What do we really know about early diagenesis of
non-marine carbonates?
Eva De Boever
a,⁎
, Alexander T. Brasier
b, Anneleen Foubert
a, Sándor Kele
caDepartment of Geosciences and Geology, University of Fribourg, Fribourg, Switzerland bSchool of Geosciences, University of Aberdeen, Old Aberdeen, Scotland, UK c
Institute for Geological and Geochemical Research, Research Centre for Astronomy and Earth Sciences, Hungarian Academy of Sciences, Budapest, Hungary
Non-marine carbonate rocks including cave, spring, stream, calcrete and lacustrine-palustrine sediments, are sus-ceptible to early diagenetic processes. These can profoundly alter the carbonate fabric and affect paleoclimatic proxies. This review integrates recent insights into diagenesis of non-marine carbonates and in particular the va-riety of early diagenetic processes, and presents a conceptual framework to address them. With ability to study at smaller and smaller scales, down to nanometers, one can now observe diagenesis taking place the moment initial precipitates have formed, and continuing thereafter. Diagenesis may affect whole rocks, but it typically starts in nano- and micro-environments. The potential for diagenetic alteration depends on the reactivity of the initial precipitate, commonly being metastable phases like vaterite, Ca-oxalates, hydrous Mg‐carbonates and aragonite with regard to the ambient fluid. Furthermore, organic compounds commonly play a crucial role in hosting these early transformations. Processes like neomorphism (inversion and recrystallization), cementation and replace-ment generally result in an overall coarsening of the fabric and homogenization of the wide range of complex, primary microtextures. If early diagenetic modifications are completed in a short time span compared to the (annual to millennial) time scale of interest, then recorded paleoenvironmental signals and trends could still ac-ceptably reflect original, depositional conditions. However, even compact, non-marine carbonate deposits may behave locally and temporarily as open systems to crystal-fluid exchange and overprinting of one or more geo-chemical proxies is not unexpected. Looking to the future, relatively few studies have examined the behaviour of promising geochemical records, such as clumped isotope thermometry and (non-conventional) stable isotopes, in well-constrained diagenetic settings. Ongoing and future in-vitro and in-situ experimental approaches will help to investigate and detangle sequences of intermediate, diagenetic products, processes and controls, and to quantify rates of early diagenesis, bridging a gap between nanoscale, molecular lab studies and the fossil field rock record of non-marine carbonates.
1. Introduction: Non-marine carbonates and early diagenesis?
Non-marine carbonate depositional environments, facies
distribu-tions, fabrics and geochemical signatures have been under the spotlight
over the past decade (
Pentecost, 2005; Fairchild et al., 2006;
Alonso-Zarza and Tanner, 2010a; Pedley and Rogerson, 2010; Brasier,
2011; Capezzuoli et al., 2014
). This is in part because they form
impor-tant continental paleoclimate archives (
Andrews, 2006; Fairchild et al.,
2006; Fairchild and Treble, 2009; Tanner, 2010; Frantz et al., 2014
).
Sev-eral non-marine carbonate fabrics have been recognized in subsurface
lithologies of economic interest (
Harris, 2000; Shiraishi et al., 2008b;
Della Porta, 2015; Ionescu et al., 2015; Wright and Barnett, 2015;
Schroeder et al., 2016; Claes et al., 2017a; Mercedes-Martín et al.,
2017
). Furthermore, many carbonate factories in non-marine
environ-ments have been studied to understand close interactions between
micro- and macrobiota (bacteria, algae, bryophytes, plants) that in
flu-ence mineral precipitation processes, pore
fluids and the resulting
car-bonate fabric (
Freytet and Verrecchia, 1998; Benzerara et al., 2006;
Bissett et al., 2008; Takashima and Kano, 2008; Bontognali et al., 2010;
Jones, 2010; Peng and Jones, 2013; Pace et al., 2016
), offering
re-searchers the chance to improve approaches to detection of ancient
and extraterrestrial life. Because diagenesis of non-marine carbonate
rocks is commonly apparent, it is surprising that studies addressing
the nature, distribution, processes and controls on early diagenesis of
terrestrial carbonates are rather scarce (
Folk and Assereto, 1976;
Kendall and Iannace, 2001; Golubi
ć et al., 2008; Armenteros, 2010;
Frisia, 2015
). This contrasts with an extensive body of literature on
dia-genesis of marine sediments. Diadia-genesis in these continental
environ-ments often starts early, from
fluids closely resembling those
⁎ Corresponding author. Tel.: +41 26 300 89 73. E-mail address:eva.deboever@unifr.ch(E. De Boever).
http://doc.rero.ch
Published in "Sedimentary Geology 361 : 25–51, 2017"
which should be cited to refer to this work.
responsible for carbonate formation, and it never really stops. This is the
inescapable conclusion from many, recent studies of
field sites and
ex-periments, as discussed here. As these early diagenetic processes and
products have so far only been tangentially addressed in continental
settings, their variety and impact on geochemical and geochronological
proxies are far from fully understood.
This review summarizes and integrates recent insights and
emerg-ing approaches to studyemerg-ing early diagenesis, leademerg-ing to the presentation
of a conceptual scheme to address these processes in non-marine
car-bonates. Burial diagenesis (deeper than the immediate subsurface) is
not included here. For that the reader is referred to several authoritative
reviews on the subject (
Tucker and Wright, 1990; Flügel, 2004; Moore
and Wade, 2013; James and Jones, 2015
).
We de
fine ‘non-marine carbonates’ here as carbonate rock deposits
that form and may be syn-depositionally transformed (
“diagenetically
altered
”) under the strong influence of meteoric waters, including
situ-ations with various degrees of mixing with seawater, evaporative or
ba-sinal
fluids (
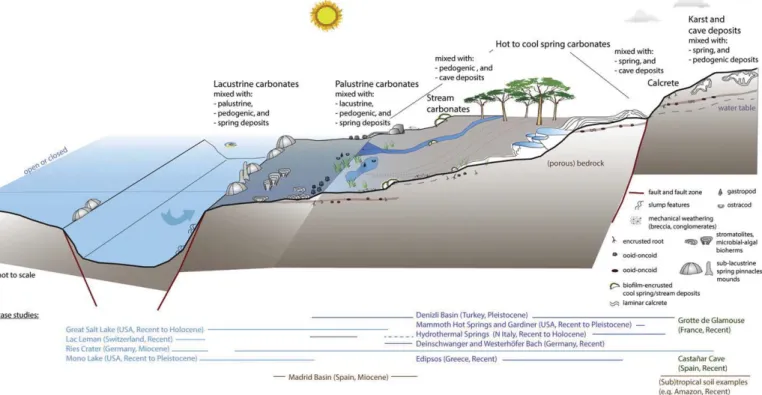
Figs. 1, 2
). Non-marine carbonates are primarily located in
continental settings, with some on islands (
Benzerara et al., 2010;
Kremer et al., 2012; Ionescu et al., 2015
). The following deposits are
in-cluded in this review under this heading (
Fig. 1
); (1) karst and cave
deposits, (2) hot and cool spring and stream carbonates, (3) calcretes
and (4) marginal, lacustrine-palustrine carbonates, including abiogenic,
microbial and biogenic deposits (
Table 1
). There is a continuum in space
and time between these different environments and their rock products
such that they are most easily addressed together under a collective
heading (
Freytet and Plaziat, 1982; Alonso-Zarza, 2003; Brasier, 2011;
Della Porta, 2015
). A limited number of reference examples that cover
this spectrum, with less focus on biogenic carbonate deposits such as
os-tracod, gastropod or bivalve beds, is selected (
Fig. 1
,
Table 1
), to
illus-trate common early diagenetic processes in these deposits and
environments.
In view of their application in paleoclimatic reconstructions, most
research has been published on Recent to Quaternary deposits.
Non-marine carbonates have however been recognized and reported from
deposits as old as the late Archaean and Palaeoproterozoic (for review
see
Brasier, 2011
). Amongst the most ancient deposits are examples of
lacustrine, pedogenic and hydrothermal spring carbonates (
Watanabe
et al., 2000; Melezhik and Fallick, 2001; Bolhar and Van Kranenkonk,
2007; Awramik and Buchheim, 2009; Fairchild et al., 2016
). Given the
focus here on the early stages of diagenesis, the examples most often
re-ferred to, range between Miocene and Recent in age (
Table 1
), with a
strong emphasis on Quaternary deposits.
2. De
fining and describing early diagenesis: The conundrum in
non-marine carbonates
‘Diagenesis’ refers to a suite of ‘post-depositional’ processes that has
an important impact on chemical, mechanical and petrophysical
prop-erties of carbonate sediments (
Bathurst, 1975; Berner, 1980; Moore
and Wade, 2013
) as well as on the preservation of depositional fabrics,
(micro)biological assemblages, geochemical and geochronological
signatures.
