HAL Id: hal-01877656
https://hal.archives-ouvertes.fr/hal-01877656
Submitted on 20 Sep 2018
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Increasing the temperature is a relevant strategy to form
microbial anodes intended to work at room temperature
Manon Oliot, Benjamin Erable, Marie-Line De Solan, Alain Bergel
To cite this version:
Manon Oliot, Benjamin Erable, Marie-Line De Solan, Alain Bergel. Increasing the temperature is a
relevant strategy to form microbial anodes intended to work at room temperature. Electrochimica
Acta, Elsevier, 2017, 258, pp.134-142. �10.1016/j.electacta.2017.10.110�. �hal-01877656�
To cite this version : Oliot, Manon and Erable, Benjamin and Solan,
Marie-Line De and Bergel, Alain Increasing the temperature is a relevant strategy to
form microbial anodes intended to work at room temperature. (2017)
Electrochimica Acta, 258. 134-142. ISSN 0013-4686
O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 20366
To link to this article: DOI :
10.1016/j.electacta.2017.10.110
URL :
http://doi.org/10.1016/j.electacta.2017.10.110
Any correspondence concerning this service should be sent to the repository
administrator:
staff-oatao@listes-diff.inp-toulouse.fr
Research Paper
Increasing the temperature is a relevant strategy to form microbial
anodes intended to work at room temperature
Manon Oliot
*, Benjamin Erable, Marie-Line De Solan, Alain Bergel
Laboratoire de G!enie Chimique CNRS ! Universit!e de Toulouse (INPT), 4 all!ee Emile Monso, 31432 Toulouse, France
Keywords:
Electroactive biofilms Bioanode
Microbial fuel cell Microbial electrochemistry Bioelectrochemical system
a b s t r a c t
Reducing the time required for the formation of microbial anodes from environmental inocula is a great challenge. The possibility of reaching this objective by increasing the temperature during the bioanode preparation was investigated here. Microbial anodes were formed at 25"C and 40"C under controlled
potential with successive acetate additions. At 25"C, around 40 days were required to perform three
acetate batches, which led to current density of 9.4 ± 2 A.m!2, while at 40"C, 20 days were sufficient to
complete three similar batches, leading to 22.9 ± 4.2 A.m!2. The bioanodes formed at 40"C revealed
three redox systems and those formed at 25"C only one. The temperature also impacted the biofilm
structure, which was less compact at 40"C. When the bioanodes formed at 40"C were switched to 25"C,
they produced current densities similar to those of bioanodes formed at 25"C; they recovered the single
redox system that was developed by the bioanodes formed at 25"C and the difference in biofilm
structures was mitigated. It is consequently fully appropriate to accelerate the formation of microbial anodes by increasing the temperatures to 40"C even if they are finally intended to operate at room
temperature.
1. Introduction
For around two decades, microbial anodes have been opening up fascinating avenues for a huge number of electrochemical pro-cesses [1e3]. They have provided electrochemists with amazing ways to oxidize various organic compounds[4,5]that could not be easily oxidized by abiotic processes. Microbial fuel cells (MFCs) were the pioneering systems in which microbial anodes were implemented[6e8]. MFCs can also be implemented in the form of self-powered biosensors[9]. Microbial anodes have also become sources of innovative technological concepts. For instance, an extremely simple device, called electro-microbial snorkel, has been derived by short-circuiting a microbial anode with a cathode[10] and has promising applications in environmental depollution [11e13]. Microbial anodes are also at the core of microbial elec-trolysis cells, which open up interesting perspectives for hydrogen production[14,15]and metal recovery[16,17].
Unfortunately, the formation of microbial anodes from natural
media often takes a long time, up to several weeks[18,19]. This is a hindrance for researchers, who need to carry out multiple experi-ments, and for engineers, who seek to shorten the start-up time of the related processes. Reducing the formation time of microbial anodes is consequently a topic of intense research, for which many different paths have been explored. Studies have generally been carried out with MFCs used as the experimental set-up, with the practical objective of reducing the MFC start-up time. The effect of the anode surface topography (roughness, porosity, etc.) has been checked[20]and surface chemistry has been widely investigated with different kinds of surface modification, including electro-chemical treatments [21,22], self-assembled monolayers [23], chemical modification[24,25], surfactant treatments[24,26], and functionalized coatings[25], which pointed out the importance of the hydrophilic/hydrophobic property of the surface[27]. From a biological standpoint, reducing the start-up time of MFCs has been attempted by changing the nature of the inoculum[28]and with various treatments of the inlet, e.g. by chemical amendment[29]or bioaugmentation with an electroactive strain[30]. Technologically, different start-up procedures[31,32]and particularly the impact of the external resistance on the start-up time [33,34] have been studied.
Temperature is a key parameter that drastically affects the growth rate of microorganisms. Increasing the temperature to 35e40"C, which is an optimal range for many microbial species
growth, may consequently be hypothesized to be a powerful lever to accelerate the formation of microbial anodes. This hypothesis has rarely been approached in the literature so far, while many studies have shown the considerable impact of temperature on MFC per-formance. Increasing the temperature has generally been observed to increase MFC power density[35e38]. The increase of current density with temperature has been confirmed over a large range of temperatures, from 4 to 35"C (23.1 mA m!2was provided at 4"C
against 93.9 mA m!2at 35"C)[39]. In the 30 to 45"C range, it has
been shown that the current produced by a microbial anode in-creases with temperature according to an Arrhenius law[40]. In contrast, a temperature higher than 50"C has been reported to
inactivate the microbial metabolism and consequently to decrease the current produced by MFCs[35,40]. It is consequently generally agreed that performance increases, reaching a maximum some-where between 35 and 45"C[35,40e42].
However, this general rule has also been observed to suffer some exceptions. For instance, higher current has been obtained with an MFC operating at lower temperature in a case where lower tem-perature mitigated the development of methanogens, which were responsible for substrate consumption to the detriment of elec-trogenic populations[43,44]. In another case, MFCs run at 10 or 20"C were observed to produce more power than those run at
35"C in long-term experiments, of around 60 weeks, because the
higher biomass accumulation at 35"C favoured the development of
non-electroactive species[45].
