HAL Id: inserm-01359632
https://www.hal.inserm.fr/inserm-01359632
Submitted on 2 Sep 2016
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
leukemia in first complete remission: a retrospective,
propensity score-weighted analysis from the ALWP of
the EBMT
Francesco Saraceni, Myriam Labopin, Norbert-Claude Gorin, Didier Blaise,
Reza Tabrizi, Liisa Volin, Jan Cornelissen, Jean-Yves Cahn, Patrice
Chevallier, Charles Craddock, et al.
To cite this version:
Francesco Saraceni, Myriam Labopin, Norbert-Claude Gorin, Didier Blaise, Reza Tabrizi, et al..
Matched and mismatched unrelated donor compared to autologous stem cell transplantation for acute
myeloid leukemia in first complete remission: a retrospective, propensity score-weighted analysis from
the ALWP of the EBMT. Journal of Hematology and Oncology, BioMed Central, 2016, 9 (1), pp.79.
�10.1186/s13045-016-0314-x�. �inserm-01359632�
R E S E A R C H
Open Access
Matched and mismatched unrelated donor
compared to autologous stem cell
transplantation for acute myeloid leukemia
in first complete remission: a retrospective,
propensity score-weighted analysis from
the ALWP of the EBMT
Francesco Saraceni
1*, Myriam Labopin
2, Norbert-Claude Gorin
2, Didier Blaise
3, Reza Tabrizi
4, Liisa Volin
5,
Jan Cornelissen
6, Jean-Yves Cahn
7, Patrice Chevallier
8, Charles Craddock
9, Depei Wu
10, Anne Huynh
11,
William Arcese
12, Mohamad Mohty
2, Arnon Nagler
13,14and Acute Leukemia Working Party (ALWP) of the European
society for Blood and Marrow Transplantation (EBMT)
Abstract
Background: Optimal post-remission strategy for patients with acute myeloid leukemia (AML) is matter of intense debate. Recent reports have shown stronger anti-leukemic activity but similar survival for allogeneic stem cell transplantation (allo-HSCT) from matched sibling donor compared to autologous transplantation (auto-HSCT); however, there is scarcity of literature confronting auto-HSCT with allo-HSCT from unrelated donor (UD-HSCT), especially mismatched UD-HSCT.
Methods: We retrospectively compared outcome of allogeneic transplantation from matched (10/10 UD-HSCT) or mismatched at a single HLA-locus unrelated donor (9/10 UD-HSCT) to autologous transplantation in patients with AML in first complete remission (CR1). A total of 2879 patients were included; 1202 patients received auto-HSCT, 1302 10/10 UD-HSCT, and 375 9/10 UD-HSCT. A propensity score-weighted analysis was conducted to control for disease risk imbalances between the groups.
Results: Matched 10/10 UD-HSCT was associated with the best leukemia-free survival (10/10 UD-HSCT vs auto-HSCT: HR 0.7, p = 0.0016). Leukemia-free survival was not statistically different between auto-HSCT and 9/10 UD-HSCT (9/10 UD-HSCT vs auto-HSCT: HR 0.8, p = 0.2). Overall survival was similar across the groups (10/10 UD-HSCT vs auto-HSCT: HR 0.98, p = 0.84; 9/10 UD-HSCT vs auto-HSCT: HR 1.1, p = 0.49). Notably, in intermediate-risk patients, OS was significantly worse for 9/10 UD-HSCT (9/10 UD-HSCT vs auto-HSCT: HR 1.6, p = 0.049), while it did not differ between auto-HSCT and 10/10 UD-HSCT (HR 0.95, p = 0.88). In favorable risk patients, auto-HSCT resulted in 3-year LFS and OS rates of 59 and 78 %, respectively.
(Continued on next page)
* Correspondence:francesco.saraceni@libero.it
1Hematology and Bone Marrow Transplantation, Polytechnic University of
Marche—Ospedali Riuniti Ancona, Via Conca 71, 60126 Ancona, Italy Full list of author information is available at the end of the article
© 2016 The Author(s). Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
(Continued from previous page)
Conclusions: Our findings suggest that in AML patients in CR1 lacking an HLA-matched sibling donor, 10/10 UD-HSCT significantly improves LFS, but this advantage does not translate in better OS compared to auto-HSCT. In intermediate-risk patients lacking a fully HLA-matched donor, auto-HSCT should be considered as a valid option, as better survival appears to be provided by auto-HSCT compared to mismatched UD-HSCT. Finally, auto-HSCT provided an encouraging outcome in patients with favorable risk AML.
Keywords: Acute myeloid leukemia (AML), Allogeneic transplantation, Matched (10/10) and mismatched (9/10) unrelated donor transplantation, Autologous transplantation, Post-remission therapy
Abbreviations: ALWP, Acute leukemia working party; AML, Acute myeloid leukemia; ATT, Average treatment effect among the treated; auto-HSCT, Autologous stem cell transplantation; BM, Bone marrow; CBF, Core-binding factor; CEBPA, CCAAT/enhancer-binding protein alpha; CR1, First complete remission; EBMT, European society for blood and marrow transplantation; ELN, European leukemia net; FLT3-ITD, fms-like tyrosine kinase-internal tandem duplication; GIMEMA, Gruppo Italiano Malattie EMatologiche dell’Adulto; GVHD, Graft-vs-host disease; GVL, Graft-vs-leukemia; LFS, Leukemia-free survival; MAC, Myeloablative; MMUD, Mismatched unrelated donor; MRD, Minimal residual disease; MSD, Matched sibling donor allo-HSCT; MUD, Matched unrelated donor; NCCN, National Comprehensive Cancer Network; NPM1, Nucleophosmin; NRM, Non-relapse mortality; OS, Overall survival; PBSCs, Peripheral blood stem cells; PS, Propensity score; RI, Relapse incidence; RIC, Reduced-intensity; TBI, Total-body irradiation; WBC, White blood cells; wtFLT3, Wild-type FLT3; 10/10 UD-HSCT, Unrelated donor transplantation matched at 10/10 HLA loci; 9/10 UD-HSCT, Unrelated donor transplantation mismatched at a single HLA-locus
Background
Optimal post-remission strategy for patients with acute myeloid leukemia (AML) is a matter of debate. Allo-geneic stem cell transplantation (allo-HSCT) is the most effective treatment to prevent leukemia relapse, and for patients lacking a matched sibling donor (MSD), transplantation from a 10/10 matched unrelated donor (MUD) is the preferred alternative [1]. The indica-tion for allo-HSCT from 9/10 unrelated donor is more controversial, and outcome according to patient and dis-ease characteristics has not been fully established yet [2].
Autologous stem cell transplantation (auto-HSCT) is an alternative approach, which was initially designed to con-solidate remission in AML patients lacking a sibling donor or unfit for allo-HSCT [3]; since then, auto-HSCT passed through alternate fortunes, and its use progressively declined following evolution of allo-HSCT protocols [1, 4, 5]. Nevertheless, auto-HSCT holds several advan-tages including low non-relapse mortality rates, ab-sence of graft-vs-host disease (GVHD) risk, lower incidence of late effects, and better quality of life for survivors compared to allo-HSCT; concerns include high relapse rate, due to the absence of graft-vs-leukemia (GVL) effect and the theoretic possibility of graft contamination by leukemic cells [6].
Recent reports [7–9] comparing allo-HSCT and auto-HSCT evidenced similar survival and concluded that auto-HSCT should still be considered as a valid alterna-tive to allo-HSCT and taken into account within AML post-remission strategies. Therefore, since transplants from unrelated donors (UD) are currently the preferred
option worldwide, and given the lack of a study con-fronting auto-HSCT with mismatched UD-HSCT, we took the advantage of the European society for blood and marrow transplantation (EBMT) data set and retrospectively compared outcome of matched (10/10 UD-HSCT) or mismatched at a single HLA-locus unre-lated donor transplantation (9/10 UD-HSCT) with auto-HSCT in patients with AML in first complete re-mission (CR1).
