HAL Id: tel-01598871
https://tel.archives-ouvertes.fr/tel-01598871
Submitted on 30 Sep 2017HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Thibaut Snollaerts
To cite this version:
Thibaut Snollaerts. Identification of new regulators for PML nuclear bodies. Biochemistry, Molecular Biology. Université Pierre et Marie Curie - Paris VI, 2016. English. �NNT : 2016PA066386�. �tel-01598871�
University Pierre and Marie Curie
Doctoral school : Complexité Du Vivant - ED515Nuclear Organization and Oncogenesis Unit (INSERM U993)
Identification of new regulators for PML Nuclear
Bodies
By Thibaut Snollaerts
Doctoral thesis of BIOCHEMISTRY AND MOLECULAR BIOLOGY
OF THE CELL
Supervised by Anne Dejean
Presented and defended publicly on September 28th 2016 In front of a jury composed of:
Dr. Laurent LE CAM INSERM research director Reviewer Dr. Dimitris XIRODIMAS CNRS research director Reviewer Dr. Mounira CHELBI-ALIX INSERM research director Examinator
Dr. Robert WEIL CNRS research director Examinator
Dr. Frederic DEVAUX UPMC university professor President Prof. Anne DEJEAN INSERM research director Thesis Director
La persévérance, c'est ce qui rend l'impossible possible, le possible probable et le probable réalisé. Perseverance is what makes the impossible possible,
the possible probable and the probable realized Robert Half
Acknowledgements
First, I would like to thank all the members of the jury: Laurent Le Cam, Dimitris Xirodimas, Mounira Chelbi-Alix, Robert Weil and Frederic Devaux who, despite a very busy schedule, took the time to take part in this thesis defense jury.
I would like to thank Anne Dejean for giving me the opportunity to be part of her research team since my master internship, for giving me the chance to meet very interesting people, and for providing me with many opportunities. Thank you for the trust you put in me to carry out this complicated project as well as for providing the best working conditions, and collaborations to realize it. Thank you for all the things I was able to learn from you, your team and the world of science in which we are involved.
I would like to thank the entire ONO team for their support during these past few, short, years:
Thank you to the past members: Adrien, the basketball player from room 306, and Pierre for your help, advices and good sense of humor; Yoon Ra for your kindness and zeness; Ricardo for bringing a little bit of Mexican spirit to the lab; Louise-Mary for all your help with the administrative paperwork and Sophie aka Fofi, aka “la personne la plus désagréable du labo, mais qui en vrai, est plus ou moins relativement super sympa”. A big thank for your help and support to you and all the people I might have met during these years.
I would like to give a special thank you to Hélène-Neyret Khan for giving me the opportunity to take over one of her projects. And also for her patience, kindness and understanding while teaching most of the techniques used for this project. Thank you for keeping contact, for supporting and helping me, even though, you moved on to another lab.
Thank you to the present members of the team : Alexandra, merci pour ton aide précieuse sur ce projet notamment à compter des points verts sans devenir folle. Merci aussi pour ton soutien et ton franc parler tout au long de ces années. Thank you Elefteria (Elma) for your support, for your friendship and all your advices over the years. Thank you for listening, understanding, supporting and for being one hell of a crazy friend. Pablo, muchas gracias for
your kindness, enthusiasm, optimism and for our scientific discussions on the project; Juan Pablo, my statistics genius, mi amigo, for all your help inside and out of the lab; Ying for sharing crazy Chinese pastries; Jack for your help, good sense of humor and support; Ilan for bringing some craziness in the lab; Oliver for your insights in the project and your good spirit; Lucas for bringing a Canadian vibe to the lab; Pierre Francois and Greg, your communicative sense of humor, for all the laughs and good times; Jacob, king of the cloning, for your help and knowledge on the project; Pierre Tiollais, for all of your stories, Friday seminar organization and of course the design of this amazing fridge, I mean building! Thank you to all the interns for making this lab so lively.
Thank you people from room 307; Agnes, qPCR master, for being there to listen and help me with my various problems. Thank you Pascal for sharing your passion of science and politics, for your availability under any circumstances, for your help, for listening and for everything else.
Thank you Florence for helping us with all of the administrative paperwork. Thank you for your good sense of humor and energy.
Merci à Chaty et Monique, qui parfois dans l’ombre, nous facilitent tant le travail. I would also like to thank all of my floor mates and in particular people from the Hepacivirus team: Barbara, the one and only unicorn from Britany; Pierick, aka the seagull, for the famous “Soirée Piscine!TM”; Dona, thank you for your support (long live ferrets and
penguins!). Thank you to all the other members of the team: Christine; Stephanie; Mila; Aurora and Patrick. I would also like to extend that thanks to the fabulous people of our building: Claudia; Iratxe; Florence; Yu; but also from Pasteur: Mathilde; Lilliana; Emmanuelle and all the people I might have missed, thank you all.
I would like to thank all my friends outside the Institute who have also supported me. Thank you Paul, for always having my back for all these years; Jessica for your support, help and Tanguy, for your good sense of humor and for making teaching so interesting; Dr David Moffet for introducing me to this crazy world of science. Thank you Raphael; Lola; Joel; Estelle; Leo; Elelta and of course all the friends that are not mentioned here.
Thank you Camille for your support, patience and understanding over the years. Thank you to your family for their support.
I would like to thank my family in the broad sense for believing in me and supporting me, especially my parents for allowing me to pursue my dreams. Thank you for your patience and unwavering support. I would also like to give a special thanks to my brothers and sister who were always supporting me no matter what. A big extra thank you for Audrey who helped me a lot with corrections. Thank you all for being here, family rocks!
I would like -to once again- thank all the people who helped me shape this thesis and make it what it is. Thank you all, family/friends for your support, it means a lot for me. Over the past years, you were always there for me, no matter the circumstances, no matter how hard or difficult the situation might have been. I am actually very proud to be one of your friend/family and I hope that I would never let you down.
Thank you for the smiles; the good and bad jokes; for coffee breaks (thank you coffee machine), for reminding me to eat and sleep (thank you to the night guard for checking that I was still alive) and for everything. I would like to thank the -80 freezer for breaking on a Sunday night at 11:30pm; penguins because they are funny, and because I have one yelling at me every time I open my fridge; and otters, because I can and I like them.
Finally, thank you reader, for taking the time to look at all the people that helped me in this project. I hope you will enjoy reading this work and find it as interesting as I did.
Collaborations
Because this work is also the result of a collaborative effort, I would like to thank our collaborators for their help by providing their expertise and tools to this project.
- Michael Howell from the “London Research Institute” and the members of the high throughput-screening platform, who helped realize the primary and secondary siRNA screening.
- Michele Pagano from the “Howard Hughes Medical Institute” of New York, who shared knowledge on the SCF complex systems and F-Box proteins, as well as, implementing the first co-immunoprecipitating screening for the F-Box.
- Florian Bassermann and his team, and in particular Vanessa Fernandez, from the School of Medicine at the Technical University of Munich who helped us with the in-vitro experiments and provided us with FBXO9 antibody.
- All the people and scientists who I had the chance to encounter at the Pasteur Institute and in conferences, which led to discussion allowing to further this project.