One classic scheme to classify diagenetic processes affecting marine
carbonate rocks, starts from the concept of diagenetic environments
or
‘realms’. These are ‘surface or subsurface zones typified by specific
processes which result in predictable and similar patterns of diagenesis
that can be studied in thin sections and by geochemical methods
’
(
Flügel, 2004
). Around typical marine carbonate systems like coral
reefs, three major realms may be recognized (
Choquette and Pray,
1970
); (i) the near-surface zone, characterized by phreatic conditions
and normal or modi
fied (marine) pore fluids (eogenetic zone), (ii) a
near-surface zone resulting from the exposure of sediments to subaerial
conditions and meteoric vadose or phreatic conditions (telogenetic
zone), and (iii) the subsurface burial realm where pore
fluids are often
a mixture of modi
fied meteoric, marine waters or chemically complex
brines as a result of long-lasting water-rock interactions (mesogenetic
zone) (
Moore, 2001
). This subdivision was practical for marine
carbon-ate fabric description, including porosity characteristics, but is no longer
widely used. An alternative, recent approach involves subdivision based
on the dominant
fluid or fluid mixtures present (
Flügel, 2004; Moore
and Wade, 2013; Swart, 2015
). This may be freshwater, marine, mixed
Fig. 1. Schematic drawing and cross-section of the location and transitions between different non-marine depositional environments and their deposits (based onFreytet and Plaziat, 1982; Arp, 1995; Pedley and Rogerson, 2010; Brasier, 2011; Wright, 2012; Della Porta, 2015) considered in this review. The extent of the reference examples ofTable 1is indicated below the cross-section.
marine-meteoric and subsurface, or burial, diagenetic
fluids (
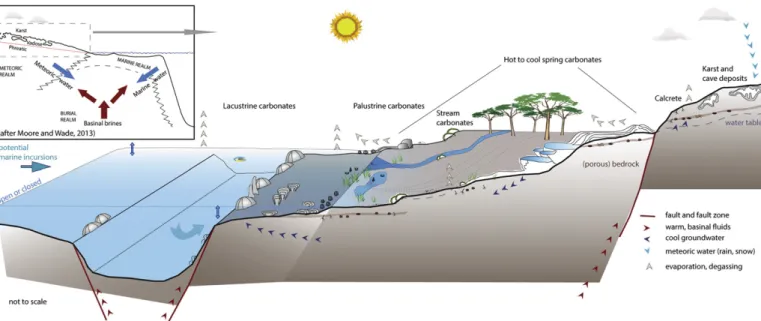
Fig. 2
). In
this view, water chemistry, water
flux, temperature and pressure of
rock-water interactions
‘drive the diagenetic processes’ (
Moore and
Wade, 2013
).
Neither of these approaches really suits or adequately allows
de-scribing early, non-marine carbonate diagenesis. Descriptions of
pro-cesses in the meteoric, vadose and phreatic zone focus solely on the
corrosion capacities of dilute, fresh waters, and limited cementation,
af-fecting previously deposited and uplifted carbonate sediments
(
Longman, 1980; Meyers and Lohmann, 1985; Armenteros, 2010;
Moore and Wade, 2013
). Further, diagenesis of non-marine carbonates
is not always driven by low ionic strength meteoric waters, as illustrated
in
Fig. 2
.
It is therefore something of an oversight that the range of early
dia-genetic conditions (
fluid composition, temperature, biology) and
pro-cesses are nearly absent from classical carbonate rock diagenesis
frameworks. The majority of non-marine carbonate deposits nucleate
and precipitate from solution
– or to a lesser extent – result from
bio-genic secretion. This makes the end of primary deposition and start of
diagenesis hard to pinpoint. Several researchers recognize this, but
few address it explicitly.
Renaut and Jones (1997)
mentioned that
‘‘all
calcite precipitated directly from spring water is considered
formational, whereas biogenic or abiogenic alteration is considered
dia-genetic
”. In practical terms, this definition is hard, if not impossible, to
apply.
Pentecost (2005)
working on travertine systems tackles the
di-lemma by de
fining the primary fabric ”as that present at the active
trav-ertine surface (including the top 1 mm of deposit)
”. However, this is also
problematic because
‘primary’ crystal nucleation and growth frequently
happen below 1 mm sediment depth in non-marine carbonate
environ-ments. For our purposes, crystalline carbonate precipitates and
carbon-ate deposits that formed at or along the initial, depositional surface from
primary pore
fluids can be considered to make up the primary fabric. In
this context,
‘early diagenesis’ refers to those rock fabric altering
pro-cesses and transformations that take place or start during and just
after deposition.
2.1. Summary of (primary) non-marine carbonate fabrics, mineralogy and
processes of crystal formation
2.1.1. Primary fabrics and mineralogy
Presumed primary, depositional fabrics of non-marine carbonates
have been rather extensively covered in several recent reviews
(
Alonso-Zarza, 2003; Fairchild et al., 2006; Brasier, 2011; Gandin and
Capezzuoli, 2014; Della Porta, 2015; Frisia, 2015; Zamanian et al.,
2016
) and volumes (
Pentecost, 2005; Alonso-Zarza and Tanner,
2010a; Pedley and Rogerson, 2010; Fairchild and Baker, 2012
), as well
as in an increasing number of individual case studies. Table A1 therefore
summarizes a number of these micro- to macrofabrics as observed in
the
field and under transmitted light microscopy, plus key literature
that offers an overview of the range of commonly reported
‘primary’,
not yet diagenetically altered, carbonate products. In many of these
cases, the presence and potential in
fluence of (early) diagenetic
pro-cesses on the fabrics is however not explicitly addressed.
Table A2 provides a summary, including references from
field and
laboratory studies, of several common carbonate mineralogies in
non-marine deposits. The initial mineralogy and fabric are important as
they will in
fluence reactivity and the so-called ‘diagenetic potential’.
Not all parameters controlling the occurrence of one or the other
miner-alogy or polymorph are yet fully resolved though (
Renaut and Jones,
1997; Kele et al., 2008; Wassenburg et al., 2012; Riechelmann et al.,
2014; Jones, 2017
) and this may in part be related to pathways and
molecular-scale controls on crystal formation that are only recently
being unveiled and addressed.
2.1.2. Recent insights into crystal formation through transient precursor
phases
High-resolution microscopic (cryo-SEM,
−TEM) and experimental
observations now exist that demonstrate that processes of crystal
nu-cleation and growth are more diverse than the conventional model of
monomer-by-monomer addition of chemical species (atom, ion,
mole-cule). New pathways are found to involve particle attachment and a
range of transient, amorphous,
‘precursor’ phases (
Radha et al., 2010;
De Yoreo et al., 2015; Purgstaller et al., 2016; Rodriguez-Blanco et al.,
2017; Van Driessche et al., 2017
). The implications of newly discovered
nano-scale crystallization processes on carbonate stability domains, on
the exact CaCO
3polymorph that forms, plus the geochemical signatures
of minerals used in environmental studies, requires further study and
quanti
fication (
Sánchez-Román et al., 2011; Demény et al., 2016;
Rodriguez-Blanco et al., 2017
).
Transformation from amorphous calcium carbonate (ACC) to
inter-mediate metastable phases appears to be a very rapid, often multistage,
reaction process taking seconds or minutes to hours, during which
sta-ble crystalline products and metastasta-ble phases may co-exist
(
Rodriguez-Blanco et al., 2011, 2012, 2017; Bots et al., 2012; Zhang
Fig. 2. Schematic drawing and cross-section showingfluids involved in formation and early diagenesis of non-marine carbonates. Early diagenesis may take place from marine influenced, evaporativefluids over freshwater and fluids that have migrated up from the deep(er) subsurface at, for example hot springs.