Methods
Study design and data collection
This is a retrospective multicenter study. Data were pro-vided, and the study design was approved by the acute leukemia working party (ALWP) of the EBMT group registry, in accordance with the EBMT guidelines for retrospective studies. EBMT is a voluntary working group of more than 500 transplant centers which are re-quired to report all consecutive stem cell transplanta-tions and follow up once a year (Additional file 1). Audits are routinely performed to determine the accur-acy of the data. We included in the analysis patients af-fected by AML older than 18 at diagnosis, who received either auto-HSCT, 10/10 UD-HSCT, or 9/10 UD-HSCT in CR1 as first transplant between January 2005 and December 2013. Patients having secondary AML were excluded. Only patients with available cytogenetic data and allelic HLA typing for A, B, C, DRB1, and DQB1 (for UD-HSCT) were included. Good risk was defined as t(8,21), inv(16)/t(16;16), or normal karyotype in the pres-ence of NPM1 mutation without fms-like tyrosine
kinase-internal tandem duplication (FLT3-ITD). Poor risk was defined as−7, abnl (17p) −5/5q−, inv(3q)/t(3;3), t(6;9), t(v;11)(v;q23), MLL rearranged except of t(9;11)(p22;q23), complex karyotype, or normal karyotype in the pres-ence of FLT3-ITD. Intermediate risk was defined as t(9;11)(p22;q23), normal karyotype without NPM1 or FLT3-ITD, or the absence of abnormalities categorized as good or poor risk [10]. One hundred and twenty pa-tients receiving auto-HSCT, 217 10/10 UD-HSCT, and 60 9/10 UD-HSCT had normal karyotype and wild-type FLT3 (wtFLT3) and were analyzed separately as “inter-mediate wtFLT3” group. Nine hundred and forty-two patients (504 auto-HSCT, 333 10/10 UD-HSCT, and 105 9/10 UD-HSCT) had normal karyotype and un-known molecular markers and were therefore assigned to the intermediate-risk group. Patients from 283 trans-plant centers were included; 83 centers reported both auto-HSCT and UD-HSCT. One thousand six hun-dred thirteen patients were transplanted in centers having reported both auto-HSCT (n = 890) and UD-HSCT (n = 723), while 1266 patients in centers having re-ported only auto-HSCT (n = 787) or UD-HSCT.
Endpoint definitions and statistical analysis
Endpoints were relapse incidence (RI), non-relapse mor-tality (NRM), leukemia-free survival (LFS), and overall survival (OS). Cumulative incidences of relapse and NRM were calculated from the date of transplant to the date of relapse or death, respectively, with the other events being the competing risk. LFS was defined as the interval from transplant to either relapse or death. OS was defined as the time between the date of transplant and the date of death.
The main patient characteristics were compared using Mann-Whitney test for quantitative variables, chi-square test, or Fisher’s exact test for categorical variables. We used propensity score (PS) weighting to control for pre-treatment imbalances on observed variables. The follow-ing factors were included in the PS model: age, year of transplant, interval diagnosis transplant, number of induction courses to reach CR1 (1 vs more than 1), and cytogenetic risk. PS estimation was performed using generalized boosted models [11]. As the research ques-tion focused on the effectiveness of 10/10 UD-HSCT or 9/10 UD-HSCT if it were to replace auto-HSCT for pa-tients having the same characteristics of those actually receiving auto-HSCT, we weighted the 10/10 UD-HSCT and 9/10 UD-HSCT groups to match the auto-HSCT group, by estimating the average treatment effect among the treated (ATT), auto-HSCT being the treated group. The ATT weights equal one for auto-HSCT, and it equals the ratio of the PS to one minus the PS in the two UD-HSCT groups. In summary, each patient that underwent UD-HSCT received a weight inversely
proportional to his probability of receiving an auto-graft. Therefore, patients receiving UD-HSCT that showed significantly different characteristics compared to average autografted patients had a low contribution in the comparisons. We checked the balance between the groups looking to ATT-weighted means. Then, we used pairwise ATTs to fit weighted Kaplan-Meier and Cox models separately for auto-HSCT vs 10/10 UD-HSCT and auto-UD-HSCT vs 9/10 UD-UD-HSCT, adjusting for stem cell source (bone marrow or peripheral blood stem cells) and conditioning regimen (total body irradiation-based or not). The same procedure was re-peated for each cytogenetic-risk group. Finally, we looked to the subgroup of patients with intermediate cytogenetics and wild-type FLT3, adding the time interval from CR1 to transplant to the PS model. All the results were checked by performing a subanalysis excluding the fourth percentile for the interval from diagnosis to transplant, obtaining consistent results. All tests were two-sided. The type I error rate was fixed at 0.05 for determination of factors associated with time to event. Analyses were performed using the R statistical software version 3.2.3; PS analysis was performed using the mnps function of the Twang package and weighted analyses using the survey pack-age [12].
Results
Patient characteristics
The total number of patients who received either auto-HSCT or UD-auto-HSCT for AML in CR1 between 2005 and 2013 and reported to the EBMT was 8943 (3161 auto-HSCT and 5782 UD-auto-HSCT). One thousand nine hun-dred and fifty-eight patients were excluded from the analysis due to incomplete data about HLA typing. Ninety-six patients were excluded as received UD-HSCT which was 8/10 HLA-matched or inferior, leading to a total number of 6889 patients available for analysis of outcome (3161 auto-HSCT, 2921 10/10 UD-HSCT, and 807 9/10 UD-HSCT). Finally, 4010 patients were subse-quently excluded due to incomplete data about cytogen-etics, leading to a final number of 2879 patients included in the propensity score model. Among them, 1202 re-ceived auto-HSCT, 1302 10/10 UD-HSCT, and 375 9/10 UD-HSCT, respectively. Median follow-up was 45, 36, and 34 months for auto-HSCT, 10/10 UD-HSCT, and 9/10 UD-HSCT, respectively. Median age at transplant was higher for 10/10 UD-HSCT (51 years) compared to 9/10 HSCT and auto-HSCT (49 years for 9/10 UD-HSCT and auto-UD-HSCT, p = 0.004). Interval from diag-nosis to transplant was longer for UD-HSCT (174 and 177 days for 10/10 UD-HSCT and 9/10 UD-HSCT, re-spectively) compared to auto-HSCT (158 days, p < 10−4). Patients who received UD-HSCT showed more frequently
poor-risk cytogenetics (16, 47, and 49 % for auto-HSCT, 10/ 10 UD-HSCT, and 9/10 UD-HSCT, respectively, p < 10−4) and were more likely to have received a total body irradiation (TBI)-based conditioning (p < 10−4). Median year of trans-plant was more recent for UD-HSCT (2010) compared to auto-HSCT (2008, p < 10−4). Stem cell source was peripheral blood stem cells for 96 % of auto-HSCT, 80 % of 10/10 UD-HSCT, and 85 % of 9/10 UD-HSCT patients (p < 10−4). Among the UD-HSCT cohort, 813 patients received a mye-loablative (MAC) conditioning (619 in the 10/10 UD-HSCT and 194 in the 9/10 UD-HSCT group, respectively), while 857 received a reduced-intensity (RIC) conditioning regimen (677 in the 10/10 UD-HSCT and 180 in the 9/10 UD-HSCT group). The characteristics of the pa-tients are summarized in Table 1.
Since patient and disease characteristics were unevenly distributed among the transplant categories (auto-HSCT, 10/10 UD-HSCT, and 9/10 UD-HSCT), we fit a propen-sity score model generating ATT-weighted means for the three groups. After weighting, group characteristics were similar in terms of all baseline covariates used for PS estimation (Table 2).
