Adherent-invasive Escherichia coli isolated from
Crohn’s disease patients induce granulomas in vitro
Sonia Meconi,1Alain Vercellone,1Florence Levillain,1 Bruno Payré,2Talal Al Saati,3Florence Capilla,3 Pierre Desreumaux,4Arlette Darfeuille-Michaud5 and Frédéric Altare1*
1Department ‘Molecular Mechanisms of Mycobacterial Infections’, IPBS, CNRS UMR5089, Toulouse, 31077, France.
2Electron Microscopy Department, Rangueil Hospital Medical School, Toulouse, France.
3Plateforme d’Histopathologie Expérimentale, INSERM IFR30, Toulouse, France.
4Unité INSERM U795 ‘Physiopathologie des maladies inflammatoires de l’intestin’, Lille, France.
5Univ Clermont 1, Pathogénie Bactérienne Intestinale, USC INRA 2018, Clermont-Ferrand, France.
Summary
Adherent-invasive Escherichia coli (AIEC) have been shown to be highly associated with ileal Crohn’s disease (CD). AIEC survive within infected macroph-ages, residing within the phagolysosomal compart-ment where they take advantage of the low pH to replicate extensively. We investigated whether, like the tuberculous bacillus which also persists within macrophages, AIEC LF82 induces the formation of granulomas, which are a common histopathological feature of CD. For this purpose, we have taken advan-tage of an in vitro model of human granulomas that we recently developed, based on blood-derived mononuclear cells. We demonstrated that AIEC LF82 induces aggregation of infected macrophages, fusion of some of them to form multinucleated giant cells and subsequent recruitment of lymphocytes. Light microscopy and scanning electron microscopy analy-sis of the cell aggregates confirmed their granuloma features. This was further confirmed by histological analysis of granuloma sections. Noteworthy, this phenomenon can be reproduced by soluble protein extracts of AIEC LF82 coated onto beads. Although the cell aggregates not completely mimic natural
CD-associated granulomas, they are very similar to early stages of epithelioid granulomas.
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease, characterized by a widespread granulomatous inflammation of the intestine. The aetiology of this disease still remains elusive, but a combination of three comple-mentary factors: genetic susceptibility, intestinal bacteria and uncontrolled tissue injury, is now recognized to be necessary for the onset and progression of this condition (Shanahan, 2002). The role of infectious agents has long been suspected, as one of the main histological charac-teristics of CD is the presence of aphthous ulcers of the mucosa, as well as epithelioid granulomas. Such granu-lomas are found associated with several infectious dis-eases involving Mycobacterium tuberculosis, Salmonella spp., Shigella spp., Yersinia enterocolitica and other pathogenic bacteria able to enter and survive within host cells (Zumla and James, 1996). Like tuberculous granu-lomas, well-circumscribed granulomas develop during CD by the accumulation of lymphocytes and macrophages, the latter maturing to form epithelioid cells (EC).
Epithelioid cells are differentiated macrophages char-acterized by a large usually oval, pale, vesicular nucleus with a clearly visible nuclear membrane. Their cytoplasm is abundant, ill-defined and slightly eosinophilic (Adams, 1974). Their function relative to that of macrophages is not completely understood. Depending on authors, EC were either described to be professional phagocytes (Cohn, 1968) or to be highly secretory cells (Papadimitriou and Spector, 1971). A more recent study of mycobacterial granulomas in the zebrafish, has shown that both types of EC may exist within granulomas in similar numbers (Bouley et al., 2001). They are now usually considered to represent the differentiation of macrophages to a new state, which is non-phagocytic and with interlocking cell membrane pseudopods and secretory functions.
Crohn’s disease patients show clinical improvement fol-lowing antibiotic therapy which decreases the luminal bacteria concentration, and this is consistent with the involvement of luminal bacteria in the physiopathology of CD (Colombel et al., 1999; Castiglione et al., 2003; Kruis, 2004; Elliott et al., 2005; Rutgeerts et al., 2005; Prantera
et al., 2006). The bacteria suspected to be involved in the
Received 18 August, 2006; revised 20 October, 2006; accepted 2 November, 2006. *For correspondence. E-mail frederic.altare@ ipbs.fr; Tel. (+33) 561 175 463; Fax (+33) 561 175 994.
onset of the disease are Mycobacterium paratuberculosis and pathogenic Escherichia coli. Conflicting results have been reported concerning M. paratuberculosis (Gaya
et al., 2004; Huggett et al., 2004). Some authors showed
that these bacteria can be isolated from tissues of patients with CD (Chiodini et al., 1984), but studies using immu-nohistochemistry detection or polymerase chain reaction techniques disagree over its aetiological role (Sanderson
et al., 1992; Dell’Isola et al., 1994; Rowbotham et al.,
1995). The identification of Mycobacterium avium ssp.
paratuberculosis in ileo-colonic mucosal biopsies from CD
patients, strengthened the possible implication of these bacteria in the formation of granulomas (Bull et al., 2003). The discrepancies between studies may be because only particular subsets of CD patients have a mycobacterial aetiology.
