Publisher’s version / Version de l'éditeur:
Accounts of Chemical Research, 31, 4, pp. 159-162, 1998-04-21
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1021/ar970057z
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
A radical account of "oxygenated Fenton chemistry"
MacFaul, Philip A.; Wayner, Danial; Ingold, Keith
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=43a01447-ce12-4795-a453-2d7be558ab41
https://publications-cnrc.canada.ca/fra/voir/objet/?id=43a01447-ce12-4795-a453-2d7be558ab41
A Radical Account of
“Oxygenated Fenton
Chemistry”
1
In a recen t Accou n t, Sawyer et al.3h ave su m m arized m u ch of th eir earlier work on wh at th ey refer to as “oxygen ated Fen ton ch em istry”. Th e overall reaction is p erfectly straigh -forward an d n on con troversial. In th e p resen ce of d ioxy-gen , a m ixtu re con tain in g an iron catalyst (ioxy-gen erally FeII), Me3COOH (or H2O2), an d a h yd rocarb on in aceton itrile at room tem p eratu re yield s m ain ly th e keton e d erived from th e h yd rocarb on togeth er with sm aller qu an tities of oth er oxid a-tion p rod u cts su ch as th e corresp on d in g alcoh ol. Th e p rop osed m ech an ism for th ese oxid ation s is an yth in g b u t straigh tforward an d d eserves to b e ch allen ged . Th e m ost rem arkab le claim3 is th at “Fen ton reagen ts do n ot produ ce [Sawyer’s italics]... free carb on rad icals...”.4 Th is ign ores a wealth of earlier work on iron / h yd rop eroxid e/ h yd rocarb on ch em istry.7 Moreover, it also ign ores classical free rad ical an d au toxid ation ch em istry8wh ich we d em on strate h erein , b y m ean s of a few carefu lly selected exp erim en ts, p rovid e b oth a sim p ler reaction m ech an ism an d on e in con cord an ce with k n ow n free rad ical kin etics. Th is (classical) m ech an ism is sh own in ab b reviated form in reaction s 1-5 and will be fu rth er elab orated wh en n ecessary.
To d istin gu ish b etween Sawyer’s n on rad ical m ech an ism an d reaction s 1-5, we have utilized four of his catalysts, viz. ferric ch loride (FeIIICl
3, 1), iron (II) tetrakis(trip h en ylp h osp h in e oxid e) (FeII(OPPh
3)4, 2), iron (II) b is(2,2′-b ip yrid yl) (FeII(b p y)2,
3), an d iron (II) b is(p icolin ate) (FeII(PA)
2, 4), an d th ree of h is su b strates, viz. cycloh exan e, eth ylb en zen e, an d cycloh exen e. Reaction s were ru n in du plicate with con stan t stirrin g at room tem p eratu re u n d er 1 atm of oxygen for 18 h (th e con d ition s given in th e origin al rep ort9sin ce th ere is little reaction with cycloh exan e after th e 3 h in d icated in Tab le 2 of th e Accou n t3). Th e reagen t con cen tration s were also th e sam e as th ose em p loyed in som e of th e p reviou s work,3,9viz. 1.0 M h yd rocarb on , 10 m M catalyst, an d 20 m M Me3COOH (TBHP) or 20 m M Ph CH2CMe2OOH (MPPH, see b elow). Th e solven ts were aceton itrile (1 an d 3), p yrid in e/ aceton itrile (1:4 m ole ratio) (2), an d p yrid in e/ acetic acid (2:1 m ole ratio) (4) as d escrib ed origin ally.9 Reaction s were q u en ch ed with an
excess of trip h en ylp h osp h in e (to con vert h yd rop eroxid es to th e corresp on d in g alcoh ols) an d an alyzed on a Hewlett-Packard 5890 Series II gas ch rom atograp h (HP Ultra 1 cross-lin ked m eth yl silicon e colu m n , 12m × 0.2 m m × 0.33 µm ; tem p eratu re p rogram : 40°C for 7 m in , 15°C/ m in to 250°C, 250°C for 5 m in ) u sin g 1,4-d ib rom ob en zen e as an in tern al stan dard. Data an alyses were p erform ed u sin g an HP Ch em -station .. Th e ab solu te p rod u ct yield s10 are sh own in b ar grap h form in Figu re 1.