Table of Contents
Acknowledgements ... 3 Collaborations ... 6 Table of Contents ... 7 Abbreviations ... 10 Introduction ... 11I) Discovery of ProMyelocytic Leukemia protein (PML) ... 11
1) Acute Promyelocytic Leukemia (APL) ... 11
2) The PML protein ... 15
3) PML Nuclear Bodies ... 19
II) Post-translational modifications ... 23
1) Diversity of post-translational modifications ... 24
2) Phosphorylation ... 26
3) Acetylation ... 29
4) SUMOylation ... 30
5) The SUMO/Ubiquitin coupled pathway ... 38
6) Ubiquitination ... 41
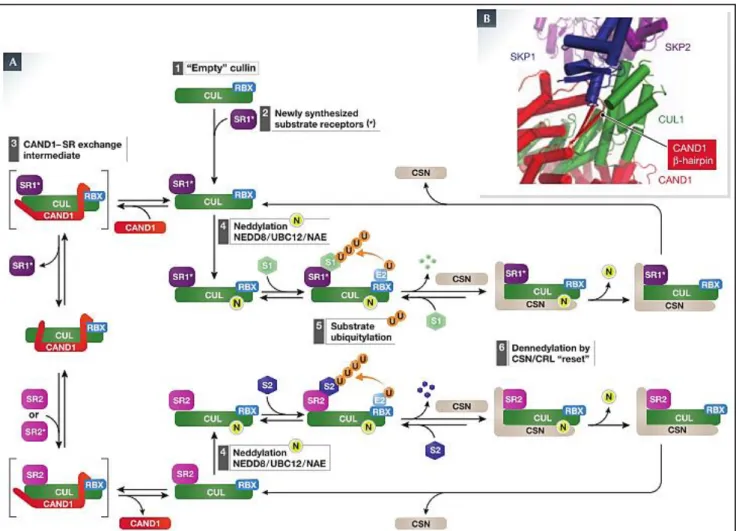
III) The SKP-Cullin-F-Box containing Complex (SCF) ... 49
1) General structure of Cullin-RING Ligases complex ... 49
2) The SCF complex and the F-Box proteins ... 50
3) Regulation of the SCF complex ... 53
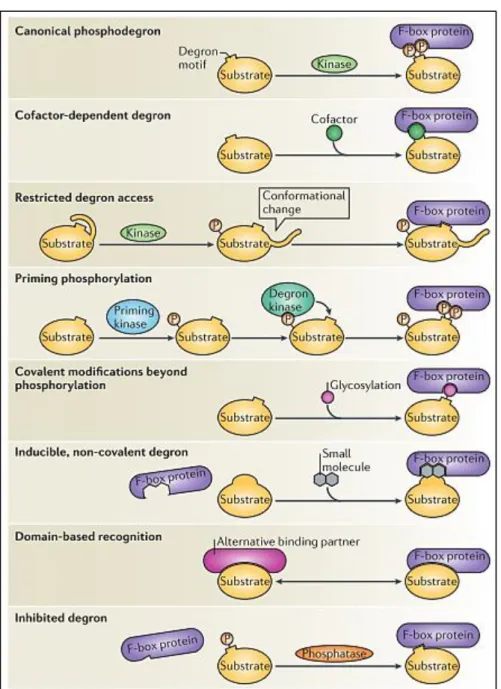
4) Substrate recognition ... 57
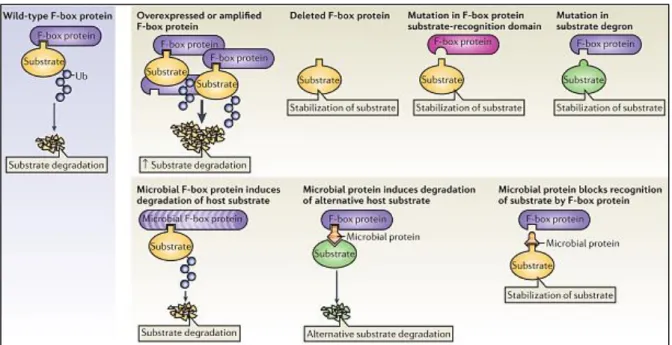
5) SCFs and diseases ... 60
6) PML and SCF ... 64
IV) PML, a tumor suppressor... 65
1) PML physiological functions ... 65
2) PML and diseases ... 66
3) PML and the apoptotic pathway ... 68
4) PML and the P53 pathway ... 68
5) PML and transcriptional regulation ... 69
6) Role of PML in DNA damage repair ... 70
7) PML and the Akt pathway ... 71
8) Cytoplasmic PML in tumorigenesis... 72
9) PML in cancers ... 73
Thesis Objective ... 74
Results ... 76
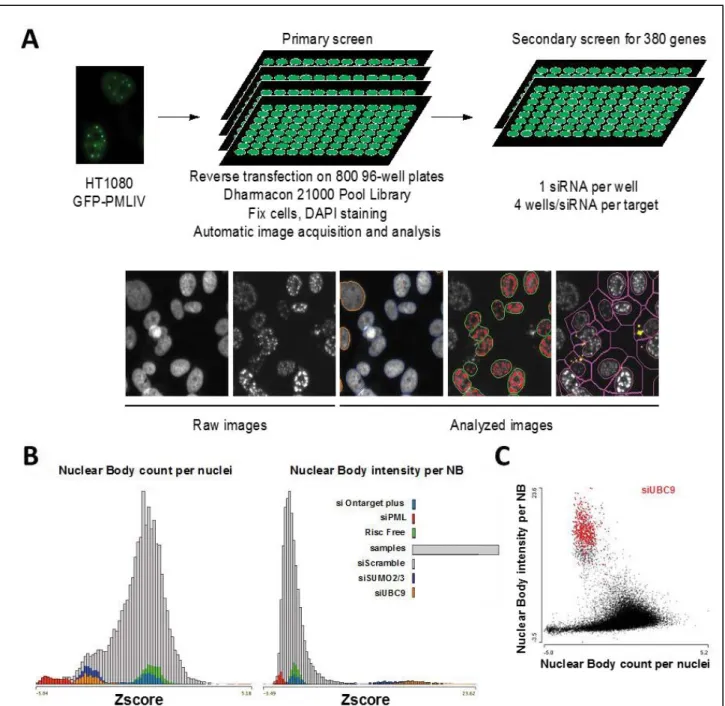
2) Primary screen results ... 79
3) Validation screen ... 80
II) Functional study of selected candidates ... 82
1) Identification of SKP1a and RBX1 ... 82
2) Manual validation of screen results ... 83
3) Depletion of SKP1a and RBX1 stabilizes PML ... 84
4) The overexpression of SKP1a and RBX1 destabilizes PML ... 86
5) RBX1 and SKP1a are both interacting with PML ... 88
6) Identification of the specific F-Box protein for PML ... 89
7) Validation of the interaction between PML and FBXO9 ... 90
8) FBXO9 interacts with all PML isoforms ... 92
9) Localization of FBXO9-PML interaction ... 93
10) SUMOylation, arsenic and PML-FBXO9 interaction ... 95
11) FBXO9 degrades PML under arsenic trioxide treatment ... 97
12) Impact of FBXO9 on the half-life of PML ... 106
13) SCFFBXO9 ubiquitinates PML in vitro ... 107
14) An attempt to localize the FBXO9 degron on PML ... 110
15) Kinase mini-screen to localize the FBXO9 degron on PML ... 112
16) Search for physiological stimuli leading to SCFFBXO9-induced PML degradation . 114 17) Possible links to diseases ... 116
Discussion and perspectives ... 120
1) SKP1a and RBX1 are members of an ubiquitination complex involved in the degradation of PML ... 120
2) The Cullin-1 is involved in SCF complexes ... 122
3) FBXO9 specifically interacts with PML ... 123
4) FBXO9 is involved in PML stability ... 123
5) Is PML degradation dependent on SUMOylation? ... 125
6) SCFFBXO9 specifically ubiquitinates PML ... 125
7) PML’s degron ... 126
8) Kinases phosphorylating PML’s degron ... 127
9) PML and FBXO9 in diseases ... 131
10) Mouse model Fbxo9 KO ... 133
11) PML and cellular differentiation ... 133
12) PML and innate immunity ... 134
13) The SCF complex: a druggable target ... 135
14) Other potential candidates to be studied ... 138
Conclusion ... 141
Material and Methods ... 142
Annexes ... 150
Annex 1: The 69 Mammalian F-Box proteins ... 150
Annex 3: Validated candidates inducing a morphological change of PML Nuclear Bodies.
... 153
Annex 4: Co-immunoprecipitation screen to identify PML interacting F-Box protein. .... 165
Annex 5: Cell lines used in mRNA mini screen. ... 166
Annex 6: FBXO9 is overexpressed in some types of breast cancers. ... 169
Annex 7: Cancer tissue PML antibody staining of Breast and lung cancers. ... 170
Bibliographical References ... 171
Table and illustration table ... 194
Abbreviations
ALT: Alternative Lenghtening of Telomere PDSM: Phosphorylation-Dependent SUMOylation Motif
APL: Acute Promelocytic Leukemia PIAS: Protein Inhibitor of Activated STAT ATR: Ataxia Telangiectasia and Rad3-related protein PML: ProMelocytic Leukemia protein ATO: Arsenic TriOxide (AS2O3, Ars) PML-NBs: PML Nuclear Bodies
ATRA: All-Trans Retinoic Acid PTMs: Post-Translational Modifications
CAND1: Cullin-Associated NEDD8-Dissociated protein 1 RARα: Retinoic Acid Receptor α
CDK1/2: Cyclin-Dependent Kinase 1/2 Rb: Retinoblastma protein
CK2: Casein Kinase 2 (α1, α2, α3, β) RBCC domain: RING B-Box Coiled-Coil domain
CRL: Cullin-Ring Ligases RBX1: Ring-BoX protein 1
CUL1: CULlin-1 RING: Really Interesting New Gene
DUBs: DeUBiquitinating enzyme RNF4: RING Finger protein 4
E1: Activation Enzyme SAE1: SUMO Activating Enzyme 1
E2: Conjugation Enzyme SAE2: SUMO Activating Enzyme 2
E3: Ligation Enzyme SCF: SKP-Cullin-F-Box containing complex ERK: Extracellular signal-Regulated Kinase SENP: SENtrin specific Proteases FBXL: F-BoX Leucine rich repeats protein shRNA: small hairpin RNA
FBXO9: F-BoX Only protein 9 SIM: SUMO Interacting Motif
FBXO: F-BoX Only protein siRNA: small interfering RNA
FBXW: F-BoX WD40 repeats protein SKP1a: S-phase Kinase-associated Protein 1
GFP: Green Fluorescent Protein STUbL: SUMO Targeted Ubiquitin Ligases
HAT: Histone Acetyl Transferase SUMO: Small Ubiquitin like MOdifier
HDAC: Histone DeACetylase TNF: Tumor Necrosis Factor
HECT: Homologous to the E6-AP Carboxyl Terminus TRIM: TRIpartite Motif-containing protein HIPK2: Homeodomain-Interacting Protein Kinase 2 Ub: Ubiquitin
IQR Inter Quartile Range UBA1: UBiquitin Activating enzyme 1
KO: Knock-Out UBC: UBiquitin Conjugating enzyme
MAPK: Mitogen-Activated Protein Kinase UBC9: UBiquitin Conjugating enzyme 9
MDM2: Murine Double Minute 2 UBD: Ubiquitin Binding Domain
MEFs: Murine Embryonic Fibroblasts UPS: Ubiquitin Proteasome System
mRNA: messenger RNA WT: Wild Type
NDSM: Negatively charged amino-acid-Dependent SUMOylation Motif
NEDD8: Neural precursor cell Expressed Developmentally Down-regulated protein 8 NES: Nuclear Export Signal
Introduction
Statistics available from the World Health Organization state that around 14 million new cases of cancer were diagnosed in 2012. They caused 8.2 million deaths worldwide. As such, tumoral diseases are recognized as being among the leading causes of morbidity and mortality worldwide and in addition are predicted to increase in number (Stewart & Wild 2014). Cancer is linked to the accumulation of genetic or epigenetic events that enable uncontrolled proliferation of cells. One type of cancer, leukemia, is caused by the malignant proliferation of cells derived from bone marrow. Leukemic cells disrupt the process called hematopoiesis and invade distant organs as well as the bloodstream. There are different forms of leukemia, which range from relatively un-impactful conditions -and therefore rarely shorten life expectancy- to highly malignant cases, for which very few therapeutic options are available to date. Leukemias are usually classified as myeloid, lymphoid, chronic or acute, depending on the phenotype of the malignant cells. However, even though each class has specific clinic-biological features, they often share common traits like anemia, hemorrhages mainly caused by the loss of platelets (thrombopenia), as well as infections related to myeloid and lymphoid deficiencies. Thanks to treatments involving inhibitors of nucleotide synthesis or DNA replication, such as DNA cross-linkers and topoisomerase inhibitors, some Acute Leukemias can be cured definitively in a great majority of patients. Most of who are children suffering from lymphoblastic leukemia. Such favorable prognosis is far less frequent in other types of acute or chronic Leukemias (de Thé et al. 2012). Acute Promyelocytic Leukemia is a well-studied disease cured through arsenic based treatment and used as a model to study PML.