Escherichia coli, are abnormally prevalent in the ileal
mucosa of CD patients, representing more than 50% of the total number of bacteria (Darfeuille-Michaud et al., 1998). In addition, some E. coli strains from CD patients were recently shown to adhere to, and to invade intestinal epithelial cells, and replicate extensively into macroph-ages in vitro, thus defining a novel pathovar of E. coli, Adherent-invasive E. coli (AIEC) (Boudeau et al., 1999; Glasser et al., 2001). The reference AIEC strain LF82 replicates within macrophages without inducing apoptosis. It resides and replicates within active macroph-age phagolysosomes, and the acidic microenvironment of the phagolysosomes is necessary for their replication. The rapid replication of LF82 bacteria within macroph-ages induces the secretion of very large amounts of TNF-a (Bringer et al., 2006).
Strains related to AIEC LF82 were found to be highly associated with ileal mucosa in CD patients (Darfeuille-Michaud et al., 2004). AIEC were identified in one-third of ileal specimens from CD patients, but only 6% of ileal controls, thus showing the high prevalence of AIEC in CD. AIEC strains were preferentially found in early recurrent lesions after surgery, suggestive of a role in the initiation of the inflammation and not only as secondary invaders. Reactivity to microbial components, including E. coli outer membrane OmpC (Landers et al., 2002), is associated with severe CD characterized by small bowel involve-ment, frequent disease progression, longer disease dura-tion and greater need for intestinal surgery (Arnott et al., 2004). Immunohistological studies have demonstrated the presence of E. coli in macrophages within the lamina
propria and in granulomatous structures (Liu et al., 1995),
and E. coli DNA was detected in 80% of microdissected granulomas from CD patients (Ryan et al., 2004).
It is therefore plausible that AIEC may penetrate the intestinal barrier, resist destruction by macrophages, and induce a strong inflammatory process, culminating with the formation of granulomas.
We therefore investigated whether AIEC induce the for-mation of granulomas, and in particular epithelioid granu-lomas resembling CD-associated granugranu-lomas. We used an in vitro human granuloma model, developed to analyse the formation and maturation of epithelioid granulomas during Mycobacterium tuberculosis infection of human peripheral blood mononuclear cells (PBMC) (Puissegur
et al., 2004). Human PBMCs incubated with live
myco-bacteria, or with mycobacterial antigens-coated synthetic beads, aggregate around the bacilli or the beads, and form complex structures consisting of lymphocytes, mac-rophages, EC and even multinucleated giant cells (MGC), typically found in tuberculous granulomas (Puissegur
et al., 2004). The cellular composition of the cellular
aggregates formed and their structural organization strongly mimics the shape of physiological epithelioid granulomas. This model is therefore a very useful tool to study the physiopathology of the inflammatory structures found in tuberculous and CD lesions.
Results
Adherent-invasive E. coli LF82 induces a granuloma-like aggregation of human PBMCs
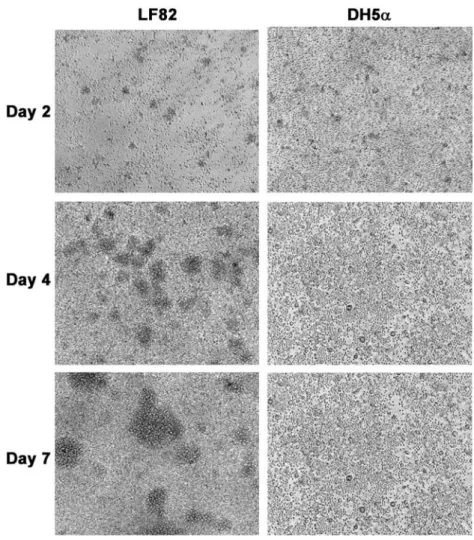
To determine whether the AIEC strain LF82 had the ability to induce a cellular aggregation of human leukocytes, we incubated PBMCs from healthy donors with either DH5a or LF82 E. coli strains. The kinetics of granuloma forma-tion was followed for 7 days, the mean time required to obtain well-circumscribed granuloma-like structures in the mycobacterium model. As shown in Fig. 1, some small cellular aggregates do form for both conditions at day 2 of incubation. These aggregates quickly disappeared before day 5 in DH5a-stimulated PBMCs, but they kept on growing in wells containing strain LF82, and had devel-oped into very large multilayer cellular structures by day 7. Thus, strain LF82-induced cellular aggregation of PBMCs. However, light microscopy analysis was unable to provide a fine characterization of the aggregates, thus precluding their classification as epithelioid granulomas, like those found in CD patients.
Adherent-invasive E. coli LF82 induces the recruitment of highly activated macrophages
We used scanning electron microscopy (SEM) to deter-mine whether the cell aggregates induced by strain LF82 were granulomas, or simply lymphocyte aggregates due to local E. coli-induced lymphocyte proliferation. After 9 days of incubation with AIEC LF82 bacteria, cell aggre-gates were collected under a light microscope, and prepared for SEM. Figure 2 shows two pictures of a representative cellular aggregate. The structures were
mainly composed of macrophages, with some lympho-cytes (arrow) in the periphery (left panel). The recruited macrophages had numerous villosities formed by their cell membrane (right panel), and thus appeared highly activated. The AIEC LF82-induced cellular aggregation
mainly involved macrophages, with some lymphocytes at the periphery of the structure. We next investigated whether the macrophages were recruited around other
E. coli-containing macrophages, or whether factors secreted by LF82 were indirectly responsible for the
Fig. 1. AIEC LF82 do induce human PBMC aggregation in vitro. Aliquots of 1¥ 106human PBMCs were incubated for 7 days with AIEC LF82 or DH5a. Representative light microscopy pictures of the culture wells after 2, 4 and 7 days of reaction are presented for each strain. Large multicellullar structures can be seen at day 4 and larger ones on day 7 for LF82-stimulation. Magnification¥200. These images are representative of seven independent experiments with unrelated healthy donors.