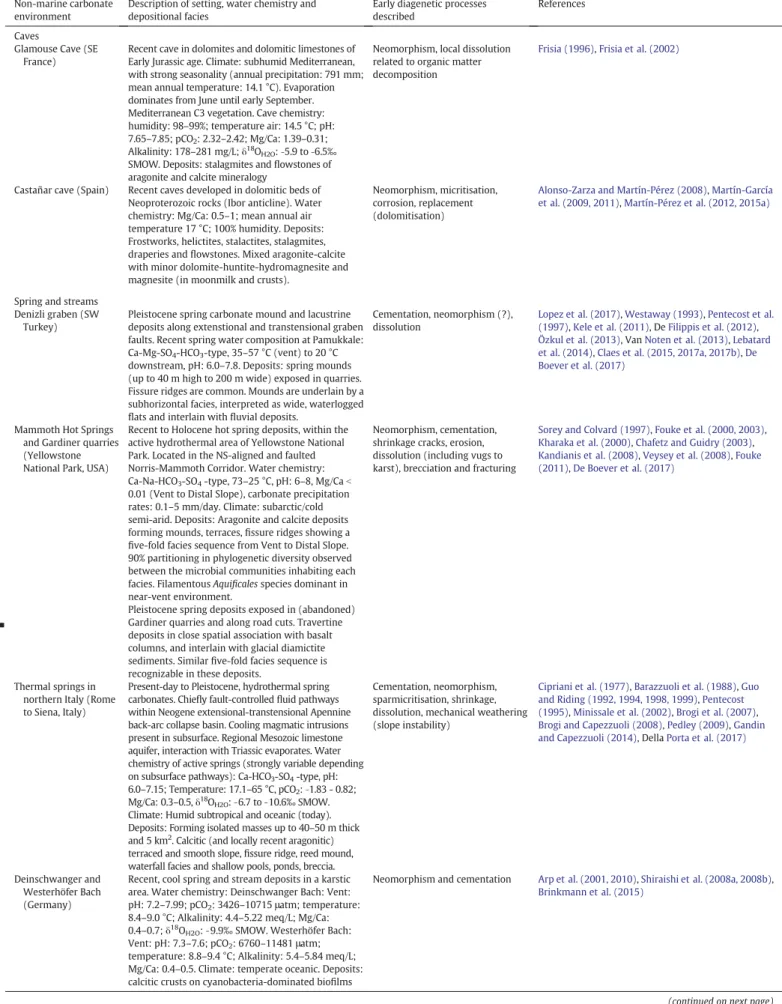
Table 1
A selection of reference case studies for each non-marine depositional environment with overview of their depositional, hydrogeochemical and (paleo)environmental characteristics that are often referred to and used in this review. They have, in general, never been buried and (early) diagenetic processes have been carefully addressed in literature or are under investigation.
Non-marine carbonate environment
Description of setting, water chemistry and depositional facies
Early diagenetic processes described
References
Marginal lacustrine and palustrine Lac Leman (Geneva,
Switzerland)
Recent monomictic, mesotrophic lake (T: 5–20 °C; Mg/Ca: 0.20–0.26). Climate: Moderate. Algal blooms occur twice a year in May and July. SW part (Geneva Bay) is well‐oxygenated and mixed due to episodic strong wind and wave action. Deposits: low‐ magnesium calcite ooid and oncoid deposits containing scattered (filamentous) algal and shell fragments (charophytes, gastropods, and pelecypods). Laminated lacustrine marls and chalk and
glaciolacustrine silty clay.
Organic matter degradation, aggrading neomorphism
Davaud and Girardclos (2001),Plee (2008), Martignier et al. (2017)
Great Salt Lake (Utah, USA)
Recent to Holocene, endorheic polymictic and stratified hypersaline lake in normal fault-bounded graben (T:b0–35 °C; pH: 7.6–8.4; salinity: 12–27%; Mg/Ca: 9–115; Alkalinity: 5.4–8.4 meq/L). Evaporative remnant of Pleistocene Lake Bonneville. Climate: semi-arid, precipitation: 125–375 mm/y. Deposits: Calcite, aragonite and dolomite. Ooidal sands, bioherms parallel to shoreline, microbialites (including stromatolites) along current lake side, ridges with spring mounds above the current lake shore, evaporates in saltflat.
Replacement, cementation; mechanical weathering, dissolution
Eardley (1938),Sandberg (1975),Currey and James (1982),Spencer et al. (1984),Currey and Oviatt (1985),Pedone and Dickson (2000),Chidsey et al. (2015),Bouton et al. (2016a, 2016b),Pace et al. (2016),Lindsay et al. (2017)
Ries Crater lake (South Germany)
Circular depression resulting from a meteoric impact (initial diameter 10 km)filled with Miocene lacustrine and submerged spring carbonates at the rim. Water chemistry: evolution of a highly alkaline, stratified soda lake (Na-Mg-CO3-SO4) to mesosaline lake
(Na-Mg-Cl-SO4) with occasional low salinity episodes
during lake high stand and high-salinity conditions prevailing at the crater rim. Climate: semi-arid to arid. Deposits: supralitoral carbonate swamps and pedogenic carbonates, spring mounds, eulitoral coated grains, microbialites (including stromatolites) and algal bioherms, speleothems
Dissolution, micritisation, shrinkage, neomorphism, replacement, cementation
Riding (1979),Arp (1995),Pache et al. (2001),Arp et al. (2013a, 2013b, 2017)
Mono Lake (CA, USA) Pleistocene to Recent, endorheic alkaline lake located in an extensional, active volcanic basin near the Sierra Nevada (T: 1.5–23 °C; pH: 9.7–10; salinity: N 90%; Alkalinity: 600–700 meq/L; Mg/Ca: 14). Climate: Mediterranean continental to subarctic. Precipitation mostly as snow (1600 mm/y). Deposits: Aragonite, Calcite and Ikaite precipitates (+assecory minerals) forming mounds or‘tufa pinnacles’ where spring waters mix with the alkaline lake waters. Mg-silica formation within decaying endolithic cyanobacterial tufa sand communities.
Replacement (ikaite to calcite), organic matter degradation and Mg-silicate formation
Shearman et al. (1989),Bischoff et al. (1993),Council and Bennett (1993),Whiticar and Suess (1998), Souza-Egipsy et al. (2005),Della Porta (2015)
Pedogenic calcrete and palustrine (Sub)tropical soils
(Bolivia, Ivory coast, Cameroon)
Amazon, Bolivia: Recent soils developed in Cretaceous to early Cenozoic sandstone formations (pH: 4.3–6.0). Climate: tropical wet and dry or savannah type to tropical rainforest type (N550 m altitude). 1300–1600 mm/y rain. Deposits: Ca-oxalates near photosynthesizing tree C. speciose as byproduct in initial, carbonate free soils.
Ivory Coast: Semidecidous, gallery forest. Climate: 1300–1500 mm/y rain, mean annual temperature: 24.5 °C. Cameroon: Gallery forest. Climate: 1800 mm/y rain, mean annual temperature: 23 °C. Deposits: Ca-oxalates and CaCO3(euhedral calcitic
rhombohedron, micritic aggregates, calcite pseudomorphoses on cellulosefibers, and parenchyma cells) in the iroko tree.
Replacement (oxalate-carbonate pathway, OCP), dissolution
Bindschedler et al. (2010),Cailleau et al. (2011, 2014)
Madrid Basin (Spain) Miocene alluvial, palustrine and lacustrine carbonate deposits with calcretes and dolocretes. Deposits: Micritic-microsparitic calcite (mudstones), siltstones and sepiolite (hydrated Mg-siliciate) with smaller amounts of illite, palygorskite and smectite formed in alluvial fans, channels, ponds and shallow lakes. Opaline chert, calcite and dolomite nodules and lenticular levels, calcified root mats, alveolar structures, fenestrae, silicified bioturbations in palustrine and pedogenic horizons.
Silicification, dolomitisation, dissolution, cementation, shrinkage cracks
Armenteros et al. (1995),Calvo et al. (1995, 1999), Bustillo et al. (2002),Alonso-Zarza (2003),Bustillo and Alonso-Zarza (2007),Bustillo (2010)
Table 1 (continued) Non-marine carbonate environment
Description of setting, water chemistry and depositional facies
Early diagenetic processes described
References
Caves
Glamouse Cave (SE France)
Recent cave in dolomites and dolomitic limestones of Early Jurassic age. Climate: subhumid Mediterranean, with strong seasonality (annual precipitation: 791 mm; mean annual temperature: 14.1 °C). Evaporation dominates from June until early September. Mediterranean C3 vegetation. Cave chemistry: humidity: 98–99%; temperature air: 14.5 °C; pH: 7.65–7.85; pCO2: 2.32–2.42; Mg/Ca: 1.39–0.31;
Alkalinity: 178–281 mg/L; δ18
OH2O:‐5.9 to ‐6.5‰
SMOW. Deposits: stalagmites andflowstones of aragonite and calcite mineralogy
Neomorphism, local dissolution related to organic matter decomposition
Frisia (1996),Frisia et al. (2002)
Castañar cave (Spain) Recent caves developed in dolomitic beds of Neoproterozoic rocks (Ibor anticline). Water chemistry: Mg/Ca: 0.5–1; mean annual air temperature 17 °C; 100% humidity. Deposits: Frostworks, helictites, stalactites, stalagmites, draperies andflowstones. Mixed aragonite-calcite with minor dolomite-huntite-hydromagnesite and magnesite (in moonmilk and crusts).
Neomorphism, micritisation, corrosion, replacement (dolomitisation)
Alonso-Zarza and Martín-Pérez (2008),Martín-García et al. (2009, 2011),Martín-Pérez et al. (2012, 2015a)
Spring and streams Denizli graben (SW
Turkey)
Pleistocene spring carbonate mound and lacustrine deposits along extenstional and transtensional graben faults. Recent spring water composition at Pamukkale: Ca-Mg-SO4-HCO3-type, 35–57 °C (vent) to 20 °C
downstream, pH: 6.0–7.8. Deposits: spring mounds (up to 40 m high to 200 m wide) exposed in quarries. Fissure ridges are common. Mounds are underlain by a subhorizontal facies, interpreted as wide, waterlogged flats and interlain with fluvial deposits.
