Zeitschrift fürPhysikalischeChemie. Bd. 201. S. 151-158(1997)
© by R. Oldenbourg Verlag,München 1997
Local Structure of
Cu2+
in the
(C2H5NH3)2MCl4:Cu2+
(M
=Cd,
)
Layer
Perovskites.
Influence of
Hydrostatic
Pressure in the
0-60
kbar
Range*
By
B. A.Moral1,
F.Rodriguez1**,
R.Valiente',
M. Moreno' and H. U. Güdel21
DCITIMAC,Facultad deCiencias,UniversidaddeCantabria,
E-39005 Santander, Spain
2 Institut füranorganische undphysikalischeChemie, Universitat Bern.
Freiestrasse3, CH-3000 Bern 9,Switzerland
(Received
August
24. 1996)Charge-transfer
spectroscopy
IHydrostatic
pressureICuClf
complex
I(C2H5NH3)2CdCl41 (C2HsNHJ2MnCh
This paper deals with the effects ofpressure on the charge-transfer spectra ofCuCl?,"
complexes
formed in Cu2+doped
(C2H5NH,)2MCl4 (M = Cd, ). Apressure-induced
redshift isobserved for the first
charge-transfer
band inboth crystals. While the shift is continuous for the Mn crystal at a rate of —40 cm"'/kbar in the 0—60 kbar range, itexperiences an abrupt jumpof —1400 cm"' around 26 kbar for the Cd crystal. Such a
discontinuous behaviour is interpreted in terms of a structural change in the CuCl¿
coordination geometry from an axially elongated octahedron to a more compressed
geometry. The present results are compared with those reported for the pure
(C2H,NH,)2CuCl4crystal.
Introduction
The aim of this work isto
investigate
the localstructureofCuCl^"
complex-esformed in the
(C2H3NH3)2MC14
(M
=Cd,
)
crystals doped
withCu2+and its
dependence
on thehydrostatic
pressurethrough
theCharge-Transfer
(CT)
spectra.
Attention ispaid
on whether the formation ofcompressed
* Presented at the 13th International
Symposium
on Electrons and Vibrations inSolids and Finite
Systems
(Jahn-TellerEffect)Berlin 1996.** Author for
152 . . Moral,F. Rodríguez, R. Valiente,M. Moreno and H. U. Giidel
CuCi6~
complexes,
unusual for chlorides[1, 2],
ispossible
ornot in theselayer perovskites,
and whether anelongated complex
can be transformedintoa
compressed
oneby
applying
hydrostatic
pressures. The selectedcrys-tals are suitable
systems
for such astudy
sincethey provide compressed
octahedron sites for
accommodating
substitutionalimpurities.
Inparticular,
theequatorial
and axial M—Cl distances of thecompressed
MCl^
octa-hedra are = 2.67 A and
Rd%
= 2.52À
for(C2H5NH3)2CdCl4 [3]
whilefor
(C2H5NH3)2MnCl4
the distances arefleq
= 2.59Á
and/?ax
= 2.47Á
[4].
In contrast, pure(CnH2n+1NH3)2CuCl4
compounds display
anin-plane
antiferrodistortive structure of
axially elongated
CuCl?r
[5].
Infact,
theelongated
octahedron is the usual coordination structure forCuCljT
eitherin pure
cupric
chlorides or indoped compounds. Exceptions
to this be-haviour are found in(enH2)MnCl4:Cu2+
[6, 7]
and,
ingeneral,
inlayer
perovskites (CnH2n+1NH3)2MnCl4:Cu2+
[8]
where acompressed
D4I,
co-ordination
geometry
wasproposed
forexplaining
the intense CTabsorption
band at 21000 cm1.
Nevertheless,
recentinvestigations performed
onisomorphous
Cdcrystals
[8, 9]
reveal thatCuCl¿"
iselongated
rather thancompressed,
eventhough
this is the actual symmetry of thereplaced
Cd2+site. In orderto
clarify
the different behaviour exhibitedby
the Cd and Mncrystals,
we have studied the effect of thehydrostatic
pressure on the CTspectra
corresponding
to these Cu2+doped
layered
perovskites.
