Publisher’s version / Version de l'éditeur:
Materials Research and Standards, 1, 9, pp. 719-723, 1961-09
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Characteristics of moisture deposition on corrosion specimens
Sereda, P. J.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=f6b43bb5-9055-4bae-a91a-b9fab36977ad https://publications-cnrc.canada.ca/fra/voir/objet/?id=f6b43bb5-9055-4bae-a91a-b9fab36977ad
Ser
THL
N21r2
no.
146
c .2
BLDG
NATIONAL
RESEARCH
COUNCIL
C A N A D A
DIVISION OF BUILDING RESEARCH
R E S E A R C H P A P E R NO. 146 O F THE
DIVISION OF BUILDING RESEARCH
OTTAWA
DECEMBER
1961P R I C E 10 C E N T S N R C 6 4 7 3
Characteristics of Moisture Deposition on
Corrosion Specimens
By P. J. SEREDA
M o l s T u I i B 1s essen- tial factor in the process of corrosion ( I ) , '
and a study of its physical character- istics, when i t is deposited us ruin or dew on the surface of metal specimens ex- posed to the atmosphere, should prove fruitful. Such information \\-ould he particularly useful when attempting to
Records of the appearance of the moisture on specimens of various metals exposed outdoors to rain or dew show that the moisture may involve a wide size range of water droplets or films and that the conditions are transient. Thus no single state represents the physical character of the moisture on the surface of an exposed metal specimen during any period of wetness.
The average temperature of the specimens during the time of wetness was less than 1 F below the air temperature. Thickness had a slight effect on the average temperature of clean specimens. This effect was cancelled
NOTE--DISCGSSION 01: T H I S I'APER when the surface was covered with corrosion products.
I S I N V I T E D , either for puhlicatiol~ or for thc attention of the nutliol. 01. authors. Ad-
dress ail commu~iications to ASTI\[ I-Iead- P. J. SEREDA, associate research officer, Division of Building Research, quarters, 1'318 Race St., Philaclclphia 8, 1% National Research Council, Ottawa, has been engaged since 1950 in the T h e boldface numbers in pare~lthescs rc- study of the behavior of water in porous systems including methods of fer t o t h e list of references amended to this . A detecting and measuring the presence of water on surfaces of materials. paper.
'I,a : ,-.,,.,,,
-
Mlll ill,, i ,1111 1111 111111111,,, ,1111 111111111 ,111 111 ,1111 1 1,111111111111 I, 111111111 ,1111 111111111111 11 ,1111 1I1II1 IIIIII,I,.September
7
96
7 719T A B L E I.-DL.:SC:IZII-'TIOI\: OF SPECI- MENS.
Thick-
ness, Surface
Metal in. Conditiol~
S t e e l . . . . . 0 . 1 Corroded Zinc.. . . 0 . 1 Corroded Stainless steel. . . 0.OG5 Shiny Steel elcctroplatcd
with zinc. . . 0.035 Clcen, m a t Copper. . . . . . 0.037 Slightly
iernislied Galvanized stccl.. . . . 0.017 Sp;~tl,olcd Aluminized steel. . . 0.030 Clean. m a t
Zinc.. . . 0 . 3 Grit blasted Zinc.. . . 0 . 2 Grit blastcd Zinc.. . . 0 . 1 Grit blastcd Zinc.. . . 0 . 0 5 Grit blastcd Zinc.. . . 0.025 Grit blastetl Steel.. . . 0 . 1 Corroded S t e e l . . . . . 0.025 Corroded
reproduce atnlospheric conditions in the laboratory.
I n this study, time-lapse photography was used t o record the appearance of rain and dew on the surface of metal specimens. The surface of the exposed specimens was photogrnphed every
3
hr starting less than 1 illin after the moisture was detected by the moisture- sensing elements developed in this laboratory (2,3,4).The tenlperatures of thc specimens were recorded during the time of wet- ness to show the effects of the different metals and different thicknesses of the same metal. The air temperature was also recorded for the same period. Experimental
Metal specimens 4 by 6 in. were ex- posed on a standard corrosion frame a t 30 deg to the horizontal. Most of the specimens had a thennocouple nlountetl on the groundward side connected t o a multipoint Speedomax recorder. The specimens are described in Tablc I.
Mounted along n ~ i t h both series of specimens there was a moisture-sensing element which consisted of a zinc plate with platinum-foil electrodes secured t o the two surfaccs in the manner described previously (2,3).
A special 35-nun camera. was mounted on a frame about 4 ft above the speci- mens. I t was shielded b y a plastic hood, and a small heater was provided inside the hood t o prevent conclensation on the lens. An electronic flash was mounted on a boom about 6 ft to the side of the specimens. I n order t h a t the light would strike the specimens tangen- tially, the axis of the light re- flector was in line with the top sur- face of the specimens. A n~echanical timer developed in this laboratory was used to provide the time-lapse, which nTas
4
hr for most of the experiments. The timer and the camera exposure and rewind mechanism were started by the relay of the amplifier in the moisture- sensing system (4).(I) Ziuc, 0.10 in. (2) Sluitiless stcel, 0.OCi:i ill. (9) Zinc-plated steel, 0.035 in.