Cementation, neomorphism (?), dissolution
Lopez et al. (2017),Westaway (1993),Pentecost et al. (1997),Kele et al. (2011), DeFilippis et al. (2012), Özkul et al. (2013), VanNoten et al. (2013),Lebatard et al. (2014),Claes et al. (2015, 2017a, 2017b),De Boever et al. (2017)
Mammoth Hot Springs and Gardiner quarries (Yellowstone National Park, USA)
Recent to Holocene hot spring deposits, within the active hydrothermal area of Yellowstone National Park. Located in the NS-aligned and faulted Norris-Mammoth Corridor. Water chemistry: Ca-Na-HCO3-SO4-type, 73–25 °C, pH: 6–8, Mg/Ca b
0.01 (Vent to Distal Slope), carbonate precipitation rates: 0.1–5 mm/day. Climate: subarctic/cold semi-arid. Deposits: Aragonite and calcite deposits forming mounds, terraces,fissure ridges showing a five-fold facies sequence from Vent to Distal Slope. 90% partitioning in phylogenetic diversity observed between the microbial communities inhabiting each facies. Filamentous Aquificales species dominant in near-vent environment.
Pleistocene spring deposits exposed in (abandoned) Gardiner quarries and along road cuts. Travertine deposits in close spatial association with basalt columns, and interlain with glacial diamictite sediments. Similarfive-fold facies sequence is recognizable in these deposits.
Neomorphism, cementation, shrinkage cracks, erosion, dissolution (including vugs to karst), brecciation and fracturing
Sorey and Colvard (1997),Fouke et al. (2000, 2003), Kharaka et al. (2000),Chafetz and Guidry (2003), Kandianis et al. (2008),Veysey et al. (2008),Fouke (2011),De Boever et al. (2017)
Thermal springs in northern Italy (Rome to Siena, Italy)
Present-day to Pleistocene, hydrothermal spring carbonates. Chiefly fault-controlled fluid pathways within Neogene extensional-transtensional Apennine back-arc collapse basin. Cooling magmatic intrusions present in subsurface. Regional Mesozoic limestone aquifer, interaction with Triassic evaporates. Water chemistry of active springs (strongly variable depending on subsurface pathways): Ca-HCO3-SO4-type, pH:
6.0–7.15; Temperature: 17.1–65 °C, pCO2:‐1.83 - 0.82;
Mg/Ca: 0.3–0.5, δ18O
H2O:‐6.7 to ‐10.6‰ SMOW.
Climate: Humid subtropical and oceanic (today). Deposits: Forming isolated masses up to 40–50 m thick and 5 km2
. Calcitic (and locally recent aragonitic) terraced and smooth slope,fissure ridge, reed mound, waterfall facies and shallow pools, ponds, breccia.
Cementation, neomorphism, sparmicritisation, shrinkage, dissolution, mechanical weathering (slope instability)
Cipriani et al. (1977),Barazzuoli et al. (1988),Guo and Riding (1992, 1994, 1998, 1999),Pentecost (1995),Minissale et al. (2002),Brogi et al. (2007), Brogi and Capezzuoli (2008),Pedley (2009),Gandin and Capezzuoli (2014), DellaPorta et al. (2017)
Deinschwanger and Westerhöfer Bach (Germany)
Recent, cool spring and stream deposits in a karstic area. Water chemistry: Deinschwanger Bach: Vent: pH: 7.2–7.99; pCO2: 3426–10715 μatm; temperature:
8.4–9.0 °C; Alkalinity: 4.4–5.22 meq/L; Mg/Ca: 0.4–0.7; δ18
OH2O:‐9.9‰ SMOW. Westerhöfer Bach:
Vent: pH: 7.3–7.6; pCO2: 6760–11481 μatm;
temperature: 8.8–9.4 °C; Alkalinity: 5.4–5.84 meq/L; Mg/Ca: 0.4–0.5. Climate: temperate oceanic. Deposits: calcitic crusts on cyanobacteria-dominated biofilms
Neomorphism and cementation Arp et al. (2001, 2010),Shiraishi et al. (2008a, 2008b), Brinkmann et al. (2015)
(continued on next page)
et al., 2012; Nielsen et al., 2014
). Lab and
field studies report ACC phases
found in close association with crystalline CaCO
3(
Jones and Peng,
2012a; Pedley, 2014
) and/or organisms (cyanobacteria) or organic
sub-stances (
Couradeau et al., 2012; Benzerara et al., 2014; Martignier et al.,
2017
). The transformation from ACC to crystalline carbonate mineral is
commonly suggested to be associated with an ACC dehydration-step
(
Rodriguez-Blanco et al., 2011; Bots et al., 2012; Demichelis et al.,
2014; De Yoreo et al., 2015
), but the mechanism seems to be in
fluenced
by, amongst others, temperature, solution pH, Mg:Ca ratios, sulfate
con-centrations and organic matter (
Rodriguez-Blanco et al., 2012; Zhang
et al., 2012; Purgstaller et al., 2016
). Micro-environmental effects are
ev-idently important for these early, nano-scale phase changes.
2.2. Early diagenesis and the role of the micro-environment
With modern techniques, one can look at increasingly smaller scales,
down to and beyond the nanometer level (
Pace et al., 2016
). Current
research has demonstrated the tight interaction between very early
(syn-depositional) carbonate recrystallisation and changes in associated
non
‐carbonate phases, including (microbial) organic matter,
Ca-oxalates and clay minerals. These processes typically take place in
‘micro-scale environments’ or ‘niches’ within primary pore spaces and
bio
films. Here we highlight a few such processes, though there are
many others in nature.
2.2.1. Ca-oxalates and their transformation into carbonate phases
Calcium oxalates are extremely common in nature, and are
particu-larly linked with fungi, lichens and vascular plants (
de Bary, 1887;
Simkiss and Wilbur, 1989; Horner and Wagner, 1995; Verrecchia
et al., 2006
). They come in various hydration states, i.e. as a
monohydrate
(whewellite,
CaC
2O
4·H
2O)
or
as
polyhydrates
(weddellite, CaC
2O
4·nH
2O, 2
≤ n ≤ 3). Dehydration rapidly causes
weddellite to transform to whewellite.
Graustein et al. (1977)
reported
that weddellite found in soils of New Mexico had dehydrated to
whewellite by the time it was measured in the laboratory.
Because of the abundance of calcium oxalates and calcium oxalate
producing organisms, it is somewhat surprising that there have been
relatively few studies linking calcium oxalate deposition with calcium
carbonate production. This may in part be due to the similar elemental
composition of calcite, whewellite and weddellite, such that structures
identi
fied as comprising Ca, C and O are assumed to be CaCO
3. This
chal-lenge was tackled by
Verrecchia et al. (1993)
with a windowless Energy
Dispersive Spectrometer (EDS) detector attached to a Scanning Electron
Microscope (SEM) that allowed comparison of the relative amounts of C
in crystals, and thus distinction between calcium oxalates and
carbon-ates. Many modern EDS detectors are able to measure relative
abun-dances of light elements including carbon without having to remove
the window, so this technique is now widely accessible.
Verrecchia et al. (1993)
used this approach to distinguish crystals of
calcite from crystals of calcium oxalate in
‘Needle Fibre Calcite’ (NFC).
This common fungal hyphae-related fabric (
Wright, 1986
), reviewed
by
Verrecchia and Verrecchia (1994)
and updated by
Cailleau et al.
(2009)
, consists of elongated crystals measuring tens to hundreds of
mi-crons in length and a few mimi-crons in width. Crystals may be randomly
stacked, or form parallel clusters, aggregates or networks (
Fig. 3
A)
(
Martín-Pérez and Ko
šir, 2017
).
Verrecchia et al. (1993)
showed that
short crystals and spikes surrounding the exterior of the calci
fied fungal
filaments in some Quaternary calcretes of Israel had compositions
bet-ter compatible with calcium oxalates than with calcite and that most
needles and mineral encrusted fungal hyphal walls had intermediate
compositions.
