Detection of Minimal Levels of Serum Anti-Mu
¨ llerian
Hormone during Follow-Up of Patients with Ovarian
Granulosa Cell Tumor by Means of a Highly Sensitive
Enzyme-Linked Immunosorbent Assay
WEN-QING LONG, VALE´ RIE RANCHIN, PATRICIA PAUTIER, CORINNE BELVILLE,
PHILIPPE DENIZOT, HE´ LE`NE CAILLA, CATHERINE LHOMME´,
JEAN-YVES PICARD, JEAN-MICHEL BIDART, AND RODOLFO REY*
Unite´ de Recherches sur l’Endocrinologie du De´veloppement, INSERM, De´partement de Biologie, Ecole Normale Supe´rieure (W.-Q.L., C.B., J.-Y.P., R.R.), 92120 Montrouge; De´partement de Biologie Clinique (J.-M.B.) and Service de Gyne´cologie (P.P., C.L.), Institut Gustave Roussy, 94805 Villejuif; and
De´partement d’Immunoanalyse, Immunotech (V.R., P.D., H.C.), 13276 Marseilles, France ABSTRACT
Granulosa cell tumors (GCT) are ovarian neoplasms that tend to recur and spread in the pelvis and the abdomen several years after
the initial treatment. Anti-Mu¨ llerian hormone (AMH) is a reliable
serum marker of these tumors. To enhance the availability and the sensitivity of serum AMH determination, we developed an ultrasen-sitive enzyme-linked immunosorbent assay. In this work we compare the results of serum AMH levels, obtained using the ultrasensitive and the traditional assays, in 31 patients with ovarian GCT followed up for up to 7 yr.
The ultrasensitive enzyme-linked immunosorbent assay has a sig-nificantly higher sensitivity than the traditional one. This resulted in the detection of low serum AMH levels, which were undetectable with the traditional assay, in several cases including one patient in whom a recurrence of a GCT had developed and two patients in whom the treatment had not been completely successful. These cases highlight the importance of the availability of a highly sensitive assay allowing evaluation with high precision of the results of treatment and to detect the recurrences of GCT at an early, preclinical stage. (J Clin Endo-crinol Metab 85: 540 –544, 2000)
G
RANULOSA cell tumors (GCT) represent 1–2% of all ovarian tumors (1), but account for 6 –10% of malig-nant tumors of the ovary (2, 3). Although the maligmalig-nant potential of GCT is relatively low, these tumors frequently recur and spread in the pelvis. Late recurrences, appearing up to 20 yr after the initial treatment, are frequent and have a poor prognosis: in two large series, the 20-yr survival rate was below 35% (4, 5).The initial treatment of GCT is mainly surgical. In ad-vanced stages or in recurrences, when the tumor is not con-fined to the ovaries, complete surgical removal may be dif-ficult, and adjuvant chemotherapy and/or radiotherapy can be used. However, as GCT have low radio- and chemosen-sitivity, the success of the treatment is dependent on an early diagnosis of the recurrence, when complete removal of the tumor is still possible (6 – 8). These data highlight the im-portance of having a reliable biochemical marker of the disease.
GCT are hormone-secreting neoplasms; they may produce steroids, such as estradiol and progesterone, and they secrete peptide hormones, such as inhibin and anti-Mu¨llerian hor-mone (AMH), also known as Mu¨llerian inhibiting substance. Because serum levels of AMH and inhibin are increased in patients with GCT of the ovary, both hormones are used as biochemical markers of the disease (9 –14).
In a previous work we showed the reliability of serum AMH as a marker of recurrent GCT (12). We have now developed a highly sensitive assay for serum AMH deter-mination, with the purpose of enhancing the detection of very low concentrations of AMH in the serum of patients followed up for GCT. We report here the results of serum AMH determination obtained in patients with GCT using the ultrasensitive assay and compare them with the results ob-tained using the traditional assay.
