Regulation of hyaluronan-stimulated VCAM-1 expression in murine renal tubular epithelial cells
Texte intégral
(2) Regulation of hyaluronan-stimulated VCAM-1 expression. Methods Reagents Tissue culture reagents were obtained from Life Technologies (Gaithersburg, MD) and chemicals from Fluka (Buchs, Switzerland) or Sigma (St Louis, MO). Fragmented HA of intermediate mol.wt derived from human umbilical cord was obtained from Fluka (Buchs, Switzerland) or ICN (Costa Mesa, CA). The PKC inhibitors GF109203X and chelerythrine chloride and the TK inhibitor genistein were obtained from RBI (Natick, MA).. Cell lines and cell culture The SV40-transformed mouse cortical tubule (MCT ) cell line was originally obtained from Dr T. Haverty [14]. MCT cells were grown in DME medium supplemented with 10% FBS, 10 mM HEPES, 100 U/ml penicillin and 100 mg/ml streptomycin. After growing cells to confluence the medium was changed to DME containing 1% FBS for 24 h. Cells were then stimulated with HA (100 mg/ml ) for 18 h in DME containing 1% FBS. PKC or TK inhibitors or vehicle were added 60 min prior to and during the 18 h stimulation period. To downregulate PKC, MCT cells were exposed to PMA at 0.75 mM for 48 h. Subsequently, MCT cells were stimulated with HA (100 mg/ml ) for 18 h in the presence of PMA, and VCAM-1 expression was then assessed by cell ELISA.. Direct cell ELISA VCAM-1 expression by MCT cells in response to HA was analysed on adherent cells by direct cell ELISA in 96-well plates as described [6 ]. After appropriate stimulation the cells were fixed with 3% paraformaldehyde and washed with PBS. Cells were then incubated with the anti-VCAM-1 mAb supernatant MK-2.7 (1550) in PBS containing 5% FCS for 1 h at 4°C. Negative controls included omission of primary mAb or use of irrelevant mAb. Cells were washed three times and incubated with peroxidase-conjugated sheep anti-rat IgG Fab fragments (Boehringer Mannheim Biochemica, Germany) at 151000 in PBS/5% FCS for 1 h at 4°C. Cells were washed again three times and were incubated with the o-phenylenediamine dihydrochloride substrate (Fast OPD tablet sets, Sigma). After 15 min, 20% v/v of concentrated sulfuric acid was added to stop the reaction. The OD in the supernatant was read at 492 nm on a Multiskan RC ELISA reader (Labsystems, Helsinki). Delta ODs were calculated by subtracting the ODs of cells incubated with irrelevant antibody from cells incubated with anti-VCAM-1 mAb. Mean±SEM were then calculated (triplicate determinations). Experiments were performed at least three times.. RNA extraction, RT–PCR and Northern blot analysis MCT cells were stimulated with HA (100 mg/ml ) for 3 h. The PKC and TK inhibitors were added 30 min prior to and during the 3 h stimulation period. Total RNA from cultured MCT cells was then extracted as described [15]. VCAM1 mRNA expression was analysed either by RT–PCR or by Northern blotting as described [6 ]. The VCAM-1 primers had the following sequence: forward primer 5∞-CCC AAG GAT CCA GAG ATT CA-3∞, reverse primer 5∞-TAA GGT GAG GGT GGC ATT TC-3∞, resulting in a 489 bp fragment. The following cycling parameters were used: denaturation at. 2131. 