced spiral CT image the treated lesion remains unenhanced, but a small nodular area of enhancement is seen close to the capsule, corresponding to vi- able tumor (arrow).
Fig. 6. Infiltrating HCC treated with PEI alone: findings on contrast-enhanced spiral CT. A A hypervascular HCC is seen in the left lobe (segment 4) of a cirrhotic liver. The lesion is poorly defined against the surrounding parenchyma. B One day after PEI, a large hypoattenuating area is seen in segment 4, indicating necrosis. The enhancing area (arrows) corresponded to residual viable tumor on fine-needle aspiration biopsy and additional treatment was needed. C Six months later, the area of necrosis has decreased in size, indicating local tumor control. However, a small secondary lesion has appeared in the fight lobe (arrow).
Fig. 7. Small HCC treated with PEI and chemoembolizafion: MR findings. A Before txeatment. On the unenhanced Tl-weighted SE image a hyperintense nodule of 2 cm is seen in the fight hepatic lobe, which corresponded to an HCC nodule. B Early findings after PEI, On the unenhanced Tl-weighted SE image obtained after chemcembofization with doxorubicin and iodized oil and PEI, the hyperintense area has become much larger, probably due to hemorrhage. C On the corresponding T2-weighted FSE image (with fat saturation), the lesion is hyperintense. This could be due to hemorrhage or fiquefactive necrosis o1" residual viable tumor. D On the gadolinium-DTPA-enhanced Tl-weighted SE image the center of the lesion lacks enhancement, but there is intense enhancement at the periphery. These findings indicate that the center of the lesion has become necrotic but that residual viable tumor may be present at the periphery. E Follow-up 3 months ",ffter additional PEI. T2-weighted FSE image at the same level as C shows a lack of signal wifffin the tumor. The lesion is almost isointense compared with the surrounding parenchyma. These findings indicate coagulative necrosis.
208 C.D. Becker et al.: Imaging of PEI
4~
...
i " : ? ;
; i Z ;
Fig. 8. Chemical portal vein thrombus following US-guided PEI. Patency of the portal vein had been documented before US-guided PEI in this patient with a HCC in segment 4, adjacent to the left portal vein. The axial US image A and the sagittal image B were obtained 24 hr after PEI and show the tumor (73, and a solid thrombus in the left portal branch adjacent to the tumor (arrows). C, D These contrast-enhanced spiral CT images were obtained 6 days after US-guided PEI, and confirm the presence of a solid floating thrombus in the left main portal branch (arrows). E - G Contrast-enhanced spiral CT was repeated 4 months later. At this time, the tumor has decreased in size E, and the thrombus has disappeared (F, G).
whereas reactive inflammation shows enhancement in
both the early and the late phase.
Investigation of the MR imaging findings after PEI
has mainly been focused on HCC [9-15]. Although the
spatial resolution of MR images is currently still infe-
rior to that of CT images and T2-weighted images often
suffer from motion artifacts, it has been shown that MR
may be used as an alternative to CT to evaluate the
effect of PEI. The signal changes observed on MR im-
ages after PEI are somewhat more complex than the
changes observed on CT scans. The MR imaging ap-
pearance of untreated HCC is variable. This may be
attributable to several factors, including magnetic field
strength, tumor size, histologic tumor grade, presence
of a capsule, and fatty or hemorrhagic intratumoral
changes. On unenhanced Tl-weighted images, HCC
may be hyperintense (Fig. 7A), hypointense, or isoin-
tense relative to surrounding liver tissue. There is no
uniform behavior after intravenous injection of gado-
linium chelates, although most lesions show at least
some enhancement [9-11]. On unenhanced, T2-
weighted images, over 80% of HCC are moderately to
strongly hyperintense, but a significant minority is iso-
or hypointense [9, 12, 13]. The MR signal changes of
HCC induced by PEI with and without concomitant
chemoembolization have been analyzed in several
studies. The signal patterns on unenhanced T1-
weighted images are variable and nonspecific [9, 14].