Studies have reported that MFCs can tolerate temperature changes, for example temperatures alternating between 18e30"C
and 6e18"C to simulate day-night cycles[41]. The MFCs produced
lower voltage at lower temperature and recovered higher perfor-mance when the temperature rose again. In one case, temperature change has been reported to have a positive impact on MFC per-formance. Starting up an MFC at low temperatures (4 and 10"C) led
to poor power production but the power improved when the MFC was first operated at a higher temperature (30"C) and then
switched to the lower temperature[46]. In contrast, another study comparing MFCs developed at 10, 20 and 35"C showed that only
the MFCs acclimated at 20"C were capable of maintaining a
con-stant steady-state voltage around 0.49 V over the 8e35"C
tem-perature range[47].
The purpose of the present study was to try to shorten the time necessary to form microbial anodes from environmental inocula by increasing the temperature during the formation phase. By analogy with the impact of temperature on MFC performance, which has shown an optimal range around 40"C, this value of the
tempera-ture was chosen with the intention to accelerate the formation of bioanodes. However, it can be feared that an efficient microbial anode formed at high temperature may not be able to operate satisfactorily at room temperature. Consequently, it is necessary to check whether a microbial anode formed rapidly at high temper-ature could remain efficient when subsequently operating at lower temperature. The question to be answered in this study was: when the final objective is to implement a microbial anode at room temperature, is it relevant to accelerate its formation by increasing the temperature, or would it be more suitable to take the time to form it at the temperature (room temperature) at which it is intended to be used?
Most studies devoted to the effect of temperature have used MFCs as the experimental set-up. In an MFC, the effect of temper-ature on the microbial anode itself is difficult to dissociate from its effect on other elements of the cell, including the cathode and the separator, which can depend on the cell configuration [40]. In
particular, the temperature impacts the cathode kinetics, which, in turn, affects the potential of the anode. It was thus observed that the 9% decrease in the power density produced by an MFC when the temperature decreased from 32"C to 20"C was mainly due a
lowering of the cathode potential[37]. Another study has pointed out the impact of the presence or absence of a separator on the effect of temperature on the anode. An MFC initially formed at 30"C maintained high performance when the temperature was
shifted to 20"C if it was equipped with a separator, while the anode
formed in the absence of separator performed poorly when the temperature was lowered[48]. The presence or not of the separator was shown to impact the potential of the anode.
The present study aimed to characterize the impact of temper-ature on the microbial anode itself. Characterizing the behaviour of each of the elements that constitute an electro-microbial reactor, by specific analytical experiments, is a pre-requisite to the launch of an engineering-based strategy for the development of the related technologies[49]. The study was therefore carried out in electro-analytical conditions [50] by using a three-electrode set-up to protect the anode from most interactions due to the other elements of the system, and particularly from variations in the kinetics of the auxiliary electrode. The potential applied to the anode was thus perfectly controlled. Such conditions have rarely been imple-mented so far to characterize the impact of temperature on the formation phase of microbial anodes.
It was chosen to use an inoculum related to soils, because the wide microbial diversity of soils[51,52]has started to be revealed as a great source of MFC electroactive microbial communities[53e56] with various applications including sensors[57]and soil remedia-tion[58]. The method implemented here, which consisted of using soil leachate as the electrolyte, has already led to efficient microbial anodes on various electrode materials including graphite [21], platinum[59]and stainless steel[60]. We thus hope that the results obtained here on the possibility of accelerating the formation of microbial anodes from a soil sample will be useful for numerous future research endeavours.
2. Materials and Methods
2.1. Microbial anode formation and electrochemical procedures Microbial anodes were formed on carbon cloth (PaxiTech SAS, Grenoble, France) of 10 cm2geometric surface area, connected with a platinum wire. The platinum wire was woven into the carbon cloth to form three stitches and the part outside the carbon struc-ture was protected by an insulating heat shrink sleeve. A platinum grid (Heraeus SAS, Germany) was used as the auxiliary electrode and a saturated calomel electrode as the reference (SCE, potential þ0.24 V/SHE). Bioanodes were formed under constant polarization at !0.20 V/SCE (VSP potentiostat, Bio-Logic SA, France). The reactors were glass bottles, which contained 650 mL of solution, covered by a screw cap with the holes required for the connection of the three electrodes. Successive additions of 20 mM sodium acetate were performed when the current felt to zero. The temperature was controlled at 40"C or 25"C with a water-bath.
Current density values were expressed with respect to the pro-jected surface area of the anodes. At the end of each batch, the acetate has been completely consumed and coulombic efficiency was calculated as the ratio of the number of electrons that crossed the electrical circuit to the total number of electrons provided by the complete oxidation of acetate to HCO3!(8 electrons per acetate
molecule). The number of electrons that crossed the electrical cir-cuit was obtained by integrating the current-time curves between two acetate additions. Integration was achieved by the EC-Lab (Bio-Logic SA, France) potentiostat software.
Firstly, microbial anodes were formed in a leachate of garden compost, which was used as both the culture medium and the inoculum as described elsewhere [18,61]. The leachate was ob-tained by filtering a mix of 1.5 L of garden compost and 2.25 L of water, containing 60 mM KCl, through a cloth with a large mesh. Initial pH was 6.8 ± 0.1. Eight reactors were run in parallel. Four were kept at 40"C and four at 25"C. After three acetate additions,
four reactors (two at 40"C and two at 25"C) were stopped and the
microbial anodes were characterized by epifluorescence and scanning electron microscopy.
The compost leachate of the four remaining reactors was replaced by a synthetic medium containing 50 mM bicarbonate buffer, 10 mL.L!1 macronutrients, 1 mL.L!1 micronutrients, 1 mL.L!1vitamins, 4.5 g.L!1KCl and 2.4 g.L!1NaH2PO4. The pH was
adjusted to 7.0 with a 37% HCl solution. The medium was vigorously deoxygenated by 5 to 10 min nitrogen bubbling before filling the reactors, which were maintained under nitrogen atmosphere dur-ing the replacement operation. The two reactors that had been started at 25"C were kept at the same temperature for four acetate
additions. The two reactors initially set up at 40"C were kept at
40"C for two acetate additions and then switched to 25"C for the
other two acetate additions. The microbial anodes were imaged by epifluorescence and scanning electron microscopy at the end of the experiments.
Cyclic voltammetry (CV) curves were recorded from !0.2 V/SCE to þ0.2 V/SCE and then back to !0.5 V/SCE at 1 mV.s!1. Three successive cycles were achieved between the upper and lower potential limits. The first cycle was generally slightly different from the two others, which were perfectly matched. Only the second cycle is presented here. The first derivative with respect to time of the catalytic CV forward scan of the second cycle was calculated in order to discern the redox species merged in the catalytic signal.