Apart
from the selective character of the CTbands,
theirhigh
oscillatorstrengths
(f~
10"1—10"2) [8]
make it suitableprobes
fordetecting
structuralchanges
around Cu2+ in diluted materials(<1%)
subjected
tohydrostatic
pressure.Interestingly,
the results obtained on these diluted systems compare wellwith those
recently
obtained in the pure(C2H5NH,)2CuCl4
crystal
[10],
in which the occurrenceofapressure-induced
phase
transition around 40 kbar wasinterpreted
in terms ofdisappearance
of thein-plane
lattice distortion ofCuCir.
Experimental
The
single crystals
of(C2H5NH3)2MnCl4
and(C2H5NH3)2CdCl4
doped
withCu2+
(about
0.2 mol%)
arethesame onesemployed
in Ref.[8].
Hydrostatic
pressure
experiments
have beenperformed
onmicrocrystals
of about100 X100X20
pm3 using
asapphire
anvil cell attached to aspecially
designed
double beamspectrometer.
Details of theexperimental
setup
aregiven
elsewhere[11].
Paraffin oil was used as pressure transmitter and thepressure was calibrated
through
the shifts of theruby
R-lines.Ruby
luminescence was excited
by
a 568 nm Coherent I-302-KKrypton
laser.Results and
discussion
Fig.
1 shows theoptical
absorption
(OA)
spectra
of thetwotitlecompounds
at
atmospheric
pressure. Ananalysis
of thecorresponding
spectra
isgiven
Local Structure ofCu2+ in the (C2H5NH,)2MCI4:Cu2tLayerPerovskites 153
6000
_I_,_I_,_I_,_ 40000 35000 30000 25000 20000
WAVENUMBER(cm1)
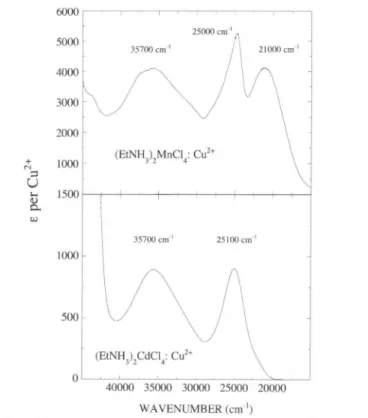
Fig.1.Optical absorptionspectraof(C2H5NH,)2MnCl4:Cu2+ and(C2H5NH,)2CdCl4:Cu2+
at
atmospheric
pressure androomtemperature.Thespectrawereobtained withpolarized
lightpropagating along
thecdirection(perpendicular
tothe layer).in Refs.
[6-9].
In bothcrystals,
theabsorption
bands have beenassigned
toCl" — Cu2+ CT transitions of the formed
CuCit
complex.
Thespectrum
of the Cd
crystal
is similar to those found inCdCl2:Cu2+, LiCl:Cu2+,
(C2H5NH,)2CuCl4
whereCuCl£
complexes display
anelongated
octa-hedron structure
[12-15].
Inparticular
the two intense bands at 25100 and 35700cm"' observed in(C2H3NH,)2CdCl4:Cu2+
areassigned
within an
elongated
complex
ofD4/, symmetry
to CT transitionsfrom the
bonding mainly
Cl_eu(7r
+rj)
and„(
+)
molecular orbitals(MO)
to theantibonding mainly
Cu2+b,g
(x2—y2)
MO,
respectively.
In(C2H5NH3)2MnCl4:Cu2+
however the intense band at 21000 cm"1 isasso-ciated with a CT transition from the
equatorial
Cl"eu(n
+a)
MO to the Cu2+a,g(3
z2-
r)
MO within acompressed
CuCl^"
complex
with the shortaxial bond
perpendicular
to thelayer
[6—8].