Subjects and Methods
Patients
Serum samples from 31 female patients with a diagnosis of GCT (27 adult-type and 4 juvenile-type) were obtained from the serum bank of the De´partement de Biologie Clinique, Institut Gustave Roussy (Villejuif, France). Diagnoses were confirmed by evaluation of histological slides in all cases. In 16 cases, patients were only studied after bilateral ovari-ectomy during the remission period of the disease. In the other 15 cases, the availability of serum samples was as follows: several samples during both progressive and remission periods of the disease in bilaterally ovariectomized patients (8 cases), several samples during progressive and remission periods in unilaterally ovariectomized young patients (2 cases), several samples in patients with progressive disease despite Received June 14, 1999. Revision received October 18, 1999. Accepted
October 22, 1999.
Address all correspondence and requests for reprints to: Dr. Rodolfo Rey, Centro de Investigaciones Endocrinolo´gicas, Hospital de Nin˜os, Gallo 1330, 1425 Buenos Aires, Argentina. E-mail: rodolforey@infovia. com.ar.
* Established researcher with the Consejo Nacional de Investiga-ciones Cientı´ficas y Te´cnicas (CONICET, Argentina) and invited pro-fessor at the Ecole Normale Supe´rieure, De´partement de Biologie, Mon-trouge, France (supported by Proce´dure PAST of the French Ministry of Education and Research).
Copyright © 2000 by The Endocrine Society
540
bilateral ovariectomy and chemotherapy or radiotherapy (4 cases), and only 1 sample during progressive disease (1 case). Serum samples were also obtained from 20 prepubertal and 22 premenopausal female sub-jects. In all cases, serum samples had been taken for a reason indepen-dent of the present study; adults were apparently healthy blood donors and children were patients free of disorders of the hypothalamic-pitu-itary-gonadal axis and of chronic illnesses.
AMH assays
Serum AMH was measured using two enzyme-linked immunosor-bent assays (ELISA): the traditional assay, which has been used in our laboratory for the past 5 yr (15), and a recently developed ultrasensitive
assay (AMH/MIS ELISA kit,1Immunotech-Coulter, Marseilles, France).
Briefly, in the traditional assay, Immulon 2 plates (Dynatech Corp., Chantilly, VA) were coated with monoclonal antibody 10.6 (gift from Dr. R. Cate, Cambridge, MA), raised against recombinant human AMH (rhAMH) (16) by an overnight incubation, at room temperature. Sera were assayed at 1:4, 1:8, 1:16, and 1:32 dilutions in PBS containing 1% BSA. As second antibody, we used polyclonal antibody L40, consisting of an IgG fraction isolated by affinity chromatography on protein A-Sepharose from serum of a rabbit immunized with recombinant human
AMH (rhAMH) (17). L40 was added to the wells at 1g/mL in PBS-1%
BSA for 1 h at room temperature. Subsequently, an alkaline phospha-tase-labeled goat anti-rabbit IgG antibody (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA) was added and incubated for a further hour before the reaction was visualized with a MRX spectro-photometer (Dynatech Corp.) at 405 nm, using paranitrophenyl phos-phate (Sigma Chimie, Saint-Quentin-Fallavier, France) as substrate. Re-sults obtained using the traditional assay in 16 of these 31 patients have been previously reported (12).