2.2. Microscopy imaging
Microbial colonization of the bioanodes was examined by epi-fluorescence microscopy. Samples were stained with acridine or-ange 0.01% (A6014 Sigma) for 10 minutes, then washed and dried at ambient temperature. The samples were imaged with a Carl Zeiss Axiomalger M2 microscope equipped for epifluorescence with an HBO 50 W ac mercury light source and the Zeiss 09 filter (excitor HP450-490, reflector FT 10, Barrier filter LP520). Images were ac-quired with a monochrome digital camera (Evolution VF) every 0.5
m
m along the axis perpendicular to the surface and the set ofimages was processed with the Axiovision®
software. For each electrode, several spots chosen at random were examined and 3 representative spots were imaged.
Biofilm structure was observed by scanning electron microscopy (SEM) with a Leo 435 VP-Carl Zeiss SMT. The bioanodes were fixed in phosphate buffer (400 mM, pH 7.4) with 4% glutaraldehyde. Samples were rinsed in phosphate buffer containing saccharose (400 mM) and were dehydrated by immersion in increasing con-centrations of acetone (50%, 70%, 100%), then in acetone and hex-amethyldisilazane (HMDS) (50:50), and finally in 100% hexamethyldisilazane. The last batch of HMDS was dried to com-plete evaporation. For each electrode, several spots chosen at random were examined and 3 representative spots were imaged.
For both epifluorescent and SEM imaging, the observations detailed in the text are the results of more than 10 observations made at different spots of each electrode surface. The images pre-sented are representative of the electrode colonization pattern and biofilm structure observed on the whole surface of each electrode.
3. Results and discussion
3.1. Microbial anode formation in garden compost leachate Microbial anodes were formed in a leachate of garden compost under controlled potential at !0.20 V/SCE [18,19]. Four reactors were implemented at 40"C and four others at 25"C. Microbial
anodes formed at 40"C produced higher current densities than
those formed at 25"C (Fig. 1). At the third oxidation peak,
22.9 ± 4.2 A.m!2was achieved at 40"C while only 9.4 ± 2 A.m!2
was observed at 25"C. It should also be noted that the current-time
curves presented a more reproducible pattern at 40"C than at
25"C. The pH values at the end of the third acetate batches were
slightly different depending on the temperature: they were 7.8 ± 0.1 at 40"C and 8.3 ± 0.3 at 25"C. The higher standard
devi-ation obtained at 40"C showed that, similarly to the current-time
curves, the temperature of 40"C improved the experimental
reproducibility.
As expected, the time required to form the microbial anodes was considerably reduced at the higher temperature. The start-up time was reduced from at least 4 days to 2.5 days and around 20 days were enough to complete the three acetate batches at 40"C,
whereas 40 days were necessary at 25"C. It is well known that
metabolic reaction rates increase with temperature. Several studies performed with MFCs have observed maximum performance at temperatures in the range of 35 to 45"C[35,40,41]. The
consider-able effect of temperature on the kinetics of the microbial anode, as shown here, was certainly an important cause of the MFC behaviour reported in the literature. The shorter formation time and higher current density observed here at 40"C were most likely due to
higher metabolic rates at higher temperature. Nevertheless, in such a complex multi-species medium, the higher current density pro-duced at higher temperature could also be due to the selection of different microbial communities[42].
Coulombic efficiencies (CEs) showed the same trend whatever the temperature (Table 1). CEs increased considerably from the first to the third oxidation peak: from 1.42 ± 0.38% to 38.2 ± 5% at 40"C
and from 3.1 ± 0.35% to 47.7 ± 5.2% at 25"C. The fact that CE values
remained low had two synergetic causes: the small surface area of the electrodes (10 cm2) in comparison with the relatively large solution volume (650 mL) on the one hand, and the richness of the medium on the other hand. As theorized by Rimboud et al.[50], an electroanalysis system must implement a working electrode of small surface area in a large volume of solution in order to favour the highest possible current density. Conversely, such a system is detrimental to CE values, particularly when non-electrochemical reactions compete for the consumption of the substrate, as was the case here. An electroanalytical device properly designed to characterize a microbial anode under the most favourable condi-tions should result in low Coulombic efficiencies when complex microbial systems are implemented[50], as observed in the present work. The compost leachate may contain various electron acceptors (nitrates, sulphates, etc.) that deflect electroactive bacteria from extracellular electron transfer to the anode. Acetate can also be consumed by non-electroactive bacteria using a soluble electron acceptor and by acetoclastic methanogenic Archaea [62]. These Archaea transform acetate into methane and CO2and they have
already been identified in significant proportions in microbial an-odes formed in soils[63].
CEs displayed lower values at 40"C than at 25"C. For instance,
at the third oxidation peak, CEs were 38.2 ± 5% at 40"C and
47.7 ± 5.2% at 25"C. The lower CEs obtained at 40"C indicate that
this temperature favoured the development of non-electroactive microorganisms, as already observed in MFCs[45], or increased the metabolic rate of the non-electroactive species to a greater
extent than that of the electroactive ones.
The anodes coming from two reactors implemented at 40"C and
two implemented at 25"C were analysed by epifluorescence and
SEM (Fig. 2). Epifluorescence images showed that the anodes developed at 25"C presented a denser colonization than those at
40"C. The colonization of the carbon cloth was more compact at
25"C and formed a homogenous biofilm that masked the carbon
cloth structure. In contrast, the carbon fibres were still apparent at 40"C and the biofilm wrapped around the fibres tightly. SEM
ob-servations at magnification x35 were consistent with the epi-fluorescence images. SEM imaging at a higher magnification (x5000) showed that biofilms formed at 25"C presented
microor-ganisms agglutinated in an abundant matrix of exopolymeric sub-stances (EPS). EPS formed a kind of lace or spider web that made observation of the individual microbial cells difficult. In contrast, cells could be observed easily in the biofilms formed at 40"C.
3.2. Microbial anode formation in synthetic medium
The compost leachate of the four remaining reactors was replaced by a synthetic medium. The procedure, which consisted of forming microbial anodes in a rich natural medium and then transferring them into a minimal synthetic medium, has already been successfully applied to different natural media[61,64]. This procedure has led to significant enhancement of the anode per-formance from the natural medium to the synthetic one. The main interest of this procedure is the possibility of preparing the mi-crobial anode firstly in a complex, rich inoculum and then imple-menting it in a clean electrolyte. For instance, in an MFC equipped with an air-cathode, transferring the microbial anode into a clean electrolyte mitigates biofouling of the cathode[61]. Relocation from natural medium to synthetic minimal medium can also be a way to design robust microbial anodes[64].