I) Discovery of ProMyelocytic Leukemia protein (PML)
1) Acute Promyelocytic Leukemia (APL)
a) The disease
The M3 subtype of Acute Myeloid Leukemia (AML-M3), also known as Acute Promyelocytic Leukemia (APL) is a rare condition, with around 100 new cases per year in France (de Thé et al. 2012), around 10% of all AML cases. It is one of the most malignant conditions due to its rapid and spontaneous evolution, as well as its sudden hemorrhages. It was first described by
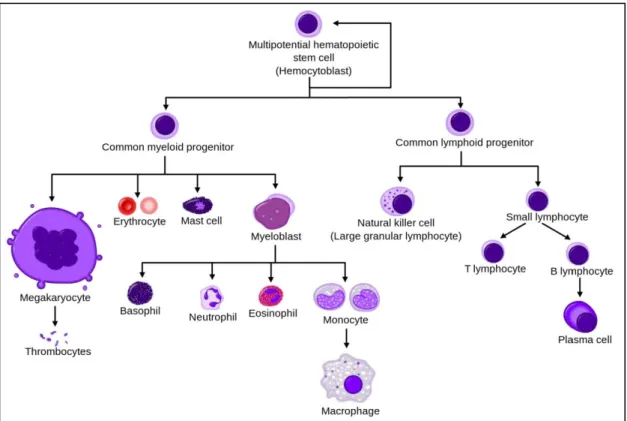
the Swedish hematologist Hillestad in 1957 (Hillestad 1957). Hemorrhages come from a coagulation disorder, due to the release of coagulation cascade activating agents from APL cells, and a low amount of platelets in the blood (de Thé et al. 2012). Normally, blood cells are produced through a process called hematopoiesis, from myeloid progenitor cells (Figure 1). In APL, however, myeloid differentiation is blocked at the promyelocyte stage causing a heavy burden of leukemia blasts (Mi et al. 2015).
Figure 1 : Hematopoietic differentiation from hematopoietic stem cells to mature cells.
(Wikipedia n.d.)
b) The genetic defect
APL is the result of specific chromosomal translocation always involving Retinoic Acid (RA) Receptor α (RARα), present on chromosome 17. In more than 98% of cases, a gene called ProMyelocytic Leukemia (PML, also known as MYL, RNF71, TRIM19 or PP8675) present on chromosome 15, can also be translocated (Kakizuka et al. 1991; de Thé et al. 1991; de Thé et al. 2012; Mi et al. 2015) (Figure 2). The second most common translocation t(11.17) is encoding for PLZF/RARα, and is clinically associated with Retinoic Acid resistant APL and a poorer prognosis (Licht et al. 1995). Promyelocytic Leukemia Zinc Finger protein (PLZF) is a transcriptional repressor and epigenetic regulator involved in hematopoietic stem cell
quiescence or natural Killer T cells formation (McConnell et al. 2015). Here however, we are interested in the translocation t(15;17) (q22;q21) resulting in the production of a fusion oncoprotein called ProMyelocytic Leukemia-Retinoic Acid Receptor α (PML/RARα) that is capable of blocking cell differentiation at the promyelocitic stage (Wang et al. 2010).
Figure 2 : PML-RARα fusion protein comes from the t(15;17) (q22;q21) translocation.
(Lo-Coco & Hasan 2014)
c) PML-RARα dominant negative effect on Retinoic Acid Receptor α
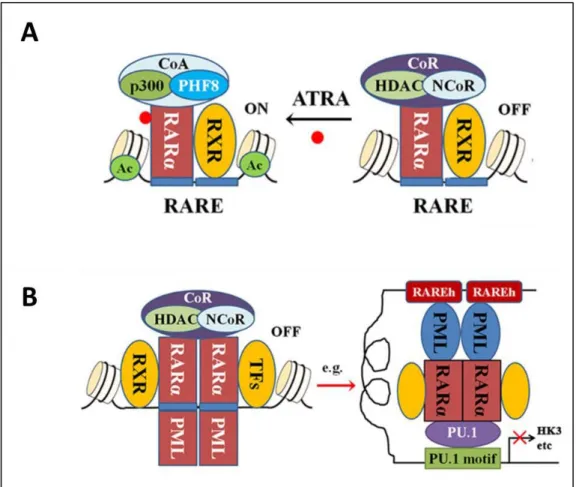
RARα is a Retinoic Acid (RA) nuclear receptor acting as a hormone dependent transcriptional switch. Retinoids are derivatives from vitamin A, and have very important effects on development, differentiation and cell proliferation, by regulating specific gene expression. RA is implicated in many types of cellular response, such as differentiation from multiple progenitors cell, as well as myeloid cells (de Thé et al. 2012). Retinoids can interact with two classes of nuclear receptor proteins: the steroid and thyroid hormone superfamily receptors, the RARs (which include RARα) and the Retinoid X Receptors (RXR). Both receptors can be activated by 9-cis-RA, but All-Trans Retinoic Acid (ATRA) only activates RARα (Lo-Coco & Hasan 2014). Under normal conditions, RARα interacts with RXR to form the RARα/RXR heterodimer that will bind typical Retinoic Acid Response Elements (RAREs) generally located in target genes promoters. In absence of RA, the heterodimer can recruit a corepressor complex (CoR) notably composed of histone deacetylases (HDACs) and will repress transcription of the target genes. However, in presence of physiological concentrations of RA (1x10-9M) (or in
presence of ATRA), a conformational change occurs and dissociation of the corepressors ensues promoting the recruitment of coactivators (CoA) with histone acetyltransferase activity
However, in APL, PML-RARα behaves as an altered RARα that can oligomerise with or without RXR. PML-RARα acts as a constitutive repressor, capable of recruiting a CoR that is not sensitive to physiological concentration of ATRA, finally leading to the characteristic block in differentiation observed in APL (Martens et al. 2010; Mi et al. 2015; Lo-Coco & Hasan 2014). In addition, PML-RARα also binds the hematopoietic transcription factor PU.1 leading to repression of genes depending on it, like glycolytic enzyme HK3 needed for hematopoietic differentiation (Wang et al. 2010; Martens et al. 2010) (Figure 3B). The PML moiety of the oncoprotein also plays a role in APL self-renewal acquisition and differentiation block, by weakening through deacetylation the P53 response to DNA damage (Mi et al. 2015), and by blocking senescence (Korf et al. 2014). However, although both the function of RARα and the effects of the PML-RARα fusion have been clearly characterized, the data available from the PML counterpart does not lead to a clear model of its function and regulation. So what do we know so far about PML?
Figure 3 : Retinoic Acid Receptor α and PML-RAR α function in normal and APL cells.
A. RAR/RXR heterodimer function in presence or not of ATRA. B. PML-RAR interaction leading to differentiation block in APL. (Mi et al. 2015)
2) The PML protein
a) PML isoforms and nomenclature
ProMelocytic Leukemia protein (PML) is a multi-faceted protein that plays key roles in cellular events under physiological and pathological conditions. PML is a strongly conserved protein expressed in all mammals, testifying of its relevant role in cell function (Cheng & Kao 2012) (Figure 4).
Figure 4 : PML protein is conserved in mammals.