An increased signal intensity within or around the le-
sion may be observed in the early phase after PEI and
can be explained by hemorrhage (Fig. 7B). High-signal
areas on T2-weighted images may correspond with
hemorrhage or liquefactive necrosis or reactive inflam-
mation but also with residual viable tumor (Fig. 7C)
[11, 15]. However, a homogeneously hypointense sig-
nal of a previously hyperintense tumor on a T2-
weighted sequence seems to correlate with coagulative
necrosis (Fig. 7E). In cases of doubt, Tl-weighted im-
ages obtained after intravenous administration of gad-
olinium chelates may be used to distinguish viable
tumor from liquefactive necrosis, since tumor shows
enhancement and necrosis does not [15] (Fig. 7D). Al-
though the necrotizing effects of PE1 and of chemoem-
bolization with iodized oil are different, the effects
observed on MR images after the combination of these
two modalities are the same as with PEI alone. Barto-
lozzi et al. [14] have shown that retention of iodized
oil after chemoembolization does not interfere with the
C.D. Becker et al.: Imaging of PEI 209
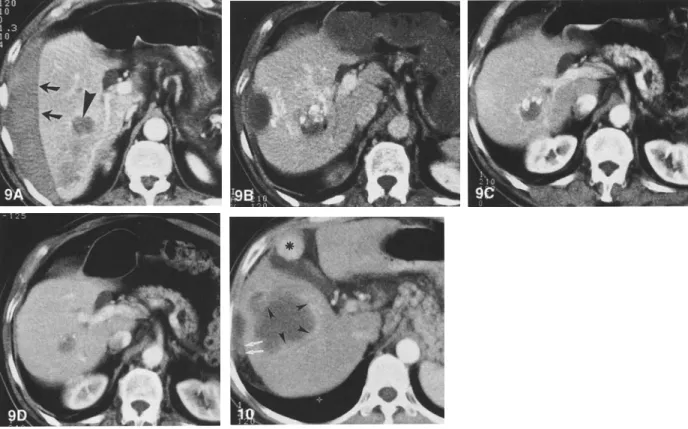
Fig. 9. Long-term follow-up after PEI of a small HCC in a noncirrhotic liver. A Contrast-enhanced spiral CT image shows a 3-cm HCC in the right lobe of a noncirrhotic liver (arrowhead). The tumor became manifest due to massive subcapsular hemorrhage (arrows) and a biopsy was done after spontaneous resolution of the hematoma. The serum alpha-fetoprotein level was significantly elevated. B Contrast-enhanced spiral CT image obtained immediately after PEI. Some densely hyperattenuating areas within the lesion are due to previous angiography with injection of iodized oil. Note the residual subcapsular hematoma. After three sessions of PEI, the alpha-fetoprotein levels became normal and remained so during further follow-up. C, D Contrast-enhanced spiral CT images show the lesion 7 months and 2.5 years after PEI; there was no evidence of tumor growth.
Fig. 10. Needle-tract seeding after PEI of HCC. Contrast-enhanced spiral CT image obtained 8 months after six PEI treatment sessions of the large HCC shown in Figure 2. At the previous puncture sites there is diffuse perihepatic infiltration corresponding with needle-tract seeding (arrows). Intrahepatically, there are multiple areas of nodular enhancement at the periphery of the treated lesion, consistent with recurrent tumor growth (arrowheads). However, biopsy of the second enhancing nodule adjacent to the gallbladder (asterisk) revealed no malignant cells.
M R signal pattern. T h i s m a y in fact b e an a d v a n t a g e o f M R i m a g i n g c o m p a r e d w i t h CT, on w h i c h r e t a i n e d io- d i z e d oil alters the attenuation v a l u e s c o n s i d e r a b l y (Figs. 4, 9). It is l i k e l y that the r e c e n t t e c h n i c a l ad- v a n c e s in the field o f M R I , such as fast and " u l t r a - f a s t " sequences, w i l l i m p r o v e the quality o f T 2 - w e i g h t e d i m a g e s as m o t i o n artifacts due to respi- ration can b e reduced.
G r a y - s c a l e U S c a n n o t r e l i a b l y d e l i n e a t e residual v i a b l e t u m o r tissue. C o l o r D o p p l e r U S has b e e n rec- o m m e n d e d for a s s e s s i n g the effect o f PEI t r e a t m e n t [16]. In our e x p e r i e n c e , h o w e v e r , o n l y a m i n o r i t y o f H C C s h o w e d an a d e q u a t e c o l o r flow signal due to h y - p e r v a s c u l a r i t y that w o u l d h a v e e n a b l e d us to d e t e c t small r e s i d u a l foci o f v i a b l e tumor. P o w e r D o p p l e r m a y e n h a n c e the a b i l i t y o f U S to d e t e c t s m a l l t u m o r foci with m o d e r a t e vascularity. T h e role o f [ 1 8 F ] f l u o r o d e o x y g l u c o s e p o s i t r o n - e m i s s i o n t o m o - g r a p h i c i m a g i n g r e m a i n s to b e d e t e r m i n e d [17]. S e l e c -
tive a r t e r i o g r a p h y has b e e n u s e d in e a r l y studies [ 1 ] but a p p e a r s too i n v a s i v e for serial f o l l o w - u p tests after PEI u n l e s s p e r f o r m e d in the c o u r s e o f c o m p l e m e n t a r y c h e m o e m b o l i z a t i o n . I f d o u b t persists r e g a r d i n g resid- ual m a l i g n a n t tissue, p e r c u t a n e o u s f i n e - n e e d l e aspira- tion b i o p s y s h o u l d a l w a y s be p e r f o r m e d .