Verrecchia et al. (1993)
envisaged that transformation of calcium
oxalate to calcite was bacterially mediated, following hypotheses of
Cromack et al. (1977)
and
Philips et al. (1987)
. Experiments have
since shown that some soil bacteria (including Ralstonia eutropha and
Xanthobacter autotrophicus) degrade oxalates, using them as a carbon
and energy source, resulting in production of carbonic acid and
ulti-mately carbonate ions that can react with locally sourced calcium to
produce calcite or vaterite (
Braissant et al., 2002
). This oxalate oxidation
locally raises the pH (
Braissant et al., 2002, 2004
), which aides
carbon-ate mineral precipitation and growth in otherwise low pH soils
(
Cailleau et al., 2011
). Calcium oxalates produced by Iroko trees of
Ivory Coast (
Cailleau et al., 2004
) and Cameroon (
Cailleau et al., 2011
)
are known to be converted into voluminous accumulations of calcium
carbonate. Here, the organic
‘litter’ produced by the trees is initially
bro-ken down by organisms including termites and fungi, releasing the
cal-cium oxalates so that they can be processed by the oxalotrophic
bacteria.
2.2.2. The role of micro-organisms and organic matter in carbonate crystal
formation
Mostly based on in-vitro laboratory experiments, recent advances
have been made in unravelling the different, microbial-related and
organic matter (degradation) processes that facilitate and localize
carbonate crystal formation. These processes fall roughly into two
cate-gories; microbial carbonate precipitation (1) as a by-product of
com-mon metabolic activities like oxygenic/anoxygenic photosynthesis,
aerobic respiration, denitri
fication, ammonification, sulfate reduction,
methanotrophy, iron reduction (
Michaelis et al., 2002; Dupraz et al.,
2004, 2009; Visscher and Stolz, 2005; Rogerson et al., 2014; Zhu and
Dittrich, 2016
); and (2) by using cell walls,
filamentous structures or
ex-tracellular organic matter (EOM) (including exex-tracellular polymeric
substances (EPS) and low-molecular weight organic carbon) (
Decho
et al., 2005
) as templates for crystals with different habits (
Shiraishi
et al., 2008a, 2008b; Dupraz et al., 2009; Arp et al., 2010; Pedley, 2014;
Rogerson et al., 2014
;
Fig. 3
B, C). These surfaces may provide a particular
micro-environment by their negatively charged functional groups (such
Table 1 (continued) Non-marine carbonate environment
Description of setting, water chemistry and depositional facies
Early diagenetic processes described
References
Edipsos hot springs (Euboea, Greece)
Recent ferrous hot springs along NNE-SSW and NW-SE to E-W extensional lineaments. Cooling magmatic intrusions present in subsurface, related to Plio-Pleistocene back-arc volcanic center. Na-CL type springfluids (T: 43.9–82.2 °C; pH: 6–6.8; TDS: 18800–33735 mg/L; Mg/Ca: 0.2–2.6; HCO3‐:
489–691 mg/L). Climate: cold semi-arid
(Mediterranean). Deposits: Spring mounds with vent to distal slope facies deposits (aragonite and calcite) including dense crystalline crusts, coated gas bubbles, rafts, shrubs, ooids, cemented microbialfilaments, allochtonous travertine together with iron oxides and minor halite, gypsum.
Neomorphism, cementation, dissolution, shrinkage
Kanellopoulos (2012, 2013),Kanellopoulos et al. (2016)
as carboxyl, amine, phosphate and hydroxyl groups) that trap or release
divalent cations (Ca
2+, Mg
2+), thereby affecting their concentration in
solution (
Dupraz and Visscher, 2005; Visscher and Stolz, 2005; Dupraz
et al., 2009; Arp et al., 2010; Roberts et al., 2013; Zhu and Dittrich,
2016
). They may as well glue particles together, such as certain
filamen-tous microbes in caves, or retain water in their framework (
Barton et al.,
2001; Barton and Northup, 2007; Jones, 2010
).
Microbial activity also encourages processes at the cell wall-pore
fluid interface during early diagenesis, in particular in association with
the degradation of organic matter (e.g. by sulfate reduction), just
below active microbial mats. When divalent cations are very locally
re-leased (
Dupraz et al., 2009; Rogerson et al., 2014; Pace et al., 2016
),
sec-ondary carbonate precipitation may occur as a result, sometimes at the
expense of primary unstable and/or amorphous phases. Experimenting
with microbial isolates from Altamira cave in Spain,
Sanchez-Moral et al.
(2003)
demonstrated that metastable phases such as vaterite and/or
monohydrocalcite formed
first. Such metastable phases can then be
eas-ily transformed into calcite phases.
Arp et al. (2003)
added to these
findings. They demonstrated that
microbialites in Satonda Crater lake (Indonesia) were formed through
passive and diffusion-controlled, EPS-mediated permineralization of
the bio
film mucus at the contact with the lake water, resulting in
micropeloidal aragonite clots. Fibrous aragonite cements then grew
into exopolymer pore spaces and voids. However, in contrast to extreme
soda lakes where EPS degradation supports carbonate precipitation, the
aragonite precipitation at Satonda results from changes in the carbonate
equilibrium driven by seasonal mixing of mixolimnion with
monimolimnion waters, and subsequent CO
2-degassing. The
Arp et al.
(2003)
study demonstrates that besides the degradation and alteration
of organic matter, extrinsic environmental conditions may govern early
diagenetic precipitation.
2.2.3. The role of Mg-silicates in secondary carbonate formation
A last, recently extensively studied process involves the intimate
in-teraction between carbonate and silicate phases during precipitation
and early diagenesis.
Warren (2016)
emphasizes that the magnitude
of detrital clastic input cannot be neglected in marginal lacustrine and
palustrine areas and that clay authigenesis in evaporitic basins is
favoured through the transformation of precursor clays. Episodic
sur-face in
flow and groundwater discharge influence pH, pCO
2, Mg/Si and
the salinity of the
fluids, resulting in the precipitation of kerolite,
stevensite, sepiolite, palygorskite, hectorite or saponite (
Warren,
2016
). Furthermore, authigenic clay minerals enriched in Mg form in
re-cent, hypersaline lacustrine and palustrine settings with high pH, as
early evaporative precipitates (
Eugster and Jones, 1979; Fisher, 1988;
Calvo et al., 1999
), where they occur in close association with
carbon-ates (
Calvo et al., 1995; Freytet and Verrecchia, 2002; Arp et al., 2003;
Kremer et al., 2012; Burne et al., 2014; Tosca and Masterson, 2014;
Pace et al., 2016
).
Experimental studies have now shown that the formation of
Mg-silicates from a primary solution evolves through poorly crystalline
in-termediates (
Jones, 1986; Tosca et al., 2011
). In particular
Tosca and
Fig. 3. Precipitation and early diagenesis in the micro-environment. (A) Porous aggregates of straight monocrystalline Needle Fibre Calcite (NFC) crystals from a moonmilk speleothem of Koselevka cave, Slovenia. SEM image, secondary electrons mode (Image: courtesy of Dr. A. Martín-Pérez;Martín-Pérez and Košir, 2017). (B) Bundles of aragonite needles radiating out-ward from a degraded Aquificales filament at the transition between the Apron-Channel and Pond Facies at New Grassy Spring (Fouke, 2011) (Mammoth Hot Springs, Yellowstone Na-tional Park, USA). SEM image, secondary electron mode. (C) Detail of B showing mineralized extracellular polymeric substances (EPS) with honeycomb structure between the aragonite needles. (Sample taken and provided by L. DeMott). (D) SEM image, secondary electron mode, of a polished thin section showing the contact between a matrix made of Mg-silicate (Mg–si) filaments and aragonite (Ar) fibre fans that seem to nucleate from this clay matrix. Mg-Mg-silicates are closely linked and interfingering with fibrous aragonite in crusts formed at the transition from marine to hypersaline conditions in the Danakil Depression (Afar, Ethiopia). Crusts occur after two incursions by the Red Sea during the Pleistocene (MIS7 and MIS5e). Although similar crusts were formed, Mg-silicates are only found in the younger of the two marine to hypersaline sequences (MIS 5e). Withb100 kyr difference in age, all Mg-silicates have been dissolved and the majority of the aragonite has been replaced by calcite in the older sequence. (Image: courtesy of Dr. D. Jaramillo-Vogel;Jaramillo-Vogel et al., 2016).
Masterson (2014)
have proven that the loss of interlayer sites and
sur-face hydration drive the change of poorly crystalline phases (Mg-silicate
‘gels’) into crystalline Mg-silicate minerals. Mg-silica precipitation may
furthermore change the brine
fluid composition, lowering the Mg/Ca
ratio of the precipitating
fluids and as such favour low Mg-calcite
pre-cipitation (
Wright and Barnett, 2015; Wright and Tosca, 2016
). This
model might explain the occurrence of both carbonates and
Mg-silicates (
Fig. 3
D) in, for example, highly alkaline lake settings such as
the Aptian Barra Velha Formation (Santos Basin, offshore Brazil;
Wright and Tosca, 2016
).
Further, poorly crystallized Mg-silicates are now also often found
and recognized in association with EOM and microbial cell walls (
Arp
et al., 2003; Bontognali et al., 2010, 2014; Kremer et al., 2012; Burne
et al., 2014; Zeyen et al., 2015
). In these cases, and tightly associated
to the processes outlined in
Section 2.2.1
, they may play a role in the
for-mation, transformation and replacement of metastable carbonate
phases.
