doi:10.1093/treephys/tpy093
Research paper
Non-invasive imaging shows no evidence of embolism repair after
drought in tree species of two genera
Brendan Choat
1,4, Markus Nolf
1, Rosana Lopez
1,3, Jennifer M.R. Peters
1, Madeline
R. Carins-Murphy
2, Danielle Creek
1and Timothy J. Brodribb
21Hawkesbury Institute for the Environment, Western Sydney University, Richmond, NSW 2753, Australia;2School of Biological Sciences, University of Tasmania, Hobart, TAS 7001, Australia;3PIAF, Institut National dela Recherche Agronomique, UCA, 63000 Clermont-Ferrand, France;4Corresponding author (b.choat@westernsydney.edu.au) orcid.org/0000-0002-9105-640X
Received May 17, 2018; accepted July 29, 2018; published online August 22, 2018; handling Editor Nathan Phillips
Drought stress can result in significant impairment of the plant hydraulic system via blockage of xylem conduits by gas emboli. Recovery after drought stress is an essential component of plant survival but is still a poorly understood process. In this study, we examined the capacity of woody species from two genera (Eucalyptus and Quercus) to refill embolized xylem vessels during a cycle of drought and recovery. Observations were made on intact plants of Eucalyptus calmudulensis, E. grandis, E. saligna and Quercus palustris using X-ray microtomography. We found no evidence of an effective xylem refilling mechanism in any of the plant species. Despite rehydration and recovery of plant water potential to near pre-drought levels, embolized vessels were not refilled up to 72 h after rewatering. In E. saligna, water droplets accumulated in previously air-filled vessels for a very small per-centage of vessels. However, no instances of complete refilling that would restore embolized vessels to hydraulic function were observed. Our observations suggest that rapid refilling of embolized vessels after drought may not be a wide spread mechanism in woody plants and that embolism formed during drought represents long term cost to the plant hydraulic system.
Keywords: cavitation, embolism, refilling, repair, xylem.
Introduction
The water transport system of terrestrial plants relies on liquid water withstanding tension created in leaves by transpiration (Dixon and Joly 1895,Debenedetti 1996,Tyree and Zimmermann 2002). This tension increases when water is limited, leading to a heightened risk of emboli (gas blockages) forming in xylem con-duits. Embolism reduces the capacity of the xylem hydraulic sys-tem to deliver water to the canopy and can lead to extensive canopy dieback and whole plant mortality (Davis et al. 2002,
Brodribb and Cochard 2009,Nardini et al. 2013,Venturas et al. 2016, Choat et al. 2018). As such, hydraulic vulnerability to embolism formation is a key ecophysiological parameter in deter-mining drought resistance and the probability of mortality events due to drought (Kursar et al. 2009, Choat 2013, Urli et al. 2013). This can be seen in the strong correlation between spe-cies vulnerability to embolism and aridity across environmental
gradients (Brodribb and Hill 1999, Choat et al. 2012, Bourne et al. 2017,Larter et al. 2017).
Recovery after drought is a crucial aspect of plant survival, but the mechanisms by which plants can restore hydraulic capacity are not well understood. Recent work has clearly shown that recovery of leaf gas exchange is closely linked to the magnitude of embolism suffered by a plant during drought (Brodribb et al. 2010, Skelton et al. 2017). Thus, the restoration of hydraulic capacity in the xylem is of paramount importance in determining how rapidly growth and productivity of woody plants recovers after drought. Two principal mechanisms have been proposed that allow plants to restore hydraulic function after drought-induced cavitation (1) refilling of embolized conduits (Salleo et al. 1996,Brodersen et al. 2010) and (2) formation of new xylem conduits (e.g.,Améglio et al. 2002, Brodribb et al. 2010). Embolism repair over short time scales is clearly
advantageous to the plant in that it would allow more rapid recovery from drought stress. Xylem refilling after winter freeze– thaw cycles is well documented in some temperate deciduous species and usually occurs in conjunction with positive root or stem pressures (Sperry et al. 1987, Utsumi et al. 1998,Cobb et al. 2007), which enable the resorption of gas bubbles into the xylem sap (Tyree and Zimmermann 2002). A significant body of work has focused on embolism repair after drought although in this case the mechanisms and dynamics are far less clear (Brodersen and McElrone 2013). A number of papers have pre-sented data that suggests embolism refilling is a routine process that occurs as part of a diurnal cycle (Canny 1997,Zwieniecki and Holbrook 1998, Taneda and Sperry 2008). While some have emphasized the role of root pressure in post drought re fill-ing (Charrier et al. 2016), there are also numerous reports of refilling in the absence of positive pressure (Bucci et al. 2003,
Stiller et al. 2005,Nardini et al. 2008,Zwieniecki et al. 2013,
Chitarra et al. 2014) and sometimes under considerable tension (e.g.,Knipfer et al. 2015a;Trifilo et al. 2015); seeNardini et al. (2017)for a detailed overview. Refilling under tension is difficult to explain in biophysical terms (Zwieniecki and Holbrook 2009) and has remained a controversial aspect of plant biology. Some authors have suggested an osmotically driven mechanism to draw water back into air-filled conduits (Tyree et al. 1999,
Hacke and Sperry 2003, Salleo et al. 2004, Brodersen et al. 2010,Nardini et al. 2011). However, others have questioned whether such a mechanism would allow successful, complete refilling of vessels and the ubiquity of refilling in woody plants (Cochard and Delzon 2013). This alternative paradigm sug-gests that high levels of cavitation are less frequent in nature and that most tree species are not capable of effective embolism repair post drought (Delzon and Cochard 2014). It is supported by some recent work showing that embolism repair did not occur post drought despite recovery in plant water status (Brodribb et al. 2010, Choat et al. 2015, Knipfer et al. 2015b). In this case, the avoidance of embolism becomes particularly important in terms of plant growth and survival, since recovery would depend solely on growth of new xylem tissue. In relation to recovery strategies, growth of new xylem tissue would be expected to incur a significantly higher carbon cost compared with embolism repair. This paradigm is consistent with recent work showing the plants generally close stomata at water poten-tials above those causing any significant embolism (Nolf et al. 2015,Martin-StPaul et al. 2017,Li et al. 2018).