The reactors initially implemented at 25"C with the compost
leachate were kept at 25"C for four additional acetate additions
(Fig. 3). The reactors initially run at 40"C with the compost leachate
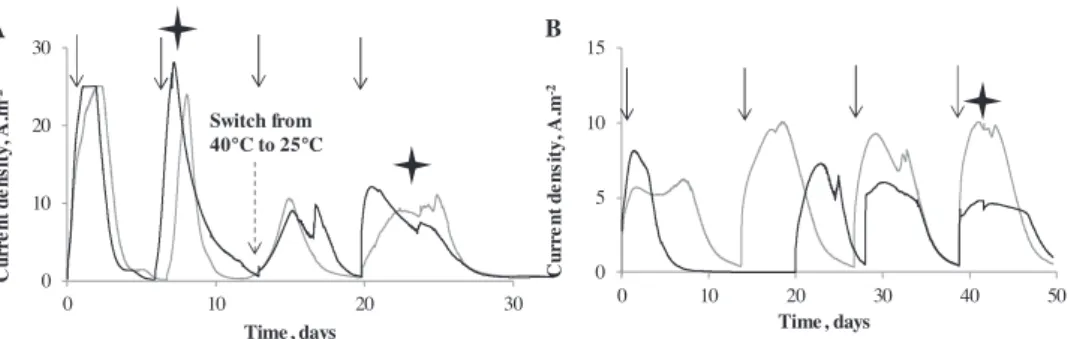
were kept at this temperature for two acetate additions and then were switched to 25"C for two acetate additions. The current
density was almost stable, or slightly decreased, after the garden compost leachate had been replaced by the synthetic medium at 25"C but increased for the anodes run at 40"C. At 40"C, the current
density produced in the garden compost leachate was 22.9 ± 4.2 A.m!2 (n ¼ 4) and it increased to 26.6 ± 1.6 A.m!2
(n ¼ 2) in the synthetic medium. pH at the end of the experiments did not show significant difference with the temperature and was 7.7 ± 0.1.
As commented above, epifluorescent and SEM imaging consis-tently showed that the biofilms formed at 25"C were thicker and
more compact with more EPS matrix, which masked the microbial cells. In contrast, microbial cells were more easily accessible for mass transfer in the biofilms formed at 40"C. Accordingly, this
difference in biofilm structures supports the idea that the biofilms formed at 40"C benefited fully from the supply of vitamins and/or
micro-nutrients because mass transfer of these compounds to the microbial cells was easy. In contrast, the compact biofilm structure of the bioanodes formed at 25"C could hamper the transfer of these
compounds to the electroactive cells, a nd so these bioanodes could not take full advantage of the supplies from the bulk.
Similarly, mass transfer limitation may affect the pH balance inside the biofilm. It is known that local acidification of the biofilm to pH values slightly lower than neutrality, due to acetate oxidation:
CH3COO!þ 4 H2O / 2 HCO3!þ 9 Hþþ 8e! (1)
is a major cause of inhibition of microbial anodes[65,66]. Here, no buffering species was added into the garden compost leachate and the pH gradient may consequently have been stiff inside the biofilms. In contrast, the synthetic medium contained 20 mM of phosphate species. The buffering effect of the synthetic medium may have been more efficient for the biofilms formed at 40"C than
for those formed at 25"C because of the greater thickness and
Fig. 1. Formation of microbial anodes under constant polarization at !0.2 V/SCE in garden compost leachate at (A) 40"C and (B) 25"C. Three successive additions of acetate 20 mM
were performed (indicated by arrows).
Table 1
Coulombic efficiencies obtained in chronoamperometry at !0.2 V/SCE in compost leachate with 4 reactors at 40"C and 4 reactors at 25"C.
40"C Average Standard deviation
1 st peak 0.85 1.5 1.43 1.91 1.4 0.4 2nd peak 30.9 28.1 26.2 25.6 27.7 2.1 3rd peak 43.6 31.5 35.5 42.3 38.2 5 25"C 1 st peak 2.8 2.7 3.5 3.4 3.1 0.4 2nd peak 30.2 37 17.1 30.7 32.6 3.1 3rd peak 52.5 52.8 40.7 44.7 47.7 5.2
compactness of the latter.
In the synthetic medium, CEs were 47.4 ± 9.4% and 42.9 ± 11.9% at 40"C and 25"C, respectively. CEs were of the same order of
magnitude as those obtained in compost leachate at the third peak, although the synthetic medium did not contain dissolved electron acceptors. It was observed that the initially limpid synthetic me-dium became brown after a few days, due to planktonic microbial growth. It cannot be absolutely excluded that traces of oxygen may have penetrated into the reactors and may have been used as electron acceptors. The medium was initially deoxygenated and the reactors were covered by a screw cap, which sealed them perfectly. Actually, the only possible source of oxygen introduction would be during the addition of the acetate solution at the beginning of the different batches. Addition was performed by slightly lifting the cap of a hole for a few seconds. Nevertheless, the possible introduction of air during this very short interval had a very limited extent and could not have supported the significant planktonic growth observed after a few days. The most likely explanation of the low CE values was the presence of acetoclastic methanogenic Archaea that consumed acetate to produce methane and carbon dioxide[62,63]. Because of the low ratio of the electrode surface area to solution volume, used here to work in suitable electroanalysis conditions [50], the effect of planktonic side-reactions was enhanced.
The anodes initially developed at 40"C and then switched to
25"C produced similar current densities, of the order of
10.2 ± 0.4 A.m!2, as to those of anodes implemented at 25"C from
the beginning of the experiment. This result showed that the bio-anodes formed at 40"C were perfectly suitable for subsequent
operation at 25"C. No loss of performance was observed with
respect to the bioanodes formed at 25"C from the beginning of the
experiments, with the same number of batches. It was thus possible to save a lot of time by developing the bioanodes at 40"C, as the
formation time was shortened by around 20 days. Consequently, the strategy of producing bioanodes in optimal conditions at high temperature and then using them in a system that operates at a less favourable temperature is a relevant one.
When the temperature was lowered from 40 to 25"C, the full
decrease of current density was observed from the first acetate batch performed at 25"C and the second acetate addition displayed
similar current values (Fig. 3A). The fact that the full extent of the current density decrease was observed from the first acetate batch indicated that current decrease was probably due to a slowing of the metabolic rates rather than evolution of the microbial com-munities. Adaptation of the microbial community by the develop-ment of different species would have required more time and would not have resulted in stable current density as soon as the temperature was changed. Even if microbial communities may adapt to temperature, it is most likely that the performance observed here for the microbial anode, in terms of current density, was due to the impact of temperature on the metabolic rates.
