Mediastinal reinforcement after induction therapy and pneumonectomy:
comparison of intercostal muscle versus diaphragm flaps
Didier Lardinois
a,*, Alexandra Horsch
a, Thorsten Krueger
b, Michael Dusmet
b, Hans-Beat Ris
ba
Division of Thoracic Surgery, University Hospital, Bern, Switzerland
b
Department of Surgery, University Hospital, Lausanne, Switzerland
Received 23 January 2001; received in revised form 24 October 2001; accepted 29 October 2001
Abstract
Objective: Prospective non-randomised comparison of full-thickness pedicled diaphragm flap with intercostal muscle flap in terms of morbidity and efficiency for bronchial stump coverage after induction therapy followed by pneumonectomy for non-small cell lung cancer (NSCLC). Methods: Between 1996 and 1998, a consecutive series of 26 patients underwent pneumonectomy following induction therapy. Half of the patients underwent mediastinal reinforcement by use of a pedicled intercostal muscle flap (IF) and half of the patients by use of a pedicled full-thickness diaphragm muscle flap (DF). Patients in both groups were matched according to age, gender, side of pneumonectomy and stage of NSCLC. Postoperative morbidity and mortality were recorded. Six months follow-up including physical examination and pulmonary function testing was performed to examine the incidence of bronchial stump fistulae, gastro-esophageal disorders or chest wall complaints. Results: There was no 30-day mortality in both groups. Complications were observed in one of 13 patients after IF and five of 13 after DF including pneumonia in two (one IF and one DF), visceral herniations in three (DF) and bronchopleural fistula in one patient (DF). There were no symptoms of gastro-esophageal reflux disease (GERD). Postoperative pulmonary function testing revealed no significant differences between the two groups. Conclusions: Pedicled intercostal and diaphragmatic muscle flaps are both valuable and effective tools for prophylactic mediastinal reinforcement following induction therapy and pneumonectomy. In our series of patients, IF seemed to be associated with a smaller operation-related morbidity than DF, although the difference was not significant. Pedicled full-thickness diaphrag-matic flaps may be indicated after induction therapy and extended pneumonectomy with pericardial resection in order to cover the stump and close the pericardial defect since they do not adversely influence pulmonary function. q 2002 Elsevier Science B.V. All rights reserved.
Keywords: Diaphragm; Intercostal muscle; Flap; Pneumonectomy; Induction therapy; Lung cancer
1. Introduction
Bronchopleural fistula after pneumonectomy remains a serious complication and represents a therapeutic challenge [1]. Several factors such as age, malnutrition, right-sided pneumonectomy, extensive resections, a long residual bron-chial stump, and preoperative steroid therapy or induction chemo- or radiotherapy were identified to increase the risk of its development [1–5]. Prevention during surgery includes the creation of a short, vascularised bronchial stump covered by tissue flaps, such as pericardium, pleura, omentum and muscle flaps (intercostal, latissimus dorsi or serratus anterior muscles) [6]. A pedicled flap of the diaphragm has also been described for this purpose [7].
Neoadjuvant induction therapy is increasingly used in
combination with resection for advanced lung cancer [8,9], which might increase the risk of bronchopleural fistula by causing immune depression and delayed wound healing [1]. In fact, a recently published report found a markedly increased incidence of bronchopleural fistulas after pneu-monectomy without bronchial stump reinforcement
follow-ing induction therapy, ranging from 8% after
chemoinduction up to 19% after radiochemoinduction [10]. Unfortunately, many of the intrathoracic tissues usually harvested for mediastinal reinforcement are either resected during resection for advanced disease or altered by induc-tion therapy and are therefore not amenable for coverage. Alternatives consist of using extrathoracic muscles such as serratus anterior and latissimus dorsi, pectoralis or rectus abdominis [6,11]. The morbidity of these muscle flaps may consist of seromas, chest wall complaints, and winged scapula [1]. The greater omentum also provides good cover-age with well vascularised tissue but requires a separate abdominal incision to be prepared [11]. The diaphragm
www.elsevier.com/locate/ejcts
1010-7940/02/$ - see front matter q 2002 Elsevier Science B.V. All rights reserved. PII: S 1 0 1 0 - 7 9 4 0 ( 0 1 ) 0 1 0 7 9 - X
* Corresponding author. Department of Surgery, Division of Thoracic Surgery, University Hospital of Zurich, CH 8091 Zurich, Switzerland. Tel.: 141-1-255-8802; fax: 141-1-255-8805.