Nevertheless,
this model is unable toexplain
the presenceofthenarrower bandat 25000 cm"'. Recentcrys-154 . . Moral,F. Rodríguez, R. Valiente,M. Moreno and H. U. Güdel
(C2H5NH3)2CdCl4:
Cu2+
1 ' ' 1 1 ' ' ' 1 I . I , I . I . I . I . I . I I i I 30000 25000 20000 WAVENUMBER(cm"1)
Fig. 2. Influence of the
hydrostatic
pressure upon the firstCl" —» Cu3+ charge transferband in(C2H5NH3)2CdCl4:Cu2+.
tal series
point
out the relevance of the Mn2+ notonly
forexplaining
theenhancement of
absorption
intensity
onpassing
from = 0 to = 1 aswell as its thermal
dependence,
but also forexplaining
the presence of the25000 cm"1 band
although
in such a case an additionalD2h
orthorhombicdistortion for the
CuCli!"
was assumed[9].
The effect of the
hydrostatic
pressure on the OA spectra of(C2H5NH3)2MCl4:Cu2+
(M
=Cd, )
is illustrated inFigs.
2 and 3. Thecorresponding
variations of thepeak
energy with pressure areplotted
inFigs.
4 and 5.Note thatin bothcrystals
the CT bands shifttolowerenergies
uponincreasing
pressure,although
thevariation,
E(P)
is rather different in each case. While a continuous redshift is observed for the 25000 and21000 cm"1 bands at shift rates, El
,
of -6.1 and -40cm"Vkbar,
respectively,
for the(C2H5NH3)2MnCl4:Cu2+,
this variation for the(C2H5NH3)2CdCl4:Cu2+
crystal undergoes
anabrupt
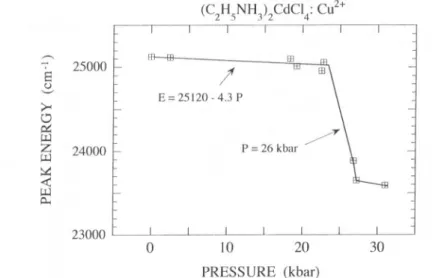
shift of -1400cm"1around 26 kbar. From
atmospheric
pressure to25kbar,
the CT bandexperi-ences a small redshift of about —100cm"1. The
steep
variation exhibitedby
the first CT band at 26 kbar isnoteworthy.
This reflects structuralchanges
of theCuCl^"
complexes
which areprobably
associated with ashortening
of thein-plane
axial bond(and
likely
alengthening
of thein-plane
shortbonds)
leading
to a morecompressed
geometry
where theshortest Cu—Cl bondsare
perpendicular
to thelayer.
Thecomparison
of thepresent
results with those obtainedby
Moritomoetal. in(C2H5NH3)2CuCl4
using
OA and Ramanspectroscopy supports
this view[10].
These authorsLocal Structure ofCu2+ in the (C,H,NH,)2MCl4:Cu2' LayerPerovskites 155
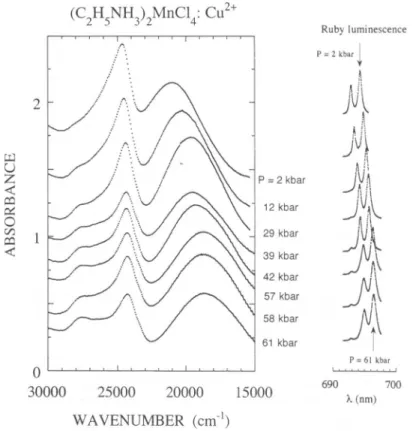
(C2H5NH3)2MnCl4:
Cu2+ 2 kbar Rubyluminescence kbarI
4
i ¡I91
P=61 kbar 690 700 .(nm) 25000 20000 15000 WAVENUMBER(cm"1)
Fig.3. Variationof theoptical
absorption
spectraof(C2H5NH,)2MnCl4:Cu2+ withhydro-staticpressure at roomtemperature in the 0
—
61 kbar range. The correspondingvariation
of the Ruby luminescence used forpressurecalibration, is shownon the rightside.