By exam in in g first th e 12 b ar grap h s from th e TBHP exp erim en ts, it is clear th at th e total p rod u ct yield in creases alon g th e series: cycloh exan e< ethylbenzene < cyclohexene an d th at, with a few excep tion s, th e total yield s for each su b strate are rou gh ly in d ep en d en t of th e catalyst. With cycloh exan e th e p rod u ct yield s are less th an or eq u al to th at of th e in itial catalyst, viz., 10 m M (overall average from ou r eigh t exp erim en ts ) 7.5 vs 10 m M from Sawyer’s et al.’s origin al fou r exp erim en ts9), with eth ylb en zen e th e average p rod u ct yield rises to 25.6 m M (vs 39.8 m M9) an d with cycloh exen e to 86.0 m M (vs 107.9 m M9). It is totally u n
-n ecessary to i-n vok e catalyst “tu r-n over” to explai-n th ese resu lts sin ce th ey can be sim ply accou n ted for on th e basis of th e relative im portan ce of reaction 5. For cycloh exan e,11eth yl-b en zen e,12an d cycloh exen e,12th e valu es for k
5are 0.048, 1.1, an d 6.1 M-1s-1, resp ectively. Th u s, reaction 5 is in sign ifican t for cycloh exan e, is relevan t for eth ylb en zen e, an d b ecom es im p ortan t for cycloh exen e. We can m ake a rou gh correction for h yd rocarb on oxid ation via tert-b u toxyl rad icals (reaction 2) b y su b tractin g th e average p rod u ct yield for cycloh exan e (7.5 m M) from th e average p rod u ct yield s for cycloh exen e (86.1 m M) an d eth ylben zen e (25.6 m M). With th is correction , th e ratio of cycloh exen e/ eth ylb en zen e p rod u cts wh ich p rob -ab ly arise m ain ly from au toxid ation , i.e. h yd rogen atom ab straction b y p eroxyl rad icals (reaction 5), is (86.1-7.5)/ (25.6-7.5) ) 4.3 (vs 3.3 from the original work9). Th e ratio of 4.3 is in rath er satisfactory agreem en t with th e ratio exp ected on th e b asis of th e relative m agn itu d es of th e k5 valu es for cycloh exen e an d eth ylb en zen e, viz.126.1/ 1.1) 5.5. We in trod u ced MPPH as a m ech an istic p rob e13 in tertalkyl h yd rop eroxid e/ iron catalyst/ alkan e system s to d istin
-Cycloh exan e
FeII+ Me3COOH f FeIII+ Me3CO•+-OH (1)
Me3CO•+ >CH2f Me3COH+ >C4H (2)
>C4H + O2f >CHOO• (3)
>CHOO•+ radical•f
>CdO (m ain)(+ >CHOH + >CHOOCMe3+ O2) (4)
Eth ylb en zen e an d Cycloh exen e
>CHOO•+ >CH2f >CHOOH + >C4H (5)
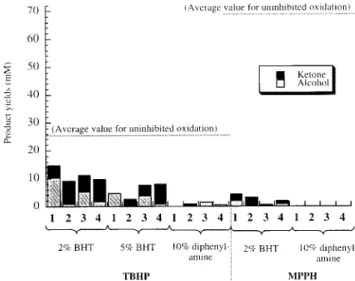
FIGURE 1. Oxidation of cyclohexane, ethylbenzene, and
cyclohex-ene by iron catalysts 1-4 and two tert-alkyl hydroperoxides, TBHP and M PPH, at room temperature under an atmosphere of oxygen for 18 h.
gu ish b etween alkan e oxid ation s via h igh -valen t iron-oxo sp ecies (as favored b y m an y oth ers) an d oxid ation s wh ich occu rred via h yd rogen atom ab straction from th e alkan e b y freely d iffu sin g alkoxyl rad icals; i.e., to d istin gu ish b etween th e reaction p ath way 6, 7 an d p ath way 8, 9 (wh ere Fenis FeII or FeIII).