Here, we investigated the capacity for short-term hydraulic recovery by means of conduit refilling in four tree species from two genera (Eucalyptus and Quercus). Eucalyptus is a large genus of evergreen tree species. It is one of the most widely planted genera globally and comprises three quarters of the area covered by native forest within Australia. Quercus palustris is a temperate deciduous species native to the northern hemisphere that is used widely for landscaping and urban greening
purposes. Both Eucalyptus and Quercus contain keystone spe-cies across their native range.
We induced embolism by withholding water from intact, pot-ted plants and monitored changes in embolism for up to 72 h after rewatering using high-resolution imaging by X-ray microto-mography (microCT). Imaging techniques such as microCT offer the advantage of making non-invasive measurements on intact plants, therefore avoiding issues associated with destructive measurements (Choat et al. 2016, 2010). These techniques have provided valuable insights into the processes of embolism formation and repair in a range of woody species (Brodersen et al. 2010,Charrier et al. 2016,Knipfer et al. 2016).
Materials and methods
Plant material
We selected three evergreen diffuse-porous species and one deciduous ring-porous species of varying ages and sizes: Eucalyptus camaldulensis (Dehnh.) and Eucalyptus grandis (W. Hill ex Maiden) (1–2 years old, ~50 cm tall), Eucalyptus saligna (Sm.) (4–5 y, ~2 m tall) and Quercus palustris (Münchh.) (3 y, ~1.5 m tall). Three plants per species were dehydrated in their pots to produce a range of water potentials between−1.7 and −6.3 MPa. For the Eucalyptus species, another 3–5 control plants from the same cohorts were kept well-watered to determine pre-drought native embolism. The experiments were conducted in October 2015 for E. camaldulensis and E. grandis, and March 2017 for E. saligna and Q. palustris.
Refilling experiment
Leaves on each plant were enclosed in plastic bags and alumin-ium foil for at least 30 min to equilibrate to stem xylem water potential (ΨX). Plant stems were scanned using X-ray
microto-mography (microCT) to record their initial embolism level, fol-lowed by stemΨXdetermination on 1–2 bagged leaves with a
pressure chamber (Model 1505D; PMS Instrument Co., Albany, OR, USA). Leaves used for water potential measurements were collected distally to, and at a distance of 20–100 cm from, the scan site. The scan site on the stem was 5–10 cm above the root collar. Scan sites were marked using correctionfluid (Liquid Paper, Newell Australia, Nobel Park, VIC, Australia), which is vis-ible in X-ray imaging. After this initial scan, plants were thor-oughly rewatered and entire plants were covered in humidified plastic bags for at least 10 h to allow for plant rehydration and recovery ofΨX. Plants were then periodically re-scanned at the
same scan site, with concurrentΨX measurement, at 10–20 h
intervals to identify any changes in embolism patterns.
MicroCT imaging and reconstruction
Plant stems were scanned using X-ray microCT at the Imaging and Medical Beamline (IMBL) of the Australian Synchrotron (Melbourne, Australia). Intact plants were positioned in the
beamline using a robotic arm (Kuka, KR1000 Titan). Samples were positioned at a distance of 1 m to the detector and rotated through 180 degrees using continuous rotation with images recorded at 0.1° angle increments. This yielded 1800 projec-tions with additional flat field and dark field images recorded before and after each scan. Exposure time at each angle was 0.45–0.60 s giving a total scan time of 18–23 min. Scans were conducted at an X-ray energy of 30 keV. The scan resolution was 6μm pixel–1for E. camaldulensis and E. grandis, and 10μm pixel–1for E. saligna and Q. palustris. Scan volumes were recon-structed using XLICT Workflow 2015 (CSIRO) using either the Gridrec or, when possible, the FBP (Paganin et al. 2002) recon-struction algorithm, which resulted in better image contrast based on X-ray phase retrieval.