Cyclic voltammetries (CVs) were recorded in turnover condi-tions, in the zones where the bioanodes produced maximum cur-rent, at different times (stars onFig. 3). All CVs showed a similar general sigmoidal shape, with similar open circuit potentials (Fig. 4A and B). The maximum current density was higher at 40"C
than at 25"C, consistently with the current recorded during
Fig. 2. Epifluorescence and scanning electron microscopy imaging of the microbial anodes formed in leachate of garden compost at 40"C (left) and 25"C (right). Representative
polarization at !0.2 V/SCE. In spite of the similarity of the general shapes, clear differences depending on temperature could be observed on the CVs. The anodes formed at 40"C reached the
maximum current plateau at around !0.1 V/SCE, while those formed at 25"C reached the maximum plateau at around !0.25 V/
SCE. After the anodes formed at 40"C had been run for two batches
at 25"C, they displayed CV curves similar to those of anodes formed
initially at 25"C; the maximum current plateau was reached at
around !0.20 V/SCE. So this characteristic was directly related to the temperature.
The first derivative of the CV curves showed another difference depending on the temperature (Fig. 4C and 4.D). The bioanodes formed at 40"C displayed two clear peaks, centred at around !0.34
and !0.20 V/SCE (solid line, Fig. 4C), and a shoulder around !0.43 V/SCE which can be interpreted as three redox sys-tems being involved in the electron transfer mechanism[67]. In contrast, the bioanodes formed at 25"C showed only the peak
centred at !0.35 V/SCE (Fig. 4D). When the bioanodes formed at 40"C were implemented at 25"C for two batches, the redox
systems at !0.43 and !0.25 V/SCE disappeared (dashed line, Fig. 4C) and the first derivative presented a single peak at !0.35 V/ SCE, similarly to the bioanodes formed at 25"C from the beginning
of the experiment.
In order to validate the approach, theoretical CV curves were reconstructed by assuming that each redox system followed Nernst-Michaelis-Menten kinetics: j ¼ jmax 1 1 þ expð!F RTðE ! EKÞÞ (2)
where j is the current density (A m!2), j
maxis the maximum current
density reached at the plateau (A.m!2), F is the Faraday constant (96 485C per mole e), R is the gas constant (8.3145 J.mol!1.K!1), T is the temperature (K), E is the applied potential (V) and the potential EKis the potential at which j ¼ jmax/2. The theoretical CV at 25"C given
by this equation when it was provided with the experimental values of jmax and EK fitted the experimental record perfectly (Fig. 5). It was thus confirmed that CV corresponded to a single redox system and that this system ensured Nernst equilibrium of
Fig. 3. Microbial anodes formed under constant polarization at !0.2 V/SCE in synthetic medium (A) at 40"C and then switched to 25"C, (B) at 25"C throughout the experiment.
Arrows indicate the successive additions of acetate 20 mM. Cyclic voltammetries were recorded at the times indicated by stars.
Fig. 4. Cyclic voltammetries in turnover conditions of (A) bioanodes formed at 40"C and then switched to 25"C and (B) bioanodes formed at 25"C from the beginning of the
the redox species at the electrode surface. This result confirmed the great interest of soil inocula for forming efficient microbial anodes. The same procedure implemented for the bioanode formed at 40"C produced a theoretical curve that clearly did not match the
experimental curve (Fig. 5). In this case, it was necessary to take the three redox systems detected by the CV first derivative into account and to add the current densities given by these three systems:
j ¼ jmax;i X3 i¼1 jmax;i 1 1 þ expð!F RTðE ! EK;iÞÞ (3)
where jmax,iand EK,icorrespond to the ith redox system. The three
values of EK,i were extracted from the CV first derivative (-0.43 V, !0.34 V and !0.20 V/SCE) and the three parameters jmax,i
were adjusted numerically in order to make the theoretical curve fit the experimental CV. The values of 5, 14.2 and 12 A.m!2 led to satisfactory fitting (Fig. 5). This result is of great importance for the basic understanding of the anode kinetics. The experimental CV of the bioanodes formed at 40"C showed a large difference with the
theoretical Nernst-Michaelis-Menten curve. Nevertheless, it must not be concluded that the interfacial electron transfer was not efficient enough to ensure Nernstian equilibrium at the interface. In contrast, the multi-system model matched the experimental CV perfectly, showing that the kinetics was the sum of three
Nernst-Fig. 5. Experimental and theoretical cyclic voltammetries of (A) bioanodes formed at 40"C and (B) bioanodes formed at 25"C. Continuous lines: experimental curves, similar to
Fig. 4; dashed lines: theoretical curve obtained with a single Nernst-Monod system; dotted line: theoretical curve obtained with three Nernst-Monod systems.
Fig. 6. Epifluorescence and scanning electron microscopy imaging of the microbial anodes formed in synthetic medium and presented inFig. 3: formed at 40"C and switched to
25"C (left) and at 25"C throughout the experiment (right). Representative views of 3 points imaged on two different electrodes for each temperature for both observation
Michaelis-Menten kinetics. Actually, the 40"C kinetics was the sum
of three redox systems, each ensuring Nernst equilibrium. The biofilm was composed of three very efficient redox systems.
Practically, if the objective of further work was to improve the anode kinetics, it would not be relevant to try to accelerate the interfacial electron transfer but, rather, to make the biofilm select for the most efficient redox pathway. The key for kinetics improvement is consequently not the interfacial electron transfer rate but the electrochemical redox system that the biofilm is able to implement. Further possible improvement of this kind of anode should concentrate on the nature of the redox systems that the biofilm develops.
These results show that the temperature has a direct impact on the redox systems involved in electron transfer. At 40"C, two
supplementary redox systems appeared, which then disappeared when the anodes were implemented at 25"C. These additional
redox systems explained the difference in the CV curves of the anodes formed at 40 and 25"C. The theoretical breakdown into
three systems indicated that the system centred at around !0.35 V/ SCE, which was present independently of the temperature, was responsible for around 14.2 A.m!2. This current density was higher than the jmax provided by the bioanodes formed at 25"C
(9.4 ± 2 A.m!2). At 25"C, the two systems centred at !0.43
and !0.20 V/SCE disappeared and the remaining single system produced less current, certainly because of slowing of the meta-bolic rate. In terms of electrochemical functionality, shifting the temperature from 40 to 25"C resulted in putting two redox systems
out of operation and slowing down the single redox system detected at 25"C, so that, when used at 25"C, the microbial anodes
formed at 40"C presented electrochemical characteristics similar
to those of anodes formed at 25"C.