Th e p rob e h yd rop eroxid e, MPPH, relies on th e fact th at β-scission of th e corresp on d in g tert-alkoxyl rad ical, reaction 10,
is m u ch too rap id (k10 ∼ 2 × 108 s-1) for th ere to b e an y h yd rogen atom ab straction from cycloh exan e (even at 1 M) an alogou s to reaction s 2 an d 9 (k2 ) k9 ) 1.2 × 106 M-1 s-1).14,15 Th u s, if th e reaction p roceed s via a m etal-b ased oxid an t (reaction s 6 an d 7), th e p rod u ct p rofile will b e u n affected b y th e u se of MPPH sin ce we h ave d em on strated th at in gen u in e 2-electron alken e oxid ation s MPPH is a p erfectly com p eten t su b stitu te for TBHP (in fact, th e ep oxi-d ation s of cycloh exen e an oxi-d cis-stilb en e were m ore efficien t with MPPH th an with TBHP).18 On th e oth er h an d , if th e reaction p roceed s via alkoxyl rad ical in term ed iates (reaction s 8 an d 9), th e very fastβ-cleavage of Ph CH2CMe2O•will lead p red om in an tly to b en zyl rad ical d erived p rod u cts. We h ave alread y ap p lied th e MPPH p rob e to a variety of h yd rop erox-ide/ iron / alkan e system s fin din g on ly alkoxyl radical ch em istry
in all cases.19 Con seq u en tly, it was n o su rp rise to d iscover th at th ere were essen tially n o cycloh exan e oxidation p rodu cts wh en MPPH was u sed in p lace of TBHP, th e m ain p rod u cts b ein g b en zald eh yd e (average yield 5.8 m M, ran ge 3.4-9.0 m M) togeth er with som e b en zyl alcoh ol (average yield 1.1 m M, ran ge 04.3 m M). These two com pounds are presum -ab ly form ed via reaction s 10, 11, an d 12.
In d ram atic con trast, th e MPPH-in d u ced oxid ation of eth yl-b en zen e an d cycloh exen e gave su yl-b stan tial q u an tities of th e corresp on din g keton es an d alcoh ols (Figu re 1).20 We attribu te th ese p rod u cts to au toxid ation of th ese two h yd rocarb on s in itiated b y th e b en zylp eroxyl rad icals, viz.
Fu rth er eviden ce th at th e ch em istry in qu estion is in itiated b y alkoxyl rad icals (reaction s 1 an d 2) was ob tain ed b y th e ad d ition of two com m ercially im p ortan t rad ical trap p in g an tioxid an ts, 2,6-d i-tert-b u tyl-4-m eth ylp h en ol (BHT) an d d ip h en ylam in e to oth erwise “n orm al” system s con tain in g 1.0 M eth ylb en zen e (EtPh ), 20 m M TBHP, or 20 m M MPPH an d 10 m M catalyst. Both of th ese an tioxid an ts, AH, react rap id ly with alkoxyl rad icals (k14BHT) 2.0 × 107M-1s-1,13k14Ph2NH) 3 × 108 M-1 s-1,21) an d with alkylp eroxyl rad icals (k
15BHT)
1.4 × 104M-1s-1,22k
15Ph2NH≈ 4 × 104M-1s-1,23). Kn owledge
of th ese an tioxid ative rate con stan ts lead s d irectly to th ree sim p le p red iction s. First, 100 m M Ph2NH sh ou ld com p letely in h ib it th e MPPH-p rom oted oxid ation s of EtPh b y trap p in g all th e b en zylp eroxyl rad icals form ed in reaction s 10 an d 11 b efore th ey can attack th e h yd rocarb on , reaction 13, i.e.