40 kbar that involves the deactivation ofthe Raman
peaks
associated with thestretching
vibrations of thein-plane
axial andequatorial
bonds of theelongated
CuCli"
complexes (Rm
= 2.98À
and = 2.28A)
[5].
Theoccurrence of such a
phase
transitionisimportant
since it wouldimply
thedisappearance
of the antiferrodistortive structuredisplayed
by
theCuCl¿
complexes
and,
consequently
achange
in themagnetic
behaviour of thecrystal
fromferromagnetic
toantiferromagnetic
should beexpected
above 40 kbar[10].
The variation of the CT energy upon pressurereported
in that work resembles those observed for thepresent
dilutedsystems
but indiffer-ent pressure ranges. The redshift of -3200cm 1 observed between 15 and
30 kbar in
(C2H5NH,)2CuCl4
is similar to the variation shown inFig.
3 for(C2H5NH1)2CdCl4:Cu2+
although
a smaller shift is observed for thepresent
case. Above 40 kbar the CT band of the purecrystal
experiences
a continu-ous redshift at a rate of -16cm"'/kbar,
analogous
to that followedby
the156 . .Moral, F. Rodríguez, R. Valiente,M.Moreno and H. U. Güdel
(C2H5NH3)2CdCl4:
Cu/+
r
0 10 20 30
PRESSURE
(kbar)
Fig.
4. Variation of thepeak energyof the first CT band in(C2H,NH1)2CdCl4:Cu24 withthe hydrostatic pressure. The straight line corresponds to the least square fit in the
0 — 25 kbar range. 24000 22000 20000 18000
(C2H5NH3)2MnCl4:
Cu:+ First CT band E=21000 -40 "T" Second CT band E=24660 -6.1 10 20 30 40 50 60 70 PRESSURE(kbar)
Fig.
5. Pressuredependence
of CT bands at 21000(C2H5NH,)2MnCl4:Cu2+. Thestraightlinesare least square fits.
and 25000cm
21000cm"1 band in
(C2H5NH3)2MnCl4:Cu2+
(Fig.
5).
Moreover the CT energy of(C2H3NH3)2CuCl4
at 90kbar,
E = 21500cm1,
is near the CTLocal Structure of Cu-' in the
(C2H5NH,)2MCl4:Cu2+ LayerPerovskites 157
These two facts
suggest
that the CT band observed in thehigh-pressure
phase
of the purecrystal
and in the Mncrystal
atatmospheric
pressure isprobably
associated withsimilarCTstates ofcompressed
CuCl4,"
complex-es of either
tetragonal
(D4I,)
ororthorhombic(D2h)
symmetry.Consequently,
the variations
experienced by
the first CT band upon pressure in the three relatedcrystals
can bereasonably explained
in terms of structuralchanges
of CuCl4"taking
into account that the effect of pressure on theelongated
CuCl4,"
complex
ismainly
to reducesignificantly
the axial Cu-Cldistance,
and
only slightly
(or
evenincrease)
thein-plane
short Cu—Cldistance,
leading
to a morecompressed
geometry
with the shortest Cu-Cl bondsperpendicular
tothelayer.
Thisanisotropie compression
of theCuCl4,-
com-plex
upon pressure ispropably responsible
for the observed CT band red-shifts. A structuralstudy
of thehigh-pressure
phase
of the pure Cucrystal
using
diffractiontechniques
would be very usefultoclarify
the coordinationgeometry
ofCuCl4,"
in thisphase.
Finally,
itmustbe noted that the narrow bandappearing
at 25000 cm"1 in the OAspectrum
of(C2H3NH3)2MnCl4:Cu2+,
is not observed in thehigh
pressurephase
of either(C2H,NH.,)2CdCl4:Cu2+
or(C2H,NH.,)2CuCl4.