flap may represent a valid alternative [12–15]. Goldstraw et al. reported its use to repair pericardial defects after exten-sive resection for pulmonary malignancies on the right side to avoid cardiac herniation [16]. In this study, we have prospectively compared the efficiency and morbidity of a full-thickness pedicled diaphragmatic flap and a pedicled intercostal flap in this respect in patients undergoing pneu-monectomy following induction therapy for stages IIIa and IIIb NSCLC.
2. Patients and methods
Between 1996 and 1998, a consecutive series of 26 patients undergoing pneumonectomy following induction chemotherapy or radiochemotherapy for stages IIIa and IIIb NSCLC were matched according to mediastinal rein-forcement by use of IF and DF (Table 1). The patients were alternately allocated to the groups IF and DF, and were prospectively compared for complications, pulmonary func-tion and gastro-esophageal symptoms.
Induction chemotherapy consisted of three courses with
cisplatin (80 mg/m2) and docetaxel (85 mg/m2) according to
the SAKK 16/96 protocol [4]. Hyperfractionated acceler-ated thoracic radiation of 30 gy and concurrent
chemother-apy (three cycles with cisplatin 60 mg/m2and vinblastin 6
mg/m2) was delivered to the patients with NSCLC IIIa
bulky, multilevel N2 disease and to the patients with stage IIIb.
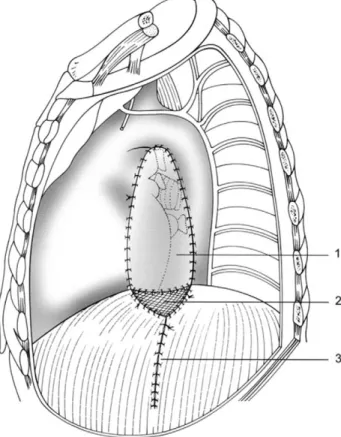
The diaphragmatic flap was fashioned with a full-thick-ness U-shape incision by use of a stapling device. The flap was based on the uncut mediastinal margin with the blood supply to the flap keeping intact by the pericardiophrenic artery or inferior phrenic arteries. The diaphragmatic pedicled flap was buttressed to the bronchial stump with interrupted sutures and sewn along its edges to the neigh-bouring tissues such as pleura or pericardium (Fig. 1). The major lateral part of the resulting diaphragmatic defect was closed with a running Prolenew suture. A mersilene mesh (polyethylen-terephthalat) was then adapted to the medial remaining part of the defect and sewn to the edge of the diaphragm and to the basal peritoneal surface of the flap (Fig. 2). The use of a mersilene mesh was introduced after
three patients presented with visceral herniations. In these patients diaphragmatic defect had been primarily comple-tely sutured with a running, non-resorbable suture. Prob-ably, excessive tension on the suture line lead to its breakdown and was followed by herniation.
The intercostal muscle flap was fashioned in the 5th inter-costal space without any removal of a rib. The periostium of the lower border of the 5th rib was incised and the flap was prepared by keeping contact with the rib to avoid injury of the intercostal blood supply. The anterior edge of the flap
Table 1
Tumor staging according to the TNM classificationa
Intercostal flap Diaphragmatic flap
Stage IIIa T2N2M0 1 (1) 2 (1) T3N1M0 3 2 T3N2M0 8 (3) 7 (4) Stage IIIb T4N1M0 1* 2*
a (): patients with stage IIIa bulky, multilevel N2 disease. *: invasion of
the vena cava superior or of the right atrium.
Fig. 1. Mediastinal reinforcement by use of a pedicled diaphragm flap following extended pneumonectomy; superior vena cava (SVC), azygos vein (AV), diaphragm flap (DF).
Fig. 2. Technique of diaphragmatic repair: (1) diaphragm flap; (2) mersi-lene mesh; and (3) suture line.
was then placed on the hilum and adapted with interrupted sutures. All the operations were performed by the same surgical team, consisting of two surgeons, independently of the muscle flap.