k15Ph2NH[Ph2NH] () 4 × 104 x 0.1 ) 4 × 103 s-1) . k13EtPh -[EtPh ] () 1.1 × 1.0 ) 1.1 s-1). Th is p red iction was con firm ed exp erim en tally with all fou r catalysts (see Figu re 2).24 Al-th ou gh BHT is on ly on e-Al-th ird as active as Ph2NH in trap p in g p eroxyl rad icals, even 20 m M BHT was su fficien t to in h ib it th e MPPH-p rom oted oxid ation of EtPh very stron gly (Figu re 2).24 Th e secon d p red iction is th at 20 m M an d 50 m M BHT sh ou ld p rovid e on ly p artial in h ib ition of th e tert-b u toxyl rad ical in itiated oxid ation of EtPh sin ce even 50 m M BHT can in tercep t on ly ca. 50% of th e tert-b u toxyl rad icals form ed in reaction 1, i.e. k14BHT[BHT] () (2.0 × 107) × 0.05 ) 1.0 × 106s-1) ≈ k2EtPh[EtPh ] () (1.05 × 106) × 1.0 ) 1.05 × 106s-1). Th e fin al p red iction is th at 100 m M d ip h en ylam in e sh ou ld p rod u ce alm ost com p lete in h ib ition of EtPh oxid ation p ro-m oted b y TBHP, i.e. k14Ph2NH[Ph2NH]) (3 × 108) × 0.1 ) 3 × 107s-1. Th e valid ity of th e secon d an d th ird p red iction s are also attested to b y th e d ata sh own in Figu re 2.24
Ou r resu lts p rovid e u n eq u ivocal p roof th at Sawyer’s “oxygen ated Fen ton ch em istry” in organ ic solven ts (an d b y im p lication in water) in volves sim p le free-rad ical-m ed iated ch em istry. It is n ot rad ical-free as Sawyer h as su ggested .3,9 Th ere are, of cou rse, n u m erou s en zym es, in clu d in g cyto-ch rom e P450s an d m eth an e m on oxygen ases, wh icyto-ch can effect alkan e oxidation s via h igh -valen t iron-oxo species. However, m im ickin g th ese en zym e with sim p le ch em ical system s is n ot a trivial u n d ertakin g an d , to ou r kn owled ge, h as n ever b een ach ieved with an iron catalyst an d a tertiary alkyl h yd rop er-oxid e.27 We con clu d e th at m ech an istic in terp retation in th is gen eral area of b iom im etic ch em istry sh ou ld on ly b e d rawn after exh au stive stu d ies u sin g a variety of exp erim en tal tests for th e in volvem en t of free rad icals.7,13,19,25-27
RtOOH+ Fenf RtOH+ Fen+2dO (6) Fen+2dO + RH f Fen+ ROH (7) RtOOH+ Fenf RtO•+ Fen+1OH (8) RtO•+ RH f RtOH+ R•98 O2 ROO•f p rod u cts (9) Ph CH2CMe2O•f Ph CH2•+ Me2CO (10) Ph CH2•+ O2f Ph CH2OO• (11) 2Ph CH2OO•f Ph CHO + Ph CH2OH+ O2 (12) Ph CH2OO•+ >CH2f Ph CH2OOH + >C4H (13)
FIGURE 2. Effect of adding antioxidants to the oxidation of
ethylbenzene by iron catalysts 1-4 and TBHP or M PPH at room temberature under an atmosphere of oxygen for 18 h.
RO•+ AH f ROH + A• (14) ROO•+ AH f ROOH + A• (15)
Commentary
W e th an k th e Association for In tern ation al Can cer Research an d th e N ation al Fou n dation for Can cer Research for partial su pport of th is w ork .
Philip A. MacFaul,2D. D. M. Wayner, and K. U. Ingold*
Steacie In stitu te for Molecu lar Scien ces, Nation al Research Cou n cil of Can ad a, Ottawa, On tario, Can ad a K1A 0R6
References
(1) Issu ed as NRCC No. 40851.
(2) NRCC Research Associate, 1995-1997.
(3) Sawyer, D. T.; Sob kowiak, A.; Matsu sh ita, T. Acc.
Ch em . Res 1996, 29, 409-416.
(4) Sawyer’s n on rad ical m ech an ism in volves in itial form ation of a p eroxy-iron(II) species (form ulated as [FeIIOOR] ign orin g th e oth er ligan d s) wh ich th en reacts with O2to form an oth er (q u ote) “h ypoth
eti-cal” in term ed iate, [FeIIIOOR(O
2)], wh ich su b se-qu en tly oxid izes alkan es to keton es. (Peroxy-iron-(III) com p lexes are well estab lish ed .5 However, at least som e of th em h ave b een d em on strated n ot to react directly with h yd rocarb on s.5a,6)
(5) See, e.g.: (a) Barton , D. H. R.; Be´vie`re, S. D.; Ch avasiri, W.; Doller, D.; Liu , W.-G.; Reib en sp ies, J. H. N ew . J. Ch em . 1992, 16, 1019-1029 and refer-en ces cited . (b ) Me´n age, S.; Wilkin son , E. C.; Qu e, L., Jr.; Fon tecave, M. An gew Ch em ., In t. Ed. En gl.
1995, 34, 203-205.
(6) Un p u blish ed stop -flow exp erim en ts from th is labo-ratory.
(7) See accom p an yin g com m en t b y Professor Ch eves Wallin g.
(8) Wallin g, C. Free Radicals in Solu tion ; Wiley: New York, 1957.
(9) Kan g, C.; Red m an , C.; Cep ak, V.; Sawyer, D. T.
