Delivery of Biomolecules into Mammalian Cells Using Anthrax Toxin
by
ARCHNES
MASSACHUSETTS INSTITUTE
Amy Ellen Rabideau OF TECHNOLOGY
B.S. Chemistry and Biology
NOV 0
9
2015
Syracuse University, 2010LIBRARIES
Submitted to the Department of Chemistryin Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy
at the
Massachusetts Institute of Technology September 2015
C 2015 Massachusetts Institute of Technology All rights reserved
Signature of Author:
Signature redacted
1)
Department of Chemistryugust 28th, 2015
Certified by:
Signature redacted
Bra ley L. Pentelute Pfizer-Laubach Career Development Professor of Chemistry Thesis Supervisor
Signature redacted
Accepted by:
Robert W. Field Haslam and Dewey Professor of Chemistry Chairman, Departmental Committee for Graduate Students
This doctoral thesis has been examined by a committee of the Department of Chemistry as
follows:
Signature redacted
Alexander M. Klibanov Novartis Professor of Chemistry and Bioengineering Thesis Committee Chair
Signature redacted
Bradley L. Pentelute Pfizer-Laubach Career Development Professor of Chemistry Thesis Supervisor
Signature redacted
John M. Essigmann William R. and Betsy P. Leitch Professor of Chemistry and Biological Engineering
Signature redacted
Barbara Imperiali Class of 1922 Professor of Chemistry and Biology
Delivery of Biomolecules into Mammalian Cells Using Anthrax Toxin
by
Amy Ellen Rabideau
Submitted to the Department of Chemistry on August 2 8th, 2015 in Partial Fulfillment of the
Requirements for the Degree of Doctor of Philosophy
Abstract
The intracellular delivery of biomolecules into mammalian cells is a major challenge due to the plasma membrane, which acts as a barrier between the extracellular environment and intracellular components. Recently, a non-toxic delivery platform derived from anthrax lethal toxin has been developed to overcome this challenge for the delivery of biomolecules into the cytosol of mammalian cells. The PA/LFN delivery platform has been used to deliver over 30 known biomolecules of diverse sequences, structures, and functionalities. Collectively, these translocation studies have helped to elucidate the translocation mechanism and to probe
intracellular biological processes.
In this thesis, the PA/LFN delivery platform was used to analyze the delivery of assorted biomolecules through the PA pore. A facile, modular ligation strategy using sortase A was developed for the conjugation of biomolecules to LFN. The biomolecules for this analysis included antibody mimic proteins with defined sizes and secondary structures, mirror image peptides and proteins, polypeptides containing non-canonical amino acids or small molecule drugs, and cyclic peptides. Our translocation analyses have led to guidelines for translocation as well as insight into design parameters for the efficient delivery of new cargos. The PA/LFN delivery platform has also been used to translocate bioactive cargos for the disruption of intracellular protein-protein interactions (PPI). The translocation efficiency and bioactivity of a tandem monobody to Bcr-Abl, an affibody to hRaf- 1, and a mirror image peptide to MDM2 were analyzed. Efficient translocation and disruption of the intended PPI in each case indicated that the delivery platform could be used to deliver bioactive cargos into cells for therapeutic utility.
As an application of this technology, the PA/LFN delivery platform was employed to analyze the intracellular stability of mixed chirality proteins. One major factor that governs a protein's stability is the N-end rule, which states that the N-terminal residue of a protein impacts its intracellular stability through the ubiquitin (Ub)/proteasome system. Utilizing the PA/LFN delivery platform, the stability of proteins containing one N-terminal D-amino acid was analyzed. In contrast to N-terminal L-amino acids, each N-terminal D-amino acid abrogates protein degradation by the N-end rule pathway.
Thesis Supervisor: Bradley L. Pentelute
Acknowledgements
Throughout my upbringing my dad emphasized the philosophy, "Learning is the journey!" I do not think I entirely understood the true meaning behind what he has been saying all these years until graduate school, where some of the most important lessons are not taught in a classroom. My graduate career has been filled with new experiences, troubleshooting, incredible colleagues, and a lot of really amazing science. I am grateful for everyone who has supported and guided me throughout this phase of my learning journey.
I am indebted to my advisor, Prof. Brad Pentelute for taking me on as his first graduate
student and exposing me the complexities of biochemistry research. I will never forget walking into our empty lab in 56-546 on my first day and thinking, "What did I get myself into?" Fortunately, in true Pentelute lab style (fast), after only a few months of ordering and organizing, we were up and running-expressing proteins, synthesizing peptides, growing cells, and writing grants. Since the beginning, Brad has encouraged me to think big and explore new ideas with confidence. He helped me realize that simple experiments are often the most successful and the more controls, the better. Under Brad's mentorship, I have developed the intuition to think critically through challenging questions.
Thank you to Prof. Alex Klibanov, my thesis chair, for his advice and helpful conversations over the past five years. I also thank Prof. John Essigmann for his encouragement and support during my transition into the Pentelute lab. Thank you to Prof. Barbara Imperiali for advising me during my first MIT experience in Summer 2009 and for her constant support throughout my graduate career.
Completing a Ph.D. would be difficult without the help of incredible lab mates. I am so grateful to have developed the translocation project with Dr. Xiaoli Liao during the first two years in the Pentelute lab. Together we expressed the lab's first proteins, worked through troublesome ligations, and got the project off the ground. I learned so much from Xiaoli-team translocation's progress would not have been possible without her help. I also thankful for all the former and current Pentelute lab members for everything they taught me and the fun times-Alex L., times-Alex M., times-Alex S., times-Alex V., Anthony, Chi, Colin, Dale, Dan C., Dan D., Daphne, Ethan, Faycal, Gizem, Guillaume, Hansol, Jingjing, Jun, Justin, Kyle, Mark, Michael, Mike, Peng, Richard, Rocco, Surin, Tatiana, Ted, Tessa, Tuang, Yekui, Yuta, Zak, and Zi-Ning. I am extremely proud of and impressed by the lab's success to date; I am excited to see what the future holds!
Graduate school would have been completely different had it not been for my colleagues and classmates. These are the people who shared in the victories, helped troubleshoot the struggles, and listened to the problems: Ali, Katya, Jingnan, Jon, Nootaree, Vinita, Whitney, and especially my biological classmates, Austin, Haritha, Kanchana, and Megan. A special thank you to Austin, a talented scientist and devoted friend, who always had time to help me think through tough problems, to eat lunch at Cosi, or to take a run around the Charles. His enthusiasm for science was infectious and I am forever grateful for all his support. My Yorktown and Syracuse friends have been extremely encouraging and always willing to talk about non-science things over the past five years as I navigated the ups and downs of graduate school: Anna, Dana, Eileen, Emily, Heather, Kaitlyn, and Rachel.
My family has been my rock and greatest support system throughout my graduate years.
No matter what time of day it is, someone is always available to listen, laugh, or advise. My best friend and sister, Erin, for always knowing exactly how to calm me down and make me smile
and for buying me my first yoga mat. My mom, for always having time to talk and listen to me and for teaching me perseverance and dependability. My dad, for the pep talks and wise words before all my big presentations and meetings and for teaching me to always think about what comes after what comes next. Indeed, learning is the journey.
