Altered Spacing of Promoter Elements Due to the Dodecamer Repeat Expansion Contributes to Reduced Expression of the Cystatin B Gene in EPM1
Texte intégral
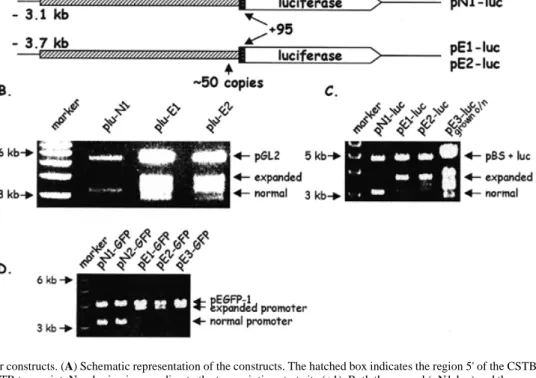
(2) 1792 Human Molecular Genetics, 1999, Vol. 8, No. 9. Figure 1. CSTB promoter constructs. (A) Schematic representation of the constructs. The hatched box indicates the region 5' of the CSTB transcript and the black box the 5'-UTR of the CSTB transcript. Numbering is according to the transcription start site (+1). Both the normal (pN1-luc) and the expanded (pE1-luc and pE2luc) clones begin from the same 5' EcoRI site, but the latter are larger due to the inclusion of ~50 copies of the dodecamer. The insert of pE1-luc was subcloned from patient GVA05 DNA and that of E2-luc from patient GVA08a DNA (5,6). (B) EcoRI and HindIII restriction digestion analyses of the normal and expanded promoters cloned into vector pGL2. The expanded promoters were unstable when cloned into pGL2 (shown here) and pGL3 (data not shown; see Materials and Methods). (C) The luciferase gene from the pGL3 vector was cloned downstream of the normal and expanded promoters in pBS. The clones were grown for 8 h and digested with EcoRI and HindIII. The expanded promoters were considerably more stable than those shown in (B). However, the clones were unstable when grown overnight (lane 5). (D) Constructs carrying the normal (pN1-GFP and pN2-GFP) or expanded CSTB promoter (pE1-GFP, pE2-GFP and pE3-GFP) in the GFP vector pEGFP-1. GFP constructs were more stable than the luciferase constructs shown in (B). Other than the exchange of the reporter genes, the GFP constructs are as shown in (A).. determine whether a promoter with an expanded dodecamer repeat would cause reduced expression of reporter genes, as was observed in white blood cells and brain of EPM1 patients (5,8). We found that the promoter containing a repeat expansion or foreign DNA fragments of similar size showed reduced transcriptional activity in certain cell lines. Terminal deletions of the CSTB promoter identified a putative activating protein 1 (AP-1) site that is likely to be implicated in the activation of transcription in these cells. RESULTS Reporter gene constructs To establish an in vitro system to study whether the altered spacing of promoter elements caused by the repeat expansion contributes to transcriptional repression of CSTB, we sought to characterize the normal and mutant CSTB ‘promoters’. A 3.2 kb fragment (including 3.1 kb of 5' upstream region and 95 nt of 5'-UTR, called hereafter ‘the promoter’) from the EcoRI site upstream of the CSTB gene to the ATG translation initiation codon was subcloned into pBS upstream of the luciferase gene (clone pN1-luc). Identical 3.8 kb fragments, except that they contained 600 bp repeat expansions (~50 dodecamer repeats), were similarly cloned from two different EPM1 patients (clones pE1-luc and pE2-luc; Fig. 1A). The constructs were assembled into the pBS vector because the expanded pro-. moters were unstable in the commercially available luciferase plasmids (Fig. 1B and C; see Materials and Methods). Luciferase assays in transient transfection experiments As mentioned above, the amount of CSTB mRNA and protein in patients homozygous for the repeat expansion is reduced in white blood cells and brain, respectively (5,8). In contrast, CSTB expression in lymphoblastoid cell lines or cultured fibroblasts from the same patients varied from reduced to approximately normal (1,3,5). In order to select the cell lines to perform our experiments, we took into consideration these differences. We aimed to identify cell lines where the mutant CSTB promoter represses expression of a reporter gene, thus mimicking the pathogenic state in vivo. Expression of the above-described luciferase constructs was examined in four different cell lines, CHP, SK-N-BE (both derived from human neuroblastomas), HeLa and cos-7 (monkey kidney cells) (Fig. 2B–E). The neuroblastoma cell lines were chosen since the EPM1 patients show neurological defects. The normal promoter in construct pN1-luc resulted in luciferase expression in all four cell lines, showing at least 100fold activation above basal transcription of the plasmid without a promoter (pGL3 basic; Fig. 2B–E). The luciferase activity of the normal construct pN1-luc with three dodecamer repeats was considered to be 100%. The transfection efficiencies were.