Fig. 2. Scanning electron microscopy analysis of an AIEC LF82-induced PBMC aggregate. Day 9 AIEC LF82 aggregates were collected and prepared for SEM analysis. Two pictures of a representative aggregate are shown at two different magnifications. On the left-hand picture (magnification¥1300), aggregates appear to contain both lymphocytes (arrow) and macrophages. A higher magnification of this aggregate (right panel, magnification¥3200) shows the numerous villosities at the macrophage cell surface, evidence of intense activity of these cells. These pictures are representative of three separate experiments with samples from unrelated individuals. Bars represent 5mm.
induction of macrophage activation and subsequent aggregation.
Adherent-invasive E. coli LF82 does mainly colocalize with macrophage aggregates
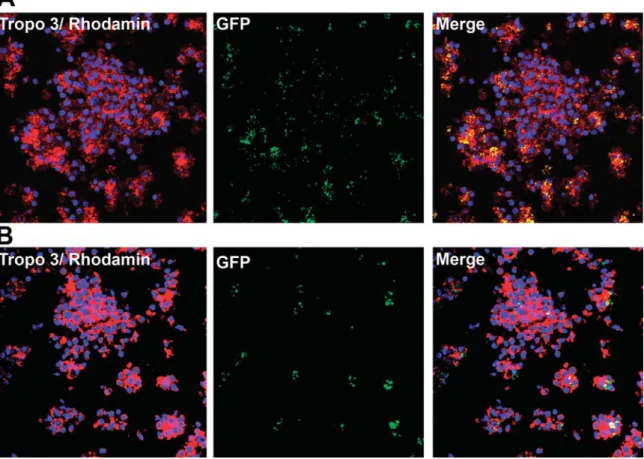
To determine whether macrophages aggregated around AIEC LF82, PBMCs were incubated with green fluores-cent protein (GFP)-expressing LF82 (Fig. 3A). As shown in the merged image, the majority of macrophages contained AIEC LF82 bacilli, and aggregated into small clumps which then seem to gather into a large aggregate. Following infection with very few bacilli per well [multiplic-ity of infection (moi): 10-4] consistent with natural
condi-tions in the gut, GFP-containing cellular aggregates were still visible on day 9 (Fig. 3B). This suggests that AIEC LF82 has a true pro-aggregation ability which is not simply the consequence of a transient hyper-activation of macrophages due to the large number of bacilli used. Interestingly, on day 9, the granulomas appeared more compact than earlier, and the bacilli had not been cleared by the cellular structures. Thus, AIEC LF82 appears to be
able to initiate an inflammatory process, giving rise to granulomas structures.
Adherent-invasive E. coli LF82-induced cell aggregates share features with CD-associated granulomas
To assess whether the LF82-induced cell aggregates do mimic CD patients’ granulomas, we analysed their struc-ture and cell type composition, comparatively to an ex vivo granuloma from a CD patient’s biopsy sample. Thin sec-tions of AIEC LF82-induced cell aggregates were analysed (Fig. 4). Typical EC and lymphocytes (Ly) can be seen within the structure and an MGC can be seen within CD granuloma (Fig. 4A). The thin section from an AIEC LF82-induced cell aggregate shows a majority of macrophages (Mf), surrounded by some lymphocytes (Ly) (Fig. 4B). Neither MGC nor EC were found in any of the sections analysed. Possibly the incubation period used in our in vitro assay was too short to allow the differentiation of recruited macrophages into EC, or alternatively, the environmental conditions of the in vitro model were sufficiently different to those in vivo which allow EC formation in granulomas.
Fig. 3. AIEC LF82 colocalize with cellular aggregates. PBMC aggregates induced by GFP-expressing AIEC LF82 (moi: 102) were collected after 2 h (A) and 9 days (B) of incubation. The aggregates were then stained with Topro-3 (nuclei) and phalloidin/rhodamin (Actin), and analysed by confocal microscopy. The granulomas (Topro3/Rhodamin), the bacteria (GFP) and a merged picture (Merge) are presented after 2H (A) and 9 days (B). This experiment was repeated three times with samples from unrelated healthy donors.
We studied the cell types recruited in more detail. Five aggregates were pooled and the aggregated cells were separated and analysed after May-Grünwald Giemsa (MGG) staining (Fig. 4C). Most cells were macrophages (Mf) or lymphocytes (Ly), but four MGC can be seen on the slide illustrated, three have two nuclei, and the largest (MGC) has around 10 nuclei. Confocal microscopy analy-sis confirmed that these MGC were not cytospin artefacts, but really cells containing several nuclei (Fig. 4D). These cells were rare, less than 1 MGC per granuloma, relative to macrophages and lymphocytes, and this may explain why MGC were not seen in the cross-section of a cell aggregate shown in Fig. 4B. The presence of rare MGC in AIEC LF82-induced aggregates is yet consistent with CD granulomas in vivo.