Bioorg. Med. Ch em . 1993, 1, 125-140.
(10) (a) Oxid ation of cycloh exan e (1 M) b y iron catalysts (10 m M) an d alkyl h yd rop eroxid es (20 m M) u n d er an atm osp h ere of oxygen . Prod u ct yield s (m M) are from d u p licate exp erim en ts; resu lts in b rackets are from Tab le 3 in ref 9. (i) TBHP. Catalyst, keton e, alcoh ol, m ixed p eroxid e: 1, 2.7, 3.4 (4.8); 4.2, 4.7 (4.2); 0.2, trace, (0). 2, 3.2, 3.9 (4.3); 1.3, 1.3 (6.1); 0, 0 (0). 3, 2.9, 4.4 (3.7); 2.6, 3.4 (4.9); 0, 0 (0). 4, 10.9, 10.7 (12); 0, 0 (0); trace, 0 (0). (ii) MPPH. Catalyst, keton e, alcoh ol, m ixed p eroxid e, b en zald eh yd e, b en zyl alcoh ol, b ib en zyl, MPPOH: 1, 0.5, 0.5; 0.9, 0.5; 0, 0; 3.8, 3.4; 4.3, 3.2; trace, trace; 0.5, 0.8. 2, 0, 0; 0, 0; 0, 0; 4.5, 6.0; 0, 0; 0, 0; 3.3, 3.5. 3, 0, 0; 0, 0; 0, 0; 5.7, 5.0; 0.4, 0; 0, 0; 0.3, trace. 4, 0, 0; 0, 0; 0, 0; 9.0, 8.7; 0.3, 0.5; 0, 0; 0.6, 0.8. (b ) Oxid ation of cycloh exen e (1 M) b y iron catalysts (10 m M) an d alkyl h ydrop eroxides (20 m M) u n der an atm osp h ere of oxygen . Prod u ct yield s (m M) are from d u p licate exp erim en ts; resu lts in b rackets are from Tab le 3 in ref 9. (i) TBHP. Catalyst, keton e, alcoh ol, ep oxide, m ixed p eroxid e: 1, 14.4, 19.9 (71); 32.7, 45.5 (69); 0.3, 0.2 (0); 0.3, 0.4 (0.5). 2, 34.1, 71.5 (60); 2.8, 6.5 (35); 0, 0 (0); 0.2, 0 (0.5). 3, 112.5, 89.5 (86); 70.0, 59.2 (60); 6.0, 4.4 (0); trace, 0 (1.8). 4, 49.5, 64.6 (45); 1.5, 2.1 (2.2); 0, 0 (0); 0, 0 (0.5). (ii) MPPH. Catalyst, keton e, alcoh ol, ep oxid e, m ixed p eroxid e, b en zal-d eh yzal-d e, b en zyl alcoh ol, b ib en zyl, MPPOH: 1, 27.7, 27.0; 49.3, 75.0; 0, 0; 0, 0; 0.1, 0; 1.3, 1.5; 0.4, 0.2; 0, 0. 2, 142.5, 168.0; 10.3, 10.7; 2.0, 2.1; 0, 0; 0, 0; trace, trace; 0, 0; 0, 0. 3, 103.9, 65.9; 82.5, 59.6; 2.0, 0.6; 0, 0; 0, 0; 4.2, 2.7; trace, 0.3; 0, 0. 4, 145.0, 158.5; 7.0, 7.5; trace, trace; 0, 0; 0, 0; 0, 0; 0, 0; 0, 0. (c) Oxidation of eth ylben zen e (1 M) by iron catalysts (10 m M) an d
alkyl h ydrop eroxides (20 m M) u n der an atm osp h ere of oxygen . Prod u ct yield s (m M) from d u p licate exp erim en ts, resu lts in b rackets are from Tab le 3 in ref 9. (i) TBHP. Catalyst, keton e, alcoh ol, m ixed p eroxid e: 1, 20.4, 18.7 (16); 10.7, 9.0 (9.2); 0, 0 (0).