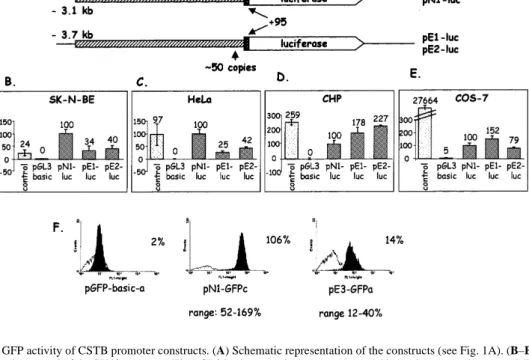
(3) Human Molecular Genetics, 1999, Vol. 8, No. 9 1793. Figure 2. Luciferase and GFP activity of CSTB promoter constructs. (A) Schematic representation of the constructs (see Fig. 1A). (B–E) The control vector contains the luciferase gene under control of the SV40 promoter. The pGL3 basic vector lacks a promoter and is used as a negative control. The numbers shown above the bars represent mean percent activity obtained from each construct in three experiments, considering that the luciferase activity from the normal promoter was 100%. (F) FACS analysis of representative isolated clones from stable transfections of SK-N-BE cells with the GFP constructs. pGFP-basic is a negative control plasmid without a promoter. One clone transfected with pN1-GFP (clone c) and one with pE3-GFP (clone a) are shown. The range of fluorescence levels obtained from five normal and four expanded individual clones are shown underneath each representative clone. Fluorescence is on the x-axis and number of cells on the yaxis. In each panel the white curve represents the basal fluorescence of the non-transfected SK-N-BE cells.. normalized using a second control plasmid (pRL; see Materials and Methods). In SK-N-BE and HeLa cells we observed a significant 2- to 4-fold reduction in luciferase activity from the constructs containing promoters with dodecamer expansions of 600 bp, compared with the normal promoter (29–42%; Fig. 2B and C). However, when the same constructs were transfected into CHP or cos-7 cells, either a small decrease or an increase in luciferase activity was observed (79–227%; Fig. 2D and E). This result is compatible with our previous observations of a reduction in CSTB mRNA associated with the dodecamer repeat expansion in certain cell types (5). Although both CHP and SK-N-BE are human neuroblastoma cell lines, the in vitro expression results differed significantly. This may be due to the different origin of the two cell lines. The CHP cells, while identified as a neuroblastoma cell line, originated from a kidney–adrenal tumor (9). The SK-NBE cell line exhibited neuronal marker enzyme activities and was established from a bone marrow metastasis of a child with disseminated neuroblastoma (10). All subsequent experiments were performed in SK-N-BE and HeLa cells, which showed reductions in promoter activity of the expanded dodecamer. GFP assays in stable transfection experiments In order to verify that the differences in luciferase activity described above were due to reduced transcription from the CSTB promoter carrying the repeat expansion and not due to variable transfection efficiencies of these clones, stably transfected cell lines were created. We inserted the same promoters in the pEGFP-1. vector carrying the modified enhanced GFP. The GFP constructs with the expanded promother were stable, unlike those of the luciferase vectors (Fig. 1D). Individual stably transfected SK-N-BE clones were selected in neomycin. PCR was performed to determine the size of the expansions carried in the isolated clones. All the clones transfected with expanded plasmids were found to have maintained large expansions of the dodecamer repeat (data not shown). The clones were analyzed using fluorescence-activated cell sorter analysis (FACS; Fig. 2C) and the results are expressed as percentages to facilitate comparison with the luciferase experiments. The mean expression of five normal clones (pN1-GFPa, pN1GFPb, pN2-GFPa, pN2-GFPb and pN2-GFPc) was considered to be 100%. The range of fluorescence was between 52 and 169%. The fluorescence emitted by all the examined clones with the expanded dodecamer was lower than that emitted by the normal clones (12–40%). Cells transfected with a GFP vector without a promoter had no detectable fluorescence. The range of fluorescence within each category of transfections is likely to be due to the different number of integrated copies of the plasmid or positional effects due to the site of integration. Insertion of heterologous DNA fragments In order to investigate whether the repeat expansion causes reduced expression in certain cells by altering the spacing of transcription factor binding sites from each other and/or the transcription initiation site(s), we substituted the repeat with heterologous fragments of bacterial and mammalian sequences of similar sizes. A 730 bp fragment of the kanamycin resistance gene and a 1000 bp fragment of the human PWP2 cDNA were cloned immediately.