Adherent-invasive E. coli LF82-extracts can mediate cell aggregation
In the mycobacterial model, in vitro granulomas can be induced either by live mycobacteria, or by mycobacterial extracts-coated artificial beads, implicating the surface
envelope in this phenomenon (Puissegur et al., 2004). CD-associated granulomas strongly resembling myco-bacterial granulomas, we thus tested whether cell surface compounds of AIEC strain LF82 are responsible for the ability of this strain to induce cell aggregation. We produced lysates of AIEC LF82 and E. coli DH5a and used the soluble proteins to coat Sepharose beads, as previously described for mycobacteria (Puissegur et al., 2004). The beads were then incubated with PBMCs for 9 days, and the samples studied by SEM. DH5a lysate-coated beads (DH5a) recruited cells very poorly, whereas AIEC LF82 lysate-coated beads (LF82) induced a very strong cell aggregation (Fig. 5). Interestingly, as with live AIEC LF82, both macrophages (Mf) and lym-phocytes (Ly) were visible in the aggregated structures. The size heterogeneity of the Sepharose beads solution used (mean size of 70mm⫾ 30) is clearly visible on the left panel, and accounts for the variations found in the sizes of the LF82 beads-induced aggregates (right panel). Together, this shows that like mycobacteria, AIEC LF82 probably contains, as yet unidentified, virulence factors that are sufficient to induce
macroph-Fig. 4. Histological analysis of AIEC LF82-induced cell aggregates. A representative day 9 cell aggregate induced by AIEC LF82 was collected and prepared for paraffin embedding. A paraffin-embedded biopsy sample from a CD patient and a cell aggregate-containing paraffin blocks were sliced into 5mm thin-sections, and stained with eosine/haematoxylin.
A. Showing a typical CD associated granuloma from the biopsy sample, presenting lymphocytes (Ly), epithelioid cells (EC) and a multinucleated giant cell (MGC). Magnification¥100.
B. Showing a representative cell aggregate in which both lymphocytes (Ly) and macrophages (Mf) are visible. Magnification¥400. C. To evaluate the different cell populations in cell aggregates induced in vitro, five aggregates were collected, pooled and the cells were recovered by cytospin, and stained with May-Grünwald Giemsa. Most cells were lymphocytes or macrophages, but MGC are also visible on the cytospin. Magnification¥200.
D. Confocal microscopy analysis of cytospined granuloma cells stained with Topro 3 (nuclei, blue) and rhodamin-phalloidin (actin, red), confirmed the intracellular localization of the nuclei associated with the MGC. Magnification¥1000. Similar results were obtained in two (B), and five (C) unrelated healthy individuals.
age aggregation, and recruit lymphocytes to the cell aggregates.
Discussion
One of the main histopathological features of CD is the development of granulomatous structures, usually found in the bowel wall and in draining lymph nodes. These granulomas in CD patients strongly resemble epithelioid granulomas found in tuberculous patients (Duchmann and Zeitz, 1999). Although the aetiology of this condition still remains elusive, the presence of such granulomatous structures, also classically found in infectious pathologies caused by Salmonella spp., Yersinia spp., Shigella spp. or
Mycobacteria, strongly supports the involvement of
infec-tious agents in the physiopathology of this disease (Zumla and James, 1996). The presence of granulomas was not associated with the severity or the presentation of the disease (Ramzan et al., 2002). Yet, CD granuloma cells mostly producing pro-inflammatory (Th1 type) cytokines, mainly consisting of IL-12 and IFN-g (Kakazu et al., 1999), it is a good argument for their involvement in the strong Th1-mediated inflammatory reaction typical of this disease.
There are various lines of evidence suggesting that
E. coli is involved in the formation of granulomas: (i) E. coli antigens are present in CD granulomas (Cartun et al., 1993; Liu et al., 1995); (ii) E. coli DNA is detected in
80% of microdissected granulomas from CD patients (Ryan et al., 2004) and (iii) diseases involving
granuloma-tous responses to E. coli have been reported in animals, such as the granulomatous colitis of the Boxer dog or Hjarre’s disease in chicken and turkeys (Hjarre and Wrambly, 1945). Indeed, E. coli was isolated from all granulomas-containing tissues biopsies analysed from Boxer dogs with colitis (van Kruiningen et al., 2005). Mucoid E. coli has been isolated from tuberculoid lesions of the caeca and liver of chicken and turkeys suffering from Hjarre’s disease. Furthermore, intramuscular inocu-lation of pure bacterial cultures or triturated diseased tissues reproduced the disease (Hjarre and Wrambly, 1945; Schofield, 1947; Morishita and Bickford, 1992).
The identification of AIEC strains in abnormal quantity within the ileal mucosa of CD patients (Darfeuille-Michaud
et al., 2004), prompted us to investigate the ability of
these invasive E. coli strains to induce a granulomatous inflammatory response.