PML protein phylogenetic tree based on maximum likelihood method with amino acid substitution (Jones-Taylor-Thornton model). Bootstrap values are displayed on branches. (Cheng & Kao 2012)
Seven PML isoforms are generated through alternative splicing of a single PML gene in the 3̍ exons. This gene is composed of a total of nine exons of which exons 7 and 8 can be divided in two (a and b) and can sometimes contain intronic sequences (Figure 5A). Because of the very high complexity of the gene alternative splicing to form PML isoforms, difficulties rose in the attempt to reach a unifying nomenclature between data banks (GeneBank, NCBI and UniProt), although finally a unified name nomenclature was adopted (Jensen et al. 2001) (Figure 5B). The result of this complex splicing generated six nuclear and one cytoplasmic isoform of PML, which could be experimentally validated. However, other isoforms can be expressed missing exons 4, 5 or 6. In these cases, a letter is added after their name depending on which exons are missing: “a” for isoforms missing exon 5, “b” for missing exons 5 and 6
missing exon 5. The longest isoform, PML I, is 882 amino acids whereas the shortest is about half of the size at 435 amino acids (PML VII) (Figure 5C) (Nisole et al. 2013).
Figure 5 : PML protein isoforms and nomenclature generated from PML gene alternative splicing. A. PML gene contains nine exons, exons 7 and 8 can be separated into “a” and “b”. Some
intronic sequences can be included. B. PML isoforms nomenclature. C. PML protein isoforms encoded by mRNA variants. Asterisks indicate the presence of an incomplete exon or intron caused by an in-frame STOP codon (Nisole et al. 2013).
b) Domains and structure of PML
All isoforms have a common sequence, encoded in exons 1 through 4, which contains the TRIpartite Motif (TRIM). TRIM proteins form a wide family of proteins involved in a variety of cellular processes such as cell differentiation, cell growth, development, apoptosis, and morphogenesis (Munir et al. 2010). More recently, an increasing number of studies are also looking into the role of TRIM proteins in immune signaling. PML is part of the TRIM family (TRIM 19) and possesses, like all TRIMs, an RBCC domain composed of a Really Interesting New Gene (RING) finger domain (R), two cysteine/histidine-rich B-box domains (B1 and B2) and an α-helical coiled-coil domain (CC) (Figure 6). TRIM proteins possess homo and hetero dimerization properties through their α-helical coiled-coil domain. However, PML seems to only homodimerize (Guan & Kao 2015; Reymond et al. 2001). Both B-boxes and RING domains (involving Cysteine and Histidine residues) require zinc ions to stabilize their structure. These structures could be used for protein-protein, protein-DNA or protein-RNA interactions. In addition, PML isoforms I to VII hold a Nuclear Localization Signal (NLS) and isoforms I to V have a SUMO interacting Motif (SIM). Only PML isoform I contains a Nuclear Export Signal (NES) (Figure 6). There are relatively few information on PML structure except from the well-studied TRIM motif meaning that structural information for more than two thirds of the protein are currently unknown.
Figure 6 : PML protein domain and structure.
PML functional domains diagram with RING (R); B-Box1 (B1); B-Box2 (B2) and coiled coil (CC) domains present in all isoforms. Nuclear Export Signal (NES) is only present in PML I while Nuclear Localization Signal (NLS) and SUMO Interacting Motif (SIM) are only present in PML I to V (Guan & Kao 2015).
c) Differential role of PML isoforms
The existence of that many different PML isoforms with such variability in their C-terminus suggests probable specific functions. Here are a few examples of specific functions discovered for PML isoforms:
PML I is the most abundant isoform (Condemine et al. 2006), interacts directly with
Herpes Simplex Virus 1 Ubiquitin ligase ICP0 (Cuchet-Lourenco et al. 2012); PML-I can also form a complex with AML1 (Acute myeloid leukemia 1 protein) leading to the transcriptional activation of genes driving myeloid cell differentiation (Nguyen et al. 2005). PML I is also the only isoform to possess a Nuclear Export Signal (NES).
PML II is involved in innate immune response as it is specifically required for the
induction of IFN-stimulated gene transcription in response to type I interferon (IFN) like NF-κB, STAT1 and CBP (Y. Chen et al. 2015). PML II is also involved in preventing Human Adenovirus type 5 infection through interferon and HSP70 (Atwan et al. 2016).
PML III is interacting with the centrosome, an organelle regulating cell cycle
progression, and controls its duplication through its interaction with Aurora B kinase (Xu et al. 2005). It is also suggested to interact and work with the tumor suppressor TIP60 (also known as Histone acetyltransferase KAT5) (Wu et al. 2009).
PML IV is probably the most studied isoform of PML, it is involved in apoptosis
regulation, DNA damage and senescence (Bischof et al. 2002; Guo et al. 2000). It was also thoroughly studied for its interaction or indirect effect on P53 as well as for its implication in immune response against viruses, such as rabies virus (Blondel et al. 2010; El Asmi et al. 2014; Ivanschitz et al. 2015).
PML V possess an α-helix in its C-terminal domain that allows for strong
homodimerization and seems to be important for recruitment of key partner proteins such as Death Domain-Associated protein 6 (DAXX) or SP100 to particular nuclear structures (Weidtkamp-Peters et al. 2008; Geng et al. 2012).
PML VI is not very well studied and the few papers available have conflicting results.
A study states that PML VI was resistant to arsenic trioxide induced degradation (Maroui et al. 2012) while another one showed that it was not the case (Hands et al. 2014).
PML VII also known as PMLc (cytoplasmic), was found to be essential for the
activation of Transforming Growth Factor Beta (TGFβ) signaling through its interaction with SMAD2/3 and Smad Anchor for Receptor Activation (SARA) (Carracedo et al. 2011; Lin et al. 2004). A recent study underlined the importance of studying this pathways as it plays a key role in some diseases like prostate cancer (Buczek et al. 2015). Cytoplasmic PML was also shown to have protective effects against viral infections through cytoplasmic sequestration of key proteins (McNally et al. 2008; Nisole et al. 2013).
Despite the high diversity of isoforms, PML function appears to be mediated mostly through the formation of complex nuclear structures involving several isoforms, called the PML Nuclear Bodies.
3) PML Nuclear Bodies
Nuclear punctate structures enriched in PML were first described to be localized around the chromatin. These spherical nuclear speckles structures were observed and given different names over the years: Kremer bodies, ND10 (Nuclear domain 10), POD (PML oncogenic domains) or PML Nuclear Bodies (PML-NBs) (Hodges et al. 1998). These structures are heterogeneous at the protein level and very dynamic as many proteins uses PML-NBs as temporary storage or as a platform to get modified and/or interact with other proteins. If PML is not present in the cell, PML-NBs do not form indicating that PML is the scaffold protein required for the formation of these structures (Lallemand-Breitenbach et al. 2010; Batty et al. 2012; Seeler & Dejean 1999). To that end the RBCC domain of PML is also essential (Shen et al. 2006; Jensen et al. 2001; Guan & Kao 2015). Another major component of PML-NBs is the protein SP100, a transcriptional regulator that also happened to be the first and major Nuclear Body associated protein described (Szostecki et al. 1990). Another common component of these bodies is DAXX which is involved in apoptosis regulation (Lallemand-Breitenbach et al. 2010). The size of these structures ranges from 0.1 to 1 µm in diameter. From 5 to 30 PML NBs can be found per nucleus depending on cell types, phase of the cell cycle, stress and nutritional conditions (Figure 7). For example, the number and size of PML NBs will increase under interferon response (Salomoni & Pandolfi 2002; Guan & Kao 2015; Everett et al. 1999).
Figure 7 : PML Nuclear Bodies.
HT1080 cell stably expressing GFP-PML IV (fluorescence microscopy). PML Nuclear Bodies appear in green while, the nucleus is stained using DAPI (blue).
The question is how do these structures form. So far, two models have been proposed to explain the genesis of PML NBs. Based on the identification of Small Ubiquitin like Modifier (SUMO) Interacting Motif (SIM) in the Carboxyl terminus of the protein and the sequence requirements in PML to observe co-localization with GFP-SUMO1, the first model proposes that the nucleation of PML-NBs depend on PML SUMOylation and non-covalent interaction between SUMOylated PML and its SIM (Shen et al. 2006). SUMOylation is a type of post-translational modification that will be discussed later. In addition, this model is comforted by the fact that PML mutants, no longer able to be modified by SUMO, do not form structured PML-NBs but rather aggregates in nucleus thus emphasizing the essential role of SUMO modification in the formation of these nuclear structures (Zhong et al. 2000) (Figure 8).
Figure 8 : PML Nuclear Body formation requires SUMO modification.
Mouse Embryonic Fibroblasts (MEFs) PML-/- immunofluorescence expressing either WT or a non-SUMOylable mutant of (PML-ΔSUMO) (Zhong et al. 2000).
However, PML VI, which does not have the SIM is still capable of forming PML NBs and polymers most likely through its RBCC domain (Shen et al. 2006) leading us to the second
model. This new model suggests a two-step process leading to PML NB formation. Firstly, Reactive Oxygen Species (ROS) such as peroxides, superoxides, hydroxyl radicals or singlet oxygen induce PML oxidation resulting in the formation of disulfide covalent bonds between PML monomers. At this point, PML NB shell becomes associated with the nuclear matrix. Moreover, this seeding step is an accurate reflection of the redox status of the cell. A second step involves UBC9 -the SUMO conjugating enzyme- recruitment causing an increase of PML SUMOylation. In the third and fourth step, a SIM-SUMO-dependent mechanism recruits SUMOylated or SIM-containing partners such as DAXX or SP100, leading to an increased interaction and mature PML NB formation (Sahin Umut et al. 2014). This model also helps to give an explanation regarding the nuclear aggregates formation by non-SUMOylable mutant or SIM deficient isoforms of PML (Figure 9).