Image analysis
Scan volumes were tilted in ImageJ 1.48 v (Schneider et al. 2012), using the‘Reslice…’ function to ensure vertical sample orientation. The precise scan location was identified in scans of the same plant by comparing patterns of microscopic air bubbles in a correctionfluid marker applied to each stem. Median projec-tions of 11 neighbouring microCT cross-secprojec-tions (110μm) were extracted for improved quantitative image analysis (Nolf et al. 2017). For each resulting median cross section, embolized vessels were identified using the ‘Threshold’ and ‘Analyze Particles’ functions in ImageJ. Then, all scans per plant were overlaid and all embolized vessels (39,110 in total) manually matched to accurately follow not only changes in total embolized vessel counts, but changes in individual vessel status (appearing water-filled or embolized). Finally, the total number of vessels (water- and air-filled) in each scanned stem was determined in one cross section per plant.
Apparent cases of refilling, i.e., vessels appearing first air-, then water-filled in subsequent scans (97 vessels in total), were cross-checked using longitudinal sections of the scan volumes. Vessel contents along the length of the vessel were examined to identify ‘false positives’ where embolized vessels appeared as water-filled in subsequent scans.
Results
Native embolism in the Eucalyptus species was between 3% and 6% of the total number of vessels per cross-section (Figure1; see Table S1 available as Supplementary Data at Tree Physiology Online). Drought treatments induced stemΨXbetween −2.17
and−3.87 MPa, causing between 10.3 ± 8.2% (E. camaldulen-sis) and 45.6 ± 21.7% (E. grandis) of vessels to embolize (Figure 1). Individual plant embolism levels ranged from 0.5% to 82.0% of vessels (see Table S2 available as Supplementary Data at Tree Physiology Online). Embolized vessels were easily
differentiated from water-filled vessel in reconstructed scan images (Figure2).
Following irrigation,ΨXrecovered in all plants except one E.
grandis tree, which was 82% embolized (Figure3). Despite this rehydration, the overall degree of embolism was unchanged or increased slightly during the recovery period (Figure 1). On average, embolism increased by 0.9± 0.3% of the total number of vessels (min:−0.02%, max: 5.4%) after rewatering (Figure2).
Looking at changes in individual vessel status rather than total numbers, a small number of vessels appeared to revert from air-filled to water-air-filled in E. saligna and Q. palustris when comparing stem cross-sections from pre- and post-rewatering (Figure 3; see Table S2 available as Supplementary Data at Tree Physiology Online). This was not observed for E. camaldulensis or E. grandis (Figure3; see Table S2 available as Supplementary Data at Tree Physiology Online). In the analysis of cross-sections, these apparent cases of refilling (<0.5% of the total vessel count per plant) were compensated by additional, cavitated vessels in the total count of embolized vessels (Figures3and4), leading to a net increase in embolism. Longitudinal sections of the scan volumes revealed that only E. saligna showed liquid re-appearing in previously embolized vessels (Figure 5). However, at least some air bubbles remained in all refilling vessels, and we did not observe any complete refilling that would restore hydraulic func-tion within the durafunc-tion of the experiment.
In Q. palustris, instances of refilling appeared to be exclusively false positives based on analysis of longitudinal sections (Figure6). These false positives included vessel junctions or perforation plates that appeared as liquid filled vessels in the transverse plane (Figure6A), small hanging columns of water that remained in ves-sels after cavitation had occurred (Figure 6B), and tyloses (Figure6C). We note that in some cases it is not possible to di ffer-entiate between tyloses and water droplets at the spatial resolution available. We identified tyloses as objects with convex shape rela-tive to vessel walls. In contrast, hanging droplets of water appeared to have convex menisci within vessels, indicating a low contact angle between liquid water and the vessel wall surface (Zwieniecki and Holbrook 2000,Kohonen 2006). Hanging droplets of water were stable between scans many hours apart indicating that they were not a result of an active refilling mechanism.
Discussion
We found embolism repair was effectively absent in four angio-sperm species following drought-induced embolism formation and subsequent irrigation. The total number of embolized ves-sels in each plant either remained unchanged or increased slightly (Figures 1 and 2) even though ΨX recovered quickly
after plant rehydration (Figure 3). These results contrast with previous reports of embolism repair after drought across a range of plant species (Salleo et al. 1996,Canny 1997, Hacke and
Sperry 2003) and add to a growing body of evidence suggest-ing that the majority of woody plants are not capable of embol-ism repair during recovery from drought (Brodribb et al. 2010,
Choat et al. 2015,Cochard et al. 2015,Knipfer et al. 2015b). This is not to say that embolism repair does not occur, for it surely does during the Spring time in many temperate deciduous species that endure freezing winters. However, it does suggest that a vigorous and effective mechanism of embolism repair is uncommon in woody plant species and may be restricted to a few capable of producing significant root pressures throughout the growing season (e.g., grapevine).