Burne et al. (2014)
, building on
Wacey et al. (2010)
, provided
petrographic evidence for stevensite growing around cyanobacterial
sheaths and in the alveolar web of thrombolite bio
film EPS in some
specimens taken from Lake Clifton (western Australia). Here, diatoms
may have acted as a local silica source, binding with Mg from the lake
water. In this case, the Mg-silicates gradually entombed the microbial
filaments on which they grew and that initially suppressed (by binding
cations) the formation of carbonates. Stevensite organomineralization
of the bio
film then allowed C and Ca activities to rise in the surrounding,
open pore space, facilitating aragonite precipitation.
Pace et al. (2016)
similarly reported the nucleation of Mg
–Si phases
on the EOM in the zone of active oxygenic photosynthesis (pH
N10)
during the initial stages of microbialite formation in Great Salt Lake
(USA). Degradation of EOM by heterotrophs (sulfate reducing bacteria)
increases alkalinity and allows the nucleation of aragonite during the
first diagenetic phase, followed by partial dissolution in the deepest
part of the mat, once advanced EOM degradation created a zone of
low pH (
Pace et al., 2016
). In this localized,
fluctuating pH zone, pockets
of partially degraded EOM bind Ca
2 +from dissolving aragonite and
Mg
2+, partly derived from the evolving amorphous Mg
\\Si phase
(de-creasing Mg:Si ratio). This joined process leads to the formation of
dolo-mite at the interface with and at the expense of (replacing) EOM,
metastable aragonite and the Mg
–Si phase (
Pace et al., 2016
).
2.3. Neomorphism
Neomorphism (
Folk, 1965
) refers to all in-situ transformations of
one mineral and itself (recrystallization) or a polymorph (inversion)
that generally take place through dissolution-reprecipitation across a
very thin
film of water. Neomorphism, and in particularly aggrading
neomorphism, is a process frequently reported to affect primary
car-bonate precipitates in non-marine environments (
Pentecost, 2005;
Fairchild et al., 2006; Arp et al., 2013a; Peng and Jones, 2013;
Martín-García et al., 2014; Frisia, 2015
).
Several studies are illustrative concerning the recognition of
pur-ported neomorphic fabric changes. Such studies usually integrated
ob-servations from recent and ancient deposits at the same locality. This
approach can be criticized because there is commonly no de
finitive
proof that the supposedly altered material was actually older than the
apparently more pristine rock, though in most cases this seems the
only practical approach to tackling early diagenesis. Good examples
in-clude
Frisia (1996)
and
Frisia et al. (2002)
who showed how aragonitic
fan layers composed of needle and ray crystals were progressively
transformed into an anhedral, equant-shaped to elongated calcite
crys-tal mosaic in a French speleothem. Similar fabrics, where
fibrous or
micritized aragonite relicts are encased in anhedral, calcite mosaics
characterize the Castañar and Basajaún Extea cave deposits (Spain)
(
Martín-García et al., 2009, 2014
) and the process is equally observed
in Recent and Holocene travertine sequences at Mammoth Hot Springs
(Yellowstone National Park, USA) (
Fouke et al., 2000; Chafetz and
Guidry, 2003
;
Fig. 4
A-C). The relics may preserve the initial mineralogy
or be merely textural. In other cases, inversion of aragonite may result in
a nearly complete destruction of the primary
fibrous fabric, leading just
to inclusion-rich elongated, calcite cements (
Arp et al., 2013a;
Martín-García et al., 2014; Frisia, 2015
).
Inversion is typically considered as a combined and co-eval
dissolution-precipitation process in the presence of a
fluid
under-saturated with respect to aragonite and under-saturated for calcite (
Frisia et
al., 2002; Domínguez-Villar et al., 2017
). This may result from short
(intra-seasonal) to longer timescale (inter-annual or permanent)
mod-i
fications of the precipitation conditions or pore fluid composition (e.g.
concentration of CO
2, water discharge, prior precipitating phases)
(
Domínguez-Villar et al., 2017
). Based on high-resolution observations,
Frisia et al. (2002)
suggested that small calcite rhombohedra
precipitat-ed initially between the aragonite neprecipitat-edles without aragonite
dissolu-tion. Those small calcite cement rhombs formed nucleation seeds for
calcite growth. The latter then took place in parallel with inversion of
aragonite when the aragonite saturation decreased. Transmission
Elec-tron Microscopy (TEM) observations by
Frisia et al. (2002)
furthermore
suggest that physical crystal defects such as twins, dislocations and
sur-face irregularities in the aragonite can have a control on the location and
kinetics of the neomorphic process. Geochemical data (
Arp et al., 2013b;
Domínguez-Villar et al., 2017
) have been used to support hypotheses of
partially open systems, with the neomorphic process occurring both
along an angstrom-thin dissolution
–re-precipitation fluid film and
within the larger, intercrystalline pores in exchange with bulk
fluid
(
Arp et al., 2013b
).
Observations on cool water spring and stream carbonates led some
authors to suggest that (aggrading) neomorphism not only affects
metastable phases like aragonite and high
‐magnesium calcite, but also
calcitic deposits (
Fig. 4
D) (
Wright et al., 1997; Janssen et al., 1999; Arp
et al., 2001; Davaud and Girardclos, 2001; Rainey and Jones, 2007
). In
their
‘neomorphic sequences’,
Freytet and Verrecchia (1998)
and
Freytet and Verrecchia (1999)
, building on previous work by
Love and
Chafetz (1988)
, showed a sequence of steps in recrystallization of
calcit-ic, micritic and sparitic envelopes of pro- and eukaryotic
filaments. They
proposed that micritic envelopes, related to Schizothrix colonies, could
evolve into
‘giant laminations’ of columnar, radial palisade crystals
with organic or micritic matter as inclusions outlining primary
lamina-tion and textures. This model was partially disputed by
Brasier et al.
(2015)
who pointed out that spar crystals may commonly be primary,
and that most crystals grow perpendicular to the surface on which
they are nucleated (i.e. here perpendicular to cyanobacterial
filaments).
While continued crystal growth seems to happen in calcitic spring and
stream environments, the production of
‘secondary’ palisade spar layers
requires that the
‘primary’ nuclei are suitably oriented for this to
happen.
Neomorphism can potentially rapidly modify the primary fabric and
texture. Several authors also suggest that any biological inclusions
pres-ent in the active deposits gradually vanish during the formation of
con-tinuous, dense calcite crusts (
Janssen et al., 1999; Arp et al., 2001; Peng
and Jones, 2013
). (Aggrading) neomorphism is therefore commonly
in-ferred
– rightly or wrongly – where rocks exhibit calcitic, coarsely
crys-talline mosaic fabrics, often consisting of anhedral-subhedral equant
(sparry) calcite (
Rainey and Jones, 2007
), or columnar calcite crystals
(
Love and Chafetz, 1988
) or radial palisade calcite with sweeping
ex-tinction (
Freytet and Verrecchia, 1999; Pache et al., 2001; Golubi
ć
et al., 2008
).
2.4. Cementation and cements
Cementation is the precipitation and growth of crystals in pores of
an existing fabric thereby decreasing the pore space (
Flügel, 2004
).
Early cements may form from waters that in
filtrate, percolate or rise
through the porous fabric and from water held in isolated voids.
Cemen-tation in relation to meteoric
fluids has classically been placed and
discussed in two different hydrological zones, the vadose zone above the
water table with both water and air in pores, and the phreatic zone
where pore spaces are saturated with water (freshwater, water of
mixed composition, saline waters) (
Fig. 2
;
Longman, 1980
). Those
zones are dynamic and may move position vertically and laterally
de-pending on the water table (
Moore and Wade, 2013
). The cementation
phenomena associated with them, including microstalactitic and
me-niscus fabrics (vadose zone;
Fig. 5
A) and pore-
filling circumgranular,
drusy and epitaxial cements (phreatic zone), have been well covered
in numerous overview and case study works (
Moore and Wade, 2013;
James and Jones, 2015
).
Cement fabrics observed in several terrestrial case studies (
Table 1
)
are, however, more diverse and often show crystal textures commonly
described from the marine realm. They consist of composite, blocky,
platy or spar calcite crystal (
Fig. 5
B;
Chafetz and Guidry, 2003; Turner
and Jones, 2005; Rainey and Jones, 2007; Jones and Renaut, 2008; Arp
et al., 2013b
); columnar, acicular, gothic arch or bladed crystals forming
isopachous calcite (
Fig. 5
C, D) and
fibrous aragonite, void lining cements
(
Chafetz et al., 1994; Arp et al., 1998; Claes et al., 2015
), radial splays
(
Barth and Chafetz, 2015
), anhedral, calcite-micrite banded crusts,
mo-saic cements and overgrowths (
Fig. 5
E) (
Rainey and Jones, 2007; Jones
and Renaut, 2008; Gandin and Capezzuoli, 2014; Claes et al., 2015
).