Figure 9 : PML Nuclear Body biogenesis model.
Reactive Oxygen Species (ROS) induce PML oligomerization through disulphide bonds. The E2 SUMO conjugating enzyme UBC9 is recruited. Finally, partners are mobilized and form the mature PML NBs (Sahin et al. 2015).
An important and increasing amount of proteins are associated to PML NBs and it has been estimated that more than 160 proteins functionally interact with PML directly or indirectly (Van Damme et al. 2010). Around 120 of these proteins were reported to physically interact with PML as shown through data obtained and analyzed from BIOGRID (http://www.thebiogrid.org/). The data suggest a potential co-regulation of some of its partners and explain PML NBs involvement in different essential cellular functions such as transcription regulation, apoptosis or stress response (Guan & Kao 2015) (Figure 10).
Figure 10 : PML interactome based on data from BIOGRID.
Data obtained from BIOGRID (http://www.thebiogrid.org/) of 120 proteins interacting with PML through affinity interactions (immunoprecipitation) followed by Western blotting experiments. The more publication referencing the interaction, the thicker the line is (Guan & Kao 2015).
Most of these interactions are made possible thanks to post-translational modifications. PML is a heavily modified protein: these modifications regulate the ability of PML to interact
with various partners and allow for stress- and signal-dependent regulation of PML or its interacting partners.
II) Post-translational modifications
As previously described the PML gene is associated with a great diversity of proteins that can be created from a few number of exons thanks to the process of alternative splicing. It is estimated that genes from the entire genome can produce in average around five to six different messenger RNAs (mRNAs). Altogether, the mRNAs comprise the transcriptome, which will encode for as many slightly different proteins. Moreover, another layer of diversification is available at the post-translational level usually by adding simple or multiple modifications through covalent bonding of short amino acids chains or molecules. This process called post-translational modifications can produce up to 10 or more different forms of the same protein. All together, they form the proteome of the cell (Figure 11). In addition to simply increasing
the variety of proteins, these modifications provide and regulate protein function and/or cellular life. They also allow the cell to adapt in a very dynamic and fined tuned way to internal and external stimulus by directly acting on existing proteins rather than producing new ones (Jensen 2004).
Figure 11 : Protein diversity explained, from Genome to Proteome.
Protein diversity obtained from a single gene thanks to alternative splicing and post-translational events. The genome is composed of around 30000 genes and could generate
1) Diversity of post-translational modifications
There is are wide range of post-translational modifications available to the cell to diversify its proteome. These modifications can be classified into two main groups. First, a chemical group, such as phosphate groups, or amino acid chains can be added on a target protein. This study will focus on the latter. On the contrary, the second group removes parts of a protein through hydrolase activity that cuts peptidic bonds found on proteins to be secreted by the cell or addressed to the membrane
These types of modifications can be found in prokaryotes but are mainly present in eukaryotes. On a biochemical level, there are five types of covalent bonds typically found which are oxidation, glycosylation, alkylation, acylation and the most common one: phosphorylation (Walsh et al. 2005) (Figure 12).
Figure 12 : Five major types of covalent modification.
Oxidation, glycosylation, alkylation, acylation and phosphorylation are the main covalent modifications (Walsh et al. 2005).
These modifications can be very diverse and can also target 15 of the 21 amino acids typically found in eukaryotes. Knowing that around 5% of the human genome codes for proteins involved in those modifications, it is not surprising to see hundreds of described covalent
modifications. Targeted proteins can gain new functions, localization or general regulation depending on the nature of the modification (Walsh et al. 2005) (Table 1).
Table 1 : Some examples of post-translational modifications.
(Walsh et al. 2005).
However, another layer of complexity is added since proteins can be modified multiple times at different sites with different modifications, each of them having a possible impact on future or existing modifications. Moreover, some modifications are targeting the same residue on the same protein leading to post-translational modification competition. In addition, these modifications can also, for the most part, be removed by specialized enzymes. This multitude of modification with infinite combination and reversibility is a major key to understand cellular life as they affect protein function. Therefore any conditions impairing this key ecosystem could lead to diseases. PML being a heavily post-translationally modified protein, it is the perfect
2) Phosphorylation
Phosphorylation is the most predominant type of modification in eukaryotes for transducing signals. Phosphates have unique properties; they are chemically versatile, are able to form mono, di or tri-esters and are very abundant on earth. Moreover, the unique size of its ionic shell and charge characteristics allows for specific and inducible protein-protein interactions (Hunter 2012). In mammals, five residues are principally targeted for phosphorylation by specific enzymes called kinases: Serine (Ser, S), Threonine (Thr, T), Tyrosine (Tyr, Y), Histidine (His, H) and Aspartic acid (Asp, D) (Walsh et al. 2005) (Figure 13).
Figure 13 : Five residues phosphorylated in mammals.
(Walsh et al. 2005)
Phosphorylation is a major regulatory mechanism for proteins and PML does not escape that observation since PML protein abundance as well as the number and size of PML NBs are depending on its phosphorylation. PML is a very heavily modified protein with around eighty potential sites for phosphorylation with forty experimentally confirmed sites (Phosphonet 2016) (Figure 14).
Figure 14 : Known PML phosphorylation sites.
Data from PhosphoSitePlus website (www.phosphosite.org/); red residues correspond to data from publications and black residues from expected sites. Zf-B_box corresponds to B-box zinc finger domain and DUF3583 stands for Domain of Unknown Function (PhosphoSitePlus 2016).
Cells use phosphorylation of PML to respond to various stimuli. PML protein possess an N-terminal region stretch enriched in prolines residues (36% of prolines between amino acids 3 and 46) usually exposed at the protein surface which participate in protein-protein interactions like signal transduction and post-translational modification (Kay et al. 2000). Within this region, a lot of residues were identified as phosphorylated in response to Epidermal Growth Factor (EGF) treatment at S8, S36, S38, S40 and T42 (Olsen et al. 2006). Extracellular signal Regulated Kinase (ERK1/2) -which is linked to EGF signaling- is probably phosphorylating site that also promotes SUMOylation at T28, S36, S38 and S40 (Hayakawa & Privalsky 2004). On the other hand, S8, S36 and S38 are phosphorylated by Homeodomain-Interacting Protein Kinase 2 (HIPK2) following DNA damage leading to the accumulation of PML protein and its SUMOylation. HIPK2 activity on PML is also required for effective pro-apoptotic activity of PML after DNA damage (Cheng & Kao 2012; Gresko et al. 2009).
In response to DNA damage like double strand breaks (DSBs), the number of PML NBs increases and multiple sites on PML protein are phosphorylated. However, this can be inhibited by caffeine or wortmannin, which are both Serine-protein Kinase ATM inhibitors. A study suggests that ATM is regulating PML NBs by phosphorylating PML directly or some of it components. (Dellaire et al. 2006). In response to gamma irradiation, DNA damage Check point Kinase 2 (CHK2) phosphorylates S117, which suggests a linked to PML-mediated apoptosis after DNA damage (Yang et al. 2002).
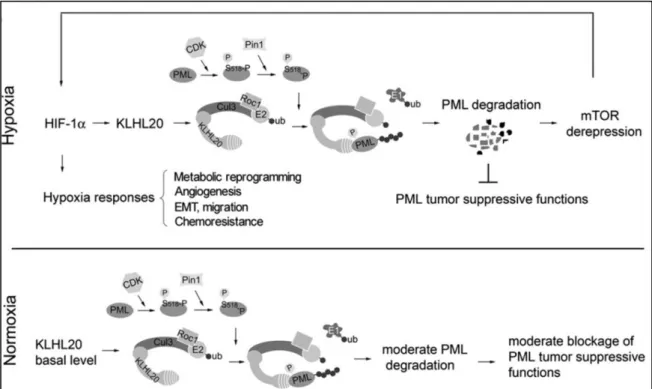
Also, PML nuclear localization requires phosphorylation at an unknown site by Ataxia Telangiectasia and Rad-3-related Kinase (ATR) (Bernardi et al. 2004). Several groups reported that the Nuclear Localization Signal (NLS) region was phosphorylated as well as the SUMO Interacting Motif (SIM) under different stimuli (Cheng & Kao 2012). The Peptidyl-prolyl cis-trans Isomerase NIMA-interacting 1 (PIN-1) mediated degradation of PML is also observed following phosphorylation at S403 and S505 by ERK2 (Lim et al. 2011). Similarly, a study showed that Cyclin-Dependent Kinase 1/2 (CDK1/2) phosphorylation of PML at S518, followed by an isomerization event mediated by PIN-1, in a prostate cancer model, was triggering PML degradation under hypoxia conditions through Cullin3-KLHL20 ubiquitin ligase as part of Hypoxia-Inducible Factor 1-alpha (HIF1α) tumor hypoxia response (Yuan et al. 2011). Under arsenic stress, PML is phosphorylated by ERK1/2 at S527 and S530 leading to PML mediated apoptosis (Hayakawa & Privalsky 2004). PML degradation is also promoted by its phosphorylation by Casein Kinase 2 at S565. This phosphorylation site (560-566,
SSSEDSE) is adjacent to a SIM (VVVI, 556-559) and is influencing interactions with SUMO (Scaglioni et al. 2006; Stehmeier & Muller 2009).