The ultrasensitive AMH/MIS ELISA is also a sandwich-type assay with two immunological steps, but using two monoclonal antibodies, 11F8 and 22A2, obtained as follows. Two female BALB/c mice were
immunized with three sc injections of 50g immunoaffinity-purified
rhAMH produced in Chinese hamster ovary cells (18). The first injection was made using complete Freund’s adjuvant; for the following injec-tions, made 3 and 4 months later, incomplete Freund’s adjuvant was
used. Nine days before death and subsequent fusion, a dose of 50g
rhAMH was injected into the peritoneum, and 6 days later, 10 g
rhAMH in NaCl solution were injected by the iv route into the tail. Spleen cell suspensions were fused with NS1 myeloma cells (four lym-phocytes/one NS1 cell) in the presence of polyethylene glycol 1000, according to Ko¨hler and Milstein (19). Two of the primary hybridomas (22A2 and 11F8) were subcloned by three rounds of limiting dilution. The isotype of these monoclonal antibodies (IgG1) was determined using IsoStrip kit (Roche Molecular Biochemicals, Mannheim, Germa-ny). The expansion of hybridomas was performed using a miniPERM Bioreactor (Heraeus, Les Ulis, France), in Hybridoma-SFM medium (Life Technologies, Inc., Cergy-Pontoise, France) without serum, at 37 C in a humid atmosphere containing 5% carbon dioxide. Monoclonal antibod-ies 22A2 and 11F8 were purified by protein A-Sepharose-4 Fast-Flow chromatography (Pharmacia Biotech, Orsay, France) from culture me-dium according to the method of Ey et al. (20). Their purity was assessed on 4 –20% SDS-PAGE (Bio-Rad Laboratories, Inc., Ivry-sur-Seine, France), and their specificity against rhAMH was determined using the traditional ELISA and replacing polyclonal antibody L40 by either monoclonal 11F8 or 22A2.
The ultrasensitive AMH/MIS ELISA was performed as follows. Twenty-five microliters of each serum sample were incubated in du-plicate for 1 h on a ready to use polystyrene plaque coated with mono-clonal antibody 22A2. Subsequently, monomono-clonal antibody 11F8, cou-pled to biotin, and a streptavidin-horseradish peroxidase complex were added and incubated for 15 min. The peroxidase substrate 3,3⬘,5,5⬘-tetramethylbenzidine was added, and the resulting color reaction was
quantified 30 min later after stopping the reaction with 50L 2 n H2SO4,
using the MRX spectrophotometer at 450 nm. A preparation of purified rhAMH was used to construct a standard curve ranging from 0.7–175 pmol/L.
The intraassay coefficient of variation of the ultrasensitive AMH/MIS
ELISA was calculated for two different AMH concentrations by assaying two serum samples (mean concentrations, 36 and 245 pmol/L, respec-tively) 12 times within the same assay; the interassay coefficient of variation was determined also for two different AMH concentrations by assaying two serum samples (mean concentrations, 31 and 257 pmol/L, respectively) in duplicate in 12 independent assays (Table 1). Three recovery tests were performed using 5 different serum samples, the original AMH concentrations of which were 16.8, 14.7, 17.7, 21.4, and 25.6 pmol/L, respectively. Ten microliters of 140 pmol/L rhAMH were
added to 100L of each sample in the first recovery test, 5 L 1400
pmol/L rhAMH were added in the second, and 10L 1400 pmol/L
rhAMH were added in the third (Table 2). A dilution test was performed using 3 different samples, in which AMH concentrations before dilution were 31.28, 23.56, and 47.56 pmol/L, respectively. Each serum sample was assayed non-diluted and diluted at 1:2, 1:4, 1:8, and 1:16 in the diluent provided with the AMH/MIS ELISA kit (Table 3).
The specificity of the AMH/MIS ELISA was assessed by assaying supraphysiological concentrations of transforming growth factor- (Sig-ma Chimie), inhibin B and activin A (R&D Systems, Inc., Minneapolis, MN), and inhibin A and inhibin pro-␣ C subunit (Oxford Bio-Innova-tion, Oxfordshire, UK).