94°C for 40 s, annealing at 58°C for 120 s, extension at 72°C for 150 s, with a terminal extension at 72°C for 7 min. The amplification was performed for 30 cycles. The housekeeping gene GAPDH was also amplified in all RNA samples as described to ensure equal RNA quantities [15]. RT–PCR products were resolved on 1% agarose gels and stained with 0.5 mg/ml ethidium bromide. Gels were then photographed with UV light. For Northern blotting, total RNA (25 mg) was electrophoresed on 1.5% agarose gels in 20 mM MOPS buffer and blotted onto nylon membranes. Membranes were hybridized overnight at 42°C with an [a-32P]dCTP labelled VCAM-1 cDNA probe as described [6 ]. Blots were then washed under stringent conditions (final wash in 0.1× SSC, 1% SDS, 62°C ) and were analysed with a PhosphorImagerB. Methylene blue staining to detect the 18S and 28S rRNA bands was performed to ensure equal loading. The presence of mRNA encoding for the atypical PKC f and l was assessed by RT–PCR, using the following primers: PKC f forward 5∞-AAG TGG GTG GAC AGT GAA GG3∞, reverse 5∞-TGC CAT CTA CTG GAG GCT CT-3∞, yielding a 418 bp fragment [16 ]; PKC l forward 5∞-AAT GGC CAC ACT TTT CAA GC-3∞, reverse 5∞-CCA CTC TCC CTG GTG TTC AT-3∞, yielding a 311 bp fragment [17]. Amplification was performed for 40 cycles, using the same cycling parameters as indicated above.. Nuclear extracts MCT cells were grown to confluence and were then stimulated with HA (100 mg/ml ) for 1 h. The PKC and TK inhibitors were added 30 min prior to and during the 1 h stimulation period. Cells were then chilled on ice and were mechanically detached and washed with PBS. Nuclear extracts were then prepared according to Schreiber et al. [18]. Cells were resuspended in 400 ml ice-cold buffer A (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF ) and incubated for 15 min and were then lysed with 25 ml of 10% NP-40. After centrifugation, the nuclear pellets were resuspended in 50 ml of ice-cold buffer C (20 mM HEPES pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF ) and rocked vigorously at 4°C for 1 h. The extracts were then centrifuged for 5 min at 4°C in a microfuge and the supernatants were frozen at −70°C until use. The protein concentration of the extracts was determined using the Bradford method (Bio-Rad, Hercules, CA).. Electrophoretic mobility shift assays (EMSA) Nuclear extracts were then analysed for the presence of NFkB using a 22mer double-stranded oligonucleotide with the sequence 5∞-AGT TGA GGG GAC TTT CCC AGG C-3∞ (Promega, Madison, WI ), the consensus sites being underlined [19]. The oligonucleotide was end-labelled with [c32P]ATP using T4 polynucleotide kinase. Extracts were then incubated for 1 h at room temperature with labelled NF-kB oligonucleotide in binding buffer (5% glycerol, 5 mM DTT, 50 mM NaCl, 50 mM KCl, 1 mM MgCl , 1 mM EDTA, 2 10 mM Tris–HCl, pH 7.5) with denatured and sonicated calf thymus DNA (50 mg/ml ) in the presence or absence of unlabelled competitor oligonucleotide. DNA–protein complexes were resolved in non-denaturing 5% acrylamide gels in 0.03× TBE buffer at 270 V. Gels were then dried and subjected to PhosphorImagerB analysis..
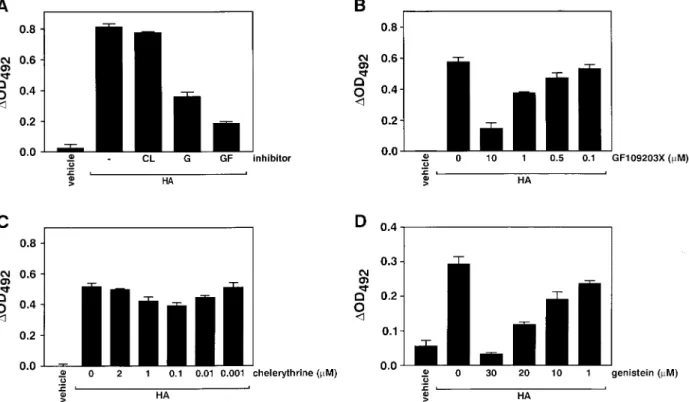