PML is also a key regulator of the cell cycle since PML overexpression in Hela cells leads to cell cycle arrest in G1/S phase whereas cell cycle progression is promoted by loss of PML (Mu et al. 1997; Wang, Delva, et al. 1998). PML phosphorylation appears to be subjected to cell cycle regulation as it is directly interacting with Aurora Kinase A (AURKA) during M phase and during G1 phase of the cell cycle. However, it is unknown if PML phosphorylation plays a direct role in cell cycle control (Cheng & Kao 2012). PML is also phosphorylated at S403 and T409 by Mitogen-Activated Kinase (MAPK) BMK1/ERK5 hereby inhibiting the activation of Cyclin-Dependent Kinase Inhibitor 1 (CDKN1A also known as p21), a key modulator of cell proliferation, through PML (Yang et al. 2010).
Phosphorylation of regions next to NLS, SIM and ubiquitination sites are very important to PML regulation and function, involving many different kinases. However, very little is known about the required stimuli leading to PML phosphorylation and coordination of other post-translational modifications inducing new specific functions (Figure 15).
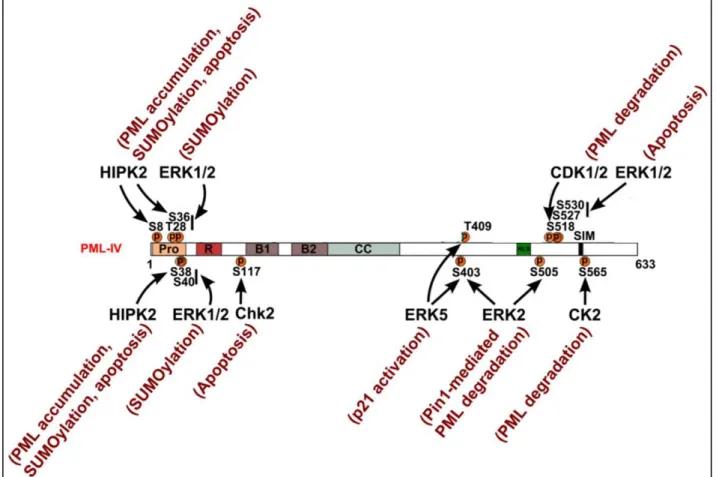
Figure 15 : Known site-specific kinases for PML phosphorylation and associated function.
Diagram showing known phosphorylated residues by corresponding kinases. Arrows indicate the targeted sites and functional consequences are annotated in red next to the corresponding kinase (Guan & Kao 2015).
There is also a great quantity of proteins that are phosphorylated in PML NBs. For example, Cellular tumor antigen p53 (or p53) a famous tumor suppressor protein involved in cell cycle regulation, is phosphorylated in PML NBs by HIPK2 following UV radiation which in turn will promote p53 acetylation by CREB-binding protein (CBP) (Hofmann et al. 2002; D’Orazi et al. 2002). These modifications enhance its pro-apoptotic and transactivation activities, as well as its ability to arrest the cell cycle. Moreover, P53 is enriched in PML NBs after DNA damage where it is stabilized by phosphorylation by CHK2, whose auto-phosphorylation is enhanced in PML NBs (Cheng & Kao 2012; Louria-Hayon et al. 2003; S. Yang et al. 2006).
3) Acetylation
regulation through histone modification (Walsh et al. 2005). PML is also acetylated by Histone acetyltransferase p300 at K487 and K515 promoting its SUMOylation and leading to apoptosis upon Trichostatin A (TSA) treatment, an inhibitor of Histone deacetylases (Hayakawa et al. 2008). PML protein abundance is increased under NAD-dependent protein deacetylase Sirtuin-1 (SIRTSirtuin-1) overexpression whereas its loss causes a decrease in PML protein accumulation. Even though SIRT1 is deacetylating PML, this effect does not appear to be linked to its deacetylating properties (Campagna et al. 2011; Miki et al. 2012). PML modification at K487 is essential for its nuclear localization, as shown through K487R mutants, but the role of acetylation at this site is not well understood yet. On the other hand, K515 acetylation site does not seem to have any effects on PML or PML NBs (Duprez et al. 1999). It is also interesting to note that some post-translational modifications, such as SUMO, can also be acetylated and may play an inhibitory role on PML NB assembly by preventing interactions with its partners such as DAXX, through their SUMO-SIM interphase thus introducing the idea of modified modifiers (Cheng & Kao 2012; Ullmann et al. 2012).
As described, phosphorylation sites for PML are numerous and important, both for its stability and function. Acetylation is important as well but to a minor extend. These modifications are often linked to another very important modification of PML that is SUMOylation.
4) SUMOylation
a) The SUMO protein
Human Small Ubiquitin-like Modifiers (SUMOs) are ~10kDa proteins that have a three-dimensional structure close to Ubiquitin, a protein used in one of the most studied protein modification system that will be described later (Figure 16). Studies found different isoforms for SUMO: SUMO1 (also known as Smt3c, PIC1, GMP1, Sentrin or Ubl1), SUMO2 (also known as Smt3a or Sentrin3), SUMO3 (also known as Smt3b or Sentrin2) and SUMO4. Ubiquitin and SUMO only share around 20% sequence identity at the protein level and display different charge distribution. All SUMO isoforms have an unstructured stretch of 10-25 amino acids at their N-terminus that is not found in any other ubiquitin related protein. It is probably used for the formation of SUMO chains (Tatham et al. 2001) (Figure 16). SUMO proteins are expressed in all eukaryotes and, in vertebrates, all SUMO isoforms are expressed in all tissues except for SUMO4 who is mainly expressed in the kidney, lymph node and spleen (Guo et al.
2004). However, it is not clear whether SUMO4 is present at the active protein level in-vivo especially since it can not be conjugated (Sinha et al. 2016; Geiss-Friedlander & Melchior 2007; Owerbach et al. 2005). Very recently, this protein family got a little bit bigger with the discovery of SUMO5, an isoform highly homologous to SUMO1, essential for PML NB formation and stability through its conjugation on PML at K160 (Liang et al. 2016).
Figure 16 : Ubiquitin and SUMO three dimensional structure comparison.
Structures obtained from crystallography (ubiquitin) and Nuclear Magnetic Resonance Spectroscopy (SUMO). Both structures share the tightly packed secondary structure made of α-helices and β-sheets along with the di-glycine motif at the C-terminus. A long flexible chain at the N-terminus is only observed in SUMO (Dohmen 2004).
All SUMO proteins need to be maturated: the immature forms carry a C-terminal stretch of amino acids (2-11) after an invariant di-glycine (Gly-Gly) motif marking the C-terminus of the mature protein. The mature form of SUMO2 is 95% identical in structure to SUMO1, but shares only 50% sequence identity with it while SUMO2 and 3 only differ from one another by three amino acids on the N-terminus. Given this similarity, they cannot be distinguished by using antibodies so they are usually referred to as SUMO2/3. Despite some functional
redundancy, SUMO1 and SUMO2/3 also display distinct functions as they are conjugated to different proteins in-vivo (Saitoh & Hinchey 2000; Vertegaal et al. 2006; Hay 2005). SUMOylation is an essential process in most eukaryotic organisms like S. cerevisiae, C. elegans, Arabidopsis thaliana and mice but not for fission yeast (Geiss-Friedlander & Melchior 2007). Absence of SUMOylation in mice causes embryonic lethality while SUMO1 haplo-insufficiency or SUMO2 deficiency induces development defect in mice (Geiss-Friedlander & Melchior 2007; Alkuraya et al. 2006; L. Wang et al. 2014). Moreover, it has been shown during the recent years that SUMO was playing an important role in many key cellular processes, such as cardiac development and function (Lee et al. 2015), but also notably in the cellular stress response (Enserink 2015).
b) Enzymatic cascade involved in SUMOylation
SUMOylation results in the formation of an isopeptide bond linking the C-terminal glycine (Gly, G) residue of the modifier protein and the lysine (Lys, K) residue of the acceptor protein. This process involves an enzymatic cascade comprised of three classes of enzymes that are very well conserved and unique to this pathway (Geiss-Friedlander & Melchior 2007). In the first step, SUMO precursor protein is processed by cysteine-specific SUMO proteases, ULPs in yeast but called SENPs (SENtrin specific Proteases) in mammals. This exposes the di-glycine motif mentioned earlier which will then be linked to the unique E1 activating heterodimer enzyme AOS1–UBA2, also known as SAE1-SAE2 dimer. The E1 will catalyze the covalent attachment of SUMO to a reactive cysteine (Cys, C) residue in SAE2 through an ATP-dependent thioesterification reaction. Through a thioester linkage, SUMO is then transferred to the cysteine residue of the unique SUMO E2 conjugating enzyme: UBC9. In vitro, the E2 enzyme is sufficient for conjugating SUMO to a lysine residue on the substrate. However, it is likely receiving help from E3 SUMO ligases in vivo. E3 ligases can serve as scaffold proteins that will bring the SUMO-charged UBC9 and the substrate in close proximity providing in the process the efficiency and specificity to the SUMOylation reaction or just stimulating the E2 enzyme (Enserink 2015; Geiss-Friedlander & Melchior 2007) (Figure 17) . There are relatively few E3 ligases identified, nine to date, the best-known class being the Protein Inhibitor of Activated STAT protein (PIAS) family. For example, PIASy was reported to interact with p53 protein and to be involved in the regulation of cellular senescence and apoptosis (Bischof et al. 2006; Nelson et al. 2001). RANBP2 is also a very well-known E3 ligase targeting SP100 and involved in nuclear import (Pichler et al. 2002). E3 ligases are very interesting to study since
they provide substrate specificity to the pathway making them good candidates for drug development.