After four batches in synthetic medium, the microbial anodes were observed by epifluorescence (Fig. 6) and SEM. Epifluorescence imaging showed no significant difference between bioanodes switched from 40 to 25"C and those formed at 25"C from the
beginning. For both conditions, biofilms were denser than those observed in the leachate of garden compost (Fig. 2) and covered the entire electrode surfaces. Differences were, however, detected with SEM. Bioanodes formed at 25"C from the beginning presented a
really thick biofilm covering the entire surface, whereas carbon fibres were still distinguishable on bioanodes firstly formed at 40"C and then switched to 25"C. The less compact structure of the
biofilm that was formed at 40"C was still visible after the bioanode
had been switched to 25"C for two batches but it tended to
disappear. The two temperatures led to different biofilm structures, which then evolved towards a similar pattern when the tempera-ture was switched to 25"C.
The conclusion on this issue may be open to debate because the biofilms formed at 40 and 25"C were compared at different ages.
As expected, forming biofilms at 40"C was much faster. The three
initial batches in compost leachate were achieved in only 20 days, against 40 to 44 days at 25"C. Then, in synthetic medium, the
biofilms formed at 40"C underwent two additional batch runs at
40"C before being switched to 25"C, so their final age was 53 days.
In comparison, the final age of the biofilms operated at 25"C for the
whole experiment was 94 days. In this context, great care must be taken when comparing the biofilm structures. Nevertheless, the biofilms aged 53 days formed at 40"C showed a more open
struc-ture (Fig. 6, SEM imaging, left) than the biofilms aged 44 days formed at 25"C (Fig. 6, SEM imaging, right). The claim that
tem-perature had a real impact on the biofilm structure is consequently sound. If the objective is to accurately characterize the impact of temperature on the biofilm structure, further studies should be performed by comparing biofilms at identical ages, instead of working at identical number of batches as we chose to do here.
4. Conclusion
As expected, the temperature of 40"C permitted the time
required to form microbial anodes to be considerably reduced. Here the formation time was reduced by 50%, from 40 to 20 days, to run three batches of acetate addition. In addition, the bioanodes formed at 40"C and then implemented at 25"C displayed similar
perfor-mance to that of the bioanodes formed initially at 25"C. The
bio-anodes formed at 40"C showed evidence of three redox systems
but, when switched to 25"C, recovered the same single system that
was developed at 25"C. It is consequently fully relevant to reduce
the formation time of microbial anodes by increasing the temper-ature, even for anodes intended to work at room temperature. Acknowledgements
This work benefited from the support of the French state, managed by the Agence Nationale de la Recherche (ANR), within the framework of the project Bioelec (ANR-13-BIME-006). Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, athttps://doi.org/10.1016/j.electacta.2017.10.110. References
[1] H. Wang, Z.J. Ren, A comprehensive review of microbial electrochemical systems as a platform technology, Biotechnol. Adv. 31 (2013) 1796e1807. [2] S. Venkata Mohan, G. Velvizhi, K. Vamshi Krishna, M. Lenin Babu, Microbial
catalyzed electrochemical systems: A bio-factory with multi-facet applica-tions, Bioresour. Technol. 165 (2014) 355e364.
[3] S. Bajracharya, M. Sharma, G. Mohanakrishna, X. Dominguez Benneton, D.P.B.T.B. Strik, P.M. Sarma, et al., An overview on emerging bio-electrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond, Renew. Energy. 98 (2016) 153e170.
[4] D. Pant, G. Van Bogaert, L. Diels, K. Vanbroekhoven, A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production, Bio-resour. Technol. 101 (2010) 1533e1543.
[5] P. Pandey, V.N. Shinde, R.L. Deopurkar, S.P. Kale, S.A. Patil, D. Pant, Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery, Appl. Energy. 168 (2016) 706e723.
[6] D.R. Bond, D.E. Holmes, L.M. Tender, D.R. Lovley, Electrode-Reducing Micro-organisms That Harvest Energy from Marine Sediments, Science. 295 (2002) 483e485.
[7] L.M. Tender, C.E. Reimers, H.A. Stecher, D.E. Holmes, D.R. Bond, D.A. Lowy, et al., Harnessing microbially generated power on the seafloor, Nat. Bio-technol. 20 (2002) 821e825.
[8] C. Santoro, C. Arbizzani, B. Erable, I. Ieropoulos, Microbial fuel cells: From fundamentals to applications. A review, J. Power Sources 356 (2017) 225e244. [9] G. Pasternak, J. Greenman, I. Ieropoulos, Self-powered, autonomous Biological Oxygen Demand biosensor for online water quality monitoring, Sens. Actua-tors B. Chem. 244 (2017) 815e822.
[10] B. Erable, L. Etcheverry, A. Bergel, From microbial fuel cell (MFC) to microbial electrochemical snorkel (MES): maximizing chemical oxygen demand (COD) removal from wastewater, Biofouling 27 (2011) 319e326.
[11] Q. Yang, H. Zhao, H. Liang, Denitrification of overlying water by microbial electrochemical snorkel, Bioresour. Technol. 197 (2015) 512e514. [12] D.R. Lovley, Live wires: direct extracellular electron exchange for bioenergy
and the bioremediation of energy-related contamination, Energy Environ. Sci. 4 (2011) 4896e4906.
[13] C.C. Viggi, E. Presta, M. Bellagamba, S. Kaciulis, S.K. Balijepalli, G. Zanaroli, et al., The Oil-Spill Snorkel: an innovative bioelectrochemical approach to accelerate hydrocarbons biodegradation in marine sediments, Front Micro-biol. 6 (2015) 881.
[14] Y. Zhang, I. Angelidaki, Microbial electrolysis cells turning to be versatile technology: Recent advances and future challenges, Water Res. 56 (2014) 11e25.
[15] M. Kitching, R. Butler, E. Marsili, Microbial bioelectrosynthesis of hydrogen: Current challenges and scale-up, Enzyme Microb. Technol. 96 (2017) 1e13. [16] H. Wang, Z.J. Ren, Bioelectrochemical metal recovery from wastewater: A
review, Water Res. 66 (2014) 219e232.