(4) 1794 Human Molecular Genetics, 1999, Vol. 8, No. 9. Figure 3. Heterologous DNA insertions. (A) Schematic representations of the constructs containing inserted foreign DNA fragments compared with the normal with three dodecamer repeats. Either a 730 bp fragment of the kanamycin resistance gene (pN1-kanF and pN1-KanR, in both orientations) or a 1000 bp fragment of the human PWP2 cDNA (pN1-PWPR) were inserted immediately in front of the three dodecamers in clone pN1-luc. (B and C) Activity of the normal, expanded and foreign DNA-containing promoters in SK-N-BE and HeLa cells.. upstream of a normal three copy repeat. The clones with the inserted foreign DNA showed a significant 2- to 4-fold reduction in luciferase activity in both SK-N-BE and HeLa cells (Fig. 3B and C). This reduction is similar to that observed with the similarly sized dodecamer repeat expansion. From these results we conclude that spacing of some transcription factor binding sites upstream of the dodecamer repeat, either relative to other transcription factor binding sites or from the transcription initiation site(s), contributes to the reduction in downstream gene expression. Deletion mapping of promoter elements To map the regions of CSTB promoter responsible for basal transcription in the SK-N-BE neuronal cells we examined a series of deletions. A 2 kb internal deletion of the normal clone, created by partial digestion with PstI, showed no significant change in transcriptional activation in SK-N-BE cells (clone pN1-luc-2; Fig. 4). This observation prompted us to test whether the –307/+109 genomic fragment retained full transcriptional activity, which was subsequently found to be the case (data not shown). We attempted to create the equivalent construct from the expanded promoter. Unfortunately, the –307/+109 promoter containing the repeat expansion was always unstable in bacteria and the repeat was lost. This –307/+109 DNA fragment is likely to contain several transcription factor binding sites necessary for transcription activation in this cell line. As not all regulatory sequences show sequence homology, searches for transcription factor binding sites that are common to the human, mouse and rat sequences were performed using sensitive matrix-based programs. Analysis of the DNA sequence of the region –307/–101 upstream of the dodecamer repeat (human CSTB, accession no. U46692) and comparable regions of the mouse (11; accession no. U59807) and rat (12; accession no. D10607) CSTB loci using these promoter prediction programs and multiple sequence alignment (13) identified the following transcription regulator binding sites in common: two SP1, two. Figure 4. Transient transfection of an internal 2 kb deletion. (A) Schematic representation of the normal (pN1-luc) and the construct with the internal deletion (pN1-luc-2). (B) Luciferase activity of the clones shown in (A).. GC, one AP-2 and one AP-1 (Fig. 5A). Deletion of the first 77 nt (–230/+109, clone pN1-luc-4) eliminated the predicted AP-2 site (Fig. 5A). This clone showed similar expression to clone pN1-luc1 (Fig. 5B). Deletion of 29 nt downstream containing a predicted and conserved AP-1 site (–201/+109, clone pN1-luc-5) showed a 2-fold reduction in luciferase activity (Fig. 5B). The deletion of this region containing this putative transcription binding site is likely to be involved in the reduction in transcription. Deletion of the entire region upstream of the dodecamer repeat (–101/+109, clone pN1-luc-3) results in a similar 2-fold transcription reduction (Fig. 5B). Extrapolating from these results, this AP-1 transcription factor binding site is likely to be involved in transcription activation in SK-N-BE cells. DISCUSSION We have previously shown that CSTB is not expressed in the white blood cells of EPM1 patients carrying the dodecamer.
(5) Human Molecular Genetics, 1999, Vol. 8, No. 9 1795. Figure 5. Refinement of the minimal CSTB promoter. (A) Multiple sequence alignment of the human –307/+112 promoter fragment and its corresponding mouse and rat sequences. The ATG translation initiation sites are in bold and the dodecamer repeat, which is not present in the mouse and rat sequences, has been reduced to one dodecamer which includes the non-shaded boxed SP1/GC sequence. The first conserved SP1/GC box just after the AP-2 site is boxed and shaded in gray. The AP-2 and AP-1 sites are also boxed and shown above the sequence. (B) Schematic representation of the region and its conserved predicted binding sites. The luciferase activity of each construct is shown on the right. Expression of the clone pN1-luc-1 (–307/+109) is considered 100%.. expansion, but is normally expressed in their lymphoblastoid cell lines and cultured fibroblasts (5). It was also shown by other investigators using immunohistochemical staining that CSTB protein was absent from the brain of EPM1 patients homozygous for the expansion (8). The expression of CSTB in cultured cell lines from patients differ between reports and varies from almost normal to undetectable (1,3,5). The repeat expansion is likely to have a direct effect on CSTB transcrip-. tion. Possible explanations for this reduced transcription include altered spacing of promoter elements, hypermethylation, altered chromatin structure and recruitment of transcription repressors on the dodecamer repeat. Here we have tested the ‘spacing’ hypothesis and showed that altered spacing near the site of the repeat expansion within the putative CSTB promoter contributes to reduced transcription of reporter genes in selected cells in vitro. These experi-.