We report evidences that the AIEC LF82 strain is able to induce the aggregation of infected human macrophages and that these aggregates recruit surrounding lymphocytes. They are also the site of the formation of MGC, previously described as the result of macrophage fusion (Anderson, 2000). The resulting cell aggregates thus strongly resemble CD-associated granulomas, most of which contain macrophages, and lymphocytes and some also contain MGC. The main difference between the AIEC LF82-induced aggregates and natural CD-associated granulomas is that the macrophages recruited in vitro did not transform into EC, but do in CD granulomas. Two main reasons may be proposed to
Fig. 5. Crude protein extracts of AIEC LF82 are sufficient to trigger PBMC aggregation. Log phase cultures of DH5a or AIEC LF82 were lysed and the soluble protein fraction was used to coat Sepharose beads. The beads were incubated with human PBMCs for 9 days, collected and prepared for SEM. Representative images for beads coated with DH5a (DH5a) and the AIEC LF82 (LF82) extracts are presented. The heterogeneity of the bead sizes is clearly visible on the left panel (large bead, small bead), as well as the presence of rare macrophages (Mf). All the beads coated with LF82 extracts are covered with cell aggregates containing macrophages (Mf) and lymphocytes (Ly). The differences in the sizes of LF82 beads-induced granulomas reflect the bead size heterogeneity. The magnification is indicated. This result is representative of three independent experiments with samples from unrelated healthy donors. Bars represent 50mm.
explain the absence of EC from AIEC LF82-induced granulomas. First, the granulomatous structures cannot be maintained in vitro for more than a couple of weeks. Such a limited period of infection could be too short for the differentiation of macrophages into EC. Second, there may be one or more factors, as yet unidentified, in CD patient’s gut required in addition to AIEC LF82 for the development of mature epithelioid granulomas.
Mutations have been identified in the NOD2-encoding gene in patients with CD, indicating a link between the innate immune response to invasive bacteria and the development of CD (Hugot et al., 2001; Ogura et al., 2001). It is therefore plausible that NOD2 variants could play a role in the formation of granulomas. NOD2 is an intracytoplasmic pattern recognition molecule sensing invading microbes through the detection of muramyl dipeptide (MDP) (Girardin et al., 2003). The expression of functional NOD2 might contribute to the clearance of bac-teria, even those that are virulent like AIEC, from intestinal epithelial cells. However, AIEC can parasitize host mac-rophages due to a dysfunction of the NOD2 innate immune surveillance mechanism and induce the develop-ment of mature epithelioid granulomas. In the context of CD, it is noteworthy that mycobacterial PIMs molecules have been shown to be Toll-like receptor-2 (TLR-2) ligands (Gilleron et al., 2003; Quesniaux et al., 2004). Bacterial peptidoglycan, another TLR-2 ligand, has been shown to interact with the intracellular NOD2 receptor, via its MDP, after degradation by cellular proteases (Girardin
et al., 2003; Inohara et al., 2003). The binding of the MDP
to NOD-2 limits the cell activation process initiated by peptidoglycan binding to TLR-2, thereby tempering the inflammatory response in normal cells (Watanabe et al., 2004). Mutations in the gene encoding NOD-2, identified in cohort studies (Hugot et al., 2001; Ogura et al., 2001), are now generally considered to be involved in the devel-opment of CD by rendering mutated cells unable to down-regulate TLR-2-initiated inflammatory processes. It is plausible that AIEC LF82 contains specific TLR-2 ligands or other TLR ligands, to be identified, able to induce an inflammatory process starting with the aggregation of macrophages: were this the case, AIEC LF82 strain would be an important cause of the disease in CD patients.
Crude protein extracts of AIEC LF82 mediated cell aggregation as well as the live bacilli, whereas protein extracts from the non-pathogenic DH5a, like the live DH5a bacteria themselves did not. This opens possibili-ties for studying the molecular process responsible for this phenomenon. Direct comparison of LF82 versus DH5a antigenic variants may lead to the identification of the bacterial inducer of the granulomatous response. This in turn would have significant implications for the improve-ment of CD therapy. These observations also indicate that cellular receptors specialized in the recognition of
bacte-rial antigens may be directly involved in the granuloma-tous response. A comparable situation has already been described in the mycobacterial model. Lipids of the myco-bacterial envelope play determinant roles in the patho-genesis of tuberculosis, and especially for the formation of granulomas (Karakousis et al., 2004). More than 35 years ago, the glycolipid trehalose-6,6′-dimycolate (TDM) was reported to be a potent granuloma-inducing factor. Intra-venous injection of mice with TDM emulsions in oil was shown to transiently induce the formation of granulomas within 1 week (Bekierkunst, 1968). In addition, phosphatidyl-myo-inositol mannosides (PIMs) have also been shown to induce granuloma formation in mice (Apostolou et al., 1999; Gilleron et al., 2001; Mempel
et al., 2002).
In conclusion, our results demonstrate that AIEC LF82 is able to initiate an inflammatory process in humans, and does so by the induction of the first stages of cell aggregation leading to the formation of granulomatous structures. We also demonstrate that this phenomenon was mediated by antigens specific to LF82. Our findings should serve as the basis for the identification of these antigens, and of the cell receptor they use to induce the inflammatory process. Identifications of these molecules may be the first step in the development of therapeutic strategies to inhibit the granuloma-inducing ability of AIEC LF82.
Experimental procedures Bacteria
Non-pathogenic E. coli K-12 strain DH5a was from ATCC. AIEC strain LF82 was isolated from the intestinal mucosa of a CD patient. All bacteria strains were cultured on liquid Luria–Bertani (LB) medium at 37°C. Single colonies were picked from LB agar Petri dishes and preculture in 5 ml of LB broth (Sigma) for 4 h at 37°C under 250 r.p.m. agitation. Then, 100 ml of LB broth culture medium was seeded with 1 ml of preculture and incubated over-night at 37°C without agitation. The bacteria were recovered by centrifugation at 4000 r.p.m. for 10 min. Bacteria were then resuspended in LB broth with 15% glycerol and stored at-80°C. LF82-GFP strain culture was cultured similarly except that 50 mg ml-1of kanamycin (Sigma) was added to the LB medium
for GFP plasmid selection.