The postoperative course of all patients of both groups was recorded by physicians who were blind to the group allocation. A clinical examination and pulmonary function testing were performed on each patient 6 months after the operation. The clinical evaluation included subjective assessment of chest wall and shoulder girdle complaints, symptoms of GERD and a physical examination of chest wall integrity and shoulder girdle function. Pulmonary func-tion was assessed, and the differences between predicted and measured postoperative FEV1 values of both groups were compared by use of the Wilcoxon-rank sum test.
Significance was accepted at P , 0:05.
3. Results
Each group consisted of 13 patients (ten male, three female). The mean age of the DF and IF patients was 62 years (range 46–73) and 59 years (range 49–70 years), respectively. A right pneumonectomy was performed in eight of 13 patients (61.5%) in both groups. The indication for pneumonectomy was a centrally localised non-small cell lung cancer in all patients.
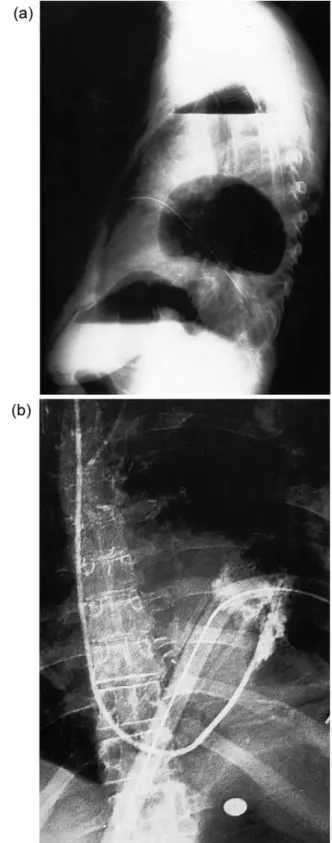
The 30-day mortality of the whole series was 0%. Complications were observed during follow-up in six patients (23%) and included atelectasis and pneumonia of the contralateral lung in one patient in each group, three visceral herniations after left pneumonectomy in the DF group (Fig. 3a, b), and one bronchial stump insufficiency in the DF group. All patients with visceral herniation under-went re-thoracotomy and closure of the diaphragmatic defect. These herniations were observed in the early phase of our experience, when the diaphragmatic defect was closed primarily without using a mersilene mesh for repair. All three patients recovered uneventfully.
Bronchial stump insufficiency was observed 2 months after surgery in a patient with synchronous NSCLC of the upper right lobe and the trachea. The patient initially under-went a right upper lobectomy with adjuvant radioche-motherapy, including 60 gy of external irradiation in combination with endotracheal brachytherapy. Twelve months later, chronic infection and destruction of the remaining middle lobe was observed and a completion right pneumonectomy was performed. The mediastinum and bronchial stump were covered with a diaphragmatic flap. A bronchopleural fistula was observed 2 months after operation. The bronchial stump was debrided and closed with a pedicled serratus anterior muscle flap. The chest cavity was left open and daily wet-to-dry dressings were done. After 4 weeks the Clagett procedure was performed and the patient had an uneventful recovery.
Six months follow-up was performed in 24/26 (92%)
patients. Chest wall complaints were observed in two patients in each group (15.4%), consisting of intermittent discomfort in one patient of each group, and constant pain in two patients. Both of the latter patients underwent
radio-Fig. 3. Postoperative chest X-ray revealing diaphragmatic herniation following left sided pneumectomy and use of a diaphragmatic flap: (a) lateral view; and (b) p.-a. view.
therapy prior to surgery. The shoulder girdle function was normal and symmetrical in 22/26 (85%) patients. In two patients in each group, the abduction of the involved upper extremity was limited to 908 and 1108, respectively. Two of these patients had undergone a postoperative radio-therapy. Clinical signs for gastro-oesophageal reflux were not noted in any patient in either group. Pulmonary function testing revealed no statistical significance in the analyse of the differences between predicted and recorded FEV1 in
both groups (P ¼ 0:7; Table 2).
4. Discussion
Induction therapy followed by resection in patients with NSCLC may require increased awareness for mediastinal reinforcement in order to prevent bronchopleural fistulae since tissues usually used for stump coverage may be altered after induction and not be suitable for this purpose [2,3,10]. Intercostal muscle flap and pedicled diaphragmatic flap have already been used to this respect.