2, 18.5, 25.1 (38); 4.5, 0.6 (16); 0, 0 (0). 3, 14.5, 13.1 (35); 11.1, 9.4 (11); 0, 0 (0). 3+ 10 m M benzaldehyde ad d ed p rior to th e ad d ition of TBHP (7.0 m M an d 5.3 m M b en zald eh yd e recovered in th e d u p licate exp erim en ts), 14.6, 13.0; 38.2, 36.5; 1.1, 1.0. 4, 28.4, 26.3 (34); 3.4, 3.1 (0); 0, 0 (0). (ii) MPPH. catalyst, keton e, alcoh ol, m ixed p eroxid e, b en zald eh yd e, b en zyl alcoh ol, b ib en zyl, MPPOH: 1, 35.4, 52.8; 19.8, 23.2; 0, 0; 0.15, trace; 0.9, 1.0; 0, trace; 0, 0.6.
2, 69.1, 46.1; 1.4, 9.5; 0, 0; trace, 0; 0, 0; 0, 0; 0, 0. 3, 52.6, 40.2; 16.8, 22.4; 0, 0; 0, 0; 1.2, 1.5; 0, 0; 0.8, 1.0.
4, 90.9, 78.8; 7.8, 4.5; 0, 0; 0, 0; 0, 0; 0, 0; 0, 0. (11) Korcek, S.; Ch en ier, J. H. B.; Howard , J. A.; In gold ,
K. U. Can . J. Ch em . 1972, 50, 2285-2297.
(12) Howard , J. A.; In gold , K. U. Can . J. Ch em . 1966, 44, 1119-1130.
(13) Aren d s, I. W. C. E.; In gold , K. U.; Wayn er, D. D. M.
J. Am . Ch em . Soc. 1995, 117, 4710-4711.
(14) Avila, D. V.; Brown , C. E.; In gold , K. U.; Lu sztyk, J.
J. Am . Ch em . Soc. 1993, 115, 466-470.
(15) Th e corresp on d in g k2(k9) valu es for eth ylb en zen e an d cycloh exen e are 1.05 × 106an d 5.7 × 106M-1 s-1, resp ectively.16Hyd rogen atom ab straction from th e h yd rop eroxid e b y th e alkoxyl rad ical (k) 8.7 × 106 M-1s-1 in CH3CN)17 can b e ign ored in th ese system s b ecau se of th e low h yd rop eroxid e con cen -tration s em p loyed .
(16) Pau l, H.; Sm all, R. D., Jr.; Scaian o, J. C. J. Am . Ch em .
Soc. 1978, 100, 4520-4527.
(17) Avila, D. V.; In gold , K. U.; Lu sztyk, J.; Green , W. H.; Procop io, D. R. J. Am . Ch em . Soc. 1995, 117, 2929 -2930.
(18) Old royd , R. D.; MacFau l, P. A.; Masch m eyer, T.; Th om as, J. M.; Sn elgrove, D. W.; In gold , K. U.; Wayn er, D. D. M. An gew . Ch em ., In t. Ed. En gl. 1996,
35, 2787-2790.
(19) Sn elgrove, D. W.; MacFau l, P. A.; In gold , K. U.; Wayn er, D. D. M. Tetrah edron Lett. 1996, 37, 823-826. Aren d s, I. W. C. E.; MacFau l, P. A.; Sn elgrove, D. W.; In gold , K. U.; Wayn er, D. D. M. N ATO ASI
Con feren ce, 1996. MacFau l, P. A.; Aren d s, I. W. C.
E.; In gold , K. U.; Wayn er, D. D. M. J. Ch em . Soc.,
Perk in Tran s. 2 1997, 135-145. MacFaul, P. A.;
In gold , K. U.; Wayn er, D. D. M.; Qu e, L., Jr. J. Am .
Ch em . Soc. 1997, 119, 10594-10598.
(20) For eth ylben zen e, Ph CHO average yield) 0.02 m M, ran ge 0-0.15 m M, PhCH2OH average yield (after worku p in th e u su al way13,19 with Ph
3P to red u ce h yd rop eroxid es to alcoh ols) 0.6 m M, ran ge 0-1.5 m M. For cycloh exen e, th e corresp on d in g valu es are 0.01, 0-0.1, 1.5, and 0-4.2 m M. The sm all yields of b en zald eh yd e in th ese reaction s are d u e to its oxid ation as was d em on strated b y th e p artial d e-stru ction of ad d ed b en zald eh yd e d u rin g th e 3/ Me3COOH-in d u ced oxid ation of eth ylb en zen e (see ref 10).
(21) MacFau l, P. A.; In gold , K. U.; Lu sztyk, J. J. Org.
Ch em . 1996, 61, 1316-1321.