( 6 ) Galvanized ~ t e e l . 0.017 ill. ( 5 ) Aluriiinizrd stecl, O.OSO in. ( 4 ) Moisture-sens~~ib element,
xitic, 0.10 in. Fig. 1. Dew fornled on specimens of different metals.
Copper-coi1stant:Ln thermocouples, wetness, being started and stopped No. 30 gage, were tspccl with Scotch by the action of the relay in the amplifier electrical tape No. 56 to the grouncl- of the moisture-sensing system.
ward side of thc specimens and were con- nected t o a Specdonlax recorder. The
rccorder was run only during the tinlc of The sensitivity of the moisture-detect-
(I) Zhic, 0.30in. (2) Zinc, 0.30iri. (3) Ziiic. ().loin. (4) Moisture-sensing clolile~it, zinc,
0.10 in.
corroded corrodetl
Fig. 2.-Dew formed on specimens of zinc and steel of different thicknesses.
Fig. 3.-Time sequence showing dew forming on the moisture-sensing element.
(1) Steel, corrodcd (2) Zinc (3) Stainless stecl
(6) C O P P C ~ (6) Galvanized steel (4) -Muminized steel
Fig. 4.-Rain drops on specimens of different metals.
ing system enabled the recording of t h e first drop of rain that fell o n the ele- ment or t h e oilset of dew before any visible nloisture was present, that is, corresponding t o a relative humidity of about 85 per cent on the surface of t h e clement. Obscrvatioiis were made for bout 2 months from August t o October. The conclusioris reported here are based on records obtained from about
350 lir of wetness. The photographs are typical.
1. When dew was deposited on clean metallic specimens, it appeared as uni- form, evenly distributed droplets which varied in size depending on t h e nature of the surface. Thc largest droplets formed on galvanized sheet metal, a n d these were never greater than about in. in diameter (Fig. I).
2. Where various thicknesses of zinc specinlens mere used, a l l with a grit-blasted surface, the deposit of dew was heavier on the thin specimens, although the average temperatures of these specimens measured during dew formation was higher and would indicate the reverse. Figure 2 shows t h e series of zinc specilllens of different thicknesses as well a s two corroded steel specimens. On the corroded steel specimens dew deposited a s a film of water tended t o
stream off the surface in the form of large droplets leaving streaks as shown on the thin steel specimen. A large dam of water accumulated a t the bottoni edge of the steel specimens. This streaming of water and dam buildup was not apparent on specimens where drop- wise condensation occurred until after the surface had been heated by the sun. 3. Figure 3 shows a series of photo- graphs taken every hour from the time moisturc was first sensed until a heavy dew was formed. When the first ex- posure was made, the panel temperature was estimated to be several degrees above the dew-point temperature of the air. Three hours elapsed from the time moisture was first sensed before any dropbts of moisture appeared on the surface. I n other cases, dew droplets appeared much earlier. After droplets of a certain size had developed, the process seemed to slow down, and this coincided with the observation that the panel temperature tended to approach the air temperature.
4. When rain fell on the surfaces of clean metal specimens, it remained there as discrete drops of all sizes until an accumulation of drops caused coalescence and streaming. The tend- ency for the water to remain as drops on the surface persisted. I n the case of corroded steel, the drops spread quickly into a film and showed none of the char- acteristics of drops that appeared on the other specimens. Figure 4 shows this effect.
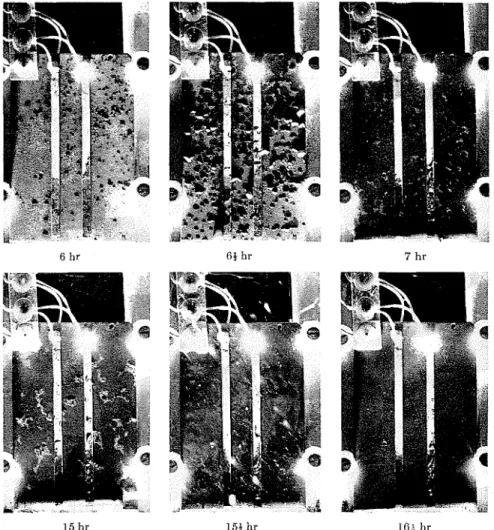
5. During any period of rainfall, the specimens were exposed to many cycles of variable moisture conditions includ- ing: drops of various sizes, streaming water, and partially dried surface show- ing only dampness and a water dam a t the bottom edge. The conditions were so variable, as shown in Fig. 5, that it was difficult to define the physical char- acter of the moisturc on the surface or to cite any average condition as represcnta- tive of the period.
Temperatures of the specinlens were recorded during the time of wetness, ancl the average hourly temperatures were obtained from these results. The aver- age hourly temperatures are given in Table I1 for the period from August 16 to September 16, when the total hours of wetness was 159, of which 66 per cent was causcd by dew.