Table of Contents
Abstract 5 Acknowledgements 7 Table of Contents 9 List of Figures 14 List of Tables 18Chapter 1: Delivery of Non-Native Cargo into Mammalian Cells Using Anthrax Lethal
Toxin 18
1.1. Introduction 19
1.2. Anthrax Lethal Toxin 23
1.3. Fusion and Conjugation Strategies 24
1.4. Methods to Study Translocation 28
1.5. Delivery of Non-Native Cargos 31
1.5.1. Natural Proteins 31
1.5.2. Engineered Protein Variants 32
1.5.3. Stabilized Protein Conjugates 34
1.5.4. Mirror Image Polypeptides 35
1.5.5. Non-Natural Peptides 36
1.5.6. Cyclic Peptides 37
1.5.7. Small Molecule Drugs 37
1.5.8. Modified Delivery System 38
1.5.9. Retargeting PA 39
1.6. Summary and Outlook 42
1.7. References 43
Chapter 2: Delivery of Antibody Mimics into Mammalian Cells via Anthrax Toxin
Protective Antigen 48
2.1. Introduction 49
2.2.2. Translocation requires a functional PA and endocytosis 54
2.2.3. Western blot analysis of cytosolic fraction 57
2.2.4. Comparison to TAT-mediated delivery 58
2.2.5. Delivery of a tandem monobody for SH2 domain of Bcr-Abl 61
2.2.6. Delivery of an affibody for Raf-1 67
2.3. Discussion 69
2.4. Experimental 74
2.4.1. Materials 74
2.4.2. One-pot sortagging reaction using Staphylococcus aureus SrtA* 74
2.4.3. Protein synthesis inhibition assay 75
2.4.4. Uptake of Lvs or TAT-HA-I to -4 in CHO-KI cells 75
2.4.5. Cytosolic protein extraction and whole cell lysate preparation 76
2.4.6. Western blot 76
2.4.7. Co-immunoprecipitation of Lv5 with Abl kinase 76
2.4.8. TUNEL assay with Lv5 in K562 cells 77
2.4.9. Transfection of HEK 293T with pcDNA3-ABRaf 77
2.4.10. Delivery of Lv6 into HEK 293T cells 78
2.4.11. Construction of plasmids for recombinant proteins and transfection 78
2.4.12. Protein expression and purification 79
2.4.13. Synthesis of TAT peptide and native chemical ligation (NCL) of TAT-DTA 80
2.4.14. Synthesis of TAT-HA-LPSTGG peptide and sortagging to G5-proteins 80
2.5. Acknowledgements 81
2.6. Appendix 82
2.6.1. LC-MS Traces 101
2.7. References 106
Chapter 3: Delivery of Mirror Image Polypeptides into Cells 110
3.1. Introduction 111
3.2. Results 113
3.2.1. Delivery of mirror image polypeptides 113
3.2.3. Translocation of a D-peptide for MDM2 118
3.2.4. Translocation of mirror image proteins 122
3.3. Discussion 125
3.4. Experimental 126
3.4.1. Materials 126
3.4.2. Solid phase peptide synthesis (Boc) 127
3.4.3. Solid phase peptide synthesis (Fmoc) 127
3.4.4. Analytical LC-MS 128
3.4.5. Preparative, semi-preparative, and analytical RP-HPLC 128
3.4.6. Synthesis of D-affibody 129
3.4.7. Synthesis of D-affibody-alkyne 131
3.4.8. Synthesis of D-GB1 131
3.4.9. Circular dichroism (CD) spectroscopy of folded proteins 133 3.4.10. Synthesis of biotinylated p53/MDM2 Inhibitor D-peptide 133
3.4.11. Construction of plasmids for recombinant proteins 134
3.4.12. Protein expression and purification 134
3.4.13. One-pot sortagging reaction using Staphylococcus aureus SrtA* 135
3.4.14. Translocation of SDvs and protein inhibition assay in CHO-Ki cells 136
3.4.15. Cytosolic protein extraction and whole cell lysate preparation for western blot 137
3.4.16. Western Blot of Svl, 2, 4, and 5 translocated into CHO-KI cells 137
3.4.17. Trypsin digestion of L- and D-Affibody 138
3.4.18. Pull down of Sv4-biotin in U-87 MG cells 138
3.4.19. Translocation of Sv4 and Sv4-biotin in U-87 MG or K562 cells 138
3.4.20. Binding interaction between SUMO-25- o9MDM2 and 4 or Sv4 139
3.5. Acknowledgements 140
3.6. Appendix 141
3.6.1. LC-MS Traces 157
3.7. References 160
Chapter 4: Translocation of Non-Canonical Polypeptides into Cells Using Protective
4.1. Introduction 164
4.2. Results 168
4.2.1. Translocation of non-canonical polypeptide cargos with backbone or side chain
modifications 168
4.2.2. Translocation of cyclic peptides 171
4.2.3. Translocation of complex small molecules 173
4.2.4. Translocation of intact cargo 176
4.3. Discussion 179
4.4. Experimental 181
4.4.1. Materials 181
4.4.2. 'H Nuclear magnetic resonance (' H NMR) 182
4.4.3. Synthesis of docetaxel-maleimide 182
4.4.4. Synthesis of doxorubicin-maleimide 183
4.4.5. Solid phase peptide synthesis (Boc) 184
4.4.6. Solid phase peptide synthesis (Fmoc) 184
4.4.7. Protein expression and purification 185
4.4.8. Sortase-mediated ligation 186
4.4.9. LC-MS analysis 186
4.4.10. Preparative, semi-preparative, and analytical RP-HPLC 187
4.4.11. Conjugation of docetaxel, doxorubicin, and MMAF to G5-LRRLRAC 187
4.4.12. Cyclization of linear peptide using native chemical ligation 188
4.4.13. Protein synthesis inhibition assay 189
4.4.14. Translocation of LDn1-1 I and cytosolic and total cell lysate extraction 190
4.4.15. Western blot of extracted material 190
4.5. Acknowledgements 191
4.6. Appendix 192
4.6.1. LC-MS Traces 200
4.7. References 204
Chapter 5: A D-Amino Acid at the N-terminus of a Protein Abrogates its Degradation by
5.1. Introduction 207
5.2. Results 209
5.2.1. Sortase A Attaches One D-Amino Acid onto the N-Terminus of LFN-DTA 209
5.2.2. One N-Terminal D-Amino Acid Stabilizes LFN-DTA to Proteasomal Degradation 209
5.2.3. Proteasomal Stabilization is Not an Artifact of the Sortag 212
5.2.4. LFN-DTA with One N-terminal D-Amino Acid is Stable In Vitro 214
5.2.5. LFN-DTA with One N-terminal D-Amino Acid is Not Ubiquitinated 214
5.2.6. N-terminal Stabilization is Not Protein-Specific 216
5.2.7. RRSPc2 is a Ras/Rap 1-Specific Endoprotease 219
5.2.8. EGFR-Targeted Delivery of RRSPc2 Interrupts the MAPK Pathway 219
5.3. Discussion 222
5.4. Experimental 225
5.4.1. Materials 225
5.4.2. Protein Expression and Purification 225
5.4.3. Fmoc Solid Phase Peptide Synthesis 226
5.4.4. Sortase A-Mediated Ligation of X-LFN-DTAut Constructs 227
5.4.5. Protein Synthesis Inhibition Assay with X-LFN-DTAmut Constructs 228
5.4.6. Translocation and Western Blot Analysis with X-LFN-DTAmut Constructs 228
5.4.7. Native Chemical Ligation of Native X-LFN-DTAmut 229
5.4.8. In Vitro Stability of X-LFN-DTAmut Constructs 230
5.4.9. Streptavidin Pulldown of Ubiquitinated Constructs 231
5.4.10. Stabilization of X-DTAmut or X-DARPin after Translocation 231
5.4.11. EGFR-Targeted Translocation of RRSPc2 233
5.5. Acknowledgements 234
5.6. Appendix 235
5.6.1. LC-MS Traces 250
List of Figures
Figure 1.1.1. Anthrax lethal toxin is comprised of two discreet components. 21
Figure 1.1.2. Translocation of biomolecules using PA/LFN delivery platform. 22
Figure 1.3.1. Modular chemistry to modify LFN or LFN-DTA at N- or C-terminus. 27 Figure 1.4.1. Representative assay results from common translocation assays. 30 Figure 1.5.1. Translocation efficiency has been analyzed for various peptides and proteins. 40 Figure 2.1.1. Delivery of antibody mimics into the cytosol by the PA/LFN system. 52 Figure 2.2.1. Control experiments validating the translocation mechanism of LDv1 and Lvl. 56 Figure 2.2.2. TAT peptide mediated translocation of DTA and antibody mimics. 60 Figure 2.2.3. Delivery of Lv5 and binding of Lv5 to Abl kinase in K562 cells. 63 Figure 2.2.4. Monitoring of apoptosis of K562 cells treated with Lv5 and PA by TUNEL assay.