(6) 1796 Human Molecular Genetics, 1999, Vol. 8, No. 9. A. B. Figure 6. Model for transcriptional repression of the CSTB gene due to the dodecamer repeat expansion. For simplicity, all other regulators that contribute to transcription are referred to as the ‘basal transcription complex’. The dodecamers are represented by hatched boxes. (A) When two or three repeats of the dodecamer are present, the transcription activator can interact with the basal transcription complex. (B) The second panel illustrates the inability of the transcription activator to interact with the complex, due to the expansion, which shifted its binding site to a non-permissive distance.. ments revealed reduced expression of reporter genes driven by a CSTB promoter containing a repeat expansion of 600 bp (~50 dodecamers) compared with a normal promoter containing three copies of the dodecamer repeat in only certain cell lines (SK-N-BE and HeLa cell lines). However, the 2- to 4-fold reduction in transcriptional activity was not as dramatic as that observed in patients. It is possible that spacing contributes to the final reduction, but the very low levels of mRNA in patients may be the result of more than one mechanism. Substitution of the expanded repeat with a foreign DNA fragment of bacterial or eukaryotic origin of similar size resulted in a similar reduction in transcription. These results suggest that altered spacing of CSTB promoter elements is implicated in this transcription reduction. Deletions of the normal promoter and comparison with the mouse and rat CSTB promoters implicate a region containing a conserved AP-1 binding site in transcription activation in SK-NBE cells. AP-1 is a family of dimeric transcription factors, consisting of either homodimers of Jun or heterodimers of Jun, Fos and activating transcription factor (ATF) (reviewed in ref. 14). Different combinations of AP-1 factors regulate expression of different genes and, therefore, have distinct biological functions. Transcription activation or repression normally occurs by interactions of transcription factors directly (or indirectly via co-activators) with the essential components of the basal transcription complex already assembled on promoters (15). We propose the following model to explain the reduced transcriptional activity of the CSTB promoter carrying the dodecamer repeat expansion (Fig. 6). Normally, activators, possibly AP-1, bind near position –207 and interact with the basal transcription complex to promote transcription of CSTB. The expansion of the dodecamer repeat displaces the activator binding site to a distance where it can no longer interact with the rest of the transcription complex. Hypermethylation of the trinucleotide repeat CGG was found to cause transcriptional repression in fragile X syndrome (16,17). Hypermethylation of the MspI/HpaII sites of CSTB alleles containing the expansion was not observed in our previous studies (5), although this finding does not formally exclude hypermethylation of other CpG islands or the repeat itself. Changes in chroma-. tin conformation due to specific DNA sequences have been shown to modulate gene expression (reviewed in ref. 18). For example, in the corticotropin-releasing hormone gene, alternating purine and pyrimidine residues (>100 bp) in an intron of the gene predisposed the sequence to adopt the left handed Z-DNA conformation (19). In CSTB the sequence consists of only C and G residues, not, however, alternating. This might induce changes in the torsional strain of DNA and facilitate conformational changes under certain physiological conditions. It is not likely that recruitment of transcription repressors by the repeat expansion is the mechanism of reduced CSTB expression, at least in the in vitro system used in this study. Our experiments demonstrate that the repeat expansion-associated reduction in CSTB expression in EPM1 is at least partially due to the altered spacing of transcription factor binding sites from each other and/or the transcription initiation site(s). While spacing contributes to the final reduction in CSTB RNA, we cannot exclude the possibility that the low levels of mRNA observed in other studies may be due to the synergistic effect of more than one mechanism. The expanded promoter did not show the same transcriptional activity in all cell lines. In CHP and COS-7 cells the transcriptional activity of the expanded promoter was disimilar or slightly increased for reasons that remain to be explored. It is possible that in CHP and COS-7 cells the basal transcription complex is composed of a different combination of transcription factors, some of which are absent or less abundant in SK-N-BE and HeLa cells, not requiring an upstream activator for transcriptional activation. It is not known whether a similar situation to our in vitro results may exist in vivo, i.e. CSTB expression is only diminished in certain tissues and cell types, and if this is related to the neuronal phenotype of EPM1. The mouse with targeted disruption of the Cstb gene has a ubiquitous absence of Cstb and shows almost exclusively neurological defects, most notably epileptic seizures. In addition, a minority of these animals show corneal degeneration and opacity (7). This defect was present in two mouse genetic backgrounds, although one of the two did not develop seizures. There are no reports of an equivalent ophthalmic defect in EPM1 patients. It is possible either that lack of CSTB protein in humans does not cause this symptom or that the repeat expansion does not alter CSTB expression in the cornea. The detailed molecular pathogenesis of EPM1, whether caused by point mutations or expansions, remains only partially defined. The CSTB point mutations are, however, direct evidence that the disease is caused by lack of functional CSTB protein (2,20). The principally neuronal phenotype of EPM1 may be a direct and specific consequence of CSTB protein function in neurons. Although the exact role of CSTB has long been studied as a cysteine protease inhibitor, its precise physiological function(s) remains unclear (21). Alternatively, it is possible that the CSTB protein has the same role in all cells but that there is a lower threshold for its absence in neurons. The CSTB knockout mouse showed that cerebellar granule cells deficient in CSTB undergo increased apoptosis and are lost (7). The same may be true in EPM1 patients, but this remains to be tested. The definition of the exact role of CSTB in these cells will be important for the discovery of new drugs and new methods of treatment of these epilepsies..