Physiological recovery after drought is a crucial process and an important determinant of survival and competitivefitness in terres-trial plants growing in water limited environments. To restore hydraulic function, plants can either grow new xylem (e.g., ring-porous species, in spring;Hacke and Sauter 1996) or repair embolized conduits by dissolving air in the presence of root or stem pressure (Cobb et al. 2007,Knipfer et al. 2015a,Charrier et al. 2016). In plants that do not exhibit root pressure, other mechanisms, based on osmotic gradients (Nardini et al. 2011) and aquaporin expression (Secchi and Zwieniecki 2010), have been suggested to allow refilling at negative pressures. However, this‘novel’ refilling in the absence of positive pressures is the least well explained and most controversial mechanism of hydraulic recovery (Cochard et al. 2015). While some previous studies have found a considerable degree of rapid refilling without root pressure (Martorell et al. 2014), these studies may have been affected by sampling or measurement artefacts such as the exci-sion artefact (Wheeler et al. 2013). This artefact leads to the high-er apparent embolism levels in samples cut from drought-stressed plants (i.e., under tension) compared with embolism levels in
rewatered plants where tension was released prior to sampling (Torres-Ruiz et al. 2015). It is noteworthy that embolism repair in the absence of root pressure has been observed far less fre-quently when non-invasive methods of observation have been employed to study this phenomenon in intact plants (Clearwater and Clark 2003,Choat et al. 2015,Knipfer et al. 2015b), but see
Zwieniecki et al. (2013).
We did not observe evidence of root pressure (xylem sap appearing when plants were detopped) after rewatering our experimental plants. There was no evidence that these species were capable of restoring hydraulic capacity rapidly, suggesting that embolism formed during drought would persist in the xylem until permanent occlusion of the embolized vessels occurred. Thus, some level of dysfunction would exist in the hydraulic sys-tem as a legacy of a given drought’s impact on the plant. This is consistent with the occurrence of‘native embolism’, which is fre-quently observed in plants growing under natural conditions (Kolb and Davis 1994,Vogt 2001). Plant recovery time in terms of growth and leaf gas exchange is closely related to the level of drought-induced embolism, as has been observed previously across a range of woody species (Brodribb et al. 2010,Urli et al. 2013,Li et al. 2016,Skelton et al. 2017). This may range from many months until full recovery in the instance that embolism spreads to a large proportion of the xylem, to a near immediate recovery in case where minor levels of embolism occur.
While we did not observe any complete refilling of embolized vessels which would restore hydraulic function, a very small per-centage of vessels in E. saligna showed water droplets accumu-lating in previously air-filled vessels (Figures 3 and 4;see Table S2 available as Supplementary Data at Tree Physiology Online). This apparent refilling process remained incomplete
Figure 1. Change in embolized vessels and water potential for control, drought and rewatered treatments in Eucalyptus camaldulensis, E. grandis, E. sal-igna and Quercus palustris. The mean stem water potential (MPa) for each treatment is shown above each bar (see Table S1 available as Supplementary Data at Tree Physiology Online). Bars correspond to mean values for each treatment with error bars showing SE (n= 3 plants). Control measurements were not available for Q. palustris.
within the time-frame of the experiment and may or may not lead to actual hydraulic recovery through complete refilling on a longer-term time-scale. This observation suggests the existence of a mechanism for moving water back into air-filled vessels without positive pressures in relatively short time (<72 h), although in this case the mechanism was ineffective in the mag-nitude of refilling. It is also possible that the fluid observed mov-ing back into embolized vessels was not water but rather some form of gel or resin deposited into the vessels as an initial stage of permanent occlusion. Further observations are required to dif-ferentiate between these two possibilities.
In our detailed analysis, we found that single cross-sections can be prone to misrepresenting vessel status in some vessels. In the case of Q. palustris, longitudinal sections of the microCT volumes showed that all suspected cases of refilling were false positives produced by fringe locations; for these particular ves-sels, the cross-section happened to be positioned at a slice where minimal movement along the sample axis would change vessel appearance, e.g., at a vessel juncture where both con-nected vessels are visible side-by-side over a few microns (com-pared to just one vessel in subsequent scans; Figure6A), near the air–water interface of a remaining (and stable) water droplet of an otherwise embolized vessel (Figure6B), or near the edge of tyloses (Figure6C). The overall effect of this artefact on PLC should generally be minor and can be minimized using median projections of multiple slices as described inNolf et al. (2017), but it is important to consider longitudinal sections when investi-gating detail of processes such as refilling of individual vessels.