An important aspect of SUMOylation is that it is a very dynamic and reversible process. SUMOylated proteins can be deSUMOylated through SENPs activity, the same enzymes used for SUMO maturation (Figure 17). These enzymes have an important functional role in the turnover and spatial regulation of SUMO (Mukhopadhyay & Dasso 2007) and are essential for many cellular processes like chromosome cohesion, mitosis or transcription (Enserink 2015).
Figure 17 : Mechanism of reversible SUMOylation.
Diagram representing the activation (E1: SAE1-SAE2), conjugation (E2: UBC9) and ligation steps leading to substrate SUMOylation. SENPs activity allows for SUMO maturation and deSUMOylation of targeted proteins. It is also important to note than the E1 and E2 are unique unlike E3 ligases.
c) Consensus motifs for SUMOylation
SUMOylation of substrates usually occurs on lysine residues in a canonical SUMO consensus motif ΨKx(D/E), in which Ψ is a large hydrophobic residue and x any amino acid followed by an acidic residue (Rodriguez et al. 2001). The hydrophobic and acidic residues promote stability of the interaction between the substrate and the E2 conjugating enzyme (Lin et al. 2002;
charged amino acid-Dependent SUMO Motifs (NDSMs) and Phosphorylation Dependent SUMO Motif (PDSMs). This last motif is an extended canonical motif in which phosphorylation, by proline-directed kinases, increase SUMOylation efficiency. PDSM and NDSM probably increase SUMOylation efficiency by increasing the stability of the interaction between UBC9 and the substrate because of the negatively charged amino acid (NDSM) or phosphate (PDSM) which will interact with charged amino acids of UBC9 (S.-H. Yang et al. 2006; Yang & Grégoire 2006). It is also important to note that although there is a canonical consensus motif, non-consensus SUMOylation sites are a relatively common event (Enserink 2015).
d) Chain formation and SIM
SUMO is capable to form polymeric chains (only with SUMO isoforms 2 and 3) through its K11 site found in a canonical SUMO consensus motif. However SUMO1 does not contain K11 and is conjugated to its substrate once or at the end of a poly-SUMO chain (Hay 2005; Tatham et al. 2001). These chains were mostly studied and characterized because of their role as an indirect degradation signal. SUMO chains can recruit conserved enzymes known as SUMO Targeted Ubiquitin ligases (STUbls). Theses E3 ubiquitin ligases can then ubiquitinate polySUMOylated substrates leading them to proteasomal degradation as it is the case for PML under arsenic trioxide induced stress (Enserink 2015; Lallemand-Breitenbach et al. 2008).
As shown, SUMO can be used as signal relay for protein degradation but most importantly, it is creating a new interface for protein-protein interactions through a particular motif called the SUMO Interacting Motif (SIM). NMR spectroscopic characterization of the interaction of SUMO and peptides derived from known substrates identified a hydrophobic core with the following consensus to identify SIM: [V/I]x[V/I] [V/I] (Song et al. 2004). Following studies confirmed the essential role of this hydrophobic core and showed that a hydrophobic pocket on SUMO was interacting with hydrophobic side chains of the SIM (Song et al. 2005). This site is also often flanked by acidic amino acid or in some cases phosphorylated residues that will interact with the lysine residue of the SUMO protein to stabilize the interaction (Stehmeier & Muller 2009). This also gives a way for the cell to control protein SUMOylation spatially and temporally as phosphorylated SIM might add specificity for appropriate substrates (Enserink 2015). SUMO can also serve as glue in a complex, stabilizing it through a cooperation of multiple weak SUMO-SIM interactions that would significantly increasing stability (Enserink 2015; Psakhye & Jentsch 2012).
SUMO plays an important role under normal conditions by maintaining cell homeostasis, promoting cell growth and proliferation whereas under stress conditions such as heat shock, DNA damage, oxidative stress but also viral infections, it is a key component in the cellular response by activating pro-survival pathways (Saitoh & Hinchey 2000; Enserink 2015).
e) PML SUMOylation and SIMs
Ran GTPase-activating protein 1 (RanGAP1) was the first SUMO target identified (Matunis et al. 1996) and was closely followed by another heavily SUMOylated protein that is PML. PML possess one SUMO-Interacting Motif (SIM) at the C-terminus of its sequence (Shen et al. 2006) and is also heavily SUMOylated by SUMO1 and SUMO2/3 (Cheng & Kao 2012). Three main SUMOylation sites on PML were identified at K65, K160 and K490 (Kamitani et al. 1998) (Table 2). Three more sites were identified for poly-SUMOylation under arsenic trioxide treatment at K380, K400 and K497 (Galisson et al. 2011). Two more potential polySUMO sites were discovered through quantitated proteomics at K226 and K616 but further confirmation is needed (Vertegaal et al. 2006) (Table2).
Table 2 : Known PML SUMOylation sites
Fluorescence microscopy data show that PML NBs and SUMO1 co-localize and that SUMOylation is essential to the maintenance of their structure and function (Müller, Matunis, et al. 1998; Gao, Cheng, et al. 2008). PML dimerization is a prerequisite for de novo assembly of PML NBs along with PML SUMOylation for recruitment of components such as SP100 or DAXX but also for the turnover and retention of PML in NBs and their integrity (Weidtkamp-Peters et al. 2008; Shen et al. 2006; Lallemand-Breitenbach et al. 2010). The importance of SUMO1 conjugation to PML for the maintenance of the integrity of PML NBs was also shown
Known and verified SUMOylation sites using Consensus Motif (CM); Inverted Consensus Motif (ICM); Hydrophobic Cluster SUMO Motif (HCSM) or Negatively charged amino-acid Dependent SUMO Motif (NDSM). Targeted lysine are shown in red (Nisole et al. 2013).
number of PML NBs was observed as well as a decrease in the amount of SUMO2/3 conjugated to PML compared to wild type cells (Evdokimov et al. 2008). SUMO3 conjugation also appears to regulate nuclear localization and formation of PML NBs when conjugated to K160 (Fu et al. 2005). Also, the SUMOylation of PML is suggested to play a role on the localization of other components of the NBs probably through SUMO-SIM interaction (Cheng & Kao 2012).
f) Regulation of PML through the SUMO pathway
PML SUMOylation seems to be dependent on the cell cycle: its SUMOylation is elevated during interphase and declines during mitosis (Everett et al. 1999). SUMOylation of PML can also be triggered through DNA damage induced chemically, for example with Adriamycin, a chemotherapeutic agent (Gresko et al. 2009). PML NBs regulate transcription through sequestration or dissociation of transcription factors. Therefore, PML SUMOylation might have both direct and indirect effects on transcriptional regulation. One example of such indirect regulation is the release from PML NBs of Signal Transducer and Activator of Transcription 3 (STAT3), due to PML deSUMOylation by SENP1 previously activated through Interleukin-6 treatment (Kawasaki et al. 2003; Ohbayashi et al. 2008). PML SUMOylation also plays a role in apoptosis regulation through PML NBs interaction with DAXX or P53 proteins (Meinecke et al. 2007; Cheng & Kao 2012).
SUMOylation of PML is controlled in part by the E3 SUMO ligases targeting it. The first identified PML E3 SUMO ligase was RAN Binding Protein 2 (RanBP2) which mediated the K490 SUMOylation, essential for PML NBs maintenance (Tatham et al. 2005; Satow et al. 2012; Saitoh et al. 2006). Another E3 ligase recently discovered for PML is Protein Inhibitor of Activated STAT1 (PIAS1) that would be responsible for the SUMOylation of K65 and K160 residues. These two sites also appear to influence Casein Kinase 2 activity on PML leading to S565 phosphorylation and subsequent degradation of PML (Rabellino et al. 2012). Histone Deacetylate 7 (HDAC7) was proposed as an E3 ligase for PML since its presence is required to keep PML SUMOylated, but there is no evidence so far that it is acting directly as a SUMO E3 ligase (Gao, Ho, et al. 2008). Recently a new SUMO2/3 E3 ligase for PML was discovered, ZNF451-1, modifying PML at established SUMOylation sites (K65/K160/K490) and involved in its stability (Koidl et al. 2016). A more indirect approach can also alter PML SUMOylation status: for example Beta-catenin was shown to prevent RanBP2 from interacting with PML thus preventing its SUMOylation (Satow et al. 2012). Finally another way to prevent PML from
interacting with its E3 ligases would be by restricting the localization of the substrate or the enzymes involved (Cheng & Kao 2012).