(22) Bu rton , G. W.; Dob a, T.; Gab e, E. J.; Hu gh es, L.; Lee, F. L.; Prasad , L.; In gold , K. U. J. Am . Ch em . Soc.
1985, 107, 7053-7065.
(23) Brown lie, I. T.; In gold , K. U. Can . J. Ch em . 1967,
48, 2419-2425.
(24) Th e effect of ad d in g an tioxid an ts (m ole p ercen t relative to su b strate) to th e oxid ation of eth ylb en -zen e (1 M) b y iron catalysts (10 m M) u n d er an
atm osp h ere of oxygen . Prod u ct yield s (m M) from du p licate exp erim en ts: (a) TBHP (20 m M). Catalyst, n o an tioxid an t, 2% BHT, 5% BHT, 10% Ph2NH: Keton e: 1, 20.4, 18.7; 3.4, 5.4; 0, 0; 0, 0. 2, 18.5, 25.1; 7.2, 9.5; 2.3, 3.2; 0.4, 1.4. 3, 14.5, 13.1; 5.8, 6.2; 5.9, 2.4; 0, 0. 4, 28.4, 26.3; 8.0, 7.8; 7.7, 6.4; 0, 0. Alcoh ol: 1, 10.7, 9.0; 10.4, 10.3; 4.8, 4.7; 0, 0. 2, 4.5, 0.6; 1.2, 0.3; 0, 0; 0, 0. 3, 11.1, 9.4; 6.2, 4.4; 4.9, 2.7; 1.1, 1.9. 4, 3.4, 3.1; 2.6, 1.0; 1.2, 0.8; 0.5, 0.1. (b ) MPPH (20 m M) + 2% BHT. Catalyst, ketone, alcohol, m ixed p eroxid e, b en zald eh yd e, b en zyl alcoh ol, b ib en zyl, MPPOH. 1, 2.6, 2.0; 2.7, 1.6; 0, 0; 2.2, 1.3; 1.8, 1.4; 0, 0; 2.8, 3.0. 2, 0.7, 5.8; 0, 0.2; 0, 0; 0.8, 1.7; 0, 0.1; 0, 0.4; 0.8, 2.0. 3, 1.4, trace; 0, 0; 0, 0; 1.6, 2.1; 0, 0; trace, 0; 4.1, 7.3. 4, 0.9, 1.2; 0, 2.0; 0, 0; 3.2, 2.8; 0, 0; 0, 0; 2.0, 2.0. (c) MPPH (20 m M)+ 10% Ph2NH. Catalyst, keton e, alcoh ol, m ixed p eroxid e, b en zald eh yd e, b en zyl alcoh ol, b ib en zyl, MPPOH: 1, 0, 0; 0, 0; 0, 0; 2.7, 2.7; 6.3, 6.4; trace, 0; 0.2, 0.2. 2, 0, 0; 0, 0; 0, 0; 12.1, 12.3; 0.4, 0; 0, 0; 0, 0. 3, 0, 0; 0, 0; 0, 0; 5.7, 6.0;
4.2, 4.3; 0, 0; 0.3, 0.2. 4, 0, 0; 0, 0; 0, 0; 11.5, 11.2; 0, 0; 0, 0; 0, 0.
(25) See, e.g., th e followin g stu d ies in n on aq u eou s system s: Grin staff, M. W.; Hill, M. G.; Lab in ger, J. A.; Gray, H. B. Scien ce 1994, 264, 1311-1313. Min isci, F.; Fon tan a, F.; Aran eo, S.; Recu p ero, F.; Zh ao, L. Syn lett 1996, 2, 119-125. Newcom b, M.; Sim akov, P. A.; Park, S.-U. Tetrah edron Lett. 1996,
37, 819-822.
(26) Peroxyl, alkoxyl, an d alkyl rad icals h ave even b een d etected b y ESR sp in -trap p in g d u rin g th e FeIIin -du ced decom p osition of TBHP in an organ ic solven t (CH2Cl2), see: Ian n on e, A.; Tom asi, A.; Can field , L. M. Rev. Ch em . In term ed. 1996, 22, 469-479. (27) In gold , K. U.; MacFau l, P. A. In Biom im etic
Oxida-tion s Catalyzed by Tran siOxida-tion Metal Com plexes;
Meu n ier, B., Ed .; Im p erial College Press: Lon d on , U.K., in p ress.
AR970057Z
Commentary