Preparation of sepharose beads coated with bacterial total extract
LF82 bacilli were cultured for 24 h, recovered by centrifugation at 4000 r.p.m. for 10 min, resuspended in PBS and lysed with a Cell Destructor machine (Retch). Bacilli extracts were recovered, fil-tered on 0.2mm filters and the protein concentration was deter-mined by the Bradford method (Bio-Rad). Aliquots corresponding to 1 mg of proteins were lyophilized (Christ Alpha1–2) and stored at-20°C. One-milligram aliquots of total extract of LF82 or DH5a
were diluted in 5 ml of coating buffer (0.1 M NaHCO3, 0.5 M NaCl) and added to 10 mg of CNBr (cyanogen bromide)-activated sepharose beads (Pharmacia). The Sepharose beads had a mean size of 70mm⫾ 30.The mixture was incubated over-night at 4°C with gentle agitation. Sepharose beads were then centrifuged for 10 min at 800 r.p.m. The supernatants were col-lected and their protein concentration determined by Bradford assay. Free CNBr sites on the beads were blocked by a 2 h incubation with 0.1 M Tris-HCl pH 8 and then washed three times in PBS with 3% penicillin-streptomycin, counted and stored at 4°C.
In vitro granuloma formation
Fresh human blood from healthy volunteers was obtained from the Etablissement Français du Sang and was diluted 1/1 (v/v) with RPMI (Gibco-BRL Life Technologies), layered gently onto a ficoll-paque solution (Amersham) and then centrifuged for 40 min at 1800 r.p.m. PBMCs were collected and washed three times in RPMI medium by 10 min centrifugation at 1800 r.p.m. Cells were counted with a Malassez cell and diluted to a concentration of 1¥ 106cells ml-1in RPMI media supplemented with 7.5%
heat-inactivated human AB serum (Sigma). A total of 1¥ 106PBMC
by well were deposited on 24 or 48 wells plates and infected with different bacterial concentrations ranging from 10 to 108bacteria well-1. The plates were then incubated during 2 h
and up to 9 days. For 7- and 9 day incubations, 20mg ml-1
gen-tamicin (Sigma) was added once the cells had aggregated to kill all extracellular bacteria. For PBMC–bead interactions, about 200 beads coated with DH5a or LF82 extract were added to each well.
Determination of AIEC localization
Bacterial localization was determined as follows. Granulomas were formed as described above except that 48-wells plates containing glass slides deposited at the bottom were used. Fluo-rescent LF82-GFP bacteria and PBMC (moi: 100) were added and the plates incubated for 2 h or 9 days. Then the slides were recovered, and the cells were fixed in 3.7% PFA and incubated with the following markers: nuclei staining with TOPRO-3 (Molecular Probes) and F-actin with rhodamin-phalloidin (Sigma). The slides were then prepared with Fluorescent Mounting Medium (Dako) and analysed under a Leica scanning confocal microscope equipped with an argon-krypton laser.
Histology
Day 9 granulomas were collected from the cell culture wells by careful pipetting, and prepared for paraffin-embedding. Granulo-mas prepared in vitro were very much smaller than tissue sec-tions, so fixed granulomas were first embedded within liquefied low-melting point agarose (2%) and then manipulated as tissue sections. Agarose blocs were fixed in 4% formol for 1 h and paraffin-embedded. Five-micrometre sections of the blocs were
deparaffinized and stained with HES, and mounted for
observation.
Alternatively, granulomas were collected, the cells were plated on a glass slide with a cytospin, fixed in cold acetone and stained with MGG.
Scanning electron microscopy
For SEM analysis, the granulomas were rapidly collected under a light microscope at various times and prepared for SEM by fixing in 2% glutaraldehyde in 0.1% phosphate buffer for 4 h. The samples were washed twice in the same buffer, and the granu-lomas were removed, dehydrated in a graded ethanol series, dried by critical point drying with EMSCOPE CPD 750 and coated with gold–palladium for 3 min at 100 Å min-1, and observed with
an S450 SEM (Hitachi) at an accelerating voltage of 15 kV.
Acknowledgements
This work was supported by grants from the Association F. Aupetit (AFA) to S.M., and from the Institut de Recherche des Maladies de l’Appareil Digestif (IRMAD, Astra Zeneca), and the Ministère de la Recherche et de la Technologie to F.A. We thank Claude Darcha, Laboratoire d’histopathologie CHRU, Clermont-Ferrand France, for providing paraffin-embedded intestine biopsy samples from CD patients.
References
Adams, D.O. (1974) The structure of mononuclear phago-cytes differentiating in vivo. I. Sequential fine and histologic studies of the effect of Bacillus Calmette-Guerin (BCG). Am J Pathol 76: 17–48.
Anderson, J.M. (2000) Multinucleated giant cells. Curr Opin Hematol 7: 40–47.