[17] Y.V. Nancharaiah, S.V. Mohan, P.N.L. Lens, Metals removal and recovery in bioelectrochemical systems: A review, Bioresour. Technol. 195 (2015)
102e114.
[18] B. Cercado, N. Byrne, M. Bertrand, D. Pocaznoi, M. Rimboud, W. Achouak, et al., Garden compost inoculum leads to microbial bioanodes with potential-independent characteristics, Bioresour. Technol. 134 (2013) 276e284. [19] D. Pocaznoi, B. Erable, L. Etcheverry, M.-L. Delia, A. Bergel, Towards an
engineering-oriented strategy for building microbial anodes for microbial fuel cells, Phys. Chem. Chem. Phys. 14 (2012) 13332e13343.
[20] C. Santoro, M. Guilizzoni, J.P.C. Baena, U. Pasaogullari, A. Casalegno, B. Li, et al., The effects of carbon electrode surface properties on bacteria attachment and start up time of microbial fuel cells, Carbon. 67 (2014) 128e139.
[21] B. Cercado-Quezada, M.-L. D!elia, A. Bergel, Electrochemical micro-structuring of graphite felt electrodes for accelerated formation of electroactive biofilms on microbial anodes, Electrochem. Commun. 13 (2011) 440e443.
[22] B. Li, J. Zhou, X. Zhou, X. Wang, B. Li, C. Santoro, et al., Surface Modification of Microbial Fuel Cells Anodes: Approaches to Practical Design, Electrochimica Acta. 134 (2014) 116e126.
[23] C. Santoro, S., Babanova, K., Artyushkova, JA., Cornejo, L., Ista, O. Bretschger, et al. Influence of anode surface chemistry on microbial fuel cell operation, Bioelectrochemistry106 (2015) 141e149.
[24] Y. Zhang, J. Jiang, Q. Zhao, Y.-Z. Gao, K. Wang, J. Ding, et al., Accelerating anodic biofilms formation and electron transfer in microbial fuel cells: Role of anionic biosurfactants and mechanism, Bioelectrochemistry 117 (2017) 48e56. [25] A. Kaur, S. Ibrahim, C.J. Pickett, I.S. Michie, R.M. Dinsdale, A.J. Guwy, et al.,
Anode modification to improve the performance of a microbial fuel cell vol-atile fatty acid biosensor, Sens. Actuators B-Chem 201 (2014) 266e273. [26] K. Guo, A.H. Soeriyadi, S.A. Patil, A. Prevoteau, S. Freguia, J.J. Gooding, et al.,
Surfactant treatment of carbon felt enhances anodic microbial electrocatalysis in bioelectrochemical systems, Electrochem. Commun. 39 (2014) 1e4. [27] K. Guo, S. Freguia, P.G. Dennis, X. Chen, B.C. Donose, J. Keller, et al., Effects of
Surface Charge and Hydrophobicity on Anodic Biofilm Formation Community Composition, and Current Generation in Bioelectrochemical Systems, Environ. Sci. Technol. 47 (2013) 7563e7570.
[28] J. Lobato, P. Canizares, F. Jesus Fernandez, M.A. Rodrigo, An evaluation of aerobic and anaerobic sludges as start-up material for microbial fuel cell systems, New Biotechnol. 29 (2012) 415e420.
[29] G. Liu, M.D. Yates, S. Cheng, D.F. Call, D. Sun, B.E. Logan, Examination of mi-crobial fuel cell start-up times with domestic wastewater and additional amendments, Bioresour. Technol. 102 (2011) 7301e7306.
[30] S. Pandit, S. Khilari, S. Roy, M.M. Ghangrekar, D. Pradhan, D. Das, Reduction of start-up time through bioaugmentation process in microbial fuel cells using an isolate from dark fermentative spent media fed anode, Water Sci. Technol. 72 (2015) 106e115.
[31] Z. Borjas, J. Manuel Ortiz, A. Aldaz, J. Feliu, A. Esteve-Nunez, Strategies for Reducing the Start-up Operation of Microbial Electrochemical Treatments of Urban Wastewater, Energies. 8 (2015) 14064e14077.
[32] H.C. Boghani, J.R. Kim, R.M. Dinsdale, A.J. Guwy, G.C. Premier, Control of power sourced from a microbial fuel cell reduces its start-up time and increases bioelectrochemical activity, Bioresour. Technol. 140 (2013) 277e285. [33] L. Rago, N. Monpart, P. Cortes, J.A. Baeza, A. Guisasola, Performance of
mi-crobial electrolysis cells with bioanodes grown at different external re-sistances, Water Sci. Technol. 73 (2016) 1129e1135.
[34] L. Zhang, J. Li, X. Zhu, D. Ye, Q. Fu, Q. Liao, Startup Performance and Anodic Biofilnn Distribution in Continuous-Flow Microbial Fuel Cells with Serpentine Flow Fields: Effects of External Resistance, Ind. Eng. Chem. Res. 56 (2017) 3767e3774.
[35] L.H. Li, Y.M. Sun, Z.H. Yuan, X.Y. Kong, Y. Li, Effect of temperature change on power generation of microbial fuel cell, Environ. Technol. 34 (2013) 1929e1934.
[36] H. Moon, I.S. Chang, B.H. Kim, Continuous electricity production from artificial wastewater using a mediator-less microbial fuel cell, Bioresour. Technol. 97 (2006) 621e627.
[37] H. Liu, S.A. Cheng, B.E. Logan, Power generation in fed-batch microbial fuel cells as a function of ionic strength temperature, and reactor configuration, Environ. Sci. Technol. 39 (2005) 5488e5493.
[38] B. Min, O.B. Rom!an, I. Angelidaki, Importance of temperature and anodic medium composition on microbial fuel cell (MFC) performance, Biotechnol. Lett 30 (2008) 1213e1218.
[39] A. Larrosa-Guerrero, K. Scott, I.M. Head, F. Mateo, A. Ginesta, F. Jose Hernan-dez-Fernandez, et al., Low temperature performance of microbial fuel cells, 13th International Conference on Process Integration, Modelling and Opti-misation for Energy Saving and Pollution Reduction. (2010) 463e468. [40] Y. Liu, V. Climent, A. Berna, J. Miguel Feliu, Effect of Temperature on the
Catalytic Ability of Electrochemically Active Biofilm as Anode Catalyst in Mi-crobial Fuel Cells, Electroanalysis 23 (2011) 387e394.
[41] Y. Zhang, J. Sun, Y. Hu, Z. Wang, S. Li, Effects of periodically alternating tem-peratures on performance of single-chamber microbial fuel cells, Int. J. Hydrog. Energy. 39 (2014) 8048e8054.