A recent study demonstrated that X-ray radiation caused dam-age to tissue of sunflower plants during scanning (Savi et al. 2017). Our plants were scanned repeatedly during experiments and there is a possibility that refilling was inhibited by damage to living cells in the xylem. However, we note that repeated scans of grapevine do not alter patterns of refilling observed in woody species such as grapevine (Brodersen et al. 2010,Knipfer et al. 2015a, 2016) and that there were no visible signs of tissue damage on our samples after scanning. In relation to Eucalyptus, refilling in stem xylem was also not observed in a recent study that employed traditional hydraulic measurements (Creek et al. 2018). Thus, based on this evidence it is unlikely that damage to tissue caused by X-ray radiation was responsible for the lack of refilling observed in our experiments. Further comparative experiments are warranted to confirm that X-ray microCT does not alter patterns of refilling during recovery from drought. This may include assessment of damage to living cells in the xylem and phloem after scanning and longer-term experiments asses-sing recovery and refilling in cohorts of plants that have not been scanned multiple times. Finally, further experiments based on field grown material will be important in resolving the uncertainty surrounding embolism refilling and confirming the generality of findings on younger, potted plants.
Conclusions
We observed no short-term hydraulic recovery in tree species of two genera after drought, suggesting that embolism repair is not routine in these species. More studies are clearly required to confirm this finding across a broader taxonomic range and to understand the ecological implications of a paradigm in which hydraulic recovery from drought does not occur rapidly via embolism repair, but rather, more gradually via growth of new xylem tissue. Recovery processes have received far less atten-tion within the field of plant hydraulics compared with the
Figure 2. Transverse slices from microCT volume showing change in embolized vessels for (A, B) control, (C, D) drought and (E, F) rewatered treatments of Eucalyptus saligna. Images represent a time series of a sin-gle stem. Embolized vessels (black) can be easily differentiated from water-filled vessel (darker grey). Virtually no change in the pattern of embolized vessels occurs between drought and rewatered treatments indicating that no refilling has occurred. Blue arrows (B) show the pos-ition of water-filled vessels that become embolized (red arrows, D) dur-ing the drought treatment and remain gas filled after rewatering (red arrows, F). The white material on the lower left surface of the stem is cor-rectionfluid applied as a marker.
Figure 3. Change in embolism and water potential after rewatering in Eucalyptus camaldulensis, E. grandis, E. saligna and Quercus palustris. Differential percentage of embolized vessels (closed symbols; % of total number of vessels per cross-section compared to initial scan at 0 h) and progression of absolute stem water potential (open symbols; MPa) over time after rewatering. Symbols within each panel correspond to individual sample plants (n= 3).
Figure 4. Changes in individual vessel status (water-filled, air-filled) between consecutive scans, following individual vessels over time in Eucalyptus camaldulensis, E. grandis, E. saligna and Quercus palustris after rewatering. Net change in the number of embolized vessels (symbols) between consecu-tive scans of the same xylem cross-sections. Whiskers indicate newly embolized vessels (upper, orange) and vessels that appeared refilled (lower, blue) since the previous scan. Distance below the X-axis indicates number of refilled vessels. Symbols correspond to individual sample plants in Figure2. X-values were minimally adjusted (d= 0.25 h) for overlapping points or whiskers at 9.5 h, 20 h and 36 h for better readability.
progression of physiological events during drought that lead to hydraulic failure and plant mortality. This recovery phase repre-sents a major knowledge gap, particularly with reference to the interaction of recovery in leaf gas exchange and xylem hydraulic function (Resco et al. 2009,Skelton et al. 2017). Further work is also required to clarify how hydraulic injury caused by one drought event may predispose plants to a greater chance of mor-tality in subsequent droughts or other disturbance events (e.g., pest outbreaks). Finally, a general lack of embolism repair in woody plants may provide a tool for study of drought impact on trees; if the level of embolism is preserved as a signal of water stress in plants it may be possible to retrospectively estimate the level of water stress that plants were exposed to during the drought. This would be important in natural drought events, which are often logistically difficult to monitor.
Supplementary Data
Supplementary Data for this article are available at Tree Physiology Online.
Acknowledgments
We thank Daniel Hausermann and Anton Maksimenko at the Imaging and Medical Beamline (IMBL) of the Australian Synchrotron for assistance with the experimental design and technical support, and Robert Skelton for assistance with measurements
Conflict of interest
None declared.
Funding
This research was supported by an ARC Future Fellowship to B.C. (FT130101115), an ARC Discovery proposal to T.J.B. and B.C. (DP170100761), a Marie Curie Fellowship to R.L. (FP7-PEOPLE-2013-IOF-624473), and beamtime allocations of the Australian Synchrotron Imaging and Medical Beamline (IMBL) to B.C. (projects 10009, 11723).