Results
Validation of the ultrasensitive AMH/MIS ELISA
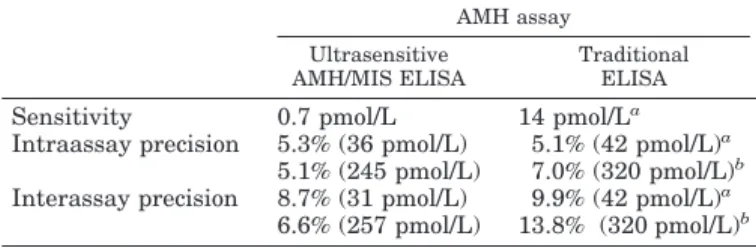
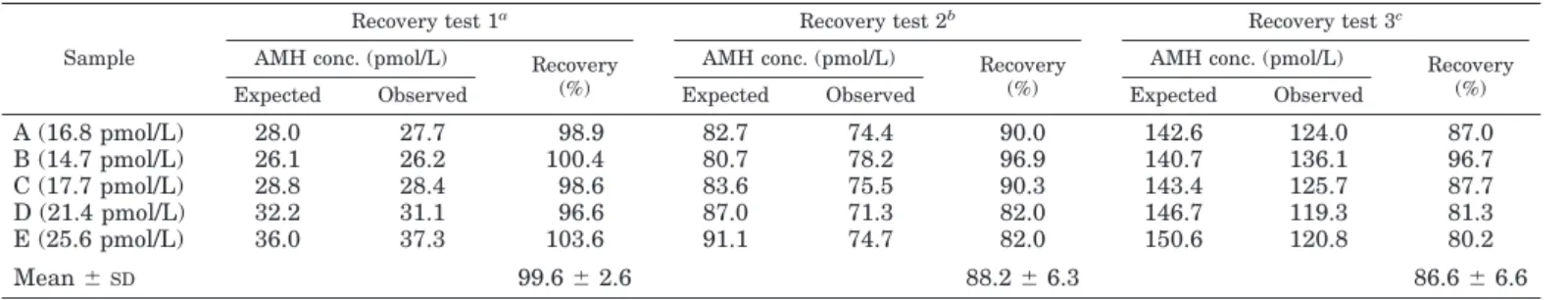
Intra- and interassay coefficients of variation showed a slightly better performance of the ultrasensitive AMH/MIS ELISA compared with the traditional assay (Table 1). The most remarkable difference was the sensitivity of the assays. The lowest AMH concentration significantly different from the zero standard with a probability of 95% was 20 times lower using the AMH/MIS ELISA (Table 1). Results of the recovery tests are shown in Table 2. The observed AMH concentration represented 80.2–103.6% of the expected val-ues. The results of the dilution tests are given in Table 3. The observed AMH concentrations reached 84.0 –104.6% of those expected. Specificity was tested by assaying samples con-taining known concentrations of the most AMH-related members of a large family of growth and differentiation factors (21): transforming growth factor-, inhibins A and B, inhibin pro-␣ C subunit, and activin A. No cross-reactivity was observed in any of the cases (data not shown). Recovery tests performed on three serum samples of known AMH concentration to which different amounts of inhibin B or activin A were added showed that the observed AMH con-centrations ranged between 92–101% of the expected values.
Serum AMH in normal women and in patients with GCT
Using the ultrasensitive AMH/MIS ELISA, the serum AMH concentration (mean⫾ sd) was 22.8 ⫾ 19.6 pmol/L (range, 0.7–73.9 pmol/L) in normal prepubertal girls and
1AMH/MIS ELISA kit is a trademark of Immunotech under INSERM
license.
TABLE 1. Comparison between the performance characteristics of the ultrasensitive and the traditional AMH assays
AMH assay Ultrasensitive
AMH/MIS ELISA
Traditional ELISA
Sensitivity 0.7 pmol/L 14 pmol/La
Intraassay precision 5.3% (36 pmol/L) 5.1% (42 pmol/L)a
5.1% (245 pmol/L) 7.0% (320 pmol/L)b
Interassay precision 8.7% (31 pmol/L) 9.9% (42 pmol/L)a
6.6% (257 pmol/L) 13.8% (320 pmol/L)b
aFrom Rey et al. (12).
bFrom Rey et al. (15).