[42] R.A. Barbato, K.L. Foley, J.A. Toro-Zapata, R.M. Jones, C.M. Reynolds, The power
of soil microbes: Sustained power production in terrestrial microbial fuel cells under various temperature regimes, Appl. Soil Ecol. 109 (2017) 14e22. [43] L. Liu, O. Tsyganova, D.-J. Lee, J.-S. Chang, A. Wang, N. Ren, Double-chamber
microbial fuel cells started up under room and low temperatures, Int. J. Hydrog. Energy. 38 (2013) 15574e15579.
[44] G.S. Jadhav, M.M. Ghangrekar, Performance of microbial fuel cell subjected to variation in pH temperature, external load and substrate concentration, Bio-resour. Technol. 100 (2009) 717e723.
[45] I.S. Michie, J.R. Kim, R.M. Dinsdale, A.J. Guwy, G.C. Premier, Operational temperature regulates anodic biofilm growth and the development of elec-trogenic activity, Appl. Microbiol. Biotechnol. 92 (2011) 419e430.
[46] S. Cheng, D. Xing, B.E. Logan, Electricity generation of single-chamber mi-crobial fuel cells at low temperatures, Biosens. Bioelectron. 26 (2011) 1913e1917.
[47] I.S. Michie, J.R. Kim, R.M. Dinsdale, A.J. Guwy, G.C. Premier, The influence of psychrophilic and mesophilic start-up temperature on microbial fuel cell system performance, Energy Environ. Sci. 4 (2011) 1011e1019.
[48] X. Zhang, P. Liang, J. Shi, J. Wei, X. Huang, Using a glass fiber separator in a single-chamber air-cathode microbial fuel cell shortens start-up time and improves anode performance at ambient and mesophilic temperatures, Bio-resour. Technol. 130 (2013) 529e535.
[49] M. Oliot, P. Chong, B. Erable, A. Bergel, Influence of the electrode size on microbial anode performance, Chem. Eng. J. (2017),https://doi.org/10.1016/ j.cej.2017.06.044.
[50] M. Rimboud, D. Pocaznoi, B. Erable, A. Bergel, Electroanalysis of microbial anodes for bioelectrochemical systems: basics, progress and perspectives, Phys. Chem. Chem. Phys. PCCP. 16 (2014) 16349e16366.
[51] V. Torsvik, R. Sorheim, J. Goksoyr, Total bacterial diversity in soil and sediment communities ! A review, J. Ind. Microbiol. 17 (1996) 170e178.
[52] B.-R. Liu, G.-M. Jia, J. Chen, G. Wang, A Review of Methods for Studying Mi-crobial Diversity in Soils, Pedosphere. 16 (2006) 18e24.
[53] H. Futamata, O. Bretschger, A. Cheung, J. Kan, R. Owen, K.H. Nealson, Adap-tation of soil microbes during establishment of microbial fuel cell consortium fed with lactate, J.Biosci. Bioeng. 115 (2013) 58e63.
[54] S.J. Dunaj, J.J. Vallino, M.E. Hines, M. Ga, C. Kobyljanec, J.N. Rooney-Varga, Relationships between Soil Organic Matter, Nutrients Bacterial Community Structure, And the Performance of Microbial Fuel Cells, Environ. Sci. Technol. 46 (2012) 1914e1922.
[55] Y.-B. Jiang, W.-H. Zhong, C. Han, H. Deng, Characterization of Electricity Generated by Soilin Microbial Fuel Cells and the Isolation of Soil Source Exoelectrogenic Bacteria, Front. Microbiol. 7 (2016) 1776.
[56] S.R.B. Arulmani, V. Jayaraj, S.R. Jebakumar, Long-term electricity production from soil electrogenic bacteria and high-content screening of biofilm forma-tion on the electrodes, J. Soils Sediments 16 (2016) 831e841.
[57] P. Cristiani, A. Franzetti, G. Bestetti, Monitoring of electro-active biofilm in soil, Electrochimica Acta. 54 (2008) 41e46.
[58] A. Dominguez-Garay, K. Boltes, A. Esteve-Nunez, Cleaning-up atrazine-polluted soil by using Microbial Electroremediating Cells, Chemosphere 161 (2016) 365e371.
[59] D. Pocaznoi, B. Erable, M.-L. D!elia, A. Bergel, Ultra microelectrodes increase the current density provided by electroactive biofilms by improving their electron transport ability, Energy Environ. Sci. 5 (2012) 5287e5296.
[60] S.F. Ketep, A. Bergel, A. Calmet, B. Erable, Stainless steel foam increases the current produced by microbial bioanodes in bioelectrochemical systems, Energy Environ. Sci. 7 (2014) 1633e1637.
[61] M. Oliot, L. Etcheverry, A. Bergel, Removable air-cathode to overcome cathode biofouling in microbial fuel cells, Bioresour. Technol. 221 (2016) 691e696. [62] B. Demirel, P. Scherer, The roles of acetotrophic and hydrogenotrophic
methanogens during anaerobic conversion of biomass to methane: a review, Rev. Environ. Sci. Biotechnol. 7 (2008) 173e190.
[63] A. Cabezas, B. Pommerenke, N. Boon, M.W. Friedrich, Geobacter, Anaero-myxobacter and Anaerolineae populations are enriched on anodes of root exudate-driven microbial fuel cells in rice field soil, Environ. Microbiol. Rep. 7 (2015) 489e497.
[64] X. Dominguez-Benetton, J.J. Godon, R. Rousseau, B. Erable, A. Bergel, M.L. D!elia, Exploring natural vs. synthetic minimal media to boost current generation with electrochemically-active marine bioanodes, J. Environ. Chem. Eng. 4 (2016) 2362e2369.
[65] C.I. Torres, A.K. Marcus, B.E. Rittmann, Proton Transport Inside the Biofilm Limits Electrical Current Generation by Anode-Respiring Bacteria, Biotechnol. Bioeng. 100 (2008) 872e881.
[66] S.C. Popat, C.I. Torres, Critical transport rates that limit the performance of microbial electrochemistry technologies, Bioresour. Technol. 215 (2016) 265e273.
[67] M. Rimboud, E. Desmond-Le Quemener, B. Erable, T. Bouchez, A. Bergel, Multi-system Nernst-Michaelis-Menten model applied to bioanodes formed from sewage sludge, Bioresour. Technol. 195 (2015) 162e169.