Outcome in the overall population
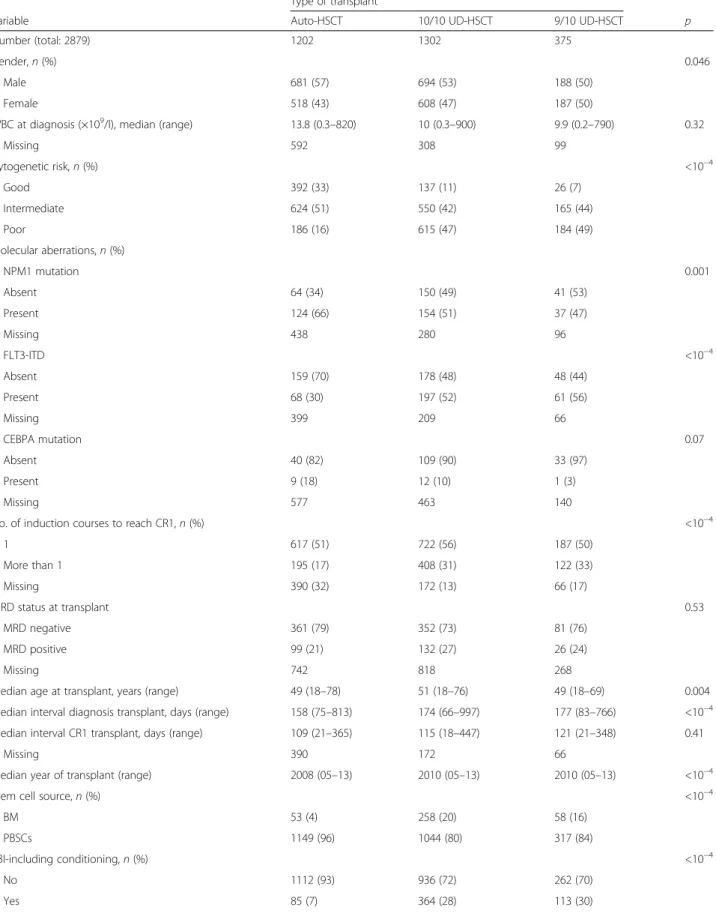
In the global population, the 3-year NRM rate was significantly lower for auto-HSCT compared to 10/10 UD-HSCT and 9/10 UD-HSCT (being 4 ± 2, 13 ± 2, and 21 ± 3 %, respectively; Fig. 1a), as evidenced by PS-weighted Cox analysis (10/10 UD-HSCT vs auto-HSCT: HR 3.1, p < 10−5, 95 % CI 2–4.7; 9/10 UD-HSCT vs auto-HSCT: HR 4.5, p < 10−5, 95 % CI 2.5–8.1, Table 3). The 3-year RI was higher following auto-HSCT (49 ± 3 %) compared to 10/10 UD-HSCT (29 ± 3 %) and 9/10 UD-HSCT (23 ± 3 %), as evidenced by PS-weighted Cox analysis (10/10 UD-HSCT vs auto-HSCT: HR 0.5, p < 10−5, 95 % CI 0.4–0.7; 9/10 UD-HSCT vs auto-HSCT: HR 0.5, p = 0.0016, 95 % CI 0.3–0.8; Fig. 1b).
Fully matched UD-HSCT was associated with the best 3-year LFS (58 ± 3 %), while LFS rates were not statisti-cally different between auto-HSCT and 9/10 UD-HSCT, being 48 ± 3 and 55 ± 3 %, respectively (10/10 vs auto-HSCT: HR 0.7, p = 0.0016, 95 % CI 0.6–0.9; 9/10 vs auto-HSCT: HR 0.8, p = 0.2, 95 % CI 0.5–1.1; Fig. 1c). The 3-year OS was not statistically different across the groups, being 64 ± 3, 63 ± 3, and 58 ± 4 % for auto-HSCT, 10/10 UD-auto-HSCT, and 9/10 UD-auto-HSCT, respect-ively (10/10 vs auto-HSCT: HR 0.98, p = 0.84, 95 % CI 0.8–1.2; 9/10 vs auto-HSCT: HR 1.1, p = 0.49, 95 % CI 0.8–1.7; Fig. 1d).
Outcome by cytogenetic risk
In the favorable risk group, we could only compare out-come of auto-HSCT to 10/10 UD-HSCT, as the number of 9/10 UD-HSCT transplants was too limited.
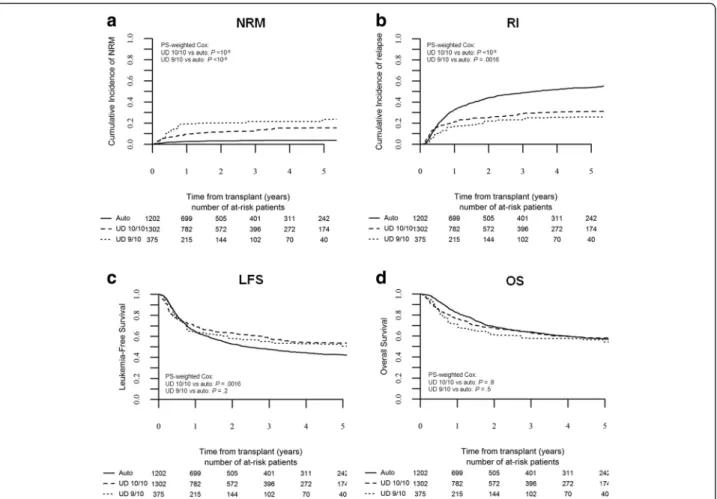
Auto-HSCT was associated with a 3-year RI rate of 36 ± 5 %, while 10/10 UD-HSCT provided a 3-year RI of 19 ± 5 %, which was significantly lower in PS-weighted Cox ana-lysis (10/10 UD-HSCT vs auto-HSCT: HR 0.5, p = 0.018, 95 % CI 0.3–0.9). There was a trend for better 3-year LFS following 10/10 UD-HSCT compared to auto-HSCT, being 72 ± 6 and 59 ± 5 %, respectively (HR 0.7, p = 0.1, 95 % CI 0.4–1.1; Fig. 2a). Overall survival at 3 years was not significantly different, being 78 ± 4 % for auto-HSCT and 77 ± 5 % for 10/10 UD-HSCT (10/10 UD-HSCT vs auto-HSCT: HR 1.1, p = 0.7, 95 % CI 0.6–2; Fig. 2b).
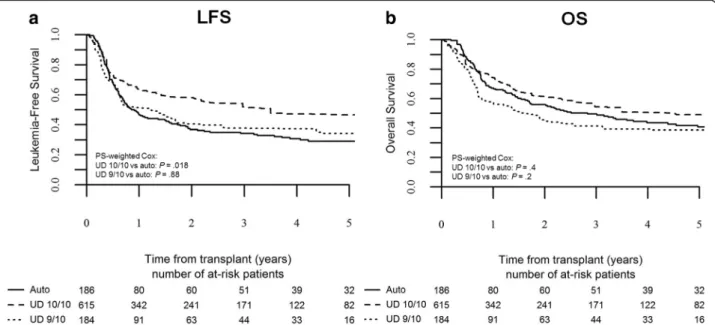
Intermediate-risk AML represented the largest sub-population in our survey and was the cohort in which the characteristics of the three groups showed the great-est overlap. In this subgroup, auto-HSCT was associated with higher relapse incidence (51 ± 4 %) compared to 10/10 UD-HSCT (30 ± 5 %) and 9/10 UD-HSCT (21 ± 4 %), as evidenced by PS-weighted Cox analysis (10/10 UD-HSCT vs auto-HSCT: HR 0.5, p < 10−5, 95 % CI 0.4– 0.7; 9/10 UD-HSCT vs auto-HSCT: HR 0.4, p = 0.004, 95 % CI 0.3–0.8). NRM rates were significantly lower for auto-HSCT compared to 10/10 HSCT and 9/10 UD-HSCT, being 4 ± 2, 16 ± 3, and 34 ± 5 %, respectively (10/10 UD-HSCT vs auto-HSCT: HR 3.6, p < 10−4, 95 % CI 2–6.4; 9/10 UD-HSCT vs auto-HSCT: HR 9.4, p < 10−5,
95 % CI 4.9–18). This translated to an advantage in terms of LFS for 10/10 UD-HSCT (54 ± 4 %) but not for 9/10 UD-HSCT (45 ± 5 %) over auto-HSCT (45 ± 4 %), as evi-denced by PS-weighted Cox analysis (10/10 UD-HSCT vs auto-HSCT: HR 0.7, p = 0.01, 95 % CI 0.6–0.9; 9/10 UD-HSCT vs auto-UD-HSCT: HR 1.1, p = 0.7, 95 % CI 0.7–1.6; Fig. 3a). Notably, 3-year OS did not differ between auto-HSCT (60 ± 4 %) and 10/10 UD-auto-HSCT (60 ± 5 %), while it was significantly lower for 9/10 UD-HSCT (48 ± 4 %), as evidenced by PS-weighted COX analysis (10/10 UD-HSCT vs auto-UD-HSCT: HR 0.98, p = 0.9, 95 % CI 0.7–1.3; 9/10 UD-HSCT vs auto-HSCT: HR 1.6, p = 0.049, 95 % CI 1.001–2.5; Fig. 3b).