However, a prospective comparison between intercostal and diaphragmatic flap for prophylactic mediastinal reinfor-cement after pneumonectomy has not been studied until now. No difference concerning wound healing, chest wall complaints and shoulder girdle function was observed between the two groups 6 months after operation. These findings do not surprise, since the surgical approach with a posterolateral thoracotomy was the same in both groups. A total of 15% of the patients complained of chest wall discomfort 6 months after the operation. This corresponds to the results obtained from our prospective study compar-ing chest wall complaints after thoracotomy and thoraco-scopy [17]. Shoulder girdle function was normal and symmetrical in 85% of the patients. Two patients in each group presented limited abduction of the involved upper extremity, probably due to postoperative radiotherapy in two patients. It has also been suggested that impaired shoulder girdle function might be related to inadequate post-operative pain control and physiotherapy rather than to the transection of chest wall muscles [18].
The rate of complications of the whole series was 23%. Major complications were visceral herniations after left pneumonectomy in three patients after diaphragmatic flaps. It was early in the series and part of the ‘learning
curve’. In subsequent patients a mersilene mesh was used to close the medial diaphragmatic defect, and no further visceral herniation was observed.
Bronchial stump insufficiency was observed in one patient in the DF group 2 months after right pneumonect-omy and induction radiochemotherapy. All other patients of both groups had an uneventful healing of their bronchial suture line, indicating that IF and DF are both efficient to prevent bronchopleural fistulas following induction therapy and pneumonectomy for NSCLC.
Our results also indicate that the use of the DF does not adversely influence the gastro-esophageal motility and pulmonary function as compared to the IF despite the fact that the ipsilateral phrenic nerve was sacrificed in all patients with a DF. This may be explained by the effect of primary closure of the diaphragmatic defect, which is comparable to diaphragmatic plication. Plication of the paralysed hemidiaphragm was described as an effective treatment for postoperative diaphragmatic paralysis after pneumonectomy [19,20]. It seems to improve contralateral hemidiaphragm function by increasing the transdiaphrag-matic pressure [21,22].
In conclusion, our results seem to indicate that intercostal muscle flaps and pedicled diaphragmatic flaps are two valu-able and effective methods in the prevention of
broncho-pleural fistulae following induction therapy and
pneumonectomy. No statistical difference could be
observed between the two groups of patients. The use of IF reinforcement may be preferred due to the easier way to fashion it. Centrally located NSCLC pre-treated by chemoradiotherapy usually requires extensive resection including pericardium in order to obtain adequate resection margins and save control of vessels. In these situation the DF has the advantage to ensure the bronchial stump rein-forcement and to cover at the same time the pericardial defect with autologous material. However, careful attention has to be given to technical details in the fashion of the diaphragmatic flap to avoid serious complications like visc-eral herniations.
References
[1] Pairolero PC, Arnold PG, Trastek VF, Meland NB, Kay PP. Post-pneumonectomy empyema. The role of intrathoracic muscle transpo-sition. J Thorac Cardiovasc Surg 1990;99:958–966 discussion 966– 968.
[2] Tildon TT, Hughes RK. Complications from preoperative irradiation therapy for lung cancer. Ann Thorac Surg 1967;3:307–326. [3] Schechter FG, Owens RR, Bryant LR. Pleural flap closure of
pericar-dial defects following intrapericarpericar-dial pneumonectomy. Ann Thorac Surg 1976;21:67–69.
[4] SIAK. Swiss Institute for Applied Cancer Research. Bern, Switzer-land.
[5] Asamura H, Naruke T, Tsuchiya R, Goya T, Kondo H, Suemasu K. Bronchopleural fistulas associated with lung cancer operations. Univariate and multivariate analysis of risk factors, management, and outcome. J Thorac Cardiovasc Surg 1992;104:1456–1464. [6] Pairolero PC, Arnold PG, Piehler JM. Intrathoracic transposition of Table 2
Pulmonary function testing 6 months after pneumonectomy and mediastinal reinforcement by use of diaphragm flaps (DF) or intercostal muscle flaps (IF) FEV1 pred (L, mean ^ 1 SD) FEV1 rec (L, mean ^ 1 SD) P-value DF 1.60 ^ 0.2 1.61 ^ 0.6 0.7a IF 1.56 ^ 0.2 1.48 ^ 0.5 a
Wilcoxon-rank sum test for comparison of D (FEV1 measured—FEV1 predicted).
extrathoracic skeletal muscle. J Thorac Cardiovasc Surg 1983;86:809–817.