(3) 2132. Results PKC and TK inhibitors block VCAM-1 cell surface expression in MCT cells To investigate the relative contribution of PKC and TK signalling pathways in the expression of VCAM-1 in response to HA, we incubated MCT cells with selective inhibitors of these pathways ( Figure 1). The broad PKC inhibitor GF109203X (acting on conventional, novel and atypical isoforms [20]) inhibited the HA-stimulated VCAM-1 expression in MCT cells significantly ( Figure 1A). The effect of GF109203X was dose-dependent between 0.1 and 10 mM, causing a maximal inhibition around 90% (Figure 1B). In four separate experiments GF109203X (10 mM ) inhibited the HA-stimulated (100 mg/ml ) VCAM-1 cell surface expression by 89.5±5.0% (mean±SEM; P<0.0001; two-tailed t-test). On the other hand the PKC inhibitor chelerythrine (acting preferentially on conventional and novel isoforms [21]) had no effect on HA-stimulated VCAM-1 expression when used between 0.001 and 2 mM (Figure 1C ). In four separate experiments chelerythrine (2 mM ) did not significantly inhibit VCAM-1 expression (10.0±9.5% inhibition; P>0.05). Together these data suggest that Ca2+- and DAG-independent (atypical ) isoforms could be involved in HA-stimulated VCAM1 expression in these cells.. A. Schawalder et al.. The TK inhibitor genistein [22] dose-dependently (1–30 mM ) inhibited the HA-stimulated VCAM-1 expression (Figure 1D). In three separate experiments genistein (25 mM ) significantly inhibited the HA-stimulated (100 mg/ml ) VCAM-1 cell surface expression by 63.9±11.2% (P<0.01). Similar results were obtained when using two different HA preparations ( Figure 1A versus B–D). We detected no toxicity at the above-mentioned inhibitor concentrations. When used for up to 18 h, there was no cell detachment, and trypan blue exclusion was intact. Furthermore, at 3 h there was no sign of mRNA degradation (see below). We also examined the combined effect of GF109203X and genistein on the HA-stimulated VCAM-1 expression by cell ELISA. When used at submaximal concentrations (GF109203X at 5 mM and genistein at 20 mM ), the combination of both inhibitors produced a complete inhibition of the HA-stimulated VCAM-1 expression (96.7±3.3% inhibition). Higher concentrations of the two inhibitors produced toxic effects on MCT cells when used together. In separate experiments we also examined the effects of GF109203X, chelerythrine and genistein on TNFa-stimulated (20 ng/ml ) VCAM-1 expression by MCT cells. GF109203X (10 mM ) but not chelerythrine (2 mM ) inhibited the TNF-a-stimulated VCAM-1 expression significantly. Genistein (25 mM ) also significantly inhibited VCAM-1 expression (data not. Fig. 1. Effect of PKC inhibitors GF109203X and chelerythrine, and of TK inhibitor genistein on cell surface VCAM-1 protein expression by MCT cells. VCAM-1 expression was assessed by direct cell ELISA. (A) MCT cells were stimulated with HA (100 mg/ml; ICN ) for 18 h. GF109203X (GF ) and genistein (G) but not chelerythrine (CL) inhibited HA-stimulated VCAM-1 expression markedly ( lane 1, vehicle; lane 2, HA (100 mg/ml ); lane 3, HA+CL (2 mM ); lane 4, HA+G (25 mM ); lane 5, HA+GF (10 mM )). (B–D) MCT cells were stimulated with HA (100 mg/ml; Fluka) for 18 h. GF109203X (B) and genistein (D) but not chelerythrine (C ) inhibited VCAM-1 expression dose-dependently. Data represent mean±SEM from typical experiments which were performed at least three times..