13.7⫾ 18.8 pmol/L (range, 0.7–74.7 pmol/L) in normal adult women. Immunoreactive AMH was undetectable, after bi-lateral ovariectomy, in all of the serum samples obtained from 15 of 16 patients who were followed up for up to 7 yr (range, 3– 81 months; median, 23 months) after treatment of GCT and in whom no signs of recurrent disease were ob-served (Table 4). In only 1 ovariectomized patient was a value of 70 pmol/L found in 1 of 5 serum samples assayed for AMH, even though no evidence of recurrence was detected. Serum AMH was readily detectable in 14 of 15 patients with a recurrent GCT, including 2 juvenile GCT and 4 bilaterally ovariectomized patients in whom routine serum samples had been assayed during follow-up when clinical signs of a recurrence were not yet evident. In 1 case, AMH was unde-tectable in the only serum sample available despite a pro-gressive disease. Sensitivity and positive predictive value of serum AMH for the presence of a GCT were 93% in our series of 15 patients with a demonstrated recurrence (Table 4). Of the 14 patients with long term follow-up, serum AMH de-clined to undetectable values after successful treatment in 8, but remained elevated in 4 others in whom the tumor could not be completely removed and/or was not responsive to chemotherapy. In the remaining 2 cases, unilateral ovariec-tomy was performed due to the young age of the patients, and low but detectable levels of AMH could be found in serum samples obtained after treatment. Considering the
serum samples studied only during the remission periods of 24 bilaterally ovariectomized patients (16 of whom were in remission during the whole follow-up period and for 8 of whom samples were available during both progressive dis-ease and remission), the specificity and negative predictive value of serum AMH reached 96% (Table 4).
Although in most cases, these results were in keeping with previously reported data using the traditional AMH assay (12), in 6 of 31 patients discordant results were observed. In a serum sample obtained from patient A 2 weeks after sur-gery for recurrence of juvenile GCT, although AMH was undetectable using the traditional assay, readily detectable levels were found with the ultrasensitive AMH/MIS ELISA (Fig. 1A), suggesting that some tumor tissue could not be extirpated. Fifteen days later, increasing serum AMH levels were detected by both assays, and a progression of the tumor was clinically confirmed. Patient B received chemotherapy for a recurrent GCT. At 1 month of treatment, although serum AMH was below the sensitivity level of the traditional assay, detectable levels, suggesting the presence of residual tumor cells, were found using the ultrasensitive AMH/MIS ELISA (Fig. 1B). Chemotherapy was continued, and serum AMH became undetectable by 6 months of treatment. Eigh-teen months later, elevated AMH levels were found in a serum sample obtained for routine control during follow-up; a new recurrence was suspected and subsequently con-firmed by ultrasound and clinical examinations. A retro-spective comparison of AMH levels using the 2 assays was performed in serum samples from patient C, in whom a recurrence of an adult-type GCT had been suspected due to elevated serum AMH using the traditional assay. When the ultrasensitive AMH/MIS ELISA was used, an elevation of AMH levels could be detected in several serum samples
TABLE 2. Validation of the ultrasensitive AMH/MIS ELISA: recovery test
Sample
Recovery test 1a Recovery test 2b Recovery test 3c
AMH conc. (pmol/L) Recovery (%)
AMH conc. (pmol/L) Recovery (%)
AMH conc. (pmol/L) Recovery (%)
Expected Observed Expected Observed Expected Observed
A (16.8 pmol/L) 28.0 27.7 98.9 82.7 74.4 90.0 142.6 124.0 87.0 B (14.7 pmol/L) 26.1 26.2 100.4 80.7 78.2 96.9 140.7 136.1 96.7 C (17.7 pmol/L) 28.8 28.4 98.6 83.6 75.5 90.3 143.4 125.7 87.7 D (21.4 pmol/L) 32.2 31.1 96.6 87.0 71.3 82.0 146.7 119.3 81.3 E (25.6 pmol/L) 36.0 37.3 103.6 91.1 74.7 82.0 150.6 120.8 80.2 Mean⫾SD 99.6⫾ 2.6 88.2⫾ 6.3 86.6⫾ 6.6
aTen microliters of 140 pmol/L rhAMH were added to 100L sample.
bFive microliters of 1400 pmol/L rhAMH were added to 100L sample.
cTen microliters of 1400 pmol/L rhAMH were added to 100L sample.