66 Figure 2.2.5. Perturbation of the MAPK signaling pathway by PA mediated delivery of an
affibody (Lv6) that targets Raf. 68
Figure 2.6.1. One-pot sortagging reaction. 82
Figure 2.6.2. Coomassie stained SDS-page gel of LDvs and Lvs obtained after sortagging and
purification. 83
Figure 2.6.3. Thermal stability of 1 OFN3 (4) and HA4 (4mut) was monitored by circular
dichroism (CD) spectroscopy. 84
Figure 2.6.4. Western blot analysis of delivered Lvs. 85
Figure 2.6.5. Coomassie stained SDS-PAGE gel of TAT-HA-I to -4 and -4mut (1 pg). 86 Figure 2.6.6. Cellular uptake of TAT-HA-I to -4 and -4mut. CHO-KI cells. 87 Figure 2.6.7. The level of protein synthesis inhibition in K562 cells. 88 Figure 2.6.8. SPR curves for SUMO-SH2 and varying concentrations of Lv5 or HA4-7c 12. 89 Figure 2.6.9. Linear relationship between signal intensity of each band and the amount of
protein loaded. 90
Figure 2.6.10. Phosphorylation analysis of Bcr-Abl. 91
Figure 2.6.11. Apoptosis measurement of K562 cells. 92
Figure 2.6.12. MTS cell viability assay. 93
Figure 3.2.1. Delivery of D-cargo sortagged onto LFN and LFN-DTA. 114 Figure 3.2.2. Translocation of mirror peptides using PA/LFN- 117
Figure 3.2.3. Translocation of a D-binder to MDM2. 121
Figure 3.2.4. Synthesis and translocation of mirror image proteins. 124 Figure 3.6.1. Immunoblot of media from CHO-KI cells treated with Svl-3. 146 Figure 3.6.2. Linear relationship between Sv4 band intensity and the amount of protein loaded.
147 Figure 3.6.3. Calibration curve for the interaction between immobilized biotin- -29 p53 and
SUMO-25- 09MDM2. 148
Figure 3.6.4. Binding interaction between SUMO-25
-'09MDM2 and 4 or Sv4. 149 Figure 3.6.5. Quantification of MDM2, p53, and p21 protein levels for U-87 MG cells. 150
Figure 3.6.6. LC-MS characterization of D-affibody synthesis. 151
Figure 3.6.7. LC-MS characterization of D-GB 1 synthesis. 152
Figure 3.6.8. CD of L- and D-affibody and L- and D-GB 1. 153
Figure 3.6.9. Trypsin digestion of L-affibody, D-affibody, Sv5-L, and Sv5-alkyne over time. 155 Figure 3.6.10. LC-MS characterization of D-affibody-alkyne and D-affibody-biotin. 156
Figure 4.1.1. Delivery of non-canonical polypeptide cargo into the cytosol. 167
Figure 4.2.1. Translocation of non-canonical peptides. 170
Figure 4.2.2. Translocation of cyclic peptides. 172
Figure 4.2.3. Translocation of small molecules. 175
Figure 4.2.4. Translocation of C-terminally biotinylated cargo. 178
Figure 4.6.1. Cyclization of L-linear peptide using native chemical ligation. 196
Figure 4.6.2. Synthesis of doxorubicin-maleimide and docetaxel-maleimide. 197
Figure 4.6.3. Western blot of total extraction of LDn1-8. 198
Figure 4.6.4. Western blot of total extraction of LDn9- 11. 199
Figure 5.2.1. Intracellular stability was monitored for X-LFN-DTA constructs delivered through
protective antigen pore. 210
Figure 5.2.2. One N-terminal D-amino acid on LFN-DTA enhances protein stability. 213
Figure 5.2.3. One N-terminal D-amino acid prevents ubiquitination of LFN-DTA. 215
Figure 5.2.5. Precision delivery of stabilized RRSPc2 through EGFR into pancreatic cancer cells
interrupts the MAPK pathway. 221
Figure 5.6.1. Translocation of X-LFN-DTAmUt constructs. 235
Figure 5.6.2. Western blot analysis of X-LFN-DTAmut constructs in CHO-KI cells. 237 Figure 5.6.3. Western blot analysis of X-LFN-DTAmut constructs delivered into HEK-293T or
HeLa cells. 238
Figure 5.6.4. Western blot analysis of sortagged and native X-LFN-DTAmut constructs. 239
Figure 5.6.5. In vitro degradation of X-K(bio)-LFN-DTAmut. 240
Figure 5.6.6. Translocation of X-K(bio)-LFN-DTAmut into CHO-Kl cells. 241 Figure 5.6.7. LFN-C* X-cargo-C is the oxidation product of LFN-C* and X-G5
-cargo-C-Ellman's. 242
Figure 5.6.8. Rate of reduction for 1-X. 243
Figure 5.6.9. Delivery of 1-X. 244
Figure 5.6.10. Translocation of LFN-DTA-RRSPc 2. 245
Figure 5.6.11. SDS-PAGE analysis of in vitro cleavage of KRas by RRSPC2 conjugates. 246 Figure 5.6.12. LC-MS analysis of in vitro cleavage of KRas by RRSPc2 conjugates. 247 Figure 5.6.13. Targeted delivery of LFN-DTA through EGFR. 248 Figure 5.6.14. Quantification of pErkl/2 levels in 3-X-treated AsPC-1 cells. 249
List of Tables
Table 1.5.1. More than 30 different non-native cargos have been delivered into the cytosol of
cells. 41
Table 2.6.1. PCR primers. 96
Table 2.6.2. Observed molecular masses of expressed protein constructs when analyzed by
LC-MS. 97
Table 2.6.3. Isolated yields of sortagging ligations from SrtA* reaction. 98 Table 2.6.4. EC5 0 values of 30-minute protein synthesis inhibition assay. 99
Table 2.6.5. List of variants. 100
Table 3.6.2. MS. Table 3.6.3. Table 3.6.4. Table 3.6.5. Table 4.6.1. Table 4.6.2. Table 4.6.3. Table 5.6.1.