(4) Regulation of hyaluronan-stimulated VCAM-1 expression. shown). These data suggest that HA and TNF-a could use similar signalling pathways.. Involvement of atypical PKC in HA-mediated VCAM-1 expression To confirm that Ca2+- and DAG-independent (atypical ) isoforms could be involved in HA-stimulated VCAM-1 expression in these cells, we used downregulation of PKC with the phorbol ester PMA. Figure 2A demonstrates that the exposure of MCT cells to 0.75 mM PMA for 48 h did not prevent the stimulation of VCAM-1 with HA. Using RT–PCR we then examined whether the atypical PKC f and l were expressed in MCT cells. Figure 2B demonstrates that both the f and the l isoform are constitutively expressed by MCT cells. HA stimulation did not change steady-state mRNA levels of these two PKC isoforms. These data together with the differential effect of GF109203X and. 2133. chelerythrine strongly suggest that atypical PKCs are involved in the HA stimulation pathway of VCAM-1. Inhibition of VCAM-1 mRNA expression by PKC and TK inhibitors We then examined the effect of PKC and TK inhibition on HA-stimulated VCAM-1 expression at the transcriptional level by RT–PCR and Northern blotting. Figure 3A demonstrates that the PKC inhibitor GF109203X (10 mM ) inhibited the HA-stimulated VCAM-1 mRNA transcript levels markedly, which is concordant with the cell surface expression. Chelerythrine (2 mM ) on the other hand did not significantly inhibit steady-state VCAM-1 mRNA expression, also in accord with the lack of inhibition of cell surface VCAM-1 protein. The TK inhibitor genistein also inhibited VCAM-1 mRNA (Figure 3B). These data suggest that the inhibition of cell surface VCAM1 expression by GF109203X and genistein could occur via interference with the transcription of the VCAM1 gene. Alternatively, the inhibitors could cause a decrease in VCAM-1 mRNA stability, leading indirectly to decreased translation. Role of NF-kB in HA-stimulated VCAM-1 expression It has been shown previously that the mouse and human VCAM-1 promoters contain NF-kB and AP-1. Fig. 2. (A) Downregulation with PMA does not prevent HA stimulation of VCAM-1. MCT cells were grown for 48 in PMA (0.75 mM ), and were then stimulated overnight for 18 h with HA (100 mg/ml; Fluka) in the presence of 0.75 mM PMA. VCAM-1 expression was assayed by cell ELISA. Data are mean±SEM from a typical experiment. (B) Messenger RNA analysis for the presence of the atypical PKC f and l in MCT cells. MCT cells were stimulated with HA (100 mg/ml for 24 h), total RNA was extracted and analysed by RT–PCR.. Fig. 3. Effect of PKC inhibitors GF109203X and chelerythrine, and effect of TK inhibitor genistein on steady-state VCAM-1 mRNA levels by MCT cells. (A) RT–PCR analysis for VCAM-1 (top) and GAPDH (bottom) in HA-stimulated (100 mg/ml; Fluka) MCT cells, demonstrating the inhibitory effect of GF109203X (10 mM ) but not chelerythrine (2 mM ). (B) Northern blot analysis for VCAM-1 (top) and methylene blue (MB) staining for 28S/18S mRNA (bottom) in HA-stimulated (100 mg/ml; Fluka) MCT cells, demonstrating the inhibitory effect of genistein (1–50 mM )..
(5) 2134. binding sites [10–12,23,24]. In a previous report we have shown that NF-kB and AP-1 are activated by HA in MCT cells, and that blocking of NF-kB with the inhibitor TPCK completely inhibited the HAstimulated VCAM-1 expression in MCT cells, suggesting that NF-kB activation plays a very important role in the expression of VCAM-1 in response to HA. To elucidate the role of PKC and TK in the HAstimulated, NF-kB-dependent VCAM-1 expression, we prepared nuclear extracts from HA-stimulated MCT cells and performed gel-shift assays for NF-kB. Interestingly, both GF109203X and genistein did not inhibit NF-kB activation in response to HA ( Figure 4), which is in striking contrast to the inhibition seen with these two inhibitors at the VCAM-1 mRNA and cell surface protein level ( Figures 1 and 3). These data, together with earlier data with TPCK suggest that. Fig. 4. EMSA for NF-kB in HA-stimulated MCT cells. The specific bands are marked with an arrow. Several additional non-specific and fainter bands are also found. (A) The PKC inhibitors GF109203X (10 mM ) and chelerythrine (2 mM ) do not inhibit the nuclear translocation of NF-kB in response to HA (100 mg/ml; Fluka). (B) The TK inhibitor genistein (30 and 50 mM ) also does not inhibit the activation of NF-kB in response to HA.. A. Schawalder et al.. NF-kB is necessary but not sufficient for VCAM-1 expression in response to HA.. Discussion Our results elucidate several important aspects in the cellular pathway of VCAM-1 activation in response to HA in MCT cells: (i) HA activates an atypical protein kinase C; (ii) HA also uses TK