TABLE 3. Validation of the ultrasensitive AMH/MIS ELISA: dilution test Sample Dilution AMH conc. Observed/ expected (%) Expected (pmol/L) Observed (pmol/L) F (31.28 pmol/L) 1/2 15.64 16.35 104.6 1/4 7.82 7.50 95.9 1/8 3.91 3.86 98.6 1/16 1.95 1.64 84.0 G (23.56 pmol/L) 1/2 11.78 11.35 96.4 1/4 5.89 5.93 100.6 1/8 2.95 3.00 101.8 1/16 1.47 1.43 97.0 H (47.56 pmol/L) 1/2 23.78 23.92 100.6 1/4 11.89 11.57 97.3 1/8 5.94 5.50 92.5 1/16 2.97 2.86 96.1 Mean⫾SD 97.1⫾ 4.9
TABLE 4. Value of serum anti-Mu¨ llerian hormone assay in the follow-up of patients with bilateral ovariectomy after a granulosa cell tumor
Granulosa cell tumor state Present (recurrence) Absent Serum AMH
Detectable 14 1
Undetectable 1 23
Sensitivity, 93% (95% confidence interval, 68 –100%); specificity, 96% (80 –100%); positive predictive value, 93% (68 –100%); negative predictive value; 96% (80 –100%).
obtained during the previous 16 months, when the tradi-tional assay still gave normal results (Fig. 1C). The recurrence was diagnosed before any clinical sign was evident and could be successfully removed. In patient D, serum AMH declined after a successful surgical extirpation of the tumor; the very low AMH levels present in serum 72 h after surgery could only be detected with the ultrasensitive assay. No AMH was detected by any of the assays in subsequent serum samples (Fig. 1D). In patients E and F, bearing juvenile-type GCT, only a unilateral ovariectomy was performed due to their young age. After surgery, although serum AMH was undetectable with the traditional assay, it oscillated between undetectable and low levels with the ultrasensitive AMH/ MIS ELISA (Fig. 1, E and F). However, serum AMH was always within the normal range, and this was interpreted as a result of normal secretion by the remaining gonad. These examples corroborate the higher sensitivity of the ultrasen-sitive AMH/MIS ELISA.
Discussion
AMH is produced exclusively by Sertoli cells in the male and by granulosa cells in the female, with certain differences. Although the testis secretes high amounts of AMH during
the fetal and prepubertal periods of life, resulting in normal serum levels between 270 – 640 pmol/L using the AMH/MIS ELISA kit (22), the ovary produces only low levels of AMH (⬍75 pmol/L) between birth and menopause. In women, the elevation of serum AMH reliably denotes the presence of GCT (10, 12, 13). However, as anti-AMH antibodies and pure AMH standards were not commercially available, and AMH determination protocols were long, requiring overnight in-cubation, only a few laboratories in the world performed AMH assays, thus precluding a widespread use of this tumor marker. We have recently developed a sandwich-type AMH ELISA with two immunological steps extending over less than 2 h, which has enabled us to evaluate testicular function in a large population of patients with intersex disorders (22). Because serum AMH levels are significantly lower in the female, our purpose was to improve the sensitivity of the assay by developing an ultrasensitive protocol. The use of two immunopurified monoclonal anti-AMH antibodies in the new assay, instead of one monoclonal and one polyclonal as was the case in our previous assay, significantly reduced the background signal, resulting in an enhancement of assay sensitivity. This ultrasensitive ELISA is capable of detecting serum AMH levels as low as 0.7 pmol/L (0.1 ng/mL). The sensitivity is significantly higher than that of our traditional
FIG. 1. Serum AMH levels, expressed as picomoles per L (left y-axis) and as nanograms per mL (right y-axis) in pa-tients with GCT of the ovary. Triangles and full lines indicate results using the ultrasensitive AMH/MIS ELISA, and dots and broken lines indicate results using the traditional assay. The limits of detection of the assays were 0.7 pmol/L for the ultrasensitive AMH/MIS ELISA and 14 pmol/L for the traditional assay. Patient A was operated on for a juvenile-type GCT recurrence, but the tumor could not be completely removed. Patient B received chemotherapy for an adult-type GCT recurrence; after 1 month of treatment, serum AMH was undetectable with the traditional as-say, but low levels were found with the ultrasensitive ELISA, suggesting the existence of residual tumor cells. At the end of the treatment, AMH was unde-tectable with both methods. Eighteen months later, elevated serum AMH was found in a routine serum determina-tion, allowing the detection of a new recurrence of GCT. Patient C, Retro-spective comparison of results obtained with both assays; serum AMH levels could be detected more than 16 months earlier with the ultrasensitive than with the traditional assay. Patient D, An adult-type GCT was successfully re-moved by surgery. In patients E and F, one ovary was removed due to juvenile-type GCT; after surgery, low AMH lev-els, detected only with the ultrasensi-tive ELISA, were interpreted as normal secretion by the remaining gonad.