References
Améglio T, Bodet C, Lacointe A, Cochard H (2002) Winter embolism, mechanisms of xylem hydraulic conductivity recovery and springtime growth patterns in walnut and peach trees. Tree Physiol 22: 1211–1220.
Bourne AE, Creek D, Peters JM, Ellsworth DS, Choat B (2017) Species climate range influences hydraulic and stomatal traits in Eucalyptus species. Ann Bot 120:123–133.
Brodersen C, McElrone A (2013) Maintenance of xylem network trans-port capacity: a review of embolism repair in vascular plants. Front Plant Sci 4:108.
Brodersen CR, McElrone AJ, Choat B, Matthews MA, Shackel KA (2010) The dynamics of embolism repair in xylem: in vivo visualizations using high-resolution computed tomography. Plant Physiol 154:1088–1095. Figure 5. Partial refilling of embolized vessels in E. saligna. Longitudinal
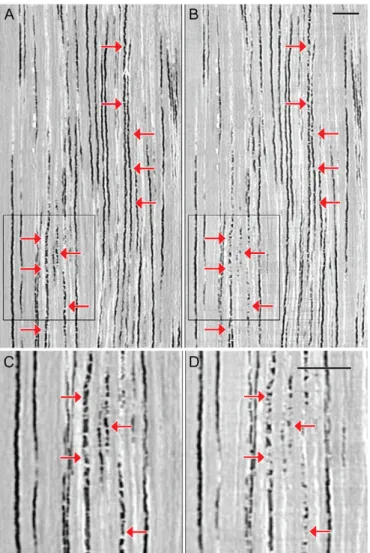
microCT sections recorded before (left) and 36 h after (right) rewater-ing. Overview (upper row) and detail view (lower row). Black boxes indi-cate location of detail crop, arrows indiindi-cate areas of pronounced change between left and right. Scale bar: 1 mm.
Figure 6. Longitudinal sections of false refilling candidates in Q. palustris. (A) Vessel juncture appearing as two embolized vessels in cross-section, and just one embolized vessel above and below (e.g., in a sub-sequent scan). (B) Small water droplets remaining in a vessel. (C) Tyloses formed in vessels after cavitation. Arrows indicate where cross-sections would intersect with vessels to produce apparent cases of refilling.
Brodribb T, Hill RS (1999) The importance of xylem constraints in the distribution of conifer species. New Phytol 143:365–372.
Brodribb TJ, Cochard H (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol 149: 575–584.
Brodribb TJ, Bowman DJMS, Nichols S, Delzon S, Burlett R (2010) Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytol 188:533–542. Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Sternberg LDL (2003)
Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: factors and mechanisms contributing to the refilling of embolized vessels. Plant Cell Environ 26:1633–1645.
Canny MJ (1997) Vessel contents during transpiration– embolisms and refilling. Am J Bot 84:1223–1230.
Charrier G, Torres-Ruiz JM, Badel E et al. (2016) Evidence for hydraulic vulnerability segmentation and lack of xylem refilling under tension. Plant Physiol 172:1657–1668.
Chitarra W, Balestrini R, Vitali M, Pagliarani C, Perrone I, Schubert A, Lovisolo C (2014) Gene expression in vessel-associated cells upon xylem embolism repair in Vitis vinifera L. petioles. Planta 239: 887–899.
Choat B (2013) Predicting thresholds of drought-induced mortality in woody plant species. Tree Physiol 33:669–671.
Choat B, Drayton WM, Brodersen C, Matthews MA, Shackel KA, Wada H, McElrone AJ (2010) Measurement of vulnerability to water stress-induced cavitation in grapevine: a comparison of four techniques applied to a long-vesseled species. Plant Cell Environ 33: 1502–1512.
Choat B, Jansen S, Brodribb TJ et al. (2012) Global convergence in the vulnerability of forests to drought. Nature 491:752–755.
Choat B, Brodersen CR, McElrone AJ (2015) Synchrotron X‐ray microto-mography of xylem embolism in Sequoia sempervirens saplings during cycles of drought and recovery. New Phytol 205:1095–1105. Choat B, Badel E, Burlett R, Delzon S, Cochard H, Jansen S (2016)
Non-invasive measurement of vulnerability to drought induced embolism by X-ray microtomography. Plant Physiol 170:273–282.
Choat B, Brodribb TJ, Brodersen CR, Duursma RA, López R, Medlyn BE (2018) Triggers of tree mortality under drought. Nature 558:531. Clearwater MJ, Clark CJ (2003) In vivo magnetic resonance imaging of
xylem vessel contents in woody lianas. Plant Cell Environ 26: 1205–1214.
Cobb AR, Choat B, Holbrook NM (2007) Dynamics of freeze-thaw embolism in Smilax rotundifolia (smilacaceae). Am J Bot 94:640–649. Cochard H, Delzon S (2013) Hydraulic failure and repair are not routine
in trees. Ann For Sci 70:659–661.