Within the intermediate-risk cohort, we further ana-lyzed the outcome of patients bearing wild-type FLT3; in this subpopulation, we could only compare auto-HSCT to 10/10 UD-HSCT, as the number of 9/10 UD-HSCT transplants was too limited to allow for propensity score weighting. RI rate was significantly higher for auto-HSCT compared to 10/10 UD-auto-HSCT, being 55 ± 10 and 31 ± 12 %, respectively (10/10 UD-HSCT vs auto-HSCT: HR 0.5, p = 0.04, 95 % CI 0.3–0.9). Matched UD-HSCT was associated with a trend for better LFS compared to auto-HSCT, being 61 ± 11 and 41 ± 8 %, respectively (10/10 UD-HSCT vs auto-HSCT: HR 0.6, p = 0.10, 95 % CI 0.4–1.1), while no significant difference was observed in terms of OS (66 ± 10 and 60 ± 8 % for 10/10 UD-HSCT and HSCT, respectively; 10/10 UD-HSCT vs auto-HSCT: HR 0.95, p = 0.88, 95 % CI 0.5–1.7).
Table 1 Patient, disease, and transplant characteristics
Type of transplant
Variable Auto-HSCT 10/10 UD-HSCT 9/10 UD-HSCT p Number (total: 2879) 1202 1302 375
Gender, n (%) 0.046
Male 681 (57) 694 (53) 188 (50) Female 518 (43) 608 (47) 187 (50)
WBC at diagnosis (×109/l), median (range) 13.8 (0.3–820) 10 (0.3–900) 9.9 (0.2–790) 0.32
Missing 592 308 99 Cytogenetic risk, n (%) <10−4 Good 392 (33) 137 (11) 26 (7) Intermediate 624 (51) 550 (42) 165 (44) Poor 186 (16) 615 (47) 184 (49) Molecular aberrations, n (%) NPM1 mutation 0.001 Absent 64 (34) 150 (49) 41 (53) Present 124 (66) 154 (51) 37 (47) Missing 438 280 96 FLT3-ITD <10−4 Absent 159 (70) 178 (48) 48 (44) Present 68 (30) 197 (52) 61 (56) Missing 399 209 66 CEBPA mutation 0.07 Absent 40 (82) 109 (90) 33 (97) Present 9 (18) 12 (10) 1 (3) Missing 577 463 140
No. of induction courses to reach CR1, n (%) <10−4
1 617 (51) 722 (56) 187 (50) More than 1 195 (17) 408 (31) 122 (33) Missing 390 (32) 172 (13) 66 (17) MRD status at transplant 0.53 MRD negative 361 (79) 352 (73) 81 (76) MRD positive 99 (21) 132 (27) 26 (24) Missing 742 818 268
Median age at transplant, years (range) 49 (18–78) 51 (18–76) 49 (18–69) 0.004 Median interval diagnosis transplant, days (range) 158 (75–813) 174 (66–997) 177 (83–766) <10−4 Median interval CR1 transplant, days (range) 109 (21–365) 115 (18–447) 121 (21–348) 0.41
Missing 390 172 66
Median year of transplant (range) 2008 (05–13) 2010 (05–13) 2010 (05–13) <10−4
Stem cell source, n (%) <10−4
BM 53 (4) 258 (20) 58 (16)
PBSCs 1149 (96) 1044 (80) 317 (84)
TBI-including conditioning, n (%) <10−4 No 1112 (93) 936 (72) 262 (70)
In the poor-risk group, RI rate was once again signifi-cantly higher for auto-HSCT compared to 10/10 UD-HSCT and 9/10 UD-UD-HSCT, being 64 ± 8, 34 ± 9, and 40 ± 9 %, respectively (10/10 UD-HSCT vs auto-HSCT: HR 0.5, p = 0.0003, 95 % CI 0.3–0.7; 9/10 UD-HSCT vs
auto-HSCT: HR 0.7, p = 0.08, 95 % CI 0.4–1.1). Fully matched UD-HSCT was associated with better LFS compared to auto-HSCT, being 52 ± 8 and 34 ± 6 %, respectively (10/10 UD-HSCT vs auto-HSCT: HR 0.7, p = 0.018, 95 % CI 0.5–0.9), while LFS was not statistically
Table 1 Patient, disease, and transplant characteristics (Continued)
Conditioning intensity, n (%)
MAC – 619 (48) 194 (52)
RIC – 677 (52) 180 (48)
Median follow-up, months (range) 45 (1–128) 36 (1–119) 25 (1–113)
Legend: BM bone marrow, CEBPA CCAAT/enhancer-binding protein alpha, CR1 first complete remission, FLT3-ITD fms-like tyrosine kinase-internal tandem duplication, MAC myeloablative, MRD minimal residual disease, NPM1 nucleophosmin, PBSCs peripheral blood stem cells, RIC reduced-intensity, TBI total-body irradiation, WBC white blood cells
Table 2 ATT-weighted means for transplant groups
Weighted means p
Variable Auto-HSCT 10/10 UD-HSCT 9/10 UD-HSCT 10/10 UD-HSCT vs auto-HSCT
9/10 UD-HSCT vs auto-HSCT Global population
Median age at transplant, years 47 46 47 0.84 1.00 Median year of transplant 2008 2008 2008 0.66 0.88 Median interval diagnosis transplant (days) 178 179 179 0.80 0.49 Good-risk cytogenetics (%) 33 31 30 1.00 1.00 Poor-risk cytogenetics (%) 15 17 19 1.00 1.00 More than 1 induction to achieve CR1 (%) 16 18 17 0.7 0.91 By cytogenetic risk
Good risk
Median age at transplant, years 44 44 n.a.a 0.96 n.a.