assay, which detected 14 pmol/L (15), and of assays per-formed by other groups, which detected 3.6 pmol/L (23, 24). The results obtained in our large series of 31 GCT patients with long-term follow-up confirm the value of serum AMH in both the evaluation of treatment and the detection of recurrences of juvenile- (13) and adult-type (10, 12) GCT. Furthermore, the use of a more sensitive assay has allowed us to improve the detection of patients with circulating AMH levels, due to the recruitment of those with serum AMH levels between 0.7–14 pmol/L.
As we had previously shown in two patients (12), two new cases of GCT recurrences were suspected at an early stage due to the elevation of serum AMH in routine determina-tions during follow-up, before any clinical sign of the disease became evident. Moreover, using the ultrasensitive AMH/ MIS ELISA, we have been able to detect low levels of serum AMH in three samples obtained from one of these patients in which no AMH could be detected with the traditional assay. Therefore, we presume that the recurrent tumor would have been suspected several months earlier if the ultrasensitive assay had been available when the earliest serum samples were taken. The ultrasensitive assay has also allowed us to detect very low serum AMH levels, suggesting the presence of residual tumor cells, 2 weeks after surgery in one patient and after the first month of chemotherapy in another patient. However, the interpretation of serum AMH levels should be performed cautiously in the first days after surgical removal of a GCT. The AMH half-life was estimated to be 48 h (25); therefore, as we show here, low levels of AMH can be detected in serum within a few days (48 h with the traditional assay and 72 h with the ultrasensitive one) after successful surgery.
Finally, the higher sensitivity of the assay also resulted in the detection of low levels of serum AMH in two young patients in whom one ovary had been preserved. Although the lowest detectable AMH levels are suggestive of a recur-rence in a bilaterally ovariectomized patient, a serum AMH concentration of up to 75 pmol/L is normal in a prepubertal girl or a premenopausal woman bearing ovaries. As no data are available on normal serum AMH in women with only one gonad, the interpretation of detectable serum AMH levels in these cases may be difficult.
In conclusion, we have developed a powerful and easy to perform ELISA for AMH determination, allowing us to eval-uate with high sensitivity the results of treatment and to detect recurrences of GCT at an early stage.
Acknowledgment
Monoclonal antibody 10.6 was a gift from Dr. R. Cate (Cambridge, MA).
References
1. Young RH, Scully RE. 1992 Endocrine tumors of the ovary. In: Sasano N, ed. Current topics in pathology. Berlin: Springer-Verlag; 114 –164.
2. Fox H, Agrawal K, Langley FA. 1975 A clinicopathologic study of 92 cases of granulosa cell tumor. Cancer. 35:231–241.
3. Fox H. 1985 Sex cord-stromal tumors of the ovary. J Pathol. 145:127–148. 4. Dempster J, Geirsson RT, Duncan ID. 1987 Survival after ovarian granulosa
and theca cell tumors. Scottish Med J. 32:38 –39.
5. Pautier P, Lhomme´ C, Culine S, et al. 1997 Adult granulosa-cell tumors of the ovary: a retrospective study of 45 cases. Int J Gynecol Cancer. 7:58 – 65. 6. Schwartz PE, Smith JP. 1976 Treatment of ovarian stromal tumors. Am J Obstet
Gynecol. 125:402– 411.