Cochard H, Delzon S, Badel E (2015) X‐ray microtomography (micro‐CT): a reference technology for high‐resolution quantification of xylem embolism in trees. Plant Cell Environ 38:201–206.
Creek D, Blackman C, Brodribb TJ, Choat B, Tissue DT (in review) Coordination between leaf, stem and root hydraulics and gas exchange in three arid-zone angiosperms during severe drought and recovery. Plant Cell and Environ.
Davis SD, Ewers FW, Sperry JS, Portwood KA, Crocker MC, Adams GC (2002) Shoot dieback during prolonged drought in Ceanothus (Rhamnaceae) chaparral of California: a possible case of hydraulic fail-ure. Am J Bot 89:820–828.
Debenedetti PG (1996) Metastable liquids: concepts and principles. Princeton Univ Pr.
Delzon S, Cochard H (2014) Recent advances in tree hydraulics high-light the ecological significance of the hydraulic safety margin. New Phytol 203:355–358.
Dixon HH, Joly J (1895) On the ascent of sap. Philos Trans R Soc Lond B Biol Sci 186:563–576.
Hacke U, Sauter J (1996) Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees. Oecologia 105:435–439.
Hacke UG, Sperry J (2003) Limits to xylem refilling under negative pres-sure in Laurus nobilis and Acer negundo. Plant Cell Environ 26: 303–311.
Knipfer T, Eustis A, Brodersen C, Walker AM, McElrone AJ (2015a) Grapevine species from varied native habitats exhibit differences in embolism formation/repair associated with leaf gas exchange and root pressure. Plant Cell Environ 38:1503–1513.
Knipfer T, Brodersen CR, Zedan A, Kluepfel DA, McElrone AJ (2015b) Patterns of drought-induced embolism formation and spread in living walnut saplings visualized using X-ray microtomography. Tree Physiol 35:744–755.
Knipfer T, Cuneo IF, Brodersen CR, McElrone AJ (2016) In situ visualiza-tion of the dynamics in xylem embolism formavisualiza-tion and removal in the absence of root pressure: a study on excised grapevine stems. Plant Physiol 171:1024–1036.
Kohonen MM (2006) Engineered wettability in tree capillaries. Langmuir 22:3148–3153.
Kolb KJ, Davis SD (1994) Drought tolerance and xylem embolism in co‐ occurring species of coastal sage and chaparral. Ecology 75: 648–659.
Kursar TA, Engelbrecht BMJ, Burke A, Tyree MT, Ei Omari B, Giraldo JP (2009) Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Funct Ecol 23: 93–102.
Larter M, Pfautsch S, Domec J-C, Trueba S, Nagalingum N, Delzon S (2017) Aridity drove the evolution of extreme embolism resistance and the radiation of conifer genus Callitris. New Phytol 215:97–112. Li S, Feifel M, Karimi Z, Schuldt B, Choat B, Jansen S (2016) Leaf gas
exchange performance and the lethal water potential offive European species during drought. Tree Physiol 36:179–192.
Li XM, Blackman CJ, Choat B, Duursma RA, Rymer PD, Medlyn BE, Tissue DT (2018) Tree hydraulic traits are coordinated and strongly linked to climate-of-origin across a rainfall gradient. Plant Cell Environ 41: 646–660.
Martin-StPaul N, Delzon S, Cochard H (2017) Plant resistance to drought depends on timely stomatal closure. Ecol Lett 20:1437–1447. Martorell S, Diaz-Espejo A, Medrano H, Ball MC, Choat B (2014) Rapid
hydraulic recovery in Eucalyptus pauciflora after drought: linkages between stem hydraulics and leaf gas exchange. Plant Cell Environ 37: 617–626.
Nardini A, Ramani M, Gortan E, Salleo S (2008) Vein recovery from embolism occurs under negative pressure in leaves of sunflower (Helianthus annuus). Physiol Plant 133:755–764.
Nardini A, Gullo MAL, Salleo S (2011) Refilling embolized xylem con-duits: is it a matter of phloem unloading? Plant Sci 180:604–611. Nardini A, Battistuzzo M, Savi T (2013) Shoot desiccation and hydraulic
failure in temperate woody angiosperms during an extreme summer drought. New Phytol 200:322–329.
Nardini A, Savi T, Trifilò P, Lo Gullo MA (2017) Drought stress and the recovery from xylem embolism in woody plants. Springer, Berlin, Heidelberg.
Nolf M, Creek D, Duursma R, Holtum J, Mayr S, Choat B (2015) Stem and leaf hydraulic properties are finely coordinated in three tropical rain forest tree species. Plant Cell Environ 38:2652–2661.
Nolf M, Lopez R, Peters JM, Flavel RJ, Koloadin LS, Young IM, Choat B (2017) Visualization of xylem embolism by X‐ray microtomography: a direct test against hydraulic measurements. New Phytol 214:890–898. Paganin D, Mayo SC, Gureyev TE, Miller PR, Wilkins SW (2002)
Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J Microsc 206:33–40.