Complications
S i d e e f f e c t s o f P E I i n c l u d e t r a n s i e n t l o c a l p a i n a n d f e v e r , e s p e c i a l l y a f t e r i n j e c t i o n o f l a r g e v o l u m e s o f e t h a n o l . P E I is a s a f e t r e a t m e n t a n d s e r i o u s , p r o c e d u r e - r e l a t e d c o m p l i c a t i o n s are rare [ 18]. A s w i t h p e r c u t a n e o u s f i n e - n e e d l e p u n c t u r e in general, p n e u - m o t h o r a x and h e m o r r h a g e are p o t e n t i a l but rare c o m - plications. S u p e r i n f e c t i o n o f necrotic t u m o r tissue is t h e o r e t i c a l l y p o s s i b l e but has not b e e n f o u n d to b e a significant c o n c e r n after PEI. D u e to its s e v e r e t h r o m -210 C.D. Becker et al.: Imaging of PEI b o g e n i c effect, e t h a n o l m a y o c c a s i o n a l l y i n d u c e a por-
tal vein thrombus. Such " c h e m i c a l " thrombi b e c o m e visible i m m e d i a t e l y after P E I (Fig. 8). T h e y are usually reversible a n d should not be c o n f u s e d with t u m o r t h r o m b u s [3].
Long-term Follow-up
PEI, if p e r f o r m e d in l i m i t e d HCC, enables effective t u m o r destruction a n d control o f local t u m o r growth (Fig. 9). T h e results o f recent l o n g - t e r m studies suggest that the overall survival rates after PEI treatment are c o m p a r a b l e to those after surgical resection, m a i n l y de- p e n d i n g o n t u m o r size a n d C h i l d ' s stage of cirrhosis [ 1 - 3 , 8, 18]. Early o b s e r v a t i o n s have suggested that P E I c o m b i n e d with c h e m o e m b o l i z a t i o n is even more effective than P E I alone [5]. R e c u r r e n t manifestations of H C C after P E I m a y occur locally or in the form o f single or m u l t i p l e intrahepatic secondary lesions (Figs. 4E, F a n d 6). Needle-tract seeding is an u n c o m m o n p h e n o m e n o n b u t has b e e n observed after PEI treatment of both H C C a n d liver metastases [ 1 9 - 2 1 ] (Fig. 10).
Summary
Since P E I is a t r e a t m e n t based o n i m a g i n g techniques, the radiologist should be familiar with the various find- ings that m a y be observed after P E I on US, CT, and M R i m a g e s i m m e d i a t e l y after treatment and d u r i n g later follow-up, A l t h o u g h US is well suited for per- f o r m i n g PEI, c o n t r a s t - e n h a n c e d C T currently is the most c o m m o n l y used i m a g i n g m e t h o d to evaluate the effect o f PEI. Residual, n o d u l a r areas of contrast en- h a n c e m e n t correlate well with residual t u m o r and war- rant additional treatment. A l t h o u g h the findings o n M R images o b t a i n e d after P E I are more complex, M R i m - aging m a y be used as an alternative to CT.