Median year of transplant 2009 2009 n.a. 0.56 n.a. Median interval diagnosis transplant (days) 186 188 n.a. 1.00 n.a. More than 1 induction to achieve CR1 (%) 0.01 0.09 n.a. 0.84 n.a. Intermediate risk
Patient age (years) 48 48 49 0.96 0.39 Year of transplant 2008 2008 2008 0.36 0.83 Interval diagnosis transplant (days) 174 181 183 0.51 0.90 More than 1 induction to achieve CR1 (%) 19 22 17 0.36 0.91 Intermediate-risk wtFLT3
Patient age (years) 46 48 n.a. 0.75 n.a. Year of transplant 2008 2009 n.a. 0.46 n.a. Interval diagnosis transplant (days) 118 115 n.a. 0.93 n.a. More than 1 induction to achieve CR1 (%) 17 21 n.a. 0.81 n.a. Poor risk
Patient age (years) 50 50 50 1.00 0.93 Year of transplant 2008 2008 2009 0.87 0.11 Interval diagnosis transplant (days) 172 170 173 1.00 0.91 More than 1 induction to achieve CR1 (%) 24 25 27 0.81 0.77
Legend: ATT average treatment effect among the treated, CR1 first complete remission, wtFLT3 wild-type FLT3
a
In good risk and intermediate wtFLT3 categories, only auto-HSCT and 10/10 UD-HSCT were analyzed, as the number of 9/10 UD-HSCT transplants resulted too limited
different between auto-HSCT and 9/10 UD-HSCT, being 34 ± 6 and 38 ± 8 % (9/10 UD-HSCT vs auto-HSCT: HR 1, p= 0.88, 95 % CI 0.7–1.5; Fig. 4a). Overall survival was not statistically different across transplant groups, being 50 ± 7, 54 ± 8, and 41 ± 8 % for auto-HSCT, 10/10 HSCT, and 9/10 HSCT, respectively (10/10 UD-HSCT vs auto-UD-HSCT: HR 0.9, p = 0.4, 95 % CI 0.6–1.2; 9/10 UD-HSCT vs auto-HSCT: HR 1.3, p = 0.2, 95 % CI 0.9–1.9; Fig. 4b).
Outcome in the global registry population (6889 patients), unadjusted
As previously stated, from the starting 6889 patients receiving auto-HSCT, 10/10 HSCT, or 9/10 UD-HSCT reported to the EBMT, 4010 patients were ex-cluded due to incomplete cytogenetic data. We herein report the unadjusted results of outcome of all AML patients receiving auto-HSCT, 10/10 UD-HSCT, or 9/10 UD-HSCT in CR1 between 2005 and 2013 included in the
EBMT registry: the 3-year LFS was 47 ± 2 % for auto-HSCT, 54 ± 2 % for 10/10 UD-auto-HSCT, and 47 ± 4 % for 9/10 UD-HSCT, while the 3-year OS was 59 ± 2, 58 ± 2, and 50 ± 4 %, respectively.
Outcome after the second transplant
Three hundred patients (25 % of the auto-HSCT group) received a subsequent allo-HSCT for leukemic relapse after auto-HSCT. Cytogenetic risk was good in 26 %, intermediate in 53 %, and poor in 21 % of the patients. With a median follow-up of 3.5 years after the second allograft, 2-year OS was 50 ± 6 %. OS was significantly affected by cytogenetic risk, being 61 ± 6 % in good risk, 45 ± 4 % in intermediate risk, and 49 ± 6 % in poor-risk patients (p = 0.019).
Conversely, 107 patients (7 % of the UD-HSCT group) underwent a second allo-HSCT for disease relapse post-first UD-HSCT transplant (79 in the 10/10 UD-HSCT group and 28 in the 9/10 UD-HSCT group). In this popu-lation, 2-year OS after second transplant was 25 ± 10 %.
Fig. 1 Outcome by type of transplant in the global population. The cumulative incidence of non-relapse mortality (a) and relapse (b) by transplant type; the probability of leukemia-free survival (c) and overall survival (d) in the global population. Kaplan-Meier curves and Cox analysis are weighted for propensity score; Cox analysis is further adjusted for kind of conditioning and stem cell source
Acute and chronic graft-vs-host disease
Incidence of grade II–IV acute graft-vs-host disease (aGVHD) in patients receiving UD-HSCT was 27 ± 2 % in 10/10 UD-HSCT and 31 ± 4 % in 9/10 UD-HSCT, with no significant difference between the two groups (p = 0.1). Cumulative incidence of chronic graft-vs-host
disease (cGVHD) at 2 years was 42 % in 10/10 UD-HSCT and 40 % in 9/10 UD-UD-HSCT with no significant difference (p = 0.7). Incidence of severe (grade 3) cGVHD was also not different between the two cohorts, being 20 ± 2 and 17 ± 4 % in 10/10 UD-HSCT and 9/10 UD-HSCT, respectively (p = 0.16).
Table 3 PS-weighted Cox analysis of transplant outcomes, adjusted for kind of conditioning and stem cell source
Type of transplant NRM RI LFS OS HR 95 % CI p HR 95 % CI p HR 95 % CI p HR 95 % CI p Global population Auto-HSCT (reference) 1 1 1 1 10/10 UD-HSCT 3.1 2–4.7 <10−5 0.5 0.4–0.7 <10−5 0.7 0.6–0.9 0.0016 0.97 0.8–1.2 0.84 9/10 UD-HSCT 4.5 2.5–8.1 <10−5 0.5 0.3–0.8 0.0016 0.8 0.6–1.1 0.227 1.1 0.8–1.7 0.49 By cytogenetic risk Good risk Auto-HSCT (reference) 1 1 1 1 10/10 UD-HSCT 1.9 0.7–5.5 0.24 0.5 0.3–0.9 0.018 0.7 0.4–1.1 0.1 1.1 0.6–2 0.7 Intermediate risk Auto-HSCT (reference) 1 1 10/10 UD-HSCT 3.6 2–6.4 <10−4 0.5 0.4–0.7 <10−5 0.7 0.6–0.9 0.01 0.98 0.7–1.3 0.9 9/10 UD-HSCT 9.4 4.9–18 <10−5 0.4 0.3–0.8 0.004 1.1 0.7–1.6 0.7 1.6 1.001–2.5 0.049 Intermediate wtFLT3 Auto-HSCT (reference) 1 1 1 1 10/10 UD-HSCT 2.8 0.8–9.8 0.11 0.5 0.29–0.98 0.04 0.6 0.4–1.1 0.10 0.95 0.53–1.7 0.88 Poor risk Auto-HSCT (reference) 1 1 1 1 10/10 UD-HSCT 6.3 2.3–17.4 0.0004 0.5 0.3–0.7 0.0003 0.7 2.3–17.4 0.0004 0.9 0.6–1.2 0.4 9/10 UD-HSCT 11.7 4–34.7 <10−5 0.7 0.4–1 0.08 1.03 0.7–1.5 0.88 1.3 0.9–1.9 0.2 Legend: wtFLT3 wild-type FLT3
Fig. 2 Leukemia-free survival and overall survival by type of transplant in good-risk patients. The probability of leukemia-free survival (a) and overall survival (b) in good-risk patients
Discussion
AML post-remission strategy remains largely debated. Different approaches are available, and recommenda-tions are quickly mutating owing to continuous refining of risk stratification [13, 14], improvements in transplant preparatory regimens and GVHD prophylaxis [5, 15], and widening of the donor pool [16]. Therefore, when counseling a patient with AML in CR1, it is often diffi-cult to make a straightforward statement.
Several randomized trials have shown significantly bet-ter LFS for auto-HSCT compared to chemotherapy as consolidation of remission in AML [17–20]. In the only prospective study conducted in the last decade, Vellenga et al. observed a reduced relapse rate following auto-HSCT when compared to chemotherapy [19]; the same group recently reported better survival following auto-HSCT in intermediate-risk AML [8]. Of note, in a recent report of a large randomized trial, Stone and colleagues
Fig. 3 Leukemia-free survival and overall survival by type of transplant in intermediate-risk patients. The probability of leukemia-free survival (a) and overall survival (b) in intermediate-risk patients
Fig. 4 Leukemia-free survival and overall survival by type of transplant in poor-risk patients. The probability of leukemia-free survival (a) and overall survival (b) in poor-risk patients
[21] showed a significant survival benefit with the addition of the multi-target kinase inhibitor midostaurin to standard chemotherapy for AML patients bearing FLT3-ITD or TKD aberrations, an important finding that hopefully will pave its way into daily clinical practice.