(7) Human Molecular Genetics, 1999, Vol. 8, No. 9 1797. MATERIALS AND METHODS Cloning of the 5' region of the human CSTB gene A 6.2 kb fragment containing the normal cystatin B gene and ~3.2 kb of sequence immediately upstream of the ATG (translation initiation site) was subcloned from cosmid Q7A12 (LL21NCO2 library; 22) into the EcoRI and HindIII sites of pBS. Two identical clones, but each containing ~600 bp of repeat expansion, were isolated from genomic EcoRI and HindIII libraries of two patients with the dodecamer repeat expansion (5). In order to transfer the CSTB 5' regions upstream of the translation initiation codon in front of the luciferase reporter gene, a HindIII site was engineered using PCR immediately upstream of the ATG. A 420 bp fragment was amplified using primers CSTB.8L (5'-ctgcaggattgcccctactccgactg-3') and CSTB.20R-Hind (5'-CCCAAGCTTGGCGGCGACGGAGGGAATC-3'), under the same PCR conditions as described before (6). The PCR products and genomic clones were digested with XcmI and HindIII and the XcmI–HindIII PCR fragment was cloned into the two genomic clones. This resulted in clones pN1prom, pN2prom (normal), pE1prom, pE2prom and pE3prom (expanded) containing 3.2 kb (3.8 kb when an expansion was present) of sequence upstream of the ATG, presumably containing the CSTB promoter. Sections of clones generated by PCR were sequenced in their entirety. The clone pE2prom contained a single C→G transversion 14 bp upstream of the initiation codon ATG. This nucleotide is in a non-conserved region of 5'UTR between the human and rodent sequences. Luciferase constructs The promoter (normal 3.2 and expanded 3.8 kb) was cloned into the EcoRI and HindIII sites of the pGL2-basic and pGL3-basic vectors carrying two different luciferase genes (Promega). However, cloning of the expanded promoters in these luciferase plasmids resulted in instability and loss of the dodecamer repeat, although we used several different bacterial hosts deficient for different recombination genes or different mutations on the same gene, short culture times and different luciferase-containing plasmids (Fig. 1B). Because the expanded repeat was stable in pBS, we transferred the luciferase gene (from plasmid pGL3) into the HindIII–SalI sites downstream of the promoters in pBS. These constructs were stable for short culture times (Fig. 1C). An additional poly(A) element for background reduction was amplified from the pGL3-basic vector by PCR using primers pGL3-pA.2L (5'-CGAGCTCACGGGAGGTACTTGGAG-3') and pGL3pA.2R (5'-CGAGCTCTATCGATAGAGAAATGTTCTG-3') and cloned upstream of the promoter in the SacI site. The clones are pN1-luc, pN2-luc (normal) and pE1-luc, pE2-luc and pE3-luc (expanded). They were transformed into the Escherichia coli strain TOP10F' (Invitrogen). A 730 bp fragment of the kanamycin gene from vector pEGFP-1 (2104–2828) was amplified using primers KAN.1L (5'-GGAATTCGGCTATGACTGGGCAC-3') and KAN.1R (5'-GGAATTCAAGAAGGCGATAGAAGGCGATG-3') and cloned in pN1-luc-1 digested with MluNI. A fragment of the human PWP2 gene (nt 1483–2789, accession no. X95263) was amplified using primers HPWP.18L (5'-GGAATTCGTGTGGTCCATGCAGAC-3') and PWP.16R (5'-CCTCTAGAGGCCAGCATCTCTTCTTC-3') and digested with EcoRI that cuts on 18L and at postion 997 in the amplified fragment. Clone pN1-KanF was. digested with EcoRI and the 1000 bp PWP2 fragment was cloned into that site substituting for the kanamycin insert. Clones pN1-luc-1 and pN1-luc-2 were produced by complete and partial digestion of clone pN1-luc with PstI, respectively, and religation. Clone pN1-luc-3 was produced from clone pN1-luc by digestion with SpeI and MluNI, end-filling and religation. For clone pN1-luc-5 a PCR fragment amplified using primers CSTB.21L (5'-GGAATTCGGCGCCCGGAAAGACGATAC-3') and CSTB.21R-Hind (5'-GTGCTGGGATTACAGGTGTGAG-3') was cloned into pN1-luc digested with EcoRI and HindIII. For clone pN1-luc-4 a fragment amplified with CSTB.8L and CSTB.20RHind was cut with EagI and HindIII and cloned