7. Stenwig JT, Hazenkamp JT, Beecham JB. 1979 Granulosa cell tumors of the ovary: a clinicopathological study of 118 cases with long-term follow-up. Gynecol Oncol. 7:136 –152.
8. Evans ATII, Gafey TA, Malkasian GDJ, Annegers JF. 1980 Clinicopatholog-ical review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol. 55:231–238.
9. Lappo¨hn RE, Burger HG, Bouma J, Bangah M, Krans M, de Bruijn HWA. 1989 Inhibin as a marker for granulosa-cell tumors. N Engl J Med. 321:790 –793. 10. Gustafson ML, Lee MM, Scully RE, et al. 1992 Mu¨llerian inhibiting substance
as a marker for ovarian sex-cord tumor. N Engl J Med. 326:466 – 471. 11. Burger HG. 1994 Inhibin as a tumour marker. Clin Endocrinol (Oxf).
41:151–153.
12. Rey R, Lhomme´ C, Marcillac I, et al. 1996 Anti-Mu¨llerian hormone as a serum marker of granulosa-cell tumors of the ovary: comparative study with serum ␣-inhibin and estradiol. Am J Obstet Gynecol. 174:958–965.
13. Silverman LA, Gitelman SE. 1996 Immunoreactive inhibin, Mullerian inhib-itory substance, and activin as biochemical markers for juvenile granulosa cell tumors. J Pediatr. 129:918 –921.
14. Petraglia F, Luisi S, Pautier P, et al. 1998 Inhibin B is the major form of inhibin/activin family secreted by granulosa cell tumors. J Clin Endocrinol Metab. 83:1029 –1032.
15. Rey R, Mebarki F, Forest MG, et al. 1994 Anti-Mu¨llerian hormone in children with androgen insensitivity. J Clin Endocrinol Metab. 79:960 –964. 16. Carre´-Euse`be D, Imbeaud S, Harbison M, New MI, Josso N, Picard JY. 1992
Variants of the anti-Mu¨llerian hormone gene in a compound heterozygote with the persistent Mu¨llerian duct syndrome and his family. Hum Genet. 90:389 –394.
17. Rey R, Al-Attar L, Louis F, et al. 1996 Testicular dysgenesis does not affect expression of anti-Mu¨llerian hormone by Sertoli cells in pre-meiotic seminif-erous tubules. Am J Pathol. 148:1689 –1698.
18. Pepinsky RB, Sinclair LK, Chow EP, et al. 1988 Proteolytic processing of Mu¨llerian inhibiting substance produces a transforming growth factor--like fragment. J Biol Chem. 263:18961–18965.
19. Ko¨hler G, Milstein C. 1975 Continuous cultures of fused cells secreting an-tibody of predefined specificity. Nature. 256:495– 497.
20. Oda S, Nishimatsu S, Murakami K, Ueno N. 1995 Molecular cloning and functional analysis of a new activin beta subunit: a dorsal mesoderm-inducing activity in Xenopus. Biochem Biophys Res Commun. 210:581–588.
21. Josso N, Cate RL, Picard JY, et al. 1993 Anti-Mu¨llerian hormone, the Jost factor. Recent Prog Horm Res. 42:1–59.
22. Rey RA, Belville C, Nihoul F, et al. 1999 Evaluation of gonadal function in 107 intersex patients by means of serum anti-Mu¨llerian hormone measurement. J Clin Endocrinol Metab. 84:627– 631.
23. Lee MM, Donahoe PK, Hasegawa T, et al. 1996 Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 81:571–576.
24. Fallat ME, Siow Y, Belker AM, Boyd JK, Yoffe S, MacLaughlin DT. 1996 The presence of Mullerian inhibiting substance in human seminal plasma. Hum Reprod. 11:2165–2169.
25. Vigier B, Tran D, Du Mesnil du Buisson F, Heyman Y, Josso N. 1983 Use of monoclonal antibody techniques to study the ontogeny of bovine anti-Mu¨l-lerian hormone. J Reprod Fertil. 69:207–214.