Observed molecular masses of expressed protein constructs when analyzed by
LC-List of variants.
Isolated yields of sortagging ligations from SrtA* reaction. EC50 values of 30-minute protein synthesis inhibition assay.
Peptides used in this investigation. List of variants.
EC50 values of 30-minute protein synthesis inhibition assay.
EC50values for X-LFN-DTAut constructs translocated in CHO-Ki cells.
142 143 144 145 192 193 195 236
Chapter 1: Delivery of Non-Native Cargo into Mammalian Cells
Using Anthrax Lethal Toxin
1.1. Introduction
Pathogenic bacteria often express protein toxins capable of delivering cytotoxic payloads into the cytosol of cells.' The cytotoxic payloads are often referred to as effector proteins and have diverse functionalities in the host cell-protease activity, modification of intracellular substrates, or interruption in cell signaling pathways-for the benefit of the bacterium. One example is anthrax lethal toxin from the gram-positive bacterium, Bacillus anthracis, which has been extensively studied for the past forty years. Thorough biophysical and biochemical analyses
have led to an increased understanding of each component as a discreet protein and together as a macromolecular nanomachine.2 Anthrax lethal toxin has evolved to deliver lethal factor (LF), a cytotoxic protein payload, into the cytosol of mammalian cells (Figure 1.1.1).3 To accomplish this transport, anthrax lethal toxin utilizes a second component called protective antigen (PA), which oligomerizes on the cell surface to form the PA pre-pore. Following endocytosis the PA pre-pore forms a ~-12
A
channel in the endosomal membrane and acts as a conduit for delivery ofLF into the Cytosol.4-6 Once in the cytosol, LF is a Zn protease that cleaves mitogen activated
protein kinase kinases (MAPKK) and causes cell death.7
While native anthrax lethal toxin expressed by B. anthracis continues to be a bioterrorism threat, in the last two decades the toxin has been modified to serve as a delivery platform for the transport of biomolecules. The PA/LFN delivery platform consists of PA and the non-toxic, N-terminal PA-binding domain of LF known as LFN (Figure 1.1.2). The delivery of cargos other than LF (i.e. non-native) such as enzymes, polypeptides comprised of non-natural amino acids, or small molecule drugs has been explored. Fusions of non-native cargos composed of canonical amino acids to LFN have been achieved through recombinant expression. Non-recombinant
methods of ligation like native chemical ligation (NCL) or enzyme-mediated ligation have been utilized to study the translocation of cargos containing non-natural functionalities.
This review provides an in-depth analysis of the PA/LFN delivery platform for the translocation of non-native cargos into the cytosol of cells. Most notably, the PA pore has been used to deliver more than 30 proteins and polypeptides containing natural and non-natural amino acids as well as small molecule drugs. Collectively, these analyses provide insight into the promiscuity of the PA pore and demonstrate its potential for the delivery of bioactive cargos to disrupt intracellular protein-protein interactions or to study biological processes. Furthermore, these studies support the current model of protein translocation through the PA pore and provide insight into design parameters for delivering new cargos efficiently.
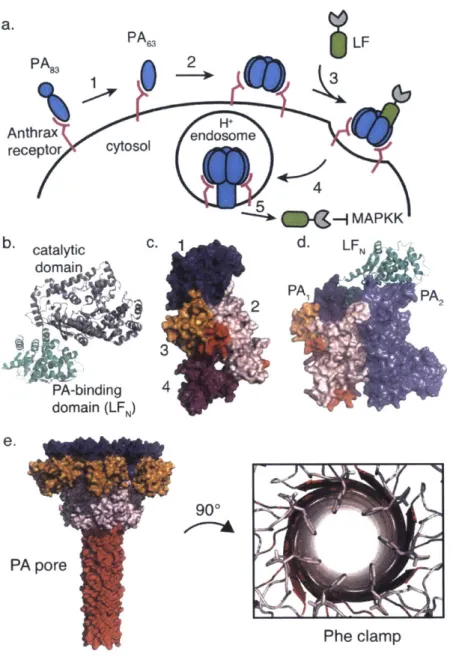
a. PA6 LF PA 8 Anthrax Hnosm receptor cytosol 4 -{MAPKK b. catalytic C. d- LFN domain 2PAI PA2 3 PA-binding 4 domain (LFN) e. 90* PA pore Phe clamp
Figure 1.1.1. Anthrax lethal toxin is comprised of two discreet components. a. Cytosolic delivery via anthrax lethal toxin is achieved by anthrax receptor recognition by PA8 3 then activation to form PA6 3 by a cell-surface furin family protease (1). Seven or eight PA63
molecules self-assemble to form the PA pre-pore (2) then LF binds (3) and the entire complex is endocytosed into the endosome (4). Acidification triggers pore formation and translocation of LF into the cytosol (5). b. Crystal structure of LF reveals two domains (pdb: 1J7N)-PA binding domain (green; LFN) and catalytic domain (gray) c. PA8 3 is composed of 4 domains (blue domain I initiates pre-pore formation, pink domain 2 and orange domain 3 are involved in forming the pore itself, and purple domain 4 is the receptor-binding domain) (pdb: 1ACC) d. LFN binds to two adjacent PA subunits (pdb: 3KWV) e. PA pore structure reveals the restrictive Phe clamp (within domain 2) at the center of the pore (pdb: 3J9C).
cargo
PA
83PA
632LFN
Anthrax
H+
receptor
cytosol
endosome
4
Figure 1.1.2. Translocation of biomolecules using PA/LFN delivery platform. The PA/LFN
delivery platform was developed such that the catalytic domain of LF could be replaced with various cargo molecules to determine their translocation efficiency through PA pore or to analyze their biological function in the cytosol of cells.