into pN1-luc-1 digested with NotI and HindIII. Transfections and luciferase activity DNA was prepared in Qiagen tips and used to transfect four mammalian cell lines: HeLa, SK-N-BE, CHP-126 (all human) and COS-7 (monkey). Transfection experiments were performed in six-well plates. Each transfection was performed in three to four replicates. The cells were co-transfected with a control vector (pRL) containing a different luciferase gene, in a 1:50 molar ratio. Cells were transfected when they reached 60–80% confluency. Cells were transfected with 500 ng of reporter DNA and either 4 (HeLa and SK-N-BE) or 3 µl (COS-7 and CHP) of LipofectACE (Gibco BRL). Both the DNA and LipofectACE were mixed with 100 µl OptiMem (Gibco BRL) and kept at room temperature for 5 min. The two mixes were combined and kept at room temperature for 10 min. During that time the cell culture medium of the cells was removed and substituted with serum-free medium. Aliquots of 800 µl of serum-free medium were added to each DNA– LipofectACE–OptiMem mix. The serum-free medium was removed from the cells and the DNA–LipofectACE–OptiMem mix was dispensed on them. The cells were incubated for 4–8 h before the transfection mix was substituted with medium containing 5% fetal bovine serum. The cells were extracted 20–24 h after transfection and both luciferase and control luciferase activities were measured with the Dual luciferase reporter assay system (Promega). All results presented are the means of two or three independent experiments, where three or four wells of cells were transfected with each vector. The mean luciferase activity of the normal promoter with three repeats was considered to be 100%. Statistical analysis was performed by comparing the mean luciferase activity of the other constructs with the control using analysis of variance followed by Scheffé’s test for multiple comparisons. GFP assay The normal and expanded promoters were cloned into the EcoRI– SalI sites of vector pEGFP-1 (Clontech). The expanded inserts were stable in this vector when grown for up to 8 h. The plasmids were linearized on the ApaLI site on the vector backbone before transfection into SK-N-BE cells in order to avoid breakage of the vector within the repeat, the GFP gene or other regulatory elements during incorporation into the genome. Forty-eight hours after transfection, medium containing 1.5 mg/ml G418 (Geneticin; Promega) was added. Stably transfected colonies were selected ~3 weeks later and analyzed in a FACS. Cells were trypsinized, washed with phosphate-buffered saline (PBS), resuspended in 100 µl PBS, stained with propidium iodide (0.2 µg; Sigma) and analyzed using a.
(8) 1798 Human Molecular Genetics, 1999, Vol. 8, No. 9. FACscan (Becton Dickinson, Lincoln Park, NY). The size of the repeat expansion integrated into the genome of these cells was examined by PCR amplification across the repeat using primers CSTB.8L (see above) and CSTB.10R (5'-GGGGTCACGTGACGCGCGGGCGGAACCAAG-3') or CSTB.8L and EGFP.1R (5'-ACACGCTGAACTTGTGGCCGTTTAC-3'). Promoter features of CSTB The human (accession no. U46692), mouse (accession no. U59807) and rat (accession no. D10607) CSTB promoter loci were compared for homologous sequences using, amongst other programs, Dotter (23), LALIGN (24,25) and GeneBee (http:// www.genebee.msu.su/services/malign_reduced.html ). As not all the regulatory sequences show sequence homology, searches for transcription factor binding sites that are common to the human, mouse and rat sequences were performed using a variety of programs, but principally MatInspector (v2.2, http://www.gsf.de/cgibin/matsearch.pl ) (13) to search the TRANSFAC database (release 3.4, http://transfac.gbf-braunschweig.de/TRANSFAC/ index.html ) (26). To facilitate multiple sequence alignments, the number of dodecamer repeats in the human sequence was reduced to only one. ACKNOWLEDGEMENTS We thank Dr Patrick Iynedjian for providing the luciferase control vector and advice with the luciferase