signalling pathways; (iii) HA activates NF-kB despite PKC and TK inhibition. Both chelerythrine and GF109203X are potent and specific inhibitors of protein kinase C [25]. The bisindolylmaleimide GF109203X inhibits PKC activity via the ATP-binding site [20]. In contrast, the benzophenanthridine alkaloid chelerythrine acts at the protein substrate binding site, does not block DAG binding to PKC and is a non-competitive inhibitor of ATP binding [21]. GF109203X inhibits all PKC subtypes, including the conventional PKCs a, bI, bII and c (Ca2+- and DAG-dependent), the novel PKCs d and e (Ca2+-independent, DAG-dependent) and the atypical PKC f (Ca2+- and DAG-independent) [25,26 ]. In one study, the IC for GF109203X was 8.4 nM for 50 the conventional PKC a and 5.8 mM for the atypical PKC f [26 ]. The concentration of 10 mM which we used should therefore have been effective on all PKC isotypes. Although chelerythrine’s inhibitory spectrum on the various PKC isoforms has not been formally determined, it is felt by most that it acts preferentially on the conventional and novel (Ca2+-dependent and -independent, DAG-dependent) PKCs, sparing the atypical forms. The IC of chelerythrine on conven50 tional PKC was found to be around 0.7 mM [21]. One report at least documents that a chicken gizzard PKC f is insensitive to chelerythrine (up to 50 mM ) [27]. Therefore, the concentration of 2 mM chelerythrine which we used should have blocked the coventional and novel but not the atypical PKC. The differential effects of GF109203X and chelerythrine can be used, together with PMA downregulation, to obtain information as to whether atypical PKCs are involved in cellular signalling. Jordan et al. have shown for example that GF109203X prevented the IL-1-induced upregulation of IL-8 in human synovial fibroblasts, whereas chelerythrine had no effect [28]. Likewise, PDGF-induced a -integrin expression 2 in human dermal fibroblasts could be inhibited completely with GF109203X, whereas chelerythrine was only partially inhibitory [29]. Using these two PKC inhibitors, it was suggested in both studies that atypical PKCs such as PKC f were involved. PKC f activation has been incriminated in many cytokine signalling processes ( TNF-a, IL-1), and our data with PMA downregulation are in agreement with such a pathway and suggest that an atypical PKC is mediating the effects of HA on VCAM-1 expression in MCT cells. Several studies have shown that TK is important in VCAM-1 expression in endothelial cells [30–32]. Few studies have been performed in epithelial cells. IFN-c.
(6) Regulation of hyaluronan-stimulated VCAM-1 expression. (typically acting through the JAK/STAT signalling cascade) does not upregulate VCAM-1 in endothelial cells by itself, but in combination with TNF-a enhances the effect of IFN-c [33]. On the other hand IFN-c is effective alone in tubular epithelial [34] and mesothelial cells [35]. This suggests that the JAK/STAT pathways could be important for epithelial VCAM-1 expression. Further studies will have to examine the involvement of specific JAKs, and will need to test the role of the STAT family of transcription factors in the HAstimulated VCAM-1 expression. It is interesting to note that GF109203X did not inhibit NF-kB activation despite blocking the HAstimulated VCAM-1 expression. Previously we have shown that blocking of NF-kB with TPCK inhibited VCAM-1 expression completely [6 ]. This suggests that NF-kB is required but not sufficient for VCAM-1 expression. A similar situation has been reported in HUVEC where PKC inhibition did not prevent NFkB translocation [36 ]. This suggests that other transcription factors are required to induce VCAM-1. In summary, we have shown that the HA-stimulated VCAM-1 expression in a murine renal tubular epithelial cell line depends on an atypical PKC and on a TK pathway. The transcription factor NF-kB is required but is not sufficient alone to stimulate VCAM-1 expression. Our data highlight the complexity of interactions between PKC, TK and nuclear transcription factors in the HA-stimulated VCAM-1 expression by epithelial cells.. 2135. 7.. 8. 9. 10.. 11. 12.. 13. 14.. 15. 16.. 