[7] Mineo TC, Ambrogi V. Early closure of the postpneumonectomy bronchopleural fistula by pedicled diaphragmatic flaps. Ann Thorac Surg 1995;60:714–715.
[8] De Leyn P, Vansteenkiste J, Deneffe G, Van Raemdonck D, Coose-mans W, Lerut T. Result of induction chemotherapy followed by surgery in patients with stage IIIA N2 NSCLC: importance of pre-treatment mediastinoscopy. Eur J Cardiothorac Surg 1999;15:608– 614.
[9] Hensing TA, Detterbeck F, Socinski MA. The role of induction ther-apy in the management of resectable non-small cell lung cancer [see comments]. Cancer Control 2000;7:45–55.
[10] Doddoli C, Thomas P, Thirion X, Sere´e Y, Giudicelli R, Fuentes P. Postoperative complications in relations with induction therapy for lung cancer. Eur J Cardiothorac Surg 2001;20:385–390.
[11] Yokomise H, Takahashi Y, Inui K, Yagi K, Mizuno H, Aoki M, Wada H, Hitomi S. Omentoplasty for postpneumonectomy bronchopleural fistulas. Eur J Cardiothorac Surg 1994;8:122–124.
[12] Mineo TC, Ambrogi V. The diaphragmatic flap: a multiuse material in thoracic surgery. J Thorac Cardiovasc Surg 1999;118:1084–1089. [13] Westaby S, Shepherd MP, Nohl-Oser HC. The use of diaphragmatic
pedicle grafts for reconstructive procedures in the esophagus and tracheobronchial tree. Ann Thorac Surg 1982;33:486–490. [14] Cohen M, Robin A, Nyhus LM. Use of the diaphragm to reinforce
anastomosis of the intestines. Surg Gynecol Obstet 1991;172:316– 318.
[15] Macoviak JA, Stephenson LW, Spielman S, Greenspan A, Likoff M, Sutton MS, Reichek N, Rashkind WJ, Edmunds Jr LH. Replacement of ventricular myocardium with diaphragmatic skeletal muscle: short-term studies. J Thorac Cardiovasc Surg 1981;81:519–527. [16] Goldstraw P, Jiao X. Pericardial repair after extensive resection:
another use for the pedicled diaphragmatic flap. Ann Thorac Surg 1996;61:1112–1114.
[17] Furrer M, Rechsteiner R, Eigenmann V, Signer C, Althaus U, Ris HB. Thoracotomy and thoracoscopy: postoperative pulmonary function, pain and chest wall complaints. Eur J Cardiothorac Surg 1997;12:82–87.
[18] Hazelrigg SR, Landreneau RJ, Boley TM, Priesmeyer M, Schmaltz RA, Nawarawong W, Johnson JA, Walls JT, Curtis JJ. The effect of muscle-sparing versus standard posterolateral thoracotomy on pulmonary function, muscle strength, and postoperative pain. J Thorac Cardiovasc Surg 1991;101:394–400 discussion 400–401. [19] Takeda S, Nakahara K, Fujii Y, Minami M, Matsuda H. Plication of
paralyzed hemidiaphragm after right sleeve pneumonectomy. Ann Thorac Surg 1994;58:1755–1757 discussion 1757–1758.
[20] Takeda S, Nakahara K, Fujii Y, Mizuta T, Matsuda H. Concomitant cardiac and pulmonary operation. Pulmonary mechanics and outcome of phrenic nerve injury. J Cardiovasc Surg (Torino) 1997;38:517–521. [21] Schwartz MZ, Filler RM. Plication of the diaphragm for symptomatic
phrenic nerve paralysis. J Pediatr Surg 1978;13:259–263.
[22] Lisboa C, Pare PD, Pertuze J, Contreras G, Moreno R, Guillemi S, Cruz E. Inspiratory muscle function in unilateral diaphragmatic paralysis. Am Rev Respir Dis 1986;134:488–492.