How these cement types relate to saturation state,
flow, water
composition (Mg/Ca) and the presence of organisms may follow similar
trends to those summarized by
Flügel (2004)
.
Cements can be found in interparticular, intercrystalline or
frame-work primary pores or as mosaics
filling small, secondary cracks.
When the host fabric is not fully lithi
fied yet, a displacive fabric may
develop, resulting in
floating grains in, for example, calcretes
(
Armenteros, 2010
).
Gandin and Capezzuoli (2014)
furthermore
re-ported of geodic cements (
Fig. 5
F) consisting of rims of gothic arch,
tri-gonal and euhedral calcite crystals. These authors linked them to the
evaporation of residual, oversaturated
fluids that had been trapped
within the primary pore space of e.g. mineralized microbial mat
lami-nae in fossil, Italian hot spring deposits. Similarly,
Arp et al. (1998)
re-port of
fibrous and botryoidal aragonite crystals that line water-filled
lenticular shrinkage voids and gas bubbles within a partly lithi
fied
mi-crobial mat.
To distinguish cements petrographically from any primary crystal
precipitate or encrustation can be challenging (
Chafetz and Guidry,
2003; Pentecost, 2005; Rainey and Jones, 2007; Jones and Renaut,
2008; Martín-Pérez et al., 2015a
). In spring carbonates and
speleothems; precipitation, neomorphism (inversion and
recrystalliza-tion) and cementation are typically a continuum (
Figs. 4
A,
5
C) and
may take place contemporaneously, at different depths (
Martín-García
et al., 2014
), or these processes may alternate through time in step
Fig. 4. Neomorphism. (A) Calcite rhomb crystals, consisting of different platelets, are encasing and growing outward from aragonite needles in an encrustedfilamentous mat sample at the transition between the Apron-Channel and Pond Facies (Fouke, 2011) of the active New Grassy Spring at Mammoth Hot Springs (Yellowstone National Park, USA). SEM image, secondary electrons mode. (Sample taken and provided by L. DeMott). (B) Transmitted light image of a large, anhedral, equant calcite mosaic. The primaryfibrous fabric of bundles of aragonite crystals became neomorphosed and gives the calcite crystals a brownish, inclusion-rich appearance. The aragonite needles seem to have been forming elongated textures that nucleated and grew from different, still faintly recognizable primary horizons (red arrows). Small-scale dissolution is indicated by black arrows. (Y-10 core, Mammoth Hot Springs, Yellowstone National Park, USA). (C) Image with crossed polars of (B) better showing thefibrous texture of the primary aragonite. (D) Neomorphic, larger radial fibrous calcite crystals build from an earlier radialfibrous calcite phase that nucleated from clotted micrite thereby obliterating the previous clotted texture (Great Salt Lake, USA). Transmitted light with crossed polars of stained thin section (Image: courtesy of Dr. P. Homewood and Dr. M. Mettraux, ongoing study).
with water chemistry changes (
Frisia et al., 2002; Jones and Renaut,
2010
). This furthermore means that otherwise
‘primary’ surface
de-posits, e.g. travertines, can also develop as
‘cements’ in cavities of
existing non-marine carbonates (
Rainey and Jones, 2007
), such as
microbial build-ups of the Green River Formation (
Seard et al., 2013
).
Where primary precipitation, neomorphism and cementation happen
simultaneously, the waters from which initial precipitates form also
mediate the
first steps of diagenesis. This means the ‘original’ and
‘diagenetic’ water geochemical signals may be indistinguishable
(
Andrews, 2006
).
2.5. Bio-erosion and micritisation
A further set of processes, having a degrading effect on initial, crystal
substrates, results from the action of organisms and/or presence of
or-ganic matter.
Schneider and Le Campion-Alsumard (1999)
demonstrat-ed, already early, the importance of biological corrosion (de
fined as
dissolution of carbonate substrata by secreted acidic substances) and
bi-ological abrasion (mechanical removal of carbonate surfaces by grazing
organisms), both de
fined as bio-erosion, by endolithic cyanobacteria
and lichens in micro-niches and/or micro-karst. The dissolved and
par-ticulate bioerosion products can be transported and deposited in
subse-quent sedimentary cycles (
Verrecchia, 2000; Kolo et al., 2007
).
Sparmicritisation (
Kahle, 1977
), as a particular process, is well-known
in marine sediments, and in lakes (
Armenteros, 2010
), resulting in micrite
envelopes of allochems (
Bathurst, 1975; Flügel, 2004
). A similar process,
mediated by the activity of cyano- and other bacteria, fungi and algae
can transform spar, columnar or
fibrous crystals to micrite or microspar
in non-marine settings (
Fig. 5
G;
Chafetz et al., 1994; Jones, 2010;
Capezzuoli et al., 2014
). Microorganisms, colonizing the surface during
periods of reduced precipitation rates (
Takashima and Kano, 2008
), or
present as endolithic communities in porous laminae of a primary crust,
are believed to break apart crystals (
Fig. 5
H) by their boring activity.
This process can be accelerated by etching from (heterotrophic) bacterial
decomposition of organic matter and the dissolution/breaking apart of
partly indurated crystals (
Arp et al., 2001; Jones, 2010; Jones and Peng,
2012b
). The effect of biogenic spar micritization is expected to decrease
- or change - with depth as light becomes limiting for phototrophic
organ-isms and the dominant microbial community will change or cease activity
(
Jones, 2010
). Further, in caves, the process of micritisation has often been
reported as
‘condensation corrosion’ (
Martín-García et al., 2011, 2014;
Martín-Pérez et al., 2012
). During a hiatus in crystal growth, or along
small inter- and intracrystalline pores and discontinuities, acidic
atmo-spheric water condenses on and dissolves the speleothem surface.
Micritisation has been held responsible for increasing
homogeniza-tion of primary shrubs surrounded by calcite spar in Quaternary
traver-tine deposits (
Guo and Riding, 1994
) and for repetitive microporous,
micritic to microsparitic (4
–10 μm grains) bands or coatings in
aragonit-ic and calcitaragonit-ic speleothems (
Martín-García et al., 2009, 2011, 2014
).
When no ghosts of a primary fabric can be recognized, together with
remnants of calci
fied filaments or endoliths, the mottled micritic fabric
can become mistaken for primary micrite laminae.
2.6. Other early diagenetic processes
A series of other, diagenetic processes are known to operate soon after
deposition. They have been well covered in literature (
James and
Choquette, 1984; Tucker and Wright, 1990; Armenteros, 2010; Moore
and Wade, 2013; James and Jones, 2015
) and will only be addressed brie
fly.
2.6.1. Replacement
Non-marine carbonate deposits can be subject to replacement
pro-cesses when the ground- and pore water chemistry and conditions
change. Some examples on the nano- to micron-scale were given in
Section 2.2
. One particular replacement process is the transformation of
ikaite to calcite (
Suess et al., 1982
). Ikaite is only metastable at the Earth's
surface and is deposited from phosphate-rich waters at temperatures
below 6
–7 °C (
Hu et al., 2014; Sánchez-Pastor et al., 2016
) (Table A2)
transforming to calcite within 5 h at temperatures of 10 °C, and even
more rapidly to calcite via vaterite at temperatures around 20 °C
(
Sánchez-Pastor et al., 2016
). Conditions of primary ikaite formation in
natural and experimental lab conditions are summarized in Table A2.
Cal-citic pseudomorphs after ikaite (CaCO
3·6H
2O) are known from recent
and ancient lakes (
Bischoff et al., 1993; Fairchild et al., 2016
), streams
(
Boch et al., 2015
) and caves (
Field et al., 2017
), and have local names
in-cluding glendonite (
Whiticar and Suess, 1998
) and thinolite (
Shearman
et al., 1989; Bischoff et al., 1993; Council and Bennett, 1993
). These are
recognised by their crystal morphology, ikaite being monoclinic. The
transformation from ikaite to calcite involves a 68% volume decrease
(
Shaikh and Shearman, 1986
). Consequently, the
final rock products are
typically porous, and can be readily recognised (
Fig. 6
A).
Some other, common, important replacement processes are
dolomitisation and silici
fication. Early diagenetic replacement of CaCO
3or silicate minerals by Mg
‐carbonates or poorly ordered dolomite,
CaMg(CO
3)
2, is associated with evaporative saline waters, lowered sulfate
concentrations and elevated Mg/Ca ratios (
Fig. 6
B, C) (Table A2). Early
dolomitisation processes often yield non-stoichiometric and poorly
or-dered Mg-rich carbonates (
Bontognali et al., 2014; Gregg et al., 2015
).