17. Acknowledgements. We thank C. Gasser for help with the illustrations. This study was supported by the Swiss National Science Foundation (grants No. 32–40390.94 and 32–50721.97 to R.P.W.), the Olga-Mayenfisch Foundation, the Hartmann-Mu¨ller Foundation and the Research Foundation of the University of Zu¨rich. B.O. is the recipient of a Postgraduate Fellowship from the University of Zu¨rich and is supported by the Swiss National Science Foundation and the Maurice E. Mu¨ller Foundation. B.B.S. is the recipient of a Federal Career Development Award from the Swiss government. R.P.W. is the recipient of a Physician Scientist Award (grant No. 32–38821.93) from the Swiss National Science Foundation.. References 1. Wu¨thrich RP. Intercellular adhesion molecules and vascular cell adhesion molecule-1 and the kidney. J Am Soc Nephrol 1992; 3: 1201–1211 2. Brady HR. Leukocyte adhesion molecules and kidney diseases. Kidney Int 1994; 45: 1285–1300 3. Qi J, Kreutzer DL, Piela-Smith TH. Fibrin induction of ICAM1 expression in human vascular endothelial cells. J Immunol 1997; 158: 1880–1886 4. Lozada C, Levin RI, Huie M et al. Identification of C1q as the heat-labile serum cofactor required for immune complexes to stimulate endothelial expression of the adhesion molecules Eselectin and intercellular and vascular cell adhesion molecules Proc Natl Acad Sci 1995; 92: 8378–8382 5. Roebuck KA, Rahman A, Lakshminarayanan V, Janakidevi K, Malik AB. H O and tumor necrosis factor-a activate intercellu2 2 lar adhesion molecule 1 (ICAM-1) gene transcription through distinct cis-regulatory elements within the ICAM-1 promoter. J Biol Chem 1995; 270: 18966–18974 6. Oertli B, Beck-Schimmer B, Fan X, Wu¨thrich RP. Mechanisms of hyaluronan-induced up-regulation of ICAM-1 and VCAM-1 expression by murine kidney tubular epithelial cells: hyaluronan. 18.. 19. 20. 21. 22. 23.. 24. 25. 26. 27.. 28.. triggers cell adhesion molecule expression through a mechanism involving activation of nuclear factor-kB and activating protein1. J Immunol 1998; 161: 3431–3437 Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kB and cytokine-inducible enhancers. FASEB J 1995; 9: 899–909 Stade BG, Messer G, Riethmu¨ller G, Johnson JP. Structural characteristics of the 5∞ region of the human ICAM-1 gene. Immunobiology 1990; 182: 79–87 Ballantyne CM, Sligh JE, Jr., Dai XY, Beaudet AL. Characterization of the murine ICAM-1 gene. Genomics 1992; 14: 1076–1080 Cybulsky MI, Fries JW, Williams AJ et al. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proc Natl Acad Sci 1991; 88: 7859–7863 Cybulsky MI, Allan-Motamed M, Collins T. Structure of the murine VCAM1 gene. Genomics 1993; 18: 387–391 Korenaga R, Ando J, Kosaki K, Isshiki M, Takada Y, Kamiya A. Negative transcriptional regulation of the VCAM-1 gene by fluid shear stress in murine endothelial cells. Am J Physiol 1997; 273: C1506–C1515 Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-kB/I-kBa autoregulatory loop in murine macrophages. J Exp Med 1996; 183: 2373–2378 Haverty TP, Kelly CJ, Hines WH et al. Characterization of a renal tubular epithelial cell line which secretes the autologous target antigen of autoimmune experimental interstitial nephritis. J Cell Biol 1988; 107: 1359–1368 Benz PS, Fan XH, Wu¨thrich RP. Enhanced tubular epithelial CD44 expression in MRL-lpr lupus nephritis. Kidney Int 1996; 50: 156–163 Goodnight J, Kazanietz MG, Blumberg PM, Mushinski JF, Mischak H. The cDNA sequence, expression pattern and protein characteristics of mouse protein kinase C f. Gene 1992; 122: 305–311 Akimoto K, Mizuno K, Osada S, Hirai S, Tanuma S, Suzuki K, Ohno S. A new member of the third class in the protein kinase C family, PKC l, expressed dominantly in an undifferentiated mouse embryonal carcinoma cell line and also in many tissues and cells. J Biol Chem 1994; 269: 12677–12683 Schreiber E, Matthias P, Mu¨ller MM, Schaffner W. Rapid detection of octamer binding proteins with mini-extracts prepared from a small number of cells. Nucleic Acids Res 1989; 17: 6419 Lenardo MJ, Baltimore D. NF-kB: a pleiotropic mediator of inducible and tissue-specific gene control. Cell 1989; 58: 227–229 Toullec D, Pianetti P, Coste H et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 1991; 266: 15771–15781 Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 1990; 172: 993–999 Akiyama T, Ishida J, Nakagawa S et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 1987; 262: 5592–5595 Kumar AG, Dai XY, Kozak CA, Mims MP, Gotto AM, Ballantyne CM. Murine VCAM-1. Molecular cloning, mapping and analysis of a truncated form. J Immunol 1994; 153: 4088–4098 Neish AS, Williams AJ, Palmer HJ, Whitley MZ, Collins T. Functional analysis of the human vascular cell adhesion molecule 1 promoter. J Exp Med 1992; 176: 1583–1593 Hofmann J. The potential for isoenzyme-selective modulation of protein kinase C. FASEB J 1997; 11: 649–669 Martiny-Baron G, Kazanietz MG, Mischak H et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go¨ 6976. J Biol Chem 1993; 268: 9194–9197 Clement-Chomienne O, Walsh MP. Identification of protein kinase C isoenzymes in smooth muscle: partial purification and characterization of chicken gizzard PKC l. Biochem Cell Biol 1996; 74: 51–65 Jordan NJ, Watson ML, Yoshimura T, Westwick J. Differential.