Resco V, Ewers BE, Sun W, Huxman TE, Weltzin JF, Williams DG (2009) Drought‐induced hydraulic limitations constrain leaf gas exchange recovery after precipitation pulses in the C3 woody legume, Prosopis velutina. New Phytol 181:672–682.
Salleo S, Gullo MAL, De Paoli D, Zippo M (1996) Xylem recovery from cavitation-induced embolism in young plants of Laurus nobilis: a pos-sible mechanism. New Phytol 132:47–56.
Salleo S, Lo Gullo MA, Trifilo P, Nardini A (2004) New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavi-tated stems of Laurus nobilis L. Plant Cell Environ 27:1065–1076. Savi T, Miotto A, Petruzzellis F, Losso A, Pacilè S, Tromba G, Mayr S,
Nardini A (2017) Drought-induced embolism in stems of sunflower: a comparison of in vivo micro-CT observations and destructive hydraulic measurements. Plant Physiol Biochem 120:24–29.
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675.
Secchi F, Zwieniecki MA (2010) Patterns of PIP gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the PIP1 aquaporin subfamily as moderators of refilling process. Plant Cell Environ 33:1285–1297.
Skelton RP, Brodribb TJ, McAdam SA, Mitchell PJ (2017) Gas exchange recovery following natural drought is rapid unless limited by loss of leaf hydraulic conductance: evidence from an evergreen woodland. New Phytol 215:1399–1412.
Sperry JS, Holbrook NM, Zimmermann MH, Tyree MT (1987) Spring fill-ing of xylem vessels in wild grapevine. Plant Physiol 83:414–417. Stiller V, Sperry JS, Lafitte R (2005) Embolized conduits of rice (Oryza
sativa, Poaceae) refill despite negative xylem pressure. Am J Bot 92: 1970–1974.
Taneda H, Sperry JS (2008) A case-study of water transport in co-occurring ring- versus diffuse-porous trees: contrasts in water-status, conducting capacity, cavitation and vessel refilling. Tree Physiol 28: 1641–1651.
Torres-Ruiz JM, Jansen S, Choat B et al. (2015) Direct X-ray microtomo-graphy observation confirms the induction of embolism upon xylem cutting under tension. Plant Physiol 167:40–43.
Trifilo P, Nardini A, Lo Gullo MA, Barbera PM, Savi T, Raimondo F (2015) Diurnal changes in embolism rate in nine dry forest trees: relationships
with species-specific xylem vulnerability, hydraulic strategy and wood traits. Tree Physiol 35:694–705.
Tyree MT, Zimmermann MH (2002) Xylem structure and the ascent of sap, 2nd edn. Springer, Berlin.
Tyree MT, Salleo S, Nardini A, Gullo MAL, Mosca R (1999) Refilling of embolized vessels in young stems of laurel. Do we need a new para-digm? Plant Physiol 120:11–22.
Urli M, Porté AJ, Cochard H, Guengant Y, Burlett R, Delzon S (2013) Xylem embolism threshold for catastrophic hydraulic failure in angio-sperm trees. Tree Physiol 33:672–683.
Utsumi Y, Sano Y, Fujikawa S, Funada R, Ohtani J (1998) Visualization of cavitated vessels in winter and refilled vessels in spring in diffuse-porous trees by cryo-scanning electron microscopy. Plant Physiol 117: 1463–1471.
Venturas M, MacKinnon ED, Dario HL, Jacobsen AL, Pratt RB, Davis SD (2016) Chaparral shrub hydraulic traits, size, and life history types relate to species mortality during California’s historic drought of 2014. PLoS One 11:e0159145.
Vogt UK (2001) Hydraulic vulnerability, vessel refilling, and seasonal courses of stem water potential of Sorbus aucuparia L. and Sambucus nigra L. J Exp Bot 52:1527–1536.
Wheeler JK, Huggett BA, Tofte AN, Rockwell FE, Holbrook NM (2013) Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell Environ 36:1938–1949.
Zwieniecki M, Melcher P, Ahrens E (2013) Analysis of spatial and tem-poral dynamics of xylem refilling in Acer rubrum L. using magnetic res-onance imaging. Front Plant Sci 4:265.
Zwieniecki MA, Holbrook NM (1998) Diurnal variation in xylem hydraulic conductivity in white ash (Fraxinus americana L.), red maple (Acer rubrum L,) and red spruce (Picea rubens Sarg,). Plant Cell Environ 21: 1173–1180.
Zwieniecki MA, Holbrook NM (2000) Bordered pit structure and vessel wall surface properties. Implications for embolism repair. Plant Physiol 123:1015–1020.
Zwieniecki MA, Holbrook NM (2009) Confronting Maxwell’s demon: biophysics of xylem embolism repair. Trends Plant Sci 14: 530–534.