Globally, donor vs no donor studies [22] and meta-analyses [23] evidenced a survival benefit for allo-HSCT over auto-HSCT in intermediate and poor cytogenetic-risk groups, but not in good-cytogenetic-risk AML, in which the high NRM rate offsets the advantage of stronger anti-leukemic activity carried by allo-HSCT [24]. Neverthe-less, donor vs no donor analyses suffered from biologic randomization bias; further, most studies combined patients receiving auto-HSCT and conventional chemo-therapy in the no donor arm and included mostly young patients that received grafts from MSD, which accounts for a minority of transplants performed today. Further-more, in some recent observations, auto-HSCT has been shown to provide similar survival to allo-HSCT from both sibling and unrelated donors [7–9]. None-theless, there is scarcity of literature confronting auto-HSCT to UD-auto-HSCT, especially mismatched unrelated donor (MMUD).
We took therefore advantage of the EBMT-ALWP registry and analyzed a large homogeneous cohort of pa-tients with AML in CR1. To mitigate the impact of the intrinsic limitations of a registry-based survey, such transplant-selection bias and disease risk imbalances be-tween the groups, we performed a propensity score ad-justed analysis, weighting transplant groups for the most significant patient characteristics, and further adjusting for kind of conditioning and stem cell source. Within this model, patients who received a UD-HSCT having significantly different characteristics compared to auto-grafted patients had a very low impact on estimation of the outcome. In addition, we analyzed separately pa-tients with good-, intermediate-, and poor-risk AML, to further elude the bias of cytogenetic risk unbalance. To better interpret the results obtained with PS-weighting analysis, it is worth noting that this model produces outcome results (i.e., LFS and OS) which, if compared to the crude (unadjusted) LFS and OS, are consistent for auto-HSCT, while better for UD-HSCT. This is a consequence of the rationale of the method itself which selects, among the UD-HSCT population, the patients which present similar characteristics to auto-HSCT patients.
Our data suggest that fully matched UD-HSCT pro-vides better leukemia control but similar survival com-pared to auto-HSCT in AML in CR1. Further, mismatched UD-HSCT appears to be associated with inferior survival compared to auto-HSCT in patients bearing intermediate-risk cytogenetics.
The widespread availability of high-resolution HLA typing has greatly improved outcome of UD transplants, and results of allo-HSCT from fully HLA-matched UD are today overlapping with MSD outcome [25, 26]. How-ever, MMUD transplants are associated with increased morbidity and mortality; in fact, higher incidence of both acute [27] and chronic [28, 29] GVHD rates have been described following mismatched transplants. In addition, NRM risk tends to increase proportionally to the number of HLA disparities [30–33], although im-proved outcome of MMUD transplants has recently been reported following RIC regimens [4]. Finally, recent developments in haploidentical transplantation are be-ginning to bring into question the choice of a mis-matched unrelated over a haploidentical donor, when available [5].
Auto-HSCT results, on the other hand, have pro-gressively improved. Switch of stem cell source from BM to peripheral blood stem cells (PBSCs) and refine-ments in preparatory regimens [15] have led to faster hematopoietic recovery, reduced mortality and satis-factory outcome; in a recent observation, Gorin et al. [34] reported a 2-year LFS of 61 % following auto-HSCT prepared with a busulfan-melphalan conditioning.
In a previous EBMT survey conducted on a cohort of patients affected by MDS or secondary AML, Al-Ali et al. [35] observed similar 3-year LFS and OS for 8/8 UD-HSCT and auto-UD-HSCT; a landmark analysis revealed bet-ter outcome with MUD-HSCT only for patients surviv-ing beyond 6 months since transplant. A more recent study by Cho et al. [36] analyzed a small population of young intermediate-risk AML patients undergoing either MSD, 8/8 UD-HSCT, or auto-HSCT; the authors re-ported an advantage in terms of LFS for 8/8 UD-HSCT over auto-HSCT, with no significant difference in OS. Similarly, in a very recent observation by Mizutani et al. [37], MUD-HSCT provided lower RI but no survival ad-vantage over auto-HSCT in patients with intermediate-risk AML in CR1.
In the current study, we observed a significantly lower NRM and higher RI for auto-HSCT compared to UD-HSCT. In the global population, auto-HSCT pro-vided an acceptable 3-year LFS rate of 48 %, which was significantly lower compared to 10/10 UD-HSCT, but not statistically different from 9/10 UD-HSCT. None-theless, the better leukemia control provided by fully matched UD-HSCT did not translate in a survival benefit, as OS at 3 years was similar for auto-HSCT and 10/10 HSCT, while slightly lower for 9/10 UD-HSCT, this difference being not statistically significant.
In the subgroup analysis stratified by cytogenetic risk, auto-HSCT provided a particularly good outcome in patients with favorable risk AML, being 3-year LFS and OS rates 59 and 78 %, respectively; those results are
consistent with previous reports [38]. There is evidence indicating that auto-HSCT is able to significantly re-duce relapse risk in AML with favorable cytogenetics, which still carry disease recurrence rates up to 35–40 % following conventional chemotherapy, with a particular risk for core-binding factor (CBF) AML with adverse prognostic features [39] or positive MRD after consolida-tion chemotherapy [40]. Further, there is data suggest-ing that in NPM1-mutated and CEBPA double-mutated (CEBPAdm) AML, the high chemosensitivity of the dis-ease might be exploited with auto-HSCT intensification [41, 42]. Awaiting for MRD-driven prospective trials comparing high-dose cytarabine and auto-HSCT in this setting, these findings support auto-HSCT as a valid strategy for consolidation of remission in patients with good-risk cytogenetics.
Intermediate risk represents the gray zone of AML guidelines. The role of allo-HSCT in these patients is not as clear as in poor-risk category [23], and it is be-coming even more controversial with the incorporating of MRD data in clinical algorithms. In 2014, auto-HSCT was removed from NCCN recommendations in intermediate-risk AML, and today, most physicians would perform allo-HSCT in this setting, supported by a clear advantage in terms of LFS over auto-HSCT and conventional chemotherapy [23, 24]. However several studies, including recent analyses [7–9], failed to ob-serve a survival advantage of allo-HSCT over auto-HSCT in intermediate-risk AML.
In our study, intermediate risk represented the largest subgroup, accounting for approximately half of all patients included in the analysis. Moreover, it was the cohort in which the characteristics of the three groups showed the greatest overlap and was therefore the main focus of our analysis. Forty-five percent of intermediate-risk patients who received auto-HSCT were alive and leukemia-free at 3 years after transplant. Further, in this subpopulation, matched UD-HSCT provided the best LFS, while no significant difference could be observed between auto-HSCT and 10/10 UD-HSCT in terms of OS. Similarly, in a subgroup analysis of patients bearing intermediate cytogenetics and wtFLT3, 10/10 UD-HSCT showed a trend for better LFS without a survival advan-tage over auto-HSCT. Notably, in the PS-weighted analysis conducted on the whole group of intermediate-risk pa-tients, auto-HSCT provided better OS compared to 9/10 UD-HSCT. In a recent report by Cornelissen et al. [8], allo-HSCT was associated with better LFS compared to auto-HSCT, but similar OS was observed in intermediate-risk patients. In that study, only MSD or 8/8 UD-HSCT were allowed in intermediate-risk group; therefore, our observa-tion of a survival advantage of auto-HSCT over MMUD in intermediate-risk AML can be interpreted as not in contra-diction with previous data.
However, the good survival following auto-HSCT should be analyzed more carefully. Indeed while, as ex-pected, OS rates of UD-HSCT were approximately 5 % higher than the respective LFS rates, in intermediate-risk patients receiving auto-HSCT, 3-year LFS was 45 %, while OS was as high as 60 %. This striking difference can be mostly explained as a consequence of successful salvage treatment for many patients relapsed after auto-HSCT. In fact, a considerably great proportion of patients who experienced disease recurrence following auto-HSCT were effectively rescued and received a subsequent allo-HSCT, which provided a 2-year OS of approximately 50 %.