Apostolou, I., Takahama, Y., Belmant, C., Kawano, T., Huerre, M., Marchal, G., et al. (1999) Murine natural killer T (NKT) cells [correction of natural killer cells] contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc Natl Acad Sci USA 96: 5141–5146.
Arnott, I.D., Landers, C.J., Nimmo, E.J., Drummond, H.E., Smith, B.K., Targan, S.R., and Satsangi, J. (2004) Sero-reactivity to microbial components in Crohn’s disease is associated with disease severity and progression, but not NOD2/CARD15 genotype. Am J Gastroenterol 99: 2376– 2384.
Bekierkunst, A. (1968) Acute granulomatous response pro-duced in mice by trehalose-6,6-dimycolate. J Bacteriol 96: 958–961.
Boudeau, J., Glasser, A.L., Masseret, E., Joly, B., and Darfeuille-Michaud, A. (1999) Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn’s disease. Infect Immun 67: 4499–4509. Bouley, D.M., Ghori, N., Mercer, K.L., Falkow, S., and Ramakrishnan, L. (2001) Dynamic nature of host– pathogen interactions in Mycobacterium marinum granulomas. Infection & Immunity 69: 7820–7831. Bringer, M.A., Glasser, A.L., Tung, C.H., Meresse, S., and
Darfeuille-Michaud, A. (2006) The Crohn’s disease-associated adherent-invasive Escherichia coli strain LF82 replicates in mature phagolysosomes within J774 macrophages. Cell Microbiol 8: 471–484.
Bull, T.J., McMinn, E.J., Sidi-Boumedine, K., Skull, A., Durkin, D., Neild, P., et al. (2003) Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals
with and without Crohn’s disease. J Clin Microbiol 41: 2915–2923.
Cartun, R.W., Van Kruiningen, H.J., Pedersen, C.A., and Berman, M.M. (1993) An immunocytochemical search for infectious agents in Crohn’s disease. Mod Pathol 6: 212– 219.
Castiglione, F., Rispo, A., Di Girolamo, E., Cozzolino, A., Manguso, F., Grassia, R., and Mazzacca, G. (2003) Anti-biotic treatment of small bowel bacterial overgrowth in patients with Crohn’s disease. Aliment Pharmacol Ther 18: 1107–1112.
Chiodini, R.J., Van Kruiningen, H.J., Thayer, W.R., Merkal, R.S., and Coutu, J.A. (1984) Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobac-terium species isolated from patients with Crohn’s disease. Dig Dis Sci 29: 1073–1079.
Cohn, Z.A. (1968) The structure and function of monocytes and macrophages. Adv Immunol 9: 163–214.
Colombel, J.F., Lemann, M., Cassagnou, M., Bouhnik, Y., Duclos, B., Dupas, J.L., et al. (1999) A controlled trial com-paring ciprofloxacin with mesalazine for the treatment of active Crohn’s disease. Groupe d’Etudes Therapeutiques des Affections Inflammatoires Digestives (GETAID). Am J Gastroenterol 94: 674–678.
Darfeuille-Michaud, A., Neut, C., Barnich, N., Lederman, E., Di Martino, P., Desreumaux, P., et al. (1998) Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology 115: 1405–1413. Darfeuille-Michaud, A., Boudeau, J., Bulois, P., Neut, C.,
Glasser, A.L., Barnich, N., et al. (2004) High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 127: 412– 421.
Dell’Isola, B., Poyart, C., Goulet, O., Mougenot, J.F., Sadoun-Journo, E., Brousse, N., et al. (1994) Detection of Myco-bacterium paratuberculosis by polymerase chain reaction in children with Crohn’s disease. J Infect Dis 169: 449–451. Duchmann, R., and Zeitz, M. (1999) Crohn’s disease. In Mucosal Immunology. al., P.L.O.e. (ed.). San Diego, CA: Academic Press, pp. 1055–1080.
Elliott, P.R., Moore, G.T., Bell, S.J., and Connell, W.R. (2005) Severe recurrent Crohn’s disease of the ileocolonic anastomosis disappearing completely with antibacterial therapy. Gut 54: 1818–1819.
Gaya, D.R., Black, R.A., and MacKenzie, J.F. (2004) Crohn’s disease and MAP. Lancet 364: 2179.
Gilleron, M., Ronet, C., Mempel, M., Monsarrat, B., Gachelin, G., and Puzo, G. (2001) Acylation state of the phosphati-dylinositol mannosides from Mycobacterium bovis bacillus Calmette Guerin and ability to induce granuloma and recruit natural killer T cells. J Biol Chem 276: 34896– 34904.
Gilleron, M., Quesniaux, V.F., and Puzo, G. (2003) Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus Calmette Guerin and myco-bacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J Biol Chem 278: 29880–29889. Girardin, S.E., Boneca, I.G., Viala, J., Chamaillard, M.,
Labigne, A., Thomas, G., et al. (2003) Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278: 8869–8872.
Glasser, A.L., Boudeau, J., Barnich, N., Perruchot, M.H., Colombel, J.F., and Darfeuille-Michaud, A. (2001) Adher-ent invasive Escherichia coli strains from patiAdher-ents with Crohn’s disease survive and replicate within macrophages without inducing host cell death. Infect Immun 69: 5529– 5537.
Hjarre, A., and Wrambly, G. (1945) Undersokninger over en med specifika granulom forlopande honssjukdom orsakad av mukoida kolibacterier (koligranuloma). Skand Veteri-nartidskr 35: 449–505.