experiments, Dr Lorenza Eder for providing the SK-N-BE and CHP cell lines, Dr Rachael Ritchie for the GFP vectors and Dr Denny Sakkas for help with the statistical analysis of results. We thank Marie-Pierre Papasavvas for assistance with cell culture and DNA extractions, Dr Colette Rossier for DNA sequencing and Roman Chrast for discussions and suggestions. This study was supported by grants from the Swiss FNRS (31.40500.94), the Swiss OFES (97.0015) and the University and Cantonal Hospital of Geneva. M.D.L. was a trainee of the Molecular and Cellular Biology graduate program of the University of Geneva Medical School. REFERENCES 1. Pennacchio, L.A., Lehesjoki, A.E., Stone, N.E., Willour, V.L., Virtaneva, K., Miao, J., D’Amato, E., Ramirez, L., Faham, M., Koskiniemi, M., Warrington, J.A., Norio, R., de la Chapelle, A., Cox, D.R. and Myers, R.M. (1996) Mutations in the gene encoding cystatin B in progressive myoclonus epilepsy (EPM1). Science, 271, 1731–1734. 2. Lalioti, M.D., Mirotsou, M., Buresi, C., Peitsch, M.C., Rossier, C., Ouazzani, R., Baldy-Moulinier, M., Bottani, A., Malafosse, A. and Antonarakis, S.E. (1997) Identification of mutations in cystatin B, the gene responsible for the Unverricht–Lundborg type of progressive myoclonus epilepsy (EPM1). Am. J. Hum. Genet., 60, 342–351. 3. Lafreniere, R.G., Rochefort, D.L., Chretien, N., Rommens, J.M., Cochius, J.I., Kalviainen, R., Nousiainen, U., Patry, G., Farrell, K., Soderfeldt, B., Federico, A., Hale, B.R., Cossio, O.H., Sorensen, T., Pouliot, M.A., Kmiec, T., Uldall, P., Janszky, J., Pranzatelli, M.R., Andermann, F., Andermann, E. and Rouleau, G.A. (1997) Unstable insertion in the 5' flanking region of the cystatin B gene is the most common mutation in progressive myoclonus epilepsy type 1, EPM1. Nature Genet., 15, 298– 302. 4. Virtaneva, K., D’Amato, E., Miao, J., Koskiniemi, M., Norio, R., Avanzini, G., Franceschetti, S., Michelucci, R., Tassinari, C.A., Omer, S., Pennacchio, L.A., Myers, R.M., Dieguez-Lucena, J.L., Krahe, R., de la Chapelle, A. and Lehesjoki, A.E. (1997) Unstable minisatellite expansion causing recessively inherited myoclonus epilepsy, EPM1. Nature Genet., 15, 393–396.. 5. Lalioti, M.D., Scott, H.S., Buresi, C., Rossier, C., Bottani, A., Morris, M.A., Malafosse, A. and Antonarakis, S.E. (1997) Dodecamer repeat expansion in cystatin B gene in progressive myoclonus epilepsy. Nature, 386, 847–851. 6. Lalioti, M.D., Scott, H.S., Genton, P., Grid, D., Ouazzani, R., M’Rabet, A., Ibrahim, S., Gouider, R., Dravet, C., Chkili, T., Bottani, A., Buresi, C., Malafosse, A. and Antonarakis, S.E. (1998) A PCR amplification method reveals instability of the dodecamer repeat in progressive myoclonus epilepsy (EPM1) and no correlation between the size of the repeat and age at onset. Am. J. Hum. Genet., 62, 842–847. 7. Pennacchio, L.A., Bouley, D.M., Higgins, K.M., Scott, M.P., Noebels, J.L. and Myers, R.M. (1998) Progressive ataxia, myoclonic epilepsy and cerebellar apoptosis in cystatin B-deficient mice. Nature Genet., 20, 251–258. 8. D’Amato, E., Haltia, M., Rinne, A., de la Chapelle, A. and Lehesjoki, A.E. (1998) Distribution of immunoreactivity for Cystatin B and Cathepsin S in the brain of progressive myoclonus epilepsy (EPM1) patients. Eur. J. Hum. Genet., 6 (Suppl. 1), 155. 9. Schlesinger, H.R., Gerson, J.M., Moorhead, P.S., Maguire, H. and Hummeler, K. (1976) Establishment and characterization of human neuroblastoma cell lines. Cancer Res., 36, 3094–3100. 10. Ciccarone, V., Spengler, B.A., Meyers, M.B., Biedler, J.L. and Ross, R.A. (1989) Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res., 49, 219–225. 11. Pennacchio, L.A. and Myers, R.M. (1996) Isolation and characterization of the mouse cystatin B gene [letter]. Genome Res., 6, 1103–1109. 12. Sato, N., Ishidoh, K., Uchiyama, Y. and Kominami, E. (1992) Structural organization of the gene encoding rat cystatin beta. Gene, 114, 257–260. 