Nevertheless, relapse incidence following auto-HSCT is disturbingly high and remains the biggest concern about this approach. We observed a 3-year cumulative RI of 51 % in intermediate-risk patients receiving an autograft. Most patients experienced disease recurrence within 2 years, but late relapses were noticed, as previ-ously reported [15, 43]. The dynamic risk stratification allowed by MRD assessment is becoming crucial in AML post-remission setting [44, 45] and might help to identify the best candidates for auto-HSCT; in fact, auto-HSCT has been already proven able to provide long-term remission in MRD-negative APL [46] and ALL [47]. In the AML setting, this concept is currently under investigation in a prospective-MRD driven clinical trial by the Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA-AML1310) which results are awaiting.
Finally, quality of life of transplant survivors should be taken into account, since leukemia cure does not always coincide with full health. Different studies highlighted the high incidence of late effects after allo-HSCT, mostly but not only related to chronic GVHD [48]. In our sur-vey, almost 40 % of UD transplant survivors experienced cGVHD, which was severe in approximately half of them. These data should be taken into consideration when comparing survival of auto-HSCT and UD-HSCT [49].
The current analysis has several limitations. First, as may occur in any multicenter registry study, the three transplant groups were unevenly balanced in terms of patient characteristics, and the retrospective design did not allow to study the reason for choosing UD-HSCT or auto-HSCT, which may vary according to physician and center strategy. We try to address those limitations fit-ting a PS-weighfit-ting model in order to control for the most significant pre-transplant covariates and further stratifying the analysis by cytogenetic risk. Additional limitations that are the consequence of being a registry-based study are missing data about molecular aberra-tions (i.e., NPM1, FLT3-ITD, and CEBPA status) and MRD status for part of the patients. However, it should
be noted that NPM1 and FLT3 status was available in approximately one third of the patients with normal karyotype, which enabled us to perform an acceptable even if not optimal risk stratification.
Conclusions
In conclusion, given the limitations of the study, in AML patients in CR1 lacking a MSD, 10/10 UD-HSCT signifi-cantly improves LFS, but this advantage does not translate in better OS compared to auto-HSCT. In intermediate-risk population, autologous transplant should be consid-ered as a valid option, especially for patients lacking a fully HLA-matched donor, as better survival appears to be provided by auto-HSCT compared to mismatched UD transplant. Further, autologous transplant provided an encouraging outcome in favorable risk AML. These data may suggest that the current strategy for manage-ment of AML in CR1 should incorporate auto-HSCT in patients with good- and intermediate-risk cytogenetics, especially for those lacking a fully HLA-matched donor. Obviously, this strategy should be examined in well-designed multicenter randomized studies incorporating MRD status and center experience as well as novel ap-proaches for post-transplantation maintenance as mid-ostaurin or other novel compounds.
Additional file
Additional file 1: Listing all EBMT members. (DOCX 17 kb) Acknowledgements
The authors would like to thank all EBMT centers for contributing patients to the study and data managers for their great work. A complete list of the members of the European Blood and Marrow Transplantation Group appears in the Additional file 1.
Funding Not applicable.
Availability of data and materials The study relies on the EBMT dataset. Authors’ contributions
FS, AN, ML, and N-CG designed the study, the synopsis of which was approved by the acute leukemia working party of the EBMT; ML performed all the statistical analysis; FS wrote the first draft of the manuscript; all co-authors contributed the data to the EBMT registry, read the manuscript, and approved the final version.
Competing interests
The authors declare that they have no competing interests. Consent for publication
Not applicable.
Ethics approval and consent to participate
Since 1990, patients provide informed consent authorizing the use of their personal information for research purposes. Data were provided, and the study design was approved by the acute leukemia working party (ALWP) of the EBMT group registry, in accordance with the EBMT guidelines for retrospective studies.
Author details
1Hematology and Bone Marrow Transplantation, Polytechnic University of
Marche—Ospedali Riuniti Ancona, Via Conca 71, 60126 Ancona, Italy.
2
ALWP-EBMT and Department of Hematology and Cell Therapy, Saint Antoine Hospital, Paris, France.3Programme de Transplantation et Therapie
Cellulaire—Institut Paoli Calmettes, Marseille, France.4CHU Bordeaux, Hôpital
Haut-Leveque, Pessac, France.5HUH, Comprehensive Cancer Center, Stem
Cell Transplantation Unit, Helsinki, Finland.6Erasmus MC-Daniel den Hoed Cancer Centre, Rotterdam, The Netherlands.7Clinique Universitaire
d’Hématologie CHU Grenoble, Grenoble, France.8Department
D’Hématologie, CHU Nantes, Nantes, France.9Centre for Clinical
Haematology, Queen Elizabeth Hospital, Birmingham, UK.10Department of Hematology, First Affiliated Hospital of Soochow University, Suzhou, China.
11CHU Department Hématologie, Hôpital de Purpan, Toulouse, France. 12Rome Transplant Network, Stem Cell Transplant Unit, Tor Vergata University
of Rome, Rome, Italy.13Chaim Sheba Medical Center, Tel-Hashomer, Israel.
14ALWP-EBMT Office, Saint Antoine Hospital, Paris, France.
Received: 18 June 2016 Accepted: 26 August 2016
References
1. Schlenk RF, Döhner K, Mack S, Stoppel M, Király F, Götze K, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol. 2010;28(30):4642–8.
2. Verneris MR, Lee SJ, Ahn KW, Wang HL, Battiwalla M, Inamoto Y, et al. HLA mismatch is associated with worse outcomes after unrelated donor reduced-intensity conditioning hematopoietic cell transplantation: an analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2015;21(10):1783–9.
3. Gorin NC, Najman A, Duhamel G. Autologous bone-marrow transplantation in acute myelocytic leukaemia. Lancet. 1977;1(8020):1050.
4. Savani BN, Labopin M, Kröger N, Finke J, Ehninger G, Niederwieser D, et al. Expanding transplant options to patients over 50 years—improved outcome after reduced intensity conditioning mismatched-unrelated donor transplantation for patients with acute myeloid leukemia: a report from the Acute Leukemia Working Party of the EBMT. Haematologica. 2016;101(6):773–80. 5. Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, et al.
Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–505.
6. Gorin NC, Giebel S, Labopin M, Savani BN, Mohty M, Nagler A. Autologous stem cell transplantation for adult acute leukemia in 2015: time to rethink? Present status and future prospects. Bone Marrow Transplant. 2015;50(12): 1495–502.
7. Keating A, DaSilva G, Perez WS, Gupta V, Cutler CS, Ballen KK, et al. Autologous blood cell transplantation versus HLA-identical sibling transplantation for acute myeloid leukemia in first complete remission: a registry study from the Center for International Blood and Marrow Transplantation Research. Haematologica. 2013;98(2):185–92.
8. Cornelissen JJ, Versluis J, Passweg JR, van Putten WLJ, Manz MG, Maertens MG, et al. Comparative therapeutic value of post-remission approaches in patients with acute myeloid leukemia aged 40–60 years. Leukemia. 2015; 29(5):1041–50.
9. Mizutani M, Hara M, Fujita H, Aoki J, Kanamori H, Ohashi K, et al. Comparable outcomes between autologous and allogeneic transplant for adult acute myeloid leukemia in first CR. Bone Marrow Transplant. 2016; 51(5):645–53.
10. Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. 11. McCaffrey D, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF.
A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388–414. 12. Ridgeway G, McCaffrey D, Griffin BA, Burgette L. Twang: toolkit for
weighting and analysis of non-equivalent groups. Available online at http://cran.rproject.org/web/packages/twang/vignettes/twang.pdf.
13. Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhäuser M, Juliusson G, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9(10):579–90.
14. Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29(9):1190–7. 15. Nagler A, Labopin M, Gorin NC, Ferrara F, Sanz MA, Wu D, et al. Intravenous
busulfan for autologous stem cell transplantation in adult patients with acute myeloid leukemia: a survey of 952 patients on behalf of the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2014;99(8):1380–6.
16. Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, Acute Leukemia Working Party (ALWP) of European Blood and Marrow Transplant (EBMT) Group, et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 2008;112(9):3574–81.
17. Burnett AK, Goldstone AH, Stevens RM, Stevens RM, Hann IM, Rees JK, et al. Randomised comparison of addition of autologous bone-marrow transplantation in intensive chemotherapy for acute myeloid leukaemia in first remission: results of MRC AML 10 Trial. Lancet. 1998;351(9104):700–8. 18. Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta
E, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339(23):1649–56.
19. Vellenga E, van Putten W, Ossenkoppele GJ, Verdonck LF, Theobald M, Cornelissen JJ, et al. Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood. 2011;118(23):6037–42.
20. Zittoun RA, Mandelli F, Willemze R, de Witte T, Labar B, Resegotti L, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995;332(4):217–23. 21. Stone RM, Mandrekar S, Sanford BL, Geyer S, Bloomfield CD, Dohner K, et al.
The multi-kinase inhibitor midostaurin (M) prolongs survival compared with placebo (P) in combination with daunorubicin (D)/cytarabine (C) induction (ind), high-dose C consolidation (consol), and as maintenance (maint) therapy in newly diagnosed acute myeloid leukemia (AML) patients (pts) age 18-60 with FLT3 mutations (muts): an international prospective randomized (rand) P-controlled double-blind trial (CALGB 10603/RATIFY [Alliance]). Blood. 2015;126:6. Abstract 6.
22. Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109(9):3658–66.
23. Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–61.
24. Suciu S, Mandelli F, de Witte T, Zittoun R, Gallo E, Labar B, et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/ GIMEMA AML-10 trial. Blood. 2003;102(4):1232–40.
25. Schetelig J, Bornhäuser M, Schmid C, Hertenstein B, Schwerdtfeger R, Martin H, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol. 2008;26(32):5183–91.
26. Gupta V, Tallman MS, He W, Logan BR, Copelan E, Gale RP, et al. Comparable survival after HLA-well-matched unrelated or matched sibling donor transplantation for acute myeloid leukemia in first remission with unfavorable cytogenetics at diagnosis. Blood. 2010;116(11):1839–48. 27. Woolfrey A, Klein JP, Haagenson M, Spellman S, Petersdorf E, Oudshoorn M,
et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):885–92.
28. Michallet M, Sobh M, Serrier C, Morisset S, Labussière H, Ducastelle S, et al. Allogeneic hematopoietic stem cell transplant for hematological malignancies from mismatched 9/10 human leukocyte antigen unrelated donors: comparison with transplants from 10/10 unrelated donors and human leukocyte antigen identical siblings. Leuk Lymphoma. 2015;56(4): 999–1003.
29. Greinix HT, Faé I, Schneider B, Rosenmayr A, Mitterschiffthaler A, Pelzmann B, et al. Impact of HLA class I high-resolution mismatches on chronic graft versus-host disease and survival of patients given hematopoietic stem cell grafts from unrelated donors. Bone Marrow Transplant. 2005;35(1):57–62. 30. Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al.
High resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–83. 31. Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A,
Horowitz M, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–30.
32. Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010; 11(7):653–60.
33. Saber W, Opie S, Rizzo JD, Zhang MJ, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012; 119(17):3908–16.
34. Gorin NC, Labopin M, Czerw T, Leibundgut K, Blaise D, et al. Autologous stem cell transplantation for adult acute myelocytic leukemia in first remission: better outcome following busulfan and melphalan compared to busulfan and cyclophosphamide: a retrospective study from the acute leukemia working party of the EBMT. Blood. 2015;126:926. Abstract 926. 35. Al-Ali HK, Brand R, van Biezen A, Finke J, Boogaerts M, Fauser AA, et al. A
retrospective comparison of autologous and unrelated donor hematopoietic cell transplantation in myelodysplastic syndrome and secondary acute myeloid leukemia: a report on behalf of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Leukemia. 2007;21(9):1945–51.
36. Cho BS, Kim JH, Yoon JH, Shin SH, Yahng SA, Lee SE, et al. Superior
transplantation outcomes of 8/8-matched unrelated donors as well as matched siblings to autologous transplantation for acute myeloid leukemia with intermediate cytogenetics in first remission. Eur J Haematol. 2013;90(5):365–74. 37. Mizutani M, Takami A, Hara M, Mizuno S, Yanada M, Ohashi K, et al. A
comparison of the outcomes of autologous and unrelated-donor transplantation in adult intermediate-risk acute myeloid leukemia patients in first complete remission. Biol Blood Marrow Transplant. 2016;22(3):S30–1. Abstract 15.
38. Gorin NC, Labopin M, Frassoni F, Milpied N, Attal M, Blaise D, et al. Identical outcome after autologous or allogeneic genoidentical hematopoietic stem cell transplantation in first remission of acute myelocytic leukemia carrying inversion 16 or t(8;21): a retrospective study from the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:3183–8. 39. Fernandez HF, Sun Z, Litzow MR, Luger SM, Paietta EM, Racevskis J, et al.
Autologous transplantation gives encouraging results for young adults with favorable-risk acute myeloid leukemia, but is not improved with
gemtuzumab ozogamicin. Blood. 2011;117(20):5306–13.
40. Mosna F, Papayannidis C, Martinelli G, Di Bona E, Bonalumi A, Tecchio C, et al. Complex karyotype, older age, and reduced first-line dose intensity determine poor survival in core binding factor acute myeloid leukemia patients with long-term follow-up. Am J Hematol. 2015;90(6):515–23. 41. Gorin NC, Labopin M, Meloni G, Pigneux A, Esteve J, Mohamad M. Impact of
FLT3 ITD/NPM1 mutation status in adult patients with acute myelocytic leukemia autografted in first remission [letter]. Haematologica. 2013;98(2):12–4. 42. Schlenk RF, Taskesen E, van Norden Y, Krauter J, Ganser A, Bullinger L, et al.
The value of allogeneic and autologous hematopoietic stem cell transplantation in prognostically favorable acute myeloid leukemia with double mutant CEBPA. Blood. 2013;122(9):1576–82.
43. Czerw T, Labopin M, Gorin NC, Giebel S, Blaise D, Meloni G, et al. Long-term follow-up of patients with acute myeloid leukemia surviving for at least 2 years after autologous stem cell transplantation: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer. 2016;122(12):1880–7.
44. Terwijn M, van Putten WL, Kelder A, van der Velden VH, Brooimans RA, Pabst T, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42 A study. J Clin Oncol. 2013;31(31):3889–97.
45. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62–70.
46. Holter Chakrabarty JL, Rubinger M, Le-Rademacher J, Wang HL, Grigg A, Selby GB, et al. Autologous is superior to allogeneic hematopoietic cell transplantation for acute promyelocytic leukemia in second complete remission. Biol Blood Marrow Transplant. 2014;20(7):1021–5. 47. Wetzler M, Watson D, Stock W, Koval G, Mulkey FA, Hoke EE, et al.
Autologous transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia achieves outcomes similar to allogeneic transplantation: results of CALGB Study 10001 (Alliance). Haematologica. 2014;99(1):111–5.
48. Savani BN, Griffith ML, Jagasia S, Lee SJ. How I treat late effects in adults after allogeneic stem cell transplantation. Blood. 2011;117(11):3002–9. 49. Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al.
Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333–8.
• We accept pre-submission inquiries
• Our selector tool helps you to find the most relevant journal
• We provide round the clock customer support
• Convenient online submission
• Thorough peer review
• Inclusion in PubMed and all major indexing services
• Maximum visibility for your research Submit your manuscript at
www.biomedcentral.com/submit