(7) 2136. 29. 30. 31. 32.. effects of protein kinase C inhibitors on chemokine production in human synovial fibroblasts. Br J Pharmacol 1996; 117: 1245–1253 Xu J, Zutter MM, Santoro SA, Clark RA. PDGF induction of a integrin gene expression is mediated by protein kinase C-f. J 2 Biol 1996; 134: 1301–1311 Cell Palmer-Crocker RL, Pober JS. IL-4 induction of VCAM-1 on endothelial cells involves activation of a protein tyrosine kinase. J Immunol 1995; 154: 2838–2845 Weber C, Negrescu E, Erl W et al. Inhibitors of protein tyrosine kinase suppress TNF-stimulated induction of endothelial cell adhesion molecules. J Immunol 1995; 155: 445–451 May MJ, Wheeler-Jones CP, Pearson JD. Effects of protein tyrosine kinase inhibitors on cytokine-induced adhesion molecule expression by human umbilical vein endothelial cells. Br J Pharmacol 1996; 118: 1761–1771. A. Schawalder et al. 33. Lechleitner S, Gille J, Johnson DR, Petzelbauer P. Interferon enhances tumor necrosis factor-induced vascular cell adhesion molecule-1 (CD106) expression in human endothelial cells by an interferon-related factor 1-dependent pathway. J Exp Med 1998; 187: 2023–2030 34. Wu¨thrich RP. Vascular cell adhesion molecule-1 ( VCAM-1) expression in murine lupus nephritis. Kidney Int 1992; 42: 903–914 35. Jonjic N, Peri G, Bernasconi S et al. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J Exp Med 1992; 176: 1165–1174 36. Deisher TA, Haddix TL, Montgomery KF, Pohlman TH, Kaushansky K, Harlan JM. The role of protein kinase C in the induction of VCAM-1 expression on human umbilical vein endothelial cells. FEBS Lett 1993; 331: 285–290 Received for publication: 4.1.99 Accepted in revised form: 27.4.99.
(8)
Figure


Documents relatifs
However, although p100 is highly expressed in a number of breast cancer cell lines, MHC Class I antigen expression was observed on all the cell lines we analysed and could be
Having a continuous flux of atoms implies using an atomic beam with a parabolic trajectory (latus rectum: 2.576 mm) and interrogating the atoms using a microwave cavity with two
Um die Rolle der Sauerstoffkonzen- tration bei der Entstehung des Gewebe- brandes zu ermitteln, und insbesondere um einen Schwellenwert zu finden, un- ter dem Entflammungen
Lecture : en 2016, 8,5 % des femmes salariées déclarent travailler habituellement trois jours ou moins par semaine dans leur emploi principal.. Champ : femmes salariées ;
Calculez chaque réponse tout au long du trajectoire... Opérations Mixtes (Pâques)
This study proposes Mg maps obtained with X-ray EDS combined with scan- ning transmission electron microscopy (STEM) of micrite crystals from the Mishrif reservoir Formation
CD44 is expressed de novo by tubular epithelial The pathogenesis of the nephritic process in kdkd cells ( TEC ) in areas of tubular injury in kdkd kidneys, mice is
Par le terme de contre-modèle à l’école, Glasman (2004, p.84) entend que les cours particuliers offrent diverses caractéristiques : une « réactivité » au sens où