Much has been published and discussed on a variety of dolomitisation
models (
Machel, 2004
). In non-marine depositional environments, early
diagenetic dolomitization may follow the sabhka playa model
(
Vasconcelos and McKenzie, 1997
) where evaporation and hypersaline
conditions create Mg-rich, dense
fluids that sink. This is a process invoked
in marginal lacustrine and palustrine settings (
Fig. 6
B, C) and in calcretes,
thereby forming
‘dolocretes’ (
Armenteros, 2010; Bontognali et al., 2010;
Richter et al., 2014
).
Arp (1995)
invoked a mixing zone model for the
dolomitisation of high
‐magnesium calcite deposits in the case of the
Ries Crater algal bioherms. Petrography and geochemistry suggested
phreatic pore water within the lake margin bioherms
fluctuated through
time, causing mixing of low ionic strength meteoric waters with saline
lake water within the pore space. Such dolomitization models are
howev-er much debated and contested when it comes to explaining large-scale
dolomitisation phenomena (
Hardie, 1987
).
Though dolomite is more rarely present in cave deposits, it has been
reported as a replacement product of metastable, hydrous phases
(hydromagnesite, huntite) (
Sánchez-Román et al., 2011; Martín-Pérez
et al., 2012, 2015a
) and as a product of dolomitization in relation to the
alteration of CaCO
3in contact with Mg-rich
fluids (
Alonso-Zarza and
Mar-tín-Pérez, 2008
). Mg-enrichment of speleothem-depositing
fluids occurs
through dissolution of adjacent dolostone lithologies, followed by
fluid
evaporation and the progressive crystallization of Ca-rich minerals,
leav-ing the residual
fluid enriched in Mg. Mg-rich carbonates have been
re-ported as forming microcrystalline to coarse, fan-shaped and spheroidal
crystals that cover aragonite textures, partly replacing the precursor
CaCO
3phase (
Fig. 6
D;
Martín-Pérez et al., 2012, 2015a
).
Early diagenetic replacement of carbonate by silica is favored in
con-ditions where a silica-charged
fluid is available (N6 mg/L SiO
2) and both
pH (~6 to
N9) and temperature are strongly fluctuating (
Fig. 6
B, C),
Fig. 5. Cements and (spar)micritisation. (A) Transmitted light image with crossed polars of micritic-sparitic layered, pendant cements lining the upper side of a cavity (Great Salt Lake, USA) (Courtesy of Dr. P. Homewood and Dr. M. Mettraux). (B) Platy calcite rhombs forming pyramidal to bipyramidal composite crystals, cementing framework porosity around moss branches in Pleistocene travertine deposits (Cakmak quarry, Denizli Basin, Turkey). SEM image, secondary electron mode. (C) Isopacheous, transparent calcite cement (red arrow) around microporous, micritic dendrite textures (Cakmak quarry, Denizli Basin, Turkey). Transmitted light. (D) Calcitic, acicular cement growing inside primary porosity of Great Salt Lake microbialites along the northeastern side of Stansbury Island. Transmitted light image (Image: courtesy of Dr. M. Vanden Berg;Chidsey et al., 2015). (E) Neomorphosed aragonite needles leaving a microporous fabric are surrounded and encased by a subhedral, equant calcite cement in Pleistocene travertine deposits (Gardiner area, USA). SEM image, secondary electron mode.(F) Transmitted light image of microbial laminites made of single or multiple micritic, organicfilms curled and folded to form open interlamina cavities lined by calcite cement. The subhedral calcite crystals are interpreted as a geodic cement resulting from the evaporation of water retained in the microbial mat. The folds apparently derive from diapiric defor-mation (Oliviera Quarry, Rapolano, Siena, Italy) (Image: courtesy of Prof. A. Gandin;Gandin and Capezzuoli, 2014). (G) Repetitive events of sparmicritisation (red arrows) of botryoidal growth radialfibrous calcite increments (Great Salt Lake, USA). Transmitted light (Image: courtesy of Dr. P. Homewood and Dr. M. Mettraux). (H) Transmitted light image of stained thin section showing possible fungal threads affecting microbialite clasts or“bedrock” (Great Salt Lake, USA) (Image: courtesy of Dr. P. Homewood and Dr. M. Mettraux, ongoing study).
thereby affecting carbonate and silica solubility (
Bustillo, 2010
). Such
conditions are found where, for example, pCO
2varies, like in cave
atmo-spheres (
Woo et al., 2008
), or where waters of different composition
and saturation state mix (saline/alkaline, meteoric waters), or where
as-cending, Si-bearing thermal waters cool down (
Bustillo, 2010
). The
dis-solved silica can be sourced from associated or extra-formational clays,
volcanic or metamorphic rocks, or even siliceous microorganisms like
diatoms within the deposits (
Bustillo et al., 2002; Burne et al., 2014
).
Sil-ica often
first forms as metastable opal A, transforming to opal CT and
then quartz, or may form directly as stable quartz (
Armenteros et al.,
1995; Bustillo et al., 2002
). Early, pseudomorphic silici
fication may
pro-tect primary features from later, burial- or subaerial exposure-related
alteration and dissolution. As such, silici
fied parts of non-marine
carbonate sequences can still hold important clues to primary
circum-stances of non-marine deposition (
Knoll et al., 2013
). Silica replacement
of evaporites (CaSO
4), for example in sabhka settings, has been
com-monly reported and the reader is referred to
Warren (2016)
for a full
re-view of the replacement processes (
Folk and Pittmann, 1971
), possible
(biogenic) silica sources and the resulting fabrics.
2.6.2. Dissolution
Local-scale dissolution is involved in processes like neomorphism
and replacement, but the dilute composition of meteoric and freshwater
may also lead to a more pervasive dissolution phase of mineral
compo-nents (
Moore and Wade, 2013
), resulting in pore enlargement (vugs)
(
Fig. 7
A), karsti
fication (
Fig. 7
B) and cavern development. Meteoric
dis-solution can be held responsible for enlarged, vuggy porosity and
trun-cation of primary features observed on a microscopic scale (
Chafetz and
Guidry, 2003; Rainey and Jones, 2007
). Episodes of dissolution or
etch-ing upon exhumation can be subtle and recurrent, contributetch-ing to
banded or layered dendrite and calcite fan crystal deposits (
Jones and
Peng, 2012b; Brasier et al., 2015
). In some cases, detailed
epi
fluorescence or optical cathodoluminescence petrography might be
needed to decipher dissolution phases between periods of precipitation
or cementation (
Rainey and Jones, 2007
;
Fig. 7
C).
2.6.3. Mechanical weathering
Additional, early, physical alteration processes may take place upon
subaerial exhumation, including desiccation (
Fig. 7
D, E), brecciation and
shrinkage (
Fig. 7
F) in, for example, calcretes, in microbial mats,
lacus-trine carbonate muds or dry ephemeral ponds (
Arp et al., 1998;
Freytet and Verrecchia, 2002; Brasier, 2011; Gandin and Capezzuoli,
2014
). Resulting fabrics, like in-situ formed pseudobreccias, horizontal
and vertical cracks and teepee formation, can also result from root
activ-ity, thermal and moisture related expansion and contraction or
crystal-lization in pores upon evaporation (
Eren, 2007; James and Jones, 2015
).
Breaking up of deposits may furthermore facilitate mechanical
trans-port and sediment re-deposition.
3. Impact of early diagenesis on carbon-, oxygen- and clumped
isotope records and paleoenvironmental interpretations
3.1. Stable carbon and oxygen isotopes in non-marine carbonates
Fig. 8
shows a compilation of carbon and oxygen stable isotopic
com-positions of carbonate sediments from the selected reference cases
introduced in
Table 1
. An overview of carbonate stable isotopic
compo-sitions characteristic of vadose and phreatic, meteoric diagenesis can be
found in numerous publications and books (
Allan and Matthews, 1982;
Morse and Mackenzie, 1990; Hoefs, 2004; Sharp, 2007; van Dongen
Fig. 6. Replacement processes. (A) Transmitted light image of calcite pseudomorphs after ikaite (Ik; large, clear nicely shaped crystals) leaving a porous fabric behind (blue resin is pore space) (north shore of Mono Lake, USA). (B) and (C) Transmitted light and cathodoluminescence image of extensively silicified dolomite (D, bright, orange-yellow luminescent). Different silica generations represent authigenic quartz (Qz, non luminescent) followed by thinly laminated chalcedony (Cad, brownish to non luminescent). Relict dolomite represent dolomitized stromatolites deposited in hypersaline lacustrine settings (Aptian, Angola;Foubert et al., 2014). (D) Spheroidal dolomite crystals withfibrous-radial texture and concentric bands cement and replace aragonite crystals (stained red) in a crust speleothem of Snežna Jama, Slovenia (Martín-Pérez et al., 2015b) (Image: courtesy of Dr. A. Martín-Pérez).