Another way to regulate the SUMOylation of PML is through the involvement of specific deSUMOylases (SENPs). In Humans, there are six SENPs, SENP-1, -2,-3,-5 and -6. The nuclear SENP1 was shown to specifically remove SUMO1 from PML (Gong et al. 2000). SENP2 isoform (SuPr-1) has also been involved on PML deSUMOylation, causing c-Jun, transactivation (Best et al. 2002). PML PolySUMO chains formed by SUMO2/3 are removed by SENP3 under mild oxidative stress causing PML NBs disruption and stimulating cell proliferation (Han et al. 2010). Just as SENP3, SENP5 deconjugates SUMO2/3 at the SUMOylation sites K160 and K490 as well as all SUMO isoforms present on K65 (Gong & Yeh 2006). SENP6 was reported to specifically remove SUMO2/3 and a loss of this SENP causes an increase of cell death as well as an increase in PML NBs (Hattersley et al. 2011; Mukhopadhyay et al. 2006). In line with MEFs experiment, removing SENP increases PML NBs, whereas knocking out SUMO1 in MEFs causes a decrease. It is rather obvious that SENPs, just as E3 ligases, are involved in PML SUMOylation dynamics however, it is still poorly understood under which conditions each component play a role and how they are coordinated (Cheng & Kao 2012) (Figure 18). Besides SENPs, other deSUMOylases exist such as Ubiquitin-specific peptidase-like protein 1 (USPL1) (Schulz et al. 2012).
Figure 18 : Known SUMOylation sites and E3 ligases of PML.
Diagram showing residues being modified. SUMO E3 ligases target is indicated with an arrow and functional consequences are indicated in red. SUMOylation resulting from arsenic trioxide stress are indicated in green. Both Mono-SUMOylation by SUMO1 and PolySUMOylation are observed at K65 while poly-SUMO chains were described at K160, K380, K400, K490 and K497. Some reports also indicate polySUMOylation at K226 and K616 but this needs to be verified (Guan & Kao 2015).
5) The SUMO/Ubiquitin coupled pathway
SUMOylation of PML is a key factor that controls PML stability in response to extracellular or intracellular stimuli. Arsenic Trioxide (AS2O3 or ATO) is a well-known drug currently used as
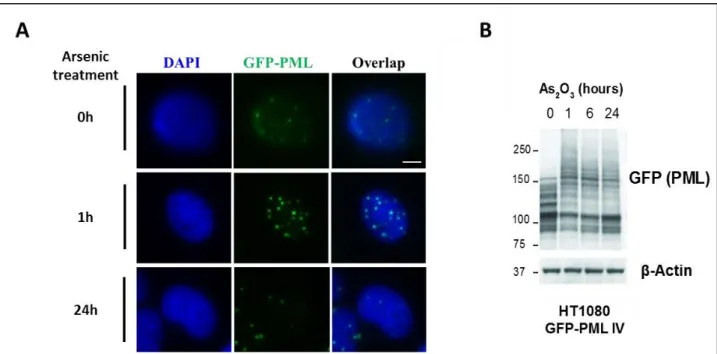
a therapeutic agent for Acute Promyelocytic Leukemia (APL) treatment. It induces an increase of PML SUMOylation about an hour after treatment, which later leads to its degradation by the proteasome. PML-RARα is also degraded in the same way (Müller, Matunis, et al. 1998) (Figure 19).
Figure 19 : PML is hyperSUMOylated and degraded under arsenic stress.
Western blot from Acute Promyelocytic Leukemia (APL) cells after treatment with 1µM Arsenic Trioxide (AS2O3) for indicated times.
Both All Trans-Retinoic Acid (ATRA) and Arsenic Trioxide (ATO) are used to treat APL as they both target Leukemic Blasts. ATRA will lead to PML-RARα, and RARα degradation and patient remission by primarily forcing differentiation by activating transcription. However, this treatment is unable to efficiently destroy Leukemia Initiating Cells (LICs) ultimately leading to disease relapse (Figure 20, right panel). On the other hand, ATO leads to PML-RARα and PML degradation and ultimately to patient remission by inducing both differentiation and apoptosis of leukemic blasts and eradication of LICs thus preventing the disease relapse (Figure 20, left panel). The vast majority of patients are now cured with a combination of both ATRA and ATO (Dos Santos et al. 2013). A study also showed that trivalent antimonials could be used instead of ATO to maybe reduce side effects such as mitochondrial toxicity or ROS (Müller, Miller, et al. 1998).
Figure 20 : Arsenic Trioxide (ATO) and All-Trans Retinoic Acid (ATRA) effects in the cure of APL.
ATRA forces differentiation but does not destroy Leukemia Initiating Cell (LICs) causing disease relapse. ATO causes apoptosis and differentiation and is able to efficiently destroy LICs. A combination of both treatments (dashed arrow) is currently used and cures most cases as they both work synergistically
(Dos Santos et al. 2013).
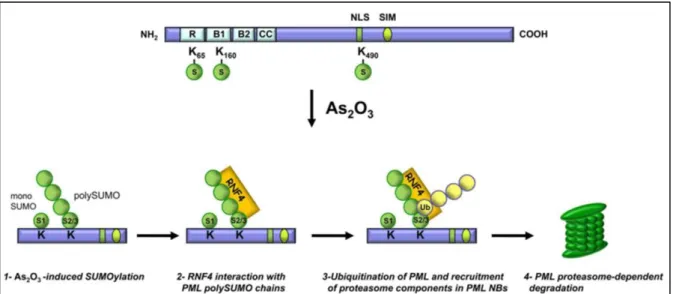
Arsenic trioxide (As2O3) directly binds to cysteine-rich zinc finger in the RBCC
domains of PML (Figure 21, top part). Arsenic trioxide binding causes a conformational change in PML that will in turn promote the interaction between PML and UBC9, the unique SUMO2 E2 conjugation enzyme (Zhang et al. 2010). This SUMOylation event can however be inhibited by calyculin, a serine/threonine phosphatase inhibitor, suggesting that some de-phosphorylation events are needed prior to PML SUMOylation, either on PML or on one of its interacting partners (Müller, Miller, et al. 1998). HyperSUMOylated PML is targeted for ubiquitination by the E3 ubiquitin ligase RNF4 (also known as SNURF) to be degraded by the proteasome. RNF4 protein contains multiple SIMs in its N-terminus and a C-terminal RING-type E3 ligase domain. These SIMs interact with SUMO2 chains of PML and allow RNF4 to ubiquitinate PML and its chains leading to its degradation (Lallemand-Breitenbach et al. 2008; Tatham et al. 2008) (Figure, 21 bottom part). SUMOylated PML also primes PML
of PML and leads to its degradation as shown in APL cells, in Non-Small Cell Lung carcinoma cells (NSCL) as well as in human primary tumor specimens (Rabellino et al. 2012).
Figure 21 : PML degradation key-step events under Arsenic trioxide (AS2O3) induced stress.
Diagram representing Arsenic Trioxide induced SUMOylation of PML and subsequent recognition of SUMO2/3 chain by RNF4 SIMs leading to Ubiquitination and degradation of PML by the proteasome (Nisole et al. 2013).
PML degradation under Arsenic Trioxide induced stress allowed the discovery of a coupled SUMO-Ubiquitin pathway in which RNF4 interacts with PML through SUMO chains leading to its ubiquitination and proteasomal degradation. Thus, ubiquitination also plays an important role on PML stability.
6) Ubiquitination
Ubiquitination is a multifaceted post-translational modification that is highly dynamic and involved in all aspects of the cell biology. Ubiquitin is a 76 amino acid protein that can be modified and is involved in many types of signal transduction leading to various cellular outcomes. The most common and remarkable one is the targeting for proteasome dependent degradation but Ubiquitination is not only about degradation. For example, P53 function can be regulated through its ubiquitination by the ubiquitin ligase E4F1 leading to cell cycle arrest specific transcriptional program (Le Cam et al. 2006).
a) Enzymatic cascade
a sophisticated three-step enzymatic cascade very similar to SUMOylation. However, the two pathways are completely independent and do not share any enzymes. First, there is the priming of ubiquitination through an ATP-dependent covalent attachment of the Ubiquitin protein to one of the two ubiquitin activating enzymes E1. Spontaneous transfer of ubiquitin to an ubiquitin conjugation enzyme E2 is facilitated by the E1-Ubiquitin intermediate. At this point, two different paths could be taken, both resulting in substrate ubiquitination. In the first option, Ubiquitin (Ub) is directly transferred to the substrate through an E3 ubiquitin ligase containing a RING domain capable of directing the E2-Ub species to its substrate. In the second option, Ubiquitin is passed on to a HECT (Homologous to the E6-AP Carboxyl Terminus) domain of an E3 ubiquitin ligase to which the E2-Ub enzymes would have been associated. Ubiquitin is then directly transferred from the E3 ligase to the substrate through a thioester bond (Lee & Diehl 2013). Ubiquitinated proteins can be recognized by Ubiquitin Binding Domains (UBDs) on receptors for example (Husnjak & Dikic 2012). Like SUMOylation, this process is reversible thanks to a specialized family of enzymes called DeUbiquitinases (DUBs) (Komander et al. 2009) (Figure 22).
Figure 22 : Ubiquitination enzymatic cascade leading to substrate degradation.
Diagram representing the consecutive activities of the three enzymes types involved in Ubiquitination: activation (E1s), conjugation (E2s) and ligation (E3s). This process is reversible thanks to deubiquitinases (DUBs). Many enzymes are involved in this process; approximate number are shown next to the type of enzyme. Note that this diagram takes degradation as an example, not all ubiquitination event lead to degradation