13. Quandt, K., Frech, K., Karas, H., Wingender, E. and Werner, T. (1995) MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res., 23, 4878–4884. 14. Karin, M., Liu, Z. and Zandi, E. (1997) AP-1 function and regulation. Curr. Opin. Cell Biol., 9, 240–246. 15. Nikolov, D.B. and Burley, S.K. (1997) RNA polymerase II transcription initiation: a structural view. Proc. Natl Acad. Sci. USA, 94, 15–22. 16. Knight, S.J., Flannery, A.V., Hirst, M.C., Campbell, L., Christodoulou, Z., Phelps, S.R., Pointon, J., Middleton-Price, H.R., Barnicoat, A. and Pembrey, M.E. (1993) Trinucleotide repeat amplification and hypermethylation of a CpG island in FRAXE mental retardation. Cell, 74, 127–134. 17. Oberle, I., Rousseau, F., Heitz, D., Kretz, C., Devys, D., Hanauer, A., Boue, J., Bertheas, M.F. and Mandel, J.L. (1991) Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science, 252, 1097–1102. 18. Struhl, K. (1996) Chromatin structure and RNA polymerase II connection: implications for transcription. Cell, 84, 179–182. 19. Wolfl, S., Martinez, C., Rich, A. and Majzoub, J.A. (1996) Transcription of the human corticotropin-releasing hormone gene in NPLC cells is correlated with Z-DNA formation. Proc. Natl Acad. Sci. USA, 93, 3664– 3668. 20. Estrada, S., Nycander, M., Hill, N.J., Craven, C.J., Waltho, J.P. and Bjork, I. (1998) The role of Gly-4 of human cystatin A (stefin A) in the binding of target proteinases. Characterization by kinetic and equilibrium methods of the interactions of cystatin A Gly-4 mutants with papain, cathepsin B, and cathepsin L. Biochemistry, 37, 7551–7560. 21. Abrahamson, M., Ritonja, A., Brown, M.A., Grubb, A., Machleidt, W. and Barrett, A.J. (1987) Identification of the probable inhibitory reactive sites of the cysteine proteinase inhibitors human cystatin C and chicken cystatin. J. Biol. Chem., 262, 9688–9694. 22. Soeda, E., Hou, D.X., Osoegawa, K., Atsuchi, Y., Yamagata, T., Shimokawa, T., Kishida, H., Okano, S. and Chumakov, I. (1995) Cosmid assembly and anchoring to human chromosome 21. Genomics, 25, 73–84. 23. Sonnhammer, E.L. and Durbin, R. (1995) A dot-matrix program with dynamic threshold control suited for genomic DNA and protein sequence analysis. Gene, 167, GC1–GC10. 24. Pearson, W.R. (1990) Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol., 183, 63–98. 25. Pearson, W.R. and Lipman, D.J. (1988) Improved tools for biological sequence comparison. Proc. Natl Acad. Sci. USA, 85, 2444–2448. 26. Heinemeyer, T., Wingender, E., Reuter, I., Hermjakob, H., Kel, A.E., Kel, O.V., Ignatieva, E.V., Ananko, E.A., Podkolodnaya, O.A., Kolpakov, F.A., Podkolodny, N.L. and Kolchanov, N.A. (1998) Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res., 26, 362–367..
(9)
Figure


Documents relatifs
The increase in GUS activities observed in the water deficit mimicking treatments in leaves of transgenic coffee plants transformed by the pHP15L, pHP16L, and pHP17L con- structs
La Conférence des Présidents d’Université demande la mise en place d’un groupe de travail, associant les représentants des universités et ceux des enseignants et
- Comme vous voulez, si je ne rentre pas en France dans un mois avec l’argent, le dossier sera envoyé à la justice française et américaine, mais comme vous dites, c’est des
Zudem wurden die führenden einschlägigen Zeitschriften in Management (Academy of Management Journal; Adminis- trative Science Quarterly; Management Science), Marketing (Journal
Calculez les mesures de chaque cercles ` a l’aide de la mesure donn´ ee.. Calcul du Rayon et Diam`etre des Cercles
Lors d’une prise de position conjointe sur les échan- tillons de médicaments, le Collège des médecins du Québec et l’Ordre des pharmaciens du Québec se sont dits sensibilisés
Prendre les moyens nécessaires pour assurer une plus grande diversité dans le rendement scolaire des élèves qui font partie d’une classe et une plus
Then, based on the proportions of abnor- mal and normal lung tissue as well as clinical data of the patients, retrieval of similar cases is enabled using a mul- timodal