1.2. Anthrax Lethal Toxin
Anthrax lethal toxin is a two-component system in which lethal factor (LF; 90 kDa) is transported into the cytosol of a host cell through protective antigen (PA83; 83 kDa) pore.2 After
nearly forty years of mechanistic and structural analyses, a model for protein translocation via anthrax lethal toxin has emerged (Figure 1.1. la). In the presence of a divalent metal ion, PA83 is recognized by and binds to either of two cell surface receptors, tumor endothelial marker 8 or capillary morphogenesis protein 2 (TEM8 or CMG2) with nanomolar or picomolar affinity, respectively.8-'0 The 1000-fold disparity in binding affinity has been attributed to non-conserved
residues of CMG2 that interact with domain 2 of PA." While the exact function of each receptor remains unknown, both TEM8 and CMG8 have been shown to regulate angiogenic processes.1 2'
13 Furthermore, the anthrax receptors are expressed on most human cells at approximately 2,000
- 50,000 receptors per cell.'4
Once PA83 (Figure 1.1.1b) is receptor-bound, a furin family protease proteolytically activates the protein by cleaving the N-terminal 20 kDa portion, leaving PA63 to oligomerize into the PA pre-pore heptamer or octamer.' 15-1" The PA pre-pore is capable of binding up to three or
four molecules of LF (Figure 1.1.1 c) between two adjacent PA6 3 subunits with 1-2 nM affinity.'8'
19 The entire complex is endocytosed and encapsulated in an endosome. Acidification of the
endosome (pH -5.5) results in a conformational rearrangement of the PA63 subunits, which leads to PA pore formation (~12
A
diameter) in the endosomal membrane.20, 21 The pH gradientgenerated between the two compartments leads to translocation of protonated LF into the cell cytosol (pH ~7.0). Translocation through PA pore is considered to be a charge state-dependent Brownian ratchet motion.22'23
Biochemical and biophysical studies have demonstrated that the structural components of anthrax lethal toxin relate to protein translocation. Mutagenesis analyses, kinetic studies, computational models, and a recent crystal structure have revealed how the N-terminal, PA binding domain of LF (1-1 4LF; LFN) interacts with two adjacent subunits of the PA pre-pore.'9'
24-26 Specifically, there are key electrostatic and hydrophobic interactions between the first
a-helix and
P-sheet
of LFN and a deep amphipathic cleft on the surface of PA (alpha clamp) that bind LF such that the N-terminal region of the protein is partially unfolded and poised for translocation from N- to C-terminus (Figure 1.1.1d).19, 27 A recent 2.9A
resolution cryogenicelectron microscopy structure was solved for the PA pore that supports the current model for protein translocation (Figure 1.1.1e).2 The structure confirmed mutagenesis studies that
predicted a narrow ring of solvent-exposed Phe427 residues in the lumen of the channel.29,30 The
ring of Phe residues, called the Phe clamp, is the most restrictive part of the pore and interacts with hydrophobic stretches. The structure also revealed negatively charged residues surrounding the Phe clamp, which are hypothesized to deprotonate the translocated protein and guide unidirectional translocation.
1.3. Fusion and Conjugation Strategies
Prior to the elucidation of the LF and PA structures, researchers utilized protein fusions to explore the translocation mechanism and to analyze the biological function of bioactive payloads inside the cytosol. The earliest work relied on recombinant expression to create fusions with the catalytic domains of select protein toxins; however, recombinant expression is not adequate for probing the delivery of cargos containing non-natural functionalities such as amino acids with inverted chirality, non-canonical side chain residues, or modified backbone structures. To work with cargos containing non-natural amino acids, semisynthetic and enzymatic
techniques like native chemical ligation (NCL) and enzyme-mediated ligation using sortase A (SrtA) have been utilized.
For protein fusions comprised of the 20 canonical amino acids, recombinant expression is often the simplest and most high yielding technique (Figure 1.3.la). However, proteins containing natural amino acids are susceptible to proteolysis and proteasomal degradation in the cytosol. Advantages of incorporating non-natural functionalities into protein fusions include stabilization to intracellular degradation, use of affinity handles, and perturbed binding affinities to target molecules. Pentelute, et al. developed a semisynthetic approach using NCL to ligate non-natural peptides onto the N-terminus of LFN in order to explore the specificity of PA with regard to translocation initiation (Figure 1.3.1 b).3' While NCL facilitates the site-specific ligation of peptides, oftentimes it is performed under denaturing conditions to achieve optimal substrate concentrations. As a result, ligated products must be purified by RP-HPLC, followed by refolding. While LFN has been found to refold well, some cargos may not refold properly, resulting in inactivity.3 1
Enzyme-mediated ligation using SrtA has allowed for the specific attachment of non-natural biomolecules under non-denaturing conditions. The catalytic domain of SrtA from
Staphylococcus aureus has been demonstrated to be useful for in vitro ligations. As a cysteine
protease and transpeptidase, SrtA ligates substrates containing an N-terminal pentaglycine tag onto molecules containing the LPXTG motif (Figure 1.3. 1c). Chen, et al. recently evolved SrtA enzymes to have -50-140-fold increase in LPETG substrate coupling activities such that ligation reactions can be carried out in less than one hour at physiological conditions with high yields.34 SrtA-mediated ligation has been used to attach antibody mimic proteins and peptides containing
non-natural functionalities on the C-terminus of LFN and LFN-DTA as well as mixed chirality peptides to the N-terminus of
LFN-Recombinant expression, NCL, and enzyme-mediated ligation each install a natural, amide linkage between LFN and the cargo of interest. While this is critical for initial analysis of translocation efficiency, there are a variety of other bioconjugation techniques that can be employed to fuse cargos onto LFN- Common examples include the genetic code expansion technique, disulfide linkage, maleimide conjugation, or click chemistry.3 5 Furthermore, combinations of techniques can be used to produce the desired construct. Recently, Ling, et al. demonstrated that SrtA can accommodate peptide thioester substrates, which can be subsequently used for NCL to form protein conjugates separated by non-natural sequences such as LFN-DTA in which the two proteins were joined together by a D-peptide linker.36
a. Recombinant Expression
E. coil
Expression
Expression Eci
vectorW W
b. Native Chemical Ligation
S'R + H2N L 0 AS'R 0 * ; S, R + + H2N 0 + H2N U H2N f
I
Expression E vector E Oil Expression - > vector HS X HS CO2H CO H-SHH NN 'H 0
c. Enzyme-Mediated Ligation
-LPSTGG
-LPSTGG
-LPSTGG
+
ORtA Ca2 SrtA + Gso + G 5 -SrtA +aG24% SrtA*LPSTGG
+
G5--LPSTG5--LPSTG
tLPSTG
5-LPSTG
AFigure 1.3.1. Modular chemistry to modify LFN or LFN-DTA at N- or C-terminus. a.
Recombinant expression in bacteria (e.g. E. coli) serves as a straightforward method to obtain fusion proteins containing canonical amino acids b. A semisynthetic platform using native chemical ligation (NCL) allows for the ligation of N-terminal cysteine biomolecules with C-terminal thioester biomolecules activated using mercaptophenylacetic acid (MPAA) c. Enzyme-mediated ligation using sortase A (SrtA) has been developed for the facile ligation of any cargo
1.4. Methods to Study Translocation
The delivery of protein and peptide cargos with non-natural functionalities has provided insight into the specificity of the PA pore, efficiency of the PA/LFN delivery platform, and a deeper understanding of the mechanism for protein translocation. Translocation efficiency of cargos at the C-terminus of LFN (or LFN-DTA) has been analyzed using three common laboratory assays: planar lipid bilayer,37 protein synthesis inhibition or cytotoxicity analysis based on enzymatic activity, 38, 39 and western blot analysis of the delivered material40. Each approach has provided new insight into key features of translocation.