Huggett, J., Dheda, K., Zumla, A., and Rook, G. (2004) Crohn’s disease and MAP. Lancet. 364: 2178; author reply 2178–2179.
Hugot, J.P., Chamaillard, M., Zouali, H., Lesage, S., Cezard, J.P., Belaiche, J., et al. (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411: 599–603.
Inohara, N., Ogura, Y., Fontalba, A., Gutierrez, O., Pons, F., Crespo, J., et al. (2003) Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem 278: 5509–5512. Kakazu, T., Hara, J., Matsumoto, T., Nakamura, S., Oshitani,
N., Arakawa, T., et al. (1999) Type 1 T-helper cell predomi-nance in granulomas of Crohn’s disease. Am J Gastroen-terol 94: 2149–2155.
Karakousis, P.C., Bishai, W.R., and Dorman, S.E. (2004) Mycobacterium tuberculosis cell envelope lipids and the host immune response. Cell Microbiol 6: 105–116. van Kruiningen, H.J., Civco, I., and Cartun, R. (2005) The
comparative importance of E. coli antigen in granuloma-tous colitis of Boxer dogs. APMIS 113: 420–425.
Kruis, W. (2004) Review article: antibiotics and probiotics in inflammatory bowel disease. Aliment Pharmacol Ther 20 (Suppl. 4): 75–78.
Landers, C.J., Cohavy, O., Misra, R., Yang, H., Lin, Y.C., Braun, J., and Targan, S.R. (2002) Selected loss of toler-ance evidenced by Crohn’s disease-associated immune responses to auto- and microbial antigens. Gastroenterol-ogy 123: 689–699.
Liu, Y., van Kruiningen, H.J., West, A.B., Cartun, R.W., Cortot, A., and Colombel, J.F. (1995) Immunocytochemical evidence of Listeria, Escherichia coli, and Streptococcus antigens in Crohn’s disease. Gastroenterology 108: 1396– 1404.
Mempel, M., Ronet, C., Suarez, F., Gilleron, M., Puzo, G., Van Kaer, L., et al. (2002) Natural killer T cells restricted by the monomorphic MHC class 1b, CD1d1 molecules behave like inflammatory cells. J Immunol 168: 365–371. Morishita, T.Y., and Bickford, A.A. (1992) Pyogranulomatous typhlitis and hepatitis of market turkeys. Avian Dis 36: 1070–1075.
Ogura, Y., Bonen, D.K., Inohara, N., Nicolae, D.L., Chen, F.F., Ramos, R., et al. (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411: 603–606.
Papadimitriou, J.M., and Spector, W.G. (1971) The origin, properties and fate of epithelioid cells. J Pathol 105: 187– 203.
Prantera, C., Lochs, H., Campieri, M., Scribano, M.L., Sturniolo, G.C., Castiglione, F., and Cottone, M. (2006) Antibiotic treatment of Crohn’s disease: results of a
multi-centre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther 23: 1117–1125. Puissegur, M.P., Botanch, C., Duteyrat, J.L., Delsol, G.,
Car-atero, C., and Altare, F. (2004) An in vitro dual model of mycobacterial granulomas to investigate the molecular interactions between mycobacteria and human host cells. Cell Microbiol 6: 423–433.
Quesniaux, V.J., Nicolle, D.M., Torres, D., Kremer, L., Guerardel, Y., Nigou, J., et al. (2004) Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by myco-bacterial lipomannans. J Immunol 172: 4425–4434. Ramzan, N.N., Leighton, J.A., Heigh, R.I., and Shapiro, M.S.
(2002) Clinical significance of granuloma in Crohn’s disease. Inflamm Bowel Dis 8: 168–173.
Rowbotham, D.S., Mapstone, N.P., Trejdosiewicz, L.K., Howdle, P.D., and Quirke, P. (1995) Mycobacterium paratuberculosis DNA not detected in Crohn’s disease tissue by fluorescent polymerase chain reaction. Gut 37: 660–667.
Rutgeerts, P., Van Assche, G., Vermeire, S., D’Haens, G., Baert, F., Noman, M., et al. (2005) Ornidazole for
pro-phylaxis of postoperative Crohn’s disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastro-enterology 128: 856–861.
Ryan, P., Kelly, R.G., Lee, G., Collins, J.K., O’Sullivan, G.C., O’Connell, J., and Shanahan, F. (2004) Bacterial DNA within granulomas of patients with Crohn’s disease – detection by laser capture microdissection and PCR. Am J Gastroenterol 99: 1539–1543.
Sanderson, J.D., Moss, M.T., Tizard, M.L., and Hermon-Taylor, J. (1992) Mycobacterium paratuberculosis DNA in Crohn’s disease tissue. Gut 33: 890–896.
Schofield, F. (1947) Hjarre and Wrambly disease in turkeys (Coli-granuloma). Can J Comp Med 11: 141–143. Shanahan, F. (2002) Crohn’s disease. Lancet 359: 62–
69.
Watanabe, T., Kitani, A., Murray, P.J., and Strober, W. (2004) NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol 5: 800–808.
Zumla, A., and James, D.G. (1996) Granulomatous infec-tions: etiology and classification. Clin Infect Dis 23: 146– 158.