References
1. Shiina S, Tagawa K, Unuma T, Fujino H, Uta Y, Niwa Y, Hata Y, Komatsu Y, Shiratori Y, Terano A, et al. (1990) Percutaneous ethanol injection therapy of hepatocellular carcinoma: Analysis of 77 patients. AJR 155:1221-1226
2. Livraghi T, Bolondi L, Lazzaroni S, Marin G, Morabito A, Ra- paccini GL, Salmi A, Torzilli G (1992) Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrho- sis. Cancer 68:925-929
3. Livraghi T, Solbiati L (1993) Percutaneous ethanol injection in liver cancer: Method and results. Semin Intervent Radiol 10:69-
77
4. Livraghi T, Vettori C, Lazzaroni S (1991) Liver metastases: Re- sults of percutaneous ethanol injection in 14 patients. Radiology 179:709-712
5. Tanaka K, Nakamura S, Numata K, Okazaki H, Endo O, Inoue S, Takamura Y, Sugiyama M, Ohaki Y (1992) Hepatocellular carcinoma: Treatment with percutaneous ethanol injection and transcatheter arterial embolization. Radiology 185:457-460 6. Livraghi T, Lazzaroni S, Pellicanb S, Ravasi S, Torzilli G, Vet-
tori C (1993) Percutaneous ethanol injection of hepatic tumors: Single-session therapy with general anesthesia. A JR 161:1065-
1069
7. Joseph FB, Baumgartner D, Bemardino ME (1993) Hepatocel- lular carcinoma: CT appearance after percutaneous ethanol in- jection. Radiology 186:553-556
8. Ebara M, Kita K, Sugiura N, Yoshikawa M, Fukuda H, Ohto M, Kondo F, Kondo Y (1995) Therapeutic effect of percutaneous ethanol injection on hepatocellular carcinoma: Evaluation with CT. Radiology 195:371-377
9. Nagel HS, Bernardino ME (1993) Contrast-enhanced MR im- aging of hepatic lesions treated with percutaneous ethanol abla- tion therapy. Radiology 189:265-270
10. Lencioni R, CarameUa D, Bartolozzi C (1993) Response of he- patocellular carcinoma to percutaneous ethanol injection: CT and MR evaluation. J Comput Assist Tomogr 17:723-729
11. Sironi S, De Cobelli F, Livraghi T, Villa G, Zanello A, Taccagni G, DelMaschio A (1994) Small hepatocellular carcinoma treated with percutaneous ethanol injection: Unenhanced and gadolin- ium-enhanced MR imaging follow-up. Radiology 192:407-412 12. Kadoya M, Matsui O, Takashima T, Nonomura A (1992) He- patocellular carcinoma: Correlation of MR imaging and histo- pathologic findings. Radiology 183:819-825
13. Sironi S, Livraghi T, Angeli E, Vanzulli A, Villa G, Colombo E, Taccagni G, DelMaschio A (1993) Small hepatocellular car- cinoma: MR follow-up of treatment with percutaneous ethanol injection. Radiology 187:119-123
14. Bartolozzi C, Lencioni R, Caramella D, Falaschi F, Cioni R, DiCoscio G (1994) Hepatocellular carcinoma: CT and MR fea- tures after transcatheter arterial embolization and percutaneous ethanol injection. Radiology 191:123-128
15. Bartolozzi C, Lencioni R, Caramella D, Mazzeo S, Ciancia EM (1994) Treatment of hepatocellular carcinoma with percutaneous ethanol injection: Evaluation with contrast-enhanced MR imag- ing. AJR 162:827-831
16. Lencioni R, Caramella D, Bartolozzi C (1995) Hepatocellular carcinoma: Use of color Doppler US to evaluate response to treatment with percutaneous ethanol injection. Radiology
194:113-118
17. Lee MJ, Mueller PR, Dawson SL, Gazelle SG, Hahn PF, Gold- berg MA, Boland GW (1995) Percutaneous ethanol injection for the treatment of hepatic tumors: Indications, mechanism of ac- tion, technique and efficacy. A JR 164:215- 220
18. Livraghi T, Giorgio A, Marin G, Salmi A, de Sit I, Bolondi L, Pompili M, Brunello F, Lazzaroni S, Torzilli G, et al. (1995) Hepatocellular carcinoma and cirrhosis in 746 patients: Long- term results of percutaneous ethanol injection. Radiology 197:101-108
19. Goletti O, De Negri F, Pucciarelli M, Sidoti F, Bertolucci A, Chiarui M, Seccia M (1992) Subcutaneous seeding after percu- taneous ethanol injection of liver metastasis. Radiology 183:785-786
20. Cedrone A, Rapaccini GL, Pompili M, Grattagliano A, Aliotta A, Trombino C (1992) Neoplastic seeding complicating percu- taneous ethanol injection for treatment of hepatocellular carci- noma. Radiology 183:787-788
21. Zerbey AL, Mueller PR, Dawson SL, Hoover HC (1994) Pleural seeding from hepatocellular carcinoma: A complication of per- cutaneous alcohol ablation. Radiology 193:81-82