Thisfeature is consistent with the
interpretation given
in[9]
for such a band asdueto an electronic transition
involving
bothCT~ — Cu2+ CT states of theCuCl4,"
complex
and the4A,4E(G)
excited state of theexchange-coupled
Mn2+neighbours, although
in such a case, an additional orthorhombicD2h
distortion was assumed forCuCl4,".
The similarpressure-induced
shiftrates measured for this band
(
=-6cm"'/kbar)
and for the4A,4E(G)
peak
inMnCl2 (
= -5cm"'/kbar) [16]
supports
thatinter-pretation.
From the pressure shift of the broad band at 21000
cm1,
weestimate that the pressure
required
to shift the first CT band from(C2H,NH,)2CdCl4:Cu2+
to(C2H5NH3)2MnCl4:Cu2+
is about 100 kbar.Summary
Hydrostatic
pressureexperiments
carried out in(C2H5NH,)2CdCl4:Cu2+
show the existence of a structural
change
in theCuCl4,"
complex
from anelongated
octahedron to anearly compressed
one at above 25 kbar. Thischange
is evidencedthrough
theabrupt
shift of —1400 cm"1undergone
by
the first CT band around that pressure. Thecomparison
of these results with those available for(C2H,NH,)2CuCl4
indicates that thehigh-pressure
structureof the
CuCl4,"
in thiscrystal
isprobably
similartothat attained for(C2H5NH,)2MnCl4:Cu2+
atatmospheric
pressure. Thepresent
hydrostatic
pressure
experiments performed
ontheseCu2+-doped
layer
perovskites
pro-vide direct epro-vidence about the redshift
undergone by
the first CT band ofCuCl4,"
complexes
upon pressure in dilutedsystems.158 . . Moral, F.Rodríguez, R. Valiente,M. Moreno and H. U. Güdel
Further work in order to
investigate
whether thesteep
redshift around 26 kbaris associatedwith a structuralphase
transition of the hostcrystal
orwhether is
just
a pure localphenomenon,
using
diffractiontechniques
isunder way.
Acknowledgments
Financial
support
fromCaja
Cantabria and CICYT(Project
No.PB92-0505)
is
acknowledged.
References1. M. A. Hitchman, CommentsInorg. Chem. 15(1994) 197.
2. D. Reinen and M. Atanasov, Magn.Res. Rev. 15 (1991) 167. 3. G.
Chapuis,
Phys. Status SolidiA43(1977) 203.4. W.
Depmeier,
ActaCrystallogr. 32(1976)303.5. J. P. Steadman and R. D. Willett,Inorg.Chim. Acta4(1970) 367. 6. U. Schmid, H. U. Güdel and R. D. Willett,Inorg. Chim. 21 (1982) 2977.
7. J. A. Aramburu and M. Moreno,J. Chim. Phys. 86(1989) 871.
8. B. Baticle,F. Rodríguezand R. Valiente, Radiât. Eff. Def.Solids 135 (1995) 89.
9. R. Valiente and F. Rodríguez,J. Phys.Chem. Solids 57(1996)571.
10. Y. Moritomo and Y. Tokura,J. Chem.
Phys.
101 (1994) 1763. 11. B. A. Moral and F.Rodríguez,
Rev. Sci. Instrum. 66(1995) 5178.12. . Kan'no, S.Naoe, S. Mukai and Y.Nakai, Solid State Commun. 13(1973) 1325. 13. S. HirakoandR. Onaka,J.
Phys.
Soc.Jpn.
51 (1982) 1255.14. S. R. Desjardins, K. W. Penfield,S. L. Cohen,R. L. Musselman andE. I.Solomon, J. Am. Chem. Soc. 105(1983)4590.
15. J. A.Aramburu and M. Moreno,J. Chem.Phys. 83(1985)6071.
16. J.C. Zahnerand H.G. Drickamer,J. Chem.Phys.35(1961) 1483.