Two facts are significant: the tem- perature of the corroded steel was in- dependent of specimen thickness, and the average temperature difference between air and specimen was small- less than a degree. This difference may, in fact, be largely duc to the difference in clevation, which was about 1 ft, be- tween the thermocouple in the screen measuring air temperature and the specimens. Geiger (5) has shown that temperature inversion near the ground
Fig. 5.-Time sequence showing moisture due to rain on moisture-sensing element. (Numbers given represent elapsed time in hours from the start of the rainfall.) can easily account for the observed
difference.
When the specinleli was free of water, as it was a t nightfall with a clear sky, the temperature diffcrcncc between the specimen and air was as much as 5 F for the corroded steel and about 2 F for thin clean specimens. This difference between specinlcns decreased as dew be-
gan fonning and the temperature of all speciincns approached the air tempera- ture. In the morning, when the sun hit the specimens, their temperatures rosc above the air temperature for zt short
time until hhey became dry. During rainfall, the teinpcrnture of the speci-
illens was the same as t h a t of the air. This may explain why the average tem- perature of the specimen for the time of wetness was very close to the air tem- perature.
Fronl series 11 rxpcriinc~nts, in \vhich tllc temperatures of a number of zinc and steel specimens were recorded dur- ing the hours of wetness, it was found that thc thickness of the metal specimen had very little effect upon the aver- age hourly temperature. The average hourly temperature for tllc period from
September 22 to October 6, representing 198 hr of wetness of which 62 per cent was caused by dew, is given in Table 111.
TABLE 11.--AVERAGE HOUItLY SPECIAIEN TEiUI'EHATUKES
FROM AUGUST 16 TO SEPTElMBER 16.n PEIZIOD
Corroded Iron Specimens
Steel Specimens
Stainless Galvanized
Thickness, in. . . . Temperature, deg Fallr . . . " ,4ir trmperature, 55.1 F.
TABLE 111.-BVERAGE I-IOUIZLY SPECIhIEN TEMPEIZATURE FOR PERIOD PIZOlM
SEPT. 22 TO OCT. 6."
~p - ~ ~ - - ~ ~ ~ ~ ~
-Steel Specimens,
Corroded Zinc Specimens
Thickness, in.. . . 0 . 1 0.025 0 . 3 0 . 2 0 . 1 0.05 0.025 Temperature, deg Fahr . . 4 9 . 5 4 9 . 8 49.9 4 9 . 9 50.0 4 9 . 8 50.2
,, Air temperature = 50.3 F.
Conclusions
This work has denlollstrated t h a t n hrn metal specimens are exposrd to the atmosphere and are wetted by rain or dew the nature of the moisture tle- posit may vary widely from droplets of various sizes t o films. The nature of the deposit is influenced not only by the atmospheric conditions and the condi- tion a n d type of surface b u t may clxtnge through repetitive cycles during any one atmospheric situation. This is particularly the case with rain when even for a steady rain condition a repetitive sequence of drop accumulation followed by drainage may take plaee, resulting in widely varying amounts of water on the surface. I n addition, northern latitudes would involve conditions of snow, hoar frost, and ice, which have not been included in this study. Thus no single steady-state moisture condition can be regarded a s typical or representa- tive of atmospheric exposure.
September
7
96
7
T h e character of the deposited mois- ture either from rain or dew on the sur- face of a corroded steel specimen is very different from t h a t on a n uncor- roded metal specimen or a metal speci- men where the corrosion products pre- sent a hard smooth surface, a s on copper. T h e average temperature of the metal specimens during the time of wetness varied from the air temperature by less than 1 F regardless of type or thickness of the specimen. Larger variations have been observed a t the time dew is first forming or a t the time the sun begins t o shine on the specimens, b u t these condi- tions a r e transitory a n d tend t o cancel one another when averages are consid- ered.
Acknowledgments:
The author is grateful t o H. F. Slade and S. E. Dods for their invaluable help in setting up the instrumentation and collecting the results. This is a contri-
bution from the Division of Building Research, National Research Council, Canada, a n d is published with the ap- proval of t h e Director of the Division.
(1) P. J. Sereda, "Atn~ospheric Factors Affecting the Corrosion of Steel,"
Industrial and Engzneming Chemistry,
Vol. 52, No. 2, 1960, pp. 157-160.
(2) P. J. Sereda, "Measurement of Sur- face Moisture-A Progress Report," ASTM BULLETIN, NO. 228, 1958, pp. 53-55.
(3) P. J. Sereda, "Measurement of Sur- face Moisture-Second Progress Re- port," ASTM BULLETIN, NO. 238,
1959, pp. 61-63.
(4) P. J. Sereda, "Measurement of Sur- face Moisture and Sulfur Dioxide Activity at Corrosion Sites," ASTM BULLETIN, NO. 346, 1960, pp. 47-48.
(5) R. Geiger, The Climate Near the
sound, Haward University Perss, Cambridge, Mass., 1950, p. 25.