As an in vitro assay, planar lipid bilayer has been used to measure the change in ion current after LFN constructs have been added to a chamber containing PA pore embedded in an artificial membrane. Electrophysiological measurements from the bilayer experiments have revealed important features of translocation initiation and the mechanism of delivery through the PA pore. Cell-based, enzymatic assays have been developed using reporter protein cargos, providing a sensitive measure of translocation into eukaryotic cells. One specific enzymatic assay relies on the activity of the A chain of diphtheria toxin (DTA), which inactivates elongation factor 2 (EF-2) through ADP ribosylation and inhibits cytosolic protein synthesis.4'
Also known as the protein synthesis inhibition assay, this assay utilizes the activity of DTA to monitor the delivery of assorted cargos fused to LFN-DTA by 3H-Leu incorporation (Figure
1.4.1 a). The Pseudomonas exotoxin A (PE) catalytic domain, which inhibits protein synthesis by a similar mechanism as DTA, has also been used as a reporter protein.41 Furthermore,
cytotoxicity assays using tetrazolium salts like MTT have been used to measure the translocation efficiency of toxic enzymatic domains. Western blot analysis has been utilized as a third method to measure translocation efficiency. After translocation, the cytosolic fraction of cells is lysed
using digitonin, a non-ionic detergent used to permeabilize the cytosolic membrane, then analyzed by western blot (Figure 1.4.1b).42 Immunostaining for LF, DTA, or biotin (if present) provides a semi-quantitative measure of translocated material. Further development of more sensitive assays is underway in order to precisely quantify the amount of material that has been translocated into the cytosol.
a.
c 1.4- 1.2-1.0. 0.8 o-0.6-8
-45 0.4-u- 0.2 0.0--14b.
-a-LF N-DTA -.- LF N-DTA, No PA -13 -12 -1 -1 b -9 -8d -7-Y-Log [Protein Concentration (M)]
PA only PA + LFN
anti-LF
anti-Erkl/2
anti-Rab
Figure 1.4.1. Representative assay results from common translocation assays. a. Protein synthesis inhibition assay measures the activity of DTA fused to LFN after translocation. After incubating cells with LFN-DTA, 3
H-Leu is added to monitor the extent of protein synthesis inhibition caused by DTA. EC50 values of conjugates can be compared with the LFN-DTA
control to determine translocation efficiency. b. Western blot analysis of the cytosolic fraction measures the amount of material delivered into the cytosol. Cytosolic extraction is achieved using a lysis buffer containing digitonin.
1.5. Delivery of Non-Native Cargos 1.5.1. Natural Proteins
Early experiments of protein translocation through PA pore monitored the delivery of protein fusions as a method to characterize the structural requirements of initiating and sustaining translocation. These studies utilized recombinant expression to generate protein fusions of LFN with the A chain of diphtheria toxin (DTA),3 8, 19,4 Pseudomonas exotoxin A
(PE),44 A chain of Shiga toxin (STA), dihydrofolate reductase (DHFR),43 and
P
lactamase (Figure 1.5.1a).45 All together these studies demonstrated that regions near the N-terminus ofLFN are important for initiating translocation into eukaryotic cells.27 The proteins however, must
be unfolded or in an extended conformation for efficient translocation, which was verified by the impeded translocation of DTA containing an artificial disulfide (Figure 1.5.1b) and LFN-DHFR bound to methotrexate.4 3 The efficient translocation of DTA, STA, PE, DHFR, and $
lactamase provided the first indication that the PA pore can accommodate non-native protein cargos. After translocation, the measured enzymatic activity for each protein cargo indicated that these proteins were folded correctly in the cytosol and recognized their intracellular substrates.
Since the development of the PA/LFN delivery platform, various protein cargos have been delivered into cells to understand their biological function. Examples include a cytotoxic T lymphocyte epitope from Listeria monocytogenes listeriolysin 0 (LLO),46' 47 Legionella pneumophila flaggellin protein,48 actin cross-linking domain (ACD) of RTX from Vibrio cholerae,49 Rho inactivation domain (RID) from Vibrio cholerae50 and a Ras/Rapl -specific
endopeptidase (RRSP) from Vibrio vulnificus. Delivery of a cytotoxic T lymphocyte LLO epitope was analyzed for its immune response activity in mice as a potential method for
immune response. In order to study the biochemical and physiological consequences of inflammasome stimulation, von Moltke, et al. delivered a Legionella pneumophila flagellin protein to stimulate the inflammasome and monitored eicosanoid release.48 Satchell and
co-workers have utilized the PA/LFN delivery platform to study the functions of various effector domains from multifunctional autoprocessing repeats in toxin (RTX) domains (MARTX) expressed by pathogenic bacteria. The bioactivity of the actin cross-linking domain (ACD) from
Vibrio cholerae was analyzed after translocation into HEp-2 cells. Cordero, et al. demonstrated
that ACD directly catalyzes the covalent cross-linking of actin providing insight into the mechanism of cell death caused by Vibrio cholerae.49 Sheahan, et al. analyzed the inactivation of Rho GTPases by the Rho Inactivation Domain (RID) from Vibrio cholera after delivery into
HEp-2 cells using the PA/LFN delivery platform.50 The bioactivity of a toxic domain within MARTX from Vibrio vulnificus whose function was previously unknown was characterized using the PA/LFN delivery platform. Antic, et al. delivered the domain into HeLa cells and demonstrated that the Ras/Rapl -specific endopeptidase (RRSP) effector domain cleaves the Switch I region of Ras and Rapi proteins and thus interferes with downstream signaling in the
MAPK pathway.5 1,5 2
1.5.2. Engineered Protein Variants
Antibodies serve as powerful tools for medical diagnostics and the treatment of disease. The use of antibodies, however, is limited to outside the cell due to their inability to cross the plasma membrane into the cytosol and the presence of disulfide crosslinks. Recently, single-domain, cysteine-free scaffold proteins have been developed as antibody mimics. The scaffolds
include monobody from the tenth type III domain of human fibronectin (1OFN3),5 3,54 affibody
the B1 domain of protein G (GBI)." Researchers have recently analyzed the translocation efficiency of these scaffolds, which can be evolved to bind extracellular receptors or intracellular
targets with high affinity.59'60
Liao, et al. recently analyzed the delivery and intracellular activity of select affibody and monobody antibody mimics using the PA/LFN delivery platform.5 9 Specifically, the researchers
analyzed the delivery and associated bioactivity of a tandem monobody designed by Koide and co-workers to bind the Src homology 2 domain (SH2) of Bcr-Abl (HA4-7cl2; KId 12 nM)61,62 and an affibody designed to bind Raf (ABRaf; Kd 100 nM) developed by Nygren and
co-workers.63 The HA4-7c12 tandem monobody conjugate was translocated into chronic myeloid
leukemia cells (K562) and intracellular binding to Bcr-Abl was confirmed by co-immunoprecipitation. The inhibition of Bcr-Abl kinase activity and induction of apoptosis was observed by TUNEL staining, which detects DNA fragmentation by labeling the termini. The ABRaf affibody conjugate was translocated in human embryonic kidney 293T (HEK 293T) cells and the interruption of the mitogen activated protein kinase (MAPK) pathway was monitored. ABRaf was found to significantly reduce the phosphorylation levels of Erkl/2 after activation with epidermal growth factor (EGF). Taken together, the HA4-7c12 and ABRaf delivery data indicate that antibody mimics can be efficiently delivered into the cell cytosol through the PA/LFN delivery platform, refold after translocation, and perturb intracellular PPIs.
The GB1 and DARPin antibody mimics have been shown to translocate efficiently into the cytosol of cells.59 These scaffolds have yet to be delivered into cells using PA pore to perturb PPIs; however, the delivery of DARPin constructs with different thermostabilities has been analyzed. Pl0ckthun and co-workers recently demonstrated that very stable DARPin constructs
(i.e. >90'C melting temperatures) cannot translocate efficiently through the PA pore.60 The researchers addressed the thermostability of DARPin by engineering constructs with reduced stability, amenable for delivery via PA pore. A similar observation was made for 1OFN3 (unpublished results) in which wild-type 1OFN3 (Tm ~88C) translocated less efficiently than HA4, a mutant 1OFN3 construct with Tm~-75'C. Together, these observations indicated that the PA pore requires destabilization of the cargo for efficient translocation.
1.5.3. Stabilized Protein Conjugates
The ubiquitin (Ub)/proteasome system is responsible for the majority of protein degradation in the cell. The N-end rule described by Varshavsky and co-workers states that the N-terminal amino acid of a protein impacts the protein's intracellular stability with regard to proteasomal degradation. Since LFN follows the N-end rule, strategies have been developed to increase cytotoxic activity and decrease immunogenicity of the translocated protein conjugates.64 Leppla and co-workers demonstrated that reductive methylation to dimethylate the epsilon amino group of lysine residues improves cytoxicity of LFN-PE conjugates by stabilizing the proteins to intracellular degradation.65 LFN-PE conjugates including those prone to degradation (i.e. contain N-terminal His residue) were reductively methylated at all 36 lysine residues using borane dimethyl amine and formaldehyde. After translocation into several eukaryotic cell lines, cytotoxicity and western blot assays revealed each methylated conjugate was stabilized to degradation. A disadvantage of this approach is non-specific methylation, which means that for substrates requiring lysine for catalysis or structural integrity, reductive methylation cannot be used for stabilization.
Stabilization of proteins using one N-terminal D-amino acid was recently demonstrated using the PA/LFN system (Chapter 5). Incorporation of N-terminal D-amino acids was achieved through SrtA ligation or NCL for a native N-terminus. Proteasomal degradation of LFN-DTA constructs containing L- or D-amino acids was screened using the protein synthesis inhibition assay as well as western blot analysis. In both assays, while constructs containing N-terminal L-amino acids followed the N-end rule, constructs with N-terminal D-L-amino acids were stabilized to degradation. We demonstrated that a protein containing one N-terminal D-amino acid is not ubiquitinated. In order to prove that this phenomenon was not protein-specific, a hindered disulfide cleavable linker was incorporated and similar observations of protein stabilization were made for DTA, DARPin, and RRSPC2. This work updated the N-end rule to include D-amino
acids as stabilizing amino acids.
1.5.4. Mirror Image Polypeptides
The biological impact of polypeptides containing amino acids with non-natural side chains, backbone composition, mirror image chirality and many others remains relatively unexplored.66 Biomolecules composed of mirror image amino acids are of particular interest for their unique biological stability and reduced immunogenicity. The translocation efficiency of mixed chirality fusions to LFN through PA pore was recently analyzed.67 Protein synthesis inhibition assays indicated that mirror image polypeptides and proteins translocate as efficiently into the cell cytosol as their L-counterparts. Western blot analysis indicated that an unstructured L-peptide cargo on the C-terminus of LFN resulted in rapid degradation of the protein conjugate in the cell cytosol; however, a conjugate containing a D-peptide cargo of the same sequence was found to be stabilized to degradation. Capping the C-terminus with two D-amino acids (D-cap)
stabilized the L-peptide cargo. Evidently, the incorporation of a short D-cap at the C-terminus of an unstructured polypeptide can provide stabilization to intracellular degradation.
Delivery of stable, bioactive cargos into the cell cytosol is an attractive application for translocation. Using the PA/LFN delivery platform, a D-peptide MDM2 antagonist (TAWYANF*EKLLR, where F* is p-CF3-D-Phe)68 was recently delivered into the glioblastoma
U-87 MG cell line, which overexpresses MDM2.67 A biotinylated form of the LFN conjugate (Kd
12.3 4.3 nM) towards MDM2 was found to interact with MDM2 after delivery into U-87 MG cells. Moreover, after delivery into U-87 MG cells, the conjugate was found to disrupt the
p53/MDM2 pathway, as evidenced by upregulation of MDM2, p53, and p21 protein levels. For
the first time, the PA/LFN delivery platform was utilized to deliver a bioactive D-peptide into the cytosol of a eukaryotic cell, where it subsequently bound the target protein and disrupted a critical PPI.
1.5.5. Non-Natural Peptides
The translocation of proteins containing non-natural moieties is of particular interest for their bioactivity, stability, or affinity properties. Conjugates containing peptide cargo with
p-alanine, N-methyl p-alanine, propargylglycine, or 2,4,5-trifluorophenylalanine modifications were found to translocate efficiently into CHO-Ki cells by western blot and protein synthesis inhibition assays.69 Thus, subtle non-natural modifications to the peptide cargo do not affect translocation efficiency through PA pore. The same conjugates with biotinylated C-termini were also found to translocate efficiently, as indicated by co-staining of DTA and streptavidin on the western blot. These results demonstrated efficient translocation of the biotin moiety and provided further support for the delivery of intact conjugates into the cytosol. There are several other
examples of translocating biotinylated cargo, which collectively confirm the efficient translocation of biotinylated protein conjugates and the accessibility of biotin as an affinity
handle.60, 67, 70
1.5.6. Cyclic Peptides
The cyclization of peptides is a useful approach to develop proteolytically stable therapeutics with large surface areas for protein binding.69 The delivery of L and D-forms of a cyclic peptide comprised of 11 amino acids was recently explored using the PA/LFN delivery platform. According to the protein synthesis inhibition assay and western blot analysis, the cyclic peptide conjugates were unable to translocate through the PA pore. Rabideau, et al. hypothesized that the cyclic peptides' constrained conformation and inability to unfold contributed to their inefficient translocation (Figure 1.5.1b). Further investigation is required to fully understand the potential of cyclic peptides as cargo of the PA/LFN delivery platform.
1.5.7. Small Molecule Drugs
To further probe translocation through PA pore, the delivery of small molecule drugs has been explored. Small molecule drugs have diverse properties, functionalities, and three-dimensional structures. The translocation of three common chemotherapeutics, doxorubicin, docetaxel, and monomethyl auristatin F (MMAF) was analyzed by Rabideau, et al.69 Analysis by protein synthesis inhibition assay as well as western blot of the cytosolic fraction indicated that small molecule drugs can translocate through the PA pore, but with limitations. Of the three molecules tested, docetaxel was unable to translocate into the cytosol through PA pore. We hypothesized that constrained or rigid molecules such as docetaxel cannot unfold or adopt a conformation amenable to translocation through the -12
A
pore (Figure 1.5. 1b).1.5.8. Modified Delivery System
The PA/LFN delivery platform has been modified in some cases for the delivery of fluorescent cargos such as small molecule probes or proteins. Using the genetic code expansion technique, Zheng et al. installed an alkynyl-pyrrolysine residue within LF at position K581 then used click chemistry to site-selectively label the protein with AlexaFluor545.7 1 The researchers monitored endocytic trafficking of the labeled protein in BHK fibroblast cells. Zornetta, et al. demonstrated the PA-mediated delivery of GFP into the cytosol fused to LF.7 The delivery of mCherry, however, proved inefficient. The researchers hypothesized that the difference in translocation was due to a higher resistance of mCherry to unfolding. The delivery of fluorescent proteins requires further investigation with respect to the cargo protein's melting temperature and the fate of the chromophore during translocation.
The PA pore is cation-selective, thus favoring the passage of protonated or neutral species. While the N-terminal 28 residues (18 are charged) are critical for initiating translocation, the incorporation of cysteic acid (pKa -1.9) in this region halts translocation (Figure 1.5.1b).73
N-terminal fusions of polycationic stretches (Lys, Arg, His) have been investigated for the delivery of DTA using PA only. Blanke, et al. and Sharma, et al. showed that DTA fused to polycationic residues rather than LFN can be delivered into cells through PA pore.74'75 Wright, et al. recently utilized PA-mediated delivery to investigate the delivery of peptide nucleic acids (PNA).76 Reporter cells containing luciferase transgenes with mutant splice sites were treated with PA and antisense PNA(Lys)8 oligomers, which bind to the mutant splice sites. The researchers
demonstrated that the PNA oligomers were delivered into cells, corrected the splice defect, and induced luciferase expression.
1.5.9. Retargeting PA
Recently, the PA/LFN delivery platform has been modified for the translocation of material into specific cells such as those that overexpress specific receptors. Collier and co-workers retargeted PA to recognize non-native receptors such as HER2 and EGFR by mutating two key residues responsible for binding the anthrax receptors and adding a new targeting domain to the C-terminus of PA.77~79 Retargeting PA to cells that overexpress specific receptors
is a method to increase the amount of material delivered into the cell. Investigations are underway to study the delivery efficacy and bioactivity of delivered non-native cargos using retargeted PA proteins.
a. Efficient translocating cargos
LF N L Affibody LFN- LFN D-Affibody DARPin DTALFL LI LFJ
GB1 N PE LFN O ® JONH, LFN a k f r p d s n v r G ONH2 HN-NH N ~ H N LFN LFN D-GB1 LFN-QOjK (-CONH, S o01y HO 1OFN3 (HA4) Q = L-amino acid - = D-amino acidb. Inefficient translocating cargos
LFN. F S
LFN
7q, V
GR
DTA with N58C+S146C disulfide fl-c.
IK E~~S5 0K ., K NK I , 0 LF N-Gi(S3(D(PCONH, S 0 0 ~YONO H yz *0& OH
Figure 1.5.1. Translocation efficiency has been analyzed for various peptides and proteins.
a. Representative protein and peptide cargos that translocated efficiently through the PA pore include DTA, PE, affibody (L and D forms), GB1 (L and D forms), DARPin, HA4, AKFRPDSNVRG peptide (L and D forms), biotinylated AKFRPDSNVRG peptide, and a doxorubicin-peptide conjugate b. Representative protein and peptide cargos that did not translocate efficiently (gray box) through the PA pore include DTA containing N58C+S146S disulfide, AKFRPDSNVRG cyclic peptide, docetaxel-peptide conjugate, and peptide containing three cysteic acid residues.
Table 1.5.1. More than 30 different non-native cargos have been delivered into the cytosol of cells. Cargos range from proteins comprised of natural or non-natural amino acids, peptides
with different functionalities, and small molecules. The cargos that could not be efficiently translocated by the PA/LFN delivery platform are highlighted in gray.
Cell Type Fusion and Citation
Non-Native Cargo conjugation strategies
Pseudomonas exotoxin A (PE) CHO Recombinant
Diphtheria toxin, A chain (DTA) CHO-KI, RAW264.7, Recombinant or Wn-,47
MC3T3, RBL-1, VERO, enzymatic or NCL
L6
A chain of Shiga toxin (STA) CHO-KI Recombinant
Listeriolysin 0 epitope (LLO) Mouse, P815 (H-2d) Recombinant
46,47
0 lactamase Mouse spleen, CHO, HeLa Recombinant
Dihydrofolate reductase (DHFR) CHO-KI, L6 Recombinant
Actin cross-linking domain (ACD) of RTX from Vibrio HEp-2 Recombinant 49
cholerae
Legionella pneumophila flagellin protein Mouse Recombinant
Rho Inactivation Domain (RID) HEp-2 Recombinant
Reductively methylated PE HN6 Recombinant, chemical 8
Green fluorescent protein (GFP) (at C-terminus of LF) BHK Recombinant _ _
KEKEKNKDENKRKDEER (ligated to N-terminus of LFN) CHO-KI NCL
Ras/Rap I-specific endopeptidase (RRSP) HeLa, HCT 116, MDA- Recombinant
MB-231, HEK 293T
Cytolethal distending toxin B (CdtB) RAW264.7, CHO-KI, Recombinant, NHS
HeLa, HN6 bioconjugation
AlexaFluor545 (conjugated to K581 of LF) J774A.1, BHK-21 Site specific click
Affibody CHO-KI, HEK 293T Enzymatic
B 1 domain of protein G (GB 1) CHO-KI Enzymatic 59
Tenth human fibronectin type three domain (IOFN3) CHO-KI Enzymatic __
Designed ankyrin repeats protein (DARPin) CHO-KI Enzymatic or
Recombinant
HA4-7c12 tandem monobody K562 Enzymatic 59
AKFRPDSNVRG peptide CHO-KI Enzymatic
akfrpdsnvrG (all D) peptide CHO-KI Enzymatic
AKFRPDSNvrG (Dcap) peptide CHO-KI Enzymatic 67
tawyanf*ekllr (all D; P is p-CF3-D-Phe) peptide U-87 MG Enzymatic
Affibody (all D) CHO-KI Enzymatic
GBl (all D) CHO-KI Enzymatic
Biotinylated affibody (all D) CHO-KI Enzymatic, click
[D-AlaKFRPDSNVRG peptide CHO-KI Enzymatic 69
[N-Me-Ala]KFRPDSNVRG peptide CHO-KI Enzymatic
[Prop-Gly]KFRPDSNVRG peptide CHO-KI Enzymatic
AK[F3-Phe]RPDSNVRG peptide CHO-Ki Enzymatic
69
AK(Cys)FRPDSNVRG peptide CHO-KI Enzymatic 69
1.6. Summary and Outlook
The PA/LFN delivery platform permits the facile delivery of biomolecules with diverse structures and functionalities into the cytosol of eukaryotic cells (Figure 1.5.1 and Table 1.5.1). The PA pore is relatively promiscuous for the delivery of non-native cargo on the C-terminus of LFN. Analyses by several groups have demonstrated that once translocation is initiated by LFN, there are a few guidelines that cargos must follow in order to gain efficient cytosolic entry through the PA pore. First, the cargo must be able to adopt an unfolded or extended conformation in the endosome. Second, non-natural moieties such as non-natural backbone structures and side chain modifications like mirror image or modified amino acids do not disrupt the translocation process. Third, cargos containing moieties with low pKa values that cannot be protonated in the endosome may inhibit translocation. Taken together, these design principles can be employed for the delivery of previously unexplored cargos such as oligonucleotides or post-translationally modified proteins.
There are several questions that remain unanswered regarding the PA/LFN delivery platform. The amount of material delivered into the cytosol varies based on the cell type (i.e. number of anthrax receptors expressed), incubation time (i.e. rate of endocytosis and receptor recycling), concentration of PA and LFN constructs, and translocation efficiency of the LFN construct. While western blot analysis provides a semi-quantitative analysis of the amount of material delivered into the cytosol, a more accurate and sensitive method will provide researchers with a better glimpse of translocation efficiency and a more precise measure of the amount of material delivered into the cytosol. Furthermore, exploration into the in vivo effects of the PA/LFN delivery platform will provide insight into the platform's efficacy